Abstract
Certain non-digestible oligosaccharides (NDO) are specifically fermented by bifidobacteria along the human gastrointestinal tract, selectively favoring their growth and the production of health-promoting metabolites. In the present study, the ability of the probiotic strain Bifidobacterium longum subsp. infantis CECT7210 (herein referred to as B. infantis IM-1®) to utilize a large range of oligosaccharides, or a mixture of oligosaccharides, was investigated. The strain was able to utilize all prebiotics screened. However, galactooligosaccharides (GOS), and GOS-containing mixtures, effectively increased its growth to a higher extent than the other prebiotics. The best synbiotic combination was used to examine the antimicrobial activity against Escherichia coli, Cronobacter sakazakii, Listeria monocytogenes and Clostridium difficile in co-culture experiments. C. difficile was inhibited by the synbiotic, but it failed to inhibit E. coli. Moreover, Cr. sakazakii growth decreased during co-culture with B. infantis IM-1®. Furthermore, adhesion experiments using the intestinal cell line HT29 showed that the strain IM-1® was able to displace some pathogens from the enterocyte layer, especially Cr. sakazakii and Salmonella enterica, and prevented the adhesion of Cr. sakazakii and Shigella sonnei. In conclusion, a new synbiotic (probiotic strain B. infantis IM-1® and GOS) appears to be a potential effective supplement for maintaining infant health. However, further studies are needed to go more deeply into the mechanisms that allow B. infantis IM-1® to compete with enteropathogens.
Keywords: probiotics, prebiotics, synbiotic, Bifidobacterium longum, enteropathogens
1. Introduction
The gastrointestinal microbiota is a complex and dynamic ecosystem that inhabits the human gut from birth, and has an important influence on human health. The gut microbiota and the mucosa themselves act as barriers against invasion by potential pathogens, promoting normal intestinal function [1,2]. The indigenous microbiota prevents bacterial colonization by competing for the adhesion to the epithelium, producing specific antimicrobial compounds such as bacteriocins, and metabolizing specific nutrients towards short chain fatty acid (SCFA) and organic acids to create a restrictive environment, which is generally unfavorable for the growth of many enteric pathogens [3,4]. For this reason, nowadays, an increasing interest in developing functional foods and dietary supplements capable of promoting human health through beneficially modulating the gut ecosystem exists.
Bifidobacteria taxa are predominant in the large bowel (109–1011 CFU/g feces), and they could represent from 3% to 7% of gut microbiota in adults [5,6,7], or even 91% in breastfed babies [8,9,10]. The well-documented health-promoting effects of the intestinal microbiota present in breastfed infants have prompted investigation into dietary approaches capable of establishing a similar microbiota structure, dominated by bifidobacteria, in formula-fed infants [11]. Indeed, it is currently accepted that nutrition and gut microbiota balancing in early life can significantly impact the immune programming development, conditioning health outcomes and the risk of suffering from chronic and inflammatory diseases in the short and long term, as recently reviewed [12]. The administration of probiotics, prebiotics, or synbiotics, which combine probiotics and prebiotics in the same formulation, are some of the dietary strategies capable to program the infant gut microbiota. Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [13], while prebiotics are substrates that are selectively utilized by host microorganisms, conferring a health benefit [14]. Among the substrates meeting the prebiotic definition criteria, non-digestible carbohydrates have been the most studied to date. These include a wide array of oligo- and polysaccharides, such as inulin, fructooligosaccharides (FOS), galactooligosaccharides/transgalactosylatedoligosaccharides (GOS/TOS), xylooligosaccharides (XOS), arabinooligosaccharides (AOS), pectic oligosaccharides (POS) or lactulose-derived galactooligosaccharides (LDGOS), among others. Furthermore, these prebiotics can exert stimulatory effects on the immune system related to the production of some organic acids and increasing of intestinal beneficial bacteria, including bifidobacteria [15,16,17]. FOS and GOS fermentation lead to the production of acetate, butyrate and propionate, compounds that have been extensively investigated for their role in the maintenance of the host-homeostasis and health [18,19,20]. Regarding the use of probiotics, Bifidobacterium strains were found to be effective in inhibiting the growth of different enteropathogens, either through the production of inhibitor compounds, i.e., such as bacteriocins, or organic acids [21,22], through competition for nutrients [23], or through competition for adhesion sites, either using mucus or enterocyte adhesion models [24,25]. However, it has to be taken into account that health-promoting effects of a given probiotic are strain-specific. In addition, synbiotic formulations, including a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host [26], can improve probiotic strains’ performance by enhancing their ability to colonize the human gut [27], or due to their effects on gut microbiota and immune modulation in early life [28].
Bifidobacterium strains originally isolated from breastfed infants have received great attention as potential probiotic strains for formula-fed infants in an attempt to act upon human components to produce beneficial functions/metabolites. Among these, the B. longum subsp. infantis CECT 7210 strain (herein referred to as B. infantis IM-1®), originally isolated from a breastfed infant feces, has previously been reported to confer protection against rotavirus infection both in animal and clinical trials [29,30,31,32], thus being an attractive probiotic strain for formula-fed infants. Indeed, whole genome comparison of B. infantis IM-1® and B. longum subsp. infantis 157F [33], the most closely related strain, identified 340 extra genomic elements in the former that could be responsible for its protective effects against rotavirus [29], although additional analyses to determine the effect of strain 157F against rotavirus should be performed to corroborate the potential relationship between these lacking genes and the protective effects conferred by the strain IM-1®. In this context, the present work aimed at identifying a suitable synbiotic formula for infants feeding by testing the B. infantis IM-1® strain with different combinations of prebiotic carbohydrates. The most attractive synbiotic combination was examined in co-cultures for their ability to inhibit the growth of enteropathogenic bacteria. Furthermore, the capacity of the bifidobacterial strain to compete with, and displace, enteropathogens, using an enterocyte adhesion assay, was also tested.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
B. infantis IM-1®, from Laboratorios Ordesa, and Bifidobacterium animalis subsp. lactis Bb12 were used in the present work. The enteropathogens Escherichia coli LMG2092, Listeria monocytogenes LMG13305, Cronobacter sakazakii LMG5740, Clostridium difficile LMG21717, Salmonella enterica subsp. enterica LMG15860, Yersinia enterocolitica LMG7889 and Shigella sonnei LMG10473 were obtained from the Belgium Coordinated Collection of Microorganisms (BCCMTM; University of Ghent, Belgium). Bifidobacteria were routinely cultured in Man, Rogosa and Sharpe broth (MRS; Merck, Darmstad, Gemany) supplemented with a 0.05% of L-cysteine (Sigma Chemical, St Louis, MO, USA) (MRSc), at 37 °C in anaerobic conditions (10% H2, 10% CO2 and 80% N2) using a chamber Mac 500 (Don Whitley Scientific, West Yorkshire, UK). For bifidobacteria/enteropathogen co-culture experiments, all the pathogens were first grown in brain heart infusion broth (BHI; Merck) at 37 °C in anaerobic conditions. For the adhesion to colonocytes, all the pathogens were grown in Gifu anaerobic medium (GAM; Nissui, Japan) to obtain the cultures for the adhesion experiments. Overall, in order to obtain a standardized culture for the various experiments, the corresponding strains were first streaked onto MRSc (Bifidobacterium) or BHI/GAM (E. coli, L. monocytogenes, Cr. sakazakii, C. difficile, S. enterica, Y. enterocolitica, Sh. sonnei) agar plates which were incubated anaerobically at 37 °C for 1–2 days. Then, a single colony was inoculated to either MRSc or BHI/GAM and grown overnight, under the same conditions. Before the utilization of these cultures for the different assays described below, cells were washed in sterile Ringer solution to prevent the carry-over of residual carbon sources from the overnight media.
2.2. Carbon Source Preferences of the Strain IM-1®
2.2.1. Growth Assays
To test the effects of the prebiotics on growth, several culture media, a wide range of bacterial inoculums and two different methodology approaches were chosen. As a basal medium, Man, Rogosa and Sharpe broth without any carbon source added (MRSF) was supplemented with 0.05% of L-cysteine hydrochloride monohydrate (MRSFc). In addition, two commercial infant formulae in powder, Blemil Plus 1 (LAC(+)) and Blemil Plus SL (LAC(-), lacking lactose) from Ordesa laboratory, were employed. The composition of LAC(+) and LAC(-) formulae are presented in Supplementary Table S1. To each of these media, FOS, FOS:Inulin mixture (50:50) and arabinogalactan were added at a final concentration of 0.8%. MRSFc basal medium and both commercial infant formulae without prebiotic supplementation were used as controls in these experiments. An overnight culture of the probiotic strain was prepared by inoculating MRSc as previously described. Before subculturing cells from this overnight into the three media supplemented with different prebiotics, cells were washed and resuspended in a Ringer solution and then added to the culture medium to a final concentration ranging from 103 to106 CFU/mL, in order to evaluate the effect of the inoculum dose. Samples were taken at 8, 24, 32 and 48 h of incubation to determine the optical density (OD) at 600 nm and/or microbial counts. Microbial counts were determined by performing tenfold serial dilutions in Ringer solution, and spreading them into MRSc agar plates which were incubated 2–3 days anaerobically at 37 °C.
2.2.2. Determination of the Best Prebiotic Oligosaccharide Mixture
Based on preliminary studies, MRSFc (10 mL) was used as a basal medium to examine the growth of B. infantis IM-1® in the absence or presence of an expanded array of prebiotic carbohydrates. The working oligosaccharide mixtures were FOS, FOS:inulin (50:50), FOS:inulin (75:25), FOS:frutalose (50:50), GOS, GOS:FOS (96:04), GOS:FOS:inulin (96:02:02), GOS:frutalose (96:04), frutalose, and frutalose:FOS:inulin (50:25:25). In accordance with previous results, the optimal bacterial inoculum, 106 CFU/mL, was used. Cultures were incubated under controlled anaerobic conditions at 37 °C for a period of 24 h. Growth of the probiotic strain was monitored by measuring the optical density (OD660 nm) at 2 h time intervals.
2.3. Inhibition of Pathogen Growth
MRSFc medium (50 mL) supplemented with the appropriate prebiotic (0.8%), selected based on the results of the test described above, was pre-reduced overnight before utilization and a cell suspension, previously washed with a sterile Ringer solution, of both probiotic and pathogenic (ratio 1:1) strains was inoculated at a final concentration of 106 CFU/mL. In addition, pathogen and bifidobacteria single cultures were conducted in parallel in the same media as co-cultures. Both co-cultures and mono-cultures were incubated anaerobically at 37 °C for 24 h. Then, tenfold dilutions of the mono and co-cultures were prepared with Ringer solution and were spread in duplicate on the following selective media: MRSc (Merck) adjusted to pH 5.4 for Bifidobacterium, clostridium difficile agar (CLO; bioMériux, Marcy l’Etoile, France) for C. difficile, chromogenic listeria agar base (CLAB; Oxoid Ltd., Hampshire, UK) for L. monocytogens and violet red bile glucose agar (VRBGA; Oxoid) for E. coli and Cr. sakazakii. Plates were incubated for 1–2 days at 37 ºC. As an additional effort to improve the selectivity of the media, plates used for pathogen enumerations were aerobically incubated, with the exception of C. difficile, to avoid the growth of bifidobacteria, especially in those samples obtained from co-cultures. Cultures were performed in triplicate for each probiotic, pathogen, or probiotic–pathogen combination.
To assay the evolution of the inhibitory activity during 24 h cultivation, new experiments were performed for those combinations that exhibited anti-pathogenic activity. One milliliter of fermentation MRSFc broth was removed at 0, 4, 8, 12, and 24 h, and was serially tenfold diluted in Ringer solution and spread on appropriate selective agar culture media to monitor the growth and inhibition of the probiotic and pathogenic strains, respectively.
2.4. Pathogen Displacement and Prevention of Pathogen’s Adhesion to Enterocytes
HT29 monolayers were used in the experiments described next, and prepared as previously described [34]. For pathogen displacement, overnight cultures of the enteropathogen strains (C. difficile, Cr. sakazakii, Y. enterocolitica, Sh. sonnei, S. enterica and E. coli) obtained as previously described, were washed twice in sterile phosphate buffered saline (PBS) solution and bacterial cells were resuspended in supplemented McCoy’s medium (10% foetal bovine serum, 3 mM L-glutamine) (MM; Sigma) without antibiotics, in a ratio of 1:10 (bacteria/enterocyte) prior to its addition to the enterocyte monolayer. The mixture was incubated for 1 h at 37 °C/5% CO2 and subsequently the monolayer was washed twice with PBS in order to remove the non-adhered pathogens. Then, an overnight culture of B. infantis IM-1® was washed twice with PBS, added to the monolayer (1:10 ratio) and incubated for 1 h, ending with a final washing step to remove unbound bacteria. Afterwards, the monolayers were trypsinized in order to release the HT29 cells and counts of the adhered bacteria were carried out by performing serial tenfold dilutions in Ringer solution and spreading in CLO plates for C. difficile and in VRBGA plates for the rest of the pathogen strains (Cr. sakazakii, Y. enterocolitica, Sh. sonnei, S. enterica and E. coli). All plates, except for those used for C. difficile counts, were incubated aerobically at 37 °C. Results were expressed as the percentage of bacteria adhered with respect to the bacteria added (% CFU adhered/CFU added). For comparison purposes, adhesion of the enteropathogens in the absence of bifidobacteria was used as reference for data normalization (% pathogen adhesion in the presence of bifidobacteria/pathogen adhesion in the absence of bifidobacteria).
To evaluate the prevention of the adhesion of the enteropathogen by the strain B. infantis IM-1®, a different experimental setup was performed. In this case, the enterocyte monolayer was first treated with the strain B. infantis IM-1®. After 1 h of incubation, non-adhered bifidobacteria were removed and the pathogen was added, incubating the co-culture for 1 h. Following trypsinization, plate counts were carried out for the pathogens by performing serial tenfold dilutions in Ringer solution and using the same selective media and incubation conditions as describe for pathogen displacement assays. Bacteria:enterocyte ratios, bacterial growth conditions and washing procedures were the same as those used for displacement assays.
Two different biological replicates (two independent cultures for each pathogen and probiotic and two independent technical replicates for each culture) were performed. The widely used probiotic B. animalis subsp. lactis Bb12 was included in the adhesion assays for comparison purposes.
2.5. Statistical Analysis
Statistical analysis of the data was performed using the 3.2.5. version of the free R software (The R Foundation, Boston, MA, USA). The differences between single and co-cultures for prebiotic preference and pathogen inhibition data were assessed using Student’s t-test. In adhesion assays, differences in pathogen adhesion among non-bifidobacterial treatment, treatment with B. animalis subsp. lactis Bb12 or treatment with B. infantis IM-1® were assessed using ANOVA tests followed by Tukey’s pairwise mean comparison.
3. Results and Discussion
3.1. Selection of Prebiotic Candidates
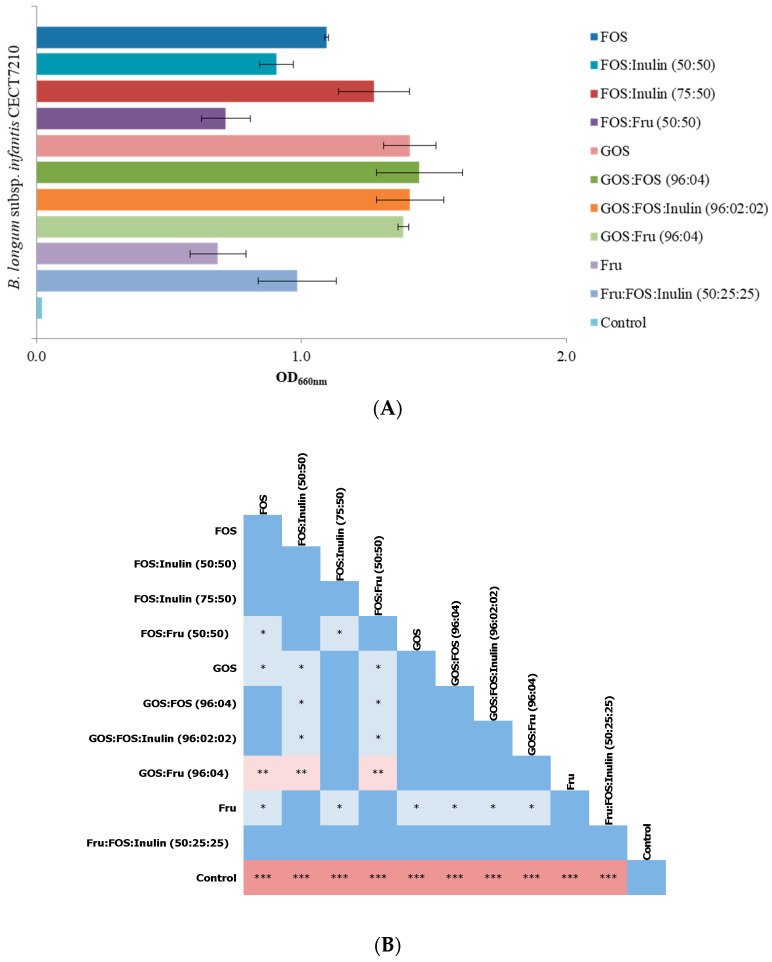
In a preliminary assay, we defined the optimal basal media formulation, dose of bacterial inoculum, and incubation period to achieve consistent and reproducible growth of B. infantis IM-1®, as well as to detect differences in the growth that could be attributed to the fermentation of the different prebiotic oligosaccharides used. For this purpose, prebiotic oligosaccharides (FOS, FOS:Inulin mixture and arabinogalactan) were tested in different media, including MRSFc (nutrient-limited medium) and two infant formulae: one containing lactose (LAC(+)) and one lacking lactose (LAC(−)) (nutrient-rich media). Using the plate count technique, we observed that B. infantis IM-1® grew well in basal medium (MRSFc), and in both infant formulae (LAC(+) and LAC(−)) supplemented with prebiotics. The values of mean log counts were 7.68 ± 0.61 log CFU/mL for MRSFc, 7.96 ± 0.11 log CFU/mL for LAC(+) and 7.40 ± 0.43 log CFU/mL for LAC(−). However, no significant differences in the number of viable cells were observed after 48 h of incubation between all cultures media supplemented with prebiotics and controls (media without prebiotics) (7.71 ± 0.75 log CFU/mL with prebiotics vs. 7.91 ± 0.32 log CFU/mL without prebiotics). Unlike this fact, a great variation on prebiotic effects was detected in the OD levels of B. infantis IM-1® measured in basal media (MRSFc), mainly with FOS and FOS:inulin in the medium, obtaining values ranging from 35 to 56 fold higher than in the non-supplemented MRSFc control medium. The highest differences between the presence or absence of prebiotics were observed at the highest bacterial inoculum concentration tested. Specifically, OD values obtained compared to no prebiotic added, were from 16- to 17-fold higher for arabinogalactan; from 35 to 46 for FOS:Inulin; and from 42 to 56 for FOS, when bacterial inoculum doses were 105 or 106 CFU/mL, respectively. Using lower doses of bacterial inoculum (103 and 104 CFU/mL), growth in MRSFc was not observed either in the absence or presence of prebiotics. In addition, incubations longer than 24 h did not result in an increase in growth of B. infantis IM-1®. In view of these results, we selected MRSFc, a bacterial dose of 106 CFU/mL, and OD600 nm after a 24 h incubation period to monitor bacterial growth in subsequent batch fermentation assays with a variety of prebiotic combinations. In this respect, the highest OD occurred with GOS and all combinations including this oligosaccharide: GOS:FOS, GOS:FOS:inulin and GOS: frutalose (Figure 1). OD of Bifidobacterium cultures also increased in the presence of FOS or FOS:inulin combinations, whereas the lowest growth was achieved in the prebiotic frutalose as a carbon source.
Figure 1.
Effect of prebiotics on the growth of B. infantis IM-1® after 24 h of incubation. (A) Optical density (OD660 nm) of the cultures of the B. infantis IM-1® strain grown in the presence of different prebiotic substrates. The represented data are means of at least three independent replicates. (B) Statistically significant differences in growth of B. infantis IM-1® in the presence of different prebiotics were determined, using Student’s t-test for each pair of substrates tested. Color key: dark blue: p > 0.05; light blue: * p < 0.05; light red: ** p < 0.01; and dark red: *** p. < 0.001. FOS, fructooligosaccharides; GOS, galactooligosaccharides; Fru; Frutalose, and control (MRSFc: Man, Rogosa and Sharpe broth with 0.05% of L-cysteine hydrochloride monohydrate. MRSFc without prebiotic supplementation).
The ability of FOS and GOS, and the mixture of both of them, to enhance the growth of bifidobacteria populations of the colonic microbiota have been previously reported [18,35,36,37]. Several reports showed the beneficial effect of prebiotic carbohydrates on the growth of probiotic strains. However, a great variability in the response of different probiotic strains to different prebiotic carbohydrates exists, suggesting that there is not a universal prebiotic to design synbiotic formulations and highlighting the fact that the ability of probiotic strains to grow in synbiotic combinations with different prebiotics may be strain-specific and must be determined independently.
3.2. B. infantis IM-1® Inhibits Growth of Enteropathogens In Vitro
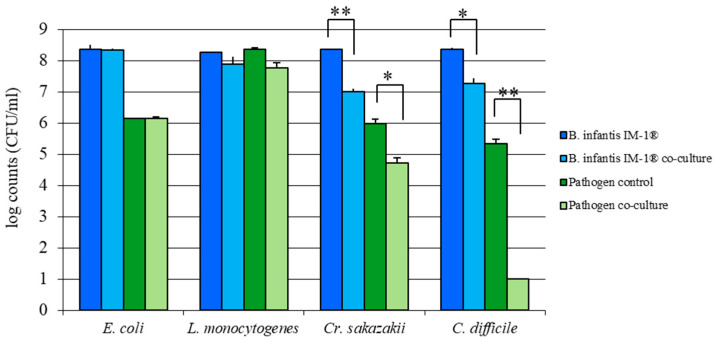
The antimicrobial activity of the strain B. infantis IM-1® against several intestinal pathogens was evaluated in vitro with co-culture assays, and data were collected after 24 h of incubation. B. infantis IM-1® was inoculated simultaneously with the enteropathogens in MRSFc supplemented with GOS (0.8% final concentration). After 24 h, the growth of pathogens and B. infantis IM-1® was determined by plating cultures on selective media and colony counting (see Methods). In co-cultures, the same sample was plated onto selective medium for probiotic strain (MRSc pH 5.4) and also onto selective medium for pathogen (CLO, CLAB or VRBGA depending on pathogen) (see Methods). Under these conditions, the greatest inhibition was observed in the growth of C. difficile during co-culture, so the decrease was of 4–5 log values with respect to the pathogen control culture (pathogenic strain grown in the absence of the bifidobacteria in the same MRSFc supplemented with GOS medium and incubated under identical conditions) (Figure 2). Furthermore, Cr. sakazakii growth was significantly inhibited by 1–2 log values during co-culture with B. infantis IM-1®, compared to single pathogen cultures. By contrast, no significant inhibition of E. coli or L. monocytogenes was exerted by B. infantis IM-1®, with identical microbial counts of these bacteria recovered when grown in mono- and co-cultures.
Figure 2.
Mean log counts (CFU/mL) and standard deviations of enteropathogenic strains (E. coli LMG 2092, L. monocytogenes LMG 13305, Cr. sakazakii LMG 5740 and C. difficile LMG 21717; pathogen co-culture) and B. infantis IM-1® strain (B. infantis IM-1® co-culture) after 24 h of co-culture in the presence of GOS. Single cultures of each bacteria type were also performed using the same media and incubation conditions (B. infantis IM-1® and pathogen control). For each pathogen–bifidobacteria combination, the statistical significance of counts between co-culture and single culture (control) was calculated using Student’s t-test (* p < 0.001, ** p < 0.0001).
B. infantis IM-1® strain has been demonstrated to prevent diarrhea episodes in formula-fed infants and to provide protection against rotavirus infection in various experimental models [30,31,32]. However, its capacity to antagonize other enteropathogens of relevance for infant health had not been explored. Our results demonstrate that B. infantis IM-1® strain is capable of reducing the growth of various enteropathogens in vitro, as previously reported for other bifidobacterial species/strains. For example, isolates belonging to the genera Bifidobacterium have been employed in the treatment of gastrointestinal diseases caused by C. difficile [38,39]. In addition, it has been previously reported that the increase in bifidobacteria populations in the presence of FOS and GOS promoted the inhibition of C. difficile in vitro [40]. Similarly, co-cultures of bifidobacteria with short chain FOS inhibited C. difficile growth [41]. On the other hand, our results are in contrast with the antipathogenic activity against E. coli observed in other species of Bifidobacterium [42,43]. Indeed, several studies have reported the existence of high variability in the antipathogenic activities exerted by different strains belonging to the same genus/species, further supporting the idea that health-promoting and probiotic traits are strain-specific and need to be evaluated individually for every probiotic candidate [44,45,46].
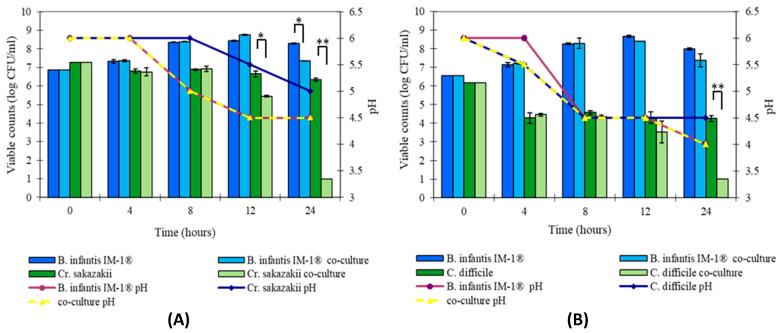
To go deeper into the inhibition activity capability of B. infantis IM-1® against C. difficile and Cr. sakazakii, new assays of co-culture were performed checking the growth decrease over time. Figure 3 represents the microbial counts (log CFU/mL) of the probiotic and pathogenic bacteria in single and co-cultures, as well as pH variations. The effect of antibacterial activity of strain B. infantis IM-1® against C. difficile growth during co-culture appears after 8 h, and no viable cells were detected after 24 h. Meanwhile, Cr. sakazakii growth showed a moderate decrease after 8–12 h in co-culture with B. infantis IM-1®, and a much greater decrease after 24 h.
Figure 3.
Evolution of microbial counts (log CFU/mL) and pH values in single and co-cultures of probiotic strain B. infantis IM-1® and enteropathogens: (A) Cr. sakazakii and (B) C. difficile. For each pathogen–bifidobacteria combination, the statistical significance of counts between co-culture and single culture (control) was calculated using Student’s t-test (* p < 0.001, ** p < 0.0001).
Similar results have been previously described [47] in Lactobacillus acidophilus LB against Salmonella typhimurium SL1344, where antipathogenic activity began after 12 h in culture. This is consistent with some reports that have demonstrated C. difficile inhibition by culture supernatants of B. longum and Bifidobacterium breve strains, an effect that was dependent on the prebiotic substrates used to grow bifidobacteria [41]. Indeed, some works have indicated that Bifidobacterium strains can be used in the treatment of Clostridium-associated diarrhea [48,49]. The selection of Bifidobacterium strains able to inhibit clostridia growth is therefore important for the development of probiotic products targeted to prevent gut colonization by C. difficile. However, the specific mechanisms responsible for the pathogen growth inhibition observed in pathogen co-cultures with Bifidobacterium strains remain to be elucidated. A possible effect of pH decrease or the production of antimicrobial substances active against pathogenic bacteria, as demonstrated for other species/strains [50], cannot be ruled out and deserves further attention.
3.3. Capability of Probiotic Strain B. Infantis IM-1® to Modify the Adhesion of Enteropathogens to HT29
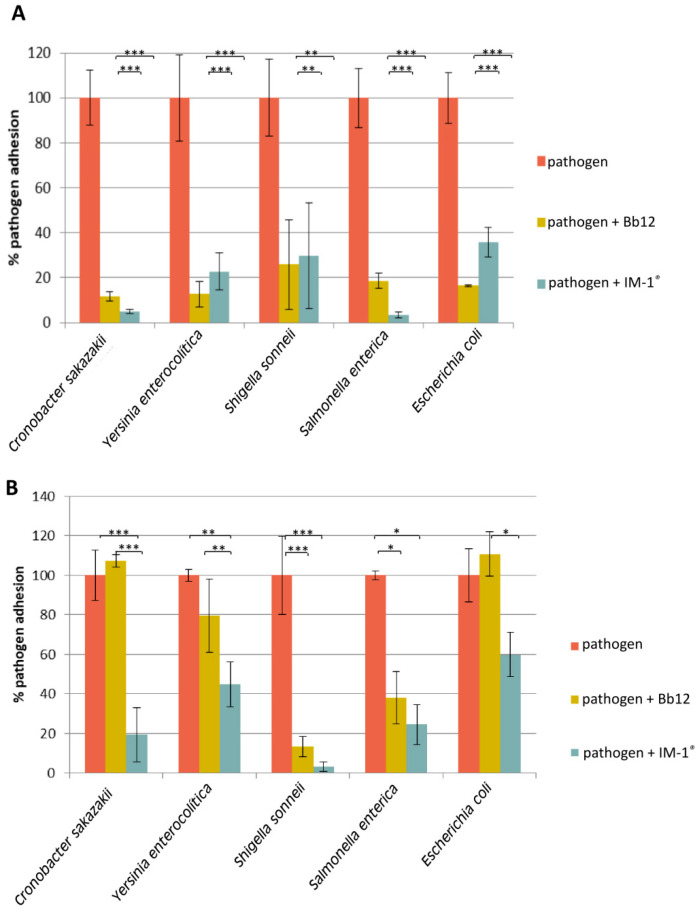
In order to determine the ability of B. infantis IM-1® to displace or to prevent the adhesion of several enteropathogens to the intestinal epithelium, adhesion assays using the enterocyte cell line HT29 were carried out. In addition, B. animalis subsp. lactis Bb12 strain was used as a probiotic reference due to the fact that it is a model probiotic strain widely used in commercial probiotic products, known to exhibit relatively high adhesion in vitro to intestinal cell models, such as the one used in the present work, and for which the capability to prevent certain pathogens adhesion has already been documented [51]. Unfortunately, C. difficile was not able to survive after the microaerophilic conditions used during the adhesion assay, and viable cells could not be recovered, thus preventing the evaluation of the strain B. infantis IM-1® capacity to prevent or displace this pathogen adhesion. The strain B. infantis IM-1® was able to displace all the other pathogenic strains tested (Figure 4A), especially Cr. sakazakii and S. enterica in a similar way to the strain B. animalis subsp. lactis Bb12. Furthermore, the previous adhesion of B. infantis IM-1® decreased the adhesion of all the pathogens to HT29 cells, although the effect was more pronounced for Sh. sonnei and Cr. sakazakii (Figure 4B). In general, B. infantis IM-1® was more effective than the reference probiotic (strain Bb12), except for Sh. sonnei and S. enterica, for which prevention of adhesion in the presence of both probiotics was similar (no statistical differences, Figure 4B).
Figure 4.
Retained pathogen adhered to HT29 cells after subsequent displacement by exposition to B. infantis IM-1® cells (A); and pathogen adhesion to HT29 cells previously exposed to B. infantis IM-1® cells (B). The results were normalized considering 100% the adhesion of the pathogen in the absence of bifidobacterial treatment. The strain B. animalis subsp. lactis Bb12 was used as a reference for comparative purposes. For each pathogen, the three treatment groups were compared to get statistical significance by using ANOVA tests, followed by Tukey pairwise test comparison (*** p < 0.001, ** p < 0.01, * p < 0.05).
Several reports have studied the effect of probiotic strains on pathogen adhesion using intestinal cell models. In this regard, different scenarios could be considered: probiotics can directly compete for adhesion sites in the intestine, or displace the already adhered pathogens, or even prevent the attachment of the pathogens when adhesion sites are blocked by the probiotic strain. Bifidobacterium strains have been able to act on these three possible scenarios [24,25,52,53,54,55,56]. However, the array of pathogens whose adhesion might be reduced by probiotic bifidobacteria, seems to be strain-dependent. In this regards, the strain B. infantis IM-1® seems to display a preferential activity on Cr. sakazakii, its inhibition effect being better than for the strain B. animalis Bb12 that was used as a control. This suggests a promising effect of the strain B. infantis IM-1® to act on Cr. sakazakii-induced infections, which are especially relevant in infants [57].
4. Conclusions
This study confirmed that the addition of a single oligosaccharide, or a mixture of oligosaccharides, has positive effects on the growth promotion of B. infantis IM-1®, a bifidobacterial strain originally isolated from the feces of a breastfed infant. Based on the results of this work, we propose that suitable synbiotic combinations including the B. infantis IM-1® probiotic strain should contain either GOS or GOS-containing mixtures. In addition, co-culture experiments confirmed the ability of B. infantis IM-1® grown in the presence of GOS to inhibit C. difficile and Cr. sakazakii when grown in co-culture. This probiotic strain was also able to prevent adhesion, and to efficiently displace some pathogens, especially Cr. sakazakii. Therefore, this work expands on the antipathogenic potential of bifidobacterial strains isolated from particular population groups (breast-fed infants) and provides the rationale to design novel functional foods and synbiotic combinations including the B. infantis IM-1® strain. Therefore, we propose that the B. infantis IM-1® strain, which had already been demonstrated to reduce diarrhea episodes in infants and to prevent rotavirus infection, also has the potential to antagonize a range of other enteropathogens in vitro. For these reasons, the results of this work support its potential benefits as a probiotic strain in infant foods specifically designed for formula-fed infants and, particularly, when formulated in synbiotic combinations including GOS or GOS-containing oligosaccharides. Further studies are needed to complete the characterization of the anti-pathogenic activity of this strain and to elucidate its mechanisms of action.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/11/3259/s1, Table S1: Nutritional composition of the two infant formulas used in the study.
Author Contributions
Conceptualization, J.A.M.-M., P.R.-M. and A.M.; methodology, L.R., A.B.F. and P.R.-M.; formal analysis, L.R., A.B.F., B.S., J.A.M.-M., M.R.-P., J.J., C.G.d.l.R.G., M.G., P.R.-M., and A.M; investigation, L.R. and A.B.F.; resources, J.A.M and A.M.; writing—original draft preparation, L.R., A.B.F., J.A.M and A.M.; writing—review and editing, L.R., A.B.F., B.S., J.A.M.-M., M.R.-P., J.J., C.G.d.l.R.G., M.G., P.R.-M., and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Laboratorios Ordesa and CDTI (Spanish Center for the Development of Industrial Technology) through the Research Contract 110108060004 (SENIFOOD PROJECT ID 2009-0001006). Publication expenses for this article has been supported by Cátedra ORDESA-University of Granada, Spain as part of Special Issue “Early Nutrition and Re-programming of Health and Disease.
Conflicts of Interest
José Antonio Moreno-Muñoz, Maria Rodríguez-Palmero and Jesús Jiménez are employees of Laboratorios Ordesa.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ducarmon Q.R., Zwittink R.D., Hornung B.V.H., van Schaik W., Young V.B., Kuijper E.J. Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 2019;83 doi: 10.1128/MMBR.00007-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada N., Chen G.Y., Inohara N., Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aw W., Fukuda S. Protective effects of bifidobacteria against enteropathogens. Microb. Biotechnol. 2019;12:1097–1100. doi: 10.1111/1751-7915.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arboleya S., Watkins C., Stanton C., Ross R.P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biavati B., Mattarelli P. The family Bifidobacteriaceae. In: Dworkin M., Fallow S., Rosenberg E., Schleifer K.H., Stackebrandt E., editors. The Prokaryotes. Springer; New York, NY, USA: 2001. pp. 1–70. [Google Scholar]
- 7.Langendijk P.S., Schut F., Jansen G.J., Raangs G.C., Kamphuis G.R., Wilkinson M.H.F., Welling G.W. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 1995;61:3069–3075. doi: 10.1128/AEM.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmsen H.J.M., Raangs G.C., He T., Degener J.E., Welling G.W. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl. Environ. Microbiol. 2002;68:2982–2990. doi: 10.1128/AEM.68.6.2982-2990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turroni F., Peano C., Pass D.A., Foroni E., Severgnini M., Claesson M.J., Kerr C., Hourihane J., Murray D., Fuligni F., et al. Diversity of bifidobacteria within the infant gut microbiota. PLoS ONE. 2012;7:e36957. doi: 10.1371/journal.pone.0036957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen H.J.M., Wildeboer-Veloo A.C.M., Raangs G.C., Wagendorp A.A., Klijn N., Bindels J.G., Welling G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Vandenplas Y., De Greef E., Veereman G. Prebiotics in infant formula. Gut Microbes. 2015;5:681–687. doi: 10.4161/19490976.2014.972237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno Villares J.M., Collado M.C., Larqué E., Leis Trabazo M.R., Sáenz De Pipaon M., Moreno Aznar L.A. The first 1000 days: An opportunity to reduce the burden of noncommunicable diseases. Nutr. Hosp. 2019;36:218–232. doi: 10.20960/nh.02453. [DOI] [PubMed] [Google Scholar]
- 13.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 14.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 15.Gibson G.R., Beatty E.R., Wang X., Cummings J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 16.Gibson G.R., McCartney A.L., Rastall R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2005;93:S31–S34. doi: 10.1079/BJN20041343. [DOI] [PubMed] [Google Scholar]
- 17.Meyer D., Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 2009;63:1277–1289. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- 18.Liu F., Li P., Chen M., Luo Y., Prabhakar M., Zheng H., He Y., Qi Q., Long H., Zhang Y., et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva J.P.B., Navegantes-Lima K.C., de Oliveira A.L.B., Rodrigues D.V.S., Gaspar S.L.F., Monteiro V.V.S., Moura D.P., Monteiro M.C. Protective mechanisms of butyrate on Inflammatory Bowel Disease. Curr. Pharm. Des. 2018;24:4154–4166. doi: 10.2174/1381612824666181001153605. [DOI] [PubMed] [Google Scholar]
- 20.Nurmi J.T., Puolakkainen P.A., Rautonen N.E. Bifidobacterium lactis sp. 420 up-regulates cyclooxygenase (Cox)-1 and down-regulates Cox-2 gene expression in a caco-2 cell culture model. Nutr. Cancer. 2005;51:83–92. doi: 10.1207/s15327914nc5101_12. [DOI] [PubMed] [Google Scholar]
- 21.Cheikhyoussef A., Pogori N., Chen W., Zhang H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: From production to their application. Int. J. Food Microbiol. 2008;125:215–222. doi: 10.1016/j.ijfoodmicro.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda S., Toh H., Taylor T.D., Ohno H., Hattori M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes. 2012;3:449–454. doi: 10.4161/gmic.21214. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Gutierrez P., de Wouters T., Werder J., Chassard C., Lacroix C. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candela M., Perna F., Carnevali P., Vitali B., Ciati R., Gionchetti P., Rizzello F., Campieri M., Brigidi P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008;125:286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Gueimonde M., Margolles A., de los Reyes-Gavilán C.G., Salminen S. Competitive exclusion of enteropathogens from human intestinal mucus by Bifidobacterium strains with acquired resistance to bile—A preliminary study. Int. J. Food Microbiol. 2007;113:228–232. doi: 10.1016/j.ijfoodmicro.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Swanson K.S., Gibson G.R., Hutkins R., Reimer R.A., Reid G., Verbeke K., Scott K.P., Holscher H.D., Azad M.B., Delzenne N.M., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020:678–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolida S., Gibson G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011;2:373–393. doi: 10.1146/annurev-food-022510-133739. [DOI] [PubMed] [Google Scholar]
- 28.Izumi H., Ehara T., Sugahara H., Matsubara T., Mitsuyama E., Nakazato Y., Tsuda M., Shimizu T., Odamaki T., Xiao J.-Z., et al. The combination of Bifidobacterium breve and three prebiotic oligosaccharides modifies gut immune and endocrine functions in neonatal mice. J. Nutr. Nutr. Immunol. 2019;149 doi: 10.1093/jn/nxy248. [DOI] [PubMed] [Google Scholar]
- 29.Chenoll E., Rivero M., Codoñer F.M., Martinez-Blanch J.F., Ramón D., Genovés S., Muñoz J.A.M. Complete genome sequence of Bifidobacterium longum subsp. infantis strain CECT 7210, a probiotic strain active against rotavirus infections. Genome Announc. 2016;3 doi: 10.1128/genomeA.00105-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muñoz J.A.M., Chenoll E., Casinos B., Bataller E., Ramón D., Genovés S., Montava R., Ribes J.M., Buesa J., Fàbrega J., et al. Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl. Environ. Microbiol. 2011;77:8775–8783. doi: 10.1128/AEM.05548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escribano J., Ferré N., Gispert-Llaurado M., Luque V., Rubio-Torrents C., Zaragoza-Jordana M., Polanco I., Codoñer F.M., Chenoll E., Morera M., et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018;83:1120–1128. doi: 10.1038/pr.2018.34. [DOI] [PubMed] [Google Scholar]
- 32.Barba-Vidal E., Castillejos L., López-Colom P., Rivero Urgell M., Moreno Muñoz J.A., Martín-Orúe S.M. Evaluation of the probiotic strain Bifidobacterium longum subsp. infantis CECT 7210 capacities to improve health status and fight digestive pathogens in a piglet model. Front. Microbiol. 2017;8:533. doi: 10.3389/fmicb.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–549. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 34.López P., Monteserín D.C., Gueimonde M., de los Reyes-Gavilán C.G., Margolles A., Suárez A., Ruas-Madiedo P. Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 2012;46:99–107. doi: 10.1016/j.foodres.2011.11.020. [DOI] [Google Scholar]
- 35.Mao B., Gu J., Li D., Cui S., Zhao J., Zhang H., Chen W. Effects of different doses of fructooligosaccharides (FOS) on the composition of mice fecal microbiota, especially the bifidobacterium composition. Nutrients. 2018;10:1105. doi: 10.3390/nu10081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteagudo-Mera A., Arthur J.C., Jobin C., Keku T., Bruno-Barcena J.M., Azcarate-Peril M.A. High purity galacto-oligosaccharides enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef. Microbes. 2016;7:247–264. doi: 10.3920/BM2015.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rada V., Nevoral J., Trojanová I., Tománková E., Šmehilová M., Killer J. Growth of infant faecal bifidobacteria and clostridia on prebiotic oligosaccharides in in vitro conditions. Anaerobe. 2008;14:205–208. doi: 10.1016/j.anaerobe.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.J., Yu W.K., Heo T.R. Identification and screening for antimicrobial activity against Clostridium difficile of Bifidobacterium and Lactobacillus species isolated from healthy infant faeces. Int. J. Antimicrob. Agents. 2003;21:340–346. doi: 10.1016/S0924-8579(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 39.Wei Y., Yang F., Wu Q., Gao J., Liu W., Liu C., Guo X., Suwal S., Kou Y., Zhang B., et al. Protective effects of bifidobacterial strains against toxigenic Clostridium difficile. Front. Microbiol. 2018;9:888. doi: 10.3389/fmicb.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopkins M.J., Macfarlane G.T. Nondigestible oligosaccharides enhance bacterial colonization resistance against Clostridium difficile in vitro. Appl. Environ. Microbiol. 2003;69:1920–1927. doi: 10.1128/AEM.69.4.1920-1927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valdés-Varela L., Hernández-Barranco A.M., Ruas-Madiedo P., Gueimonde M. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front. Microbiol. 2016;7:738. doi: 10.3389/fmicb.2016.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drakoularakou A., Wells A., Robinsons R., Rastall R., Gibson G., Mccartney A. Acid and bile tolerance, adhesion properties and anti-pathogenic effects of three potential probiotic strains. Int. J. Probiotics Prebiotics. 2007;2:185–194. [Google Scholar]
- 43.Fooks L.J., Gibson G.R. Mixed culture fermentation studies on the effects of synbiotics on the human intestinal pathogens Campylobacter jejuni and Escherichia coli. Anaerobe. 2003;9:231–242. doi: 10.1016/S1075-9964(03)00043-X. [DOI] [PubMed] [Google Scholar]
- 44.Bevilacqua L., Ovidi M., Di Mattia E., Trovatelli L.D., Canganella F. Screening of Bifidobacterium strains isolated from human faeces for antagonistic activities against potentially bacterial pathogens. Microbiol. Res. 2003;158:179–185. doi: 10.1078/0944-5013-00192. [DOI] [PubMed] [Google Scholar]
- 45.Toure R., Kheadr E., Lacroix C., Moroni O., Fliss I. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J. Appl. Microbiol. 2003;95:1058–1069. doi: 10.1046/j.1365-2672.2003.02085.x. [DOI] [PubMed] [Google Scholar]
- 46.Zinedine A., Faid M. Isolation and characterization of strains of bifidobacteria with probiotic properties in vitro. World J. Dairy Food Sci. 2007;2:28–34. [Google Scholar]
- 47.Coconnier M.H., Liévin V., Bernet-Camard M.F., Hudault S., Servin A.L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 1997;41:1046–1052. doi: 10.1128/AAC.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombel J.F., Cortot A., Neut C., Romond C. Yoghurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet. 1987;330:43. doi: 10.1016/S0140-6736(87)93078-9. [DOI] [PubMed] [Google Scholar]
- 49.Plummer S., Weaver M., Harris J., Dee P., Hunter J. Clostridium difficile pilot study: Effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int. Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- 50.Servin A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. Fems Microbiol. Rev. 2004;28:405–440. doi: 10.1016/j.femsre.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Collado M.C., Meriluoto J., Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett. Appl. Microbiol. 2007;45:454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]
- 52.Candela M., Seibold G., Vitali B., Lachenmaier S., Eikmanns B.J., Brigidi P. Real-time PCR quantification of bacterial adhesion to Caco-2 cells: Competition between bifidobacteria and enteropathogens. Res. Microbiol. 2005;156:887–895. doi: 10.1016/j.resmic.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Collado M.C., Grześkowiak Ł., Salminen S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007;55:260–265. doi: 10.1007/s00284-007-0144-8. [DOI] [PubMed] [Google Scholar]
- 54.Moroni O., Kheadr E., Boutin Y., Lacroix C., Fliss I. Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl. Environ. Microbiol. 2006;72:6894–6901. doi: 10.1128/AEM.00928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inturri R., Stivala A., Furneri P.M., Blandino G. Growth and Adhesion to HT-29 Cells Inhibition of Gram-Negatives by Bifidobacterium longum BB536 e Lactobacillus rhamnosus HN001 alone and in combination. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4943–4949. [PubMed] [Google Scholar]
- 56.Serafini F., Strati F., Ruas-Madiedo P., Turroni F., Foroni E., Duranti S., Milano F., Perotti A., Viappiani A., Guglielmetti S., et al. Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe. 2013;21:9–17. doi: 10.1016/j.anaerobe.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Healy B., Cooney S., O′Brien S., Iversen C., Whyte P., Nally J., Callanan J.J., Fanning S. Cronobacter (Enterobacter sakazakii): An opportunistic foodborne pathogen. Foodborne Pathog. Dis. 2010;7:339–350. doi: 10.1089/fpd.2009.0379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.