Abstract
Cryptococcus neoformans is a basidiomycete human fungal pathogen causing lethal meningoencephalitis, mainly in immunocompromised patients. Oxidoreductases are a class of enzymes that catalyze redox, playing a crucial role in biochemical reactions. In this study, we identified one Cryptococcus oxidoreductase-like protein-encoding gene OLP1 and investigated its role in the sexual reproduction and virulence of C. neoformans. Gene expression patterns analysis showed that the OLP1 gene was expressed in each developmental stage of Cryptococcus, and the Olp1 protein was located in the cytoplasm of Cryptococcus cells. Although it produced normal major virulence factors such as melanin and capsule, the olp1Δ mutants showed growth defects on the yeast extract peptone dextrose (YPD) medium supplemented with lithium chloride (LiCl) and 5-fluorocytosine (5-FC). The fungal mating analysis showed that Olp1 is also essential for fungal sexual reproduction, as olp1Δ mutants show significant defects in hyphae growth and basidiospores production during bisexual reproduction. The fungal nuclei imaging showed that during the bilateral mating of olp1Δ mutants, the nuclei failed to undergo meiosis after fusion in the basidia, indicating that Olp1 is crucial for regulating meiosis during mating. Moreover, Olp1 was also found to be required for fungal virulence in C. neoformans, as the olp1Δ mutants showed significant virulence attenuation in a murine inhalation model. In conclusion, our results showed that the oxidoreductase-like protein Olp1 is required for both fungal sexual reproduction and virulence in C. neoformans.
Keywords: Cryptococcus neoformans, oxidoreductases-like protein, Olp1, sexual reproduction, virulence
1. Introduction
Cryptococcus neoformans is a globally distributed fungal pathogen causing life-threatening cryptococcal meningitis in both immunocompromised and immunocompetent patients [1]. An updated analysis recently showed that C. neoformans could cause nearly 300,000 infections and almost 200,000 deaths worldwide each year [2,3]. Initially, C. neoformans was proposed to contain three varieties, namely, C. neoformans var. grubii, C. neoformans var. neoformans, and C. neoformans var. gattii [4]. In 2002, the variety C. neoformans var. gattii was classified as an independent species C. gattii based on improved molecular methods [5]. Recently, some researchers proposed to recognize the two varieties of current C. neoformans (C. neoformans var. grubii and C. neoformans var. neoformans) as two separate species (C. neoformans and C. deneoformans) and split C. gattii into a total of five species (C. gattii, C. bacillisporus, C. deuterogattii, C. tetragattii, and C. decagattii) [6]. However, others recommend using the “C. neoformans species complex” and “C. gattii species complex” to recognize genetic diversity without creating the nomenclatural instability [7].
As a basidiomycetes yeast fungus, C. neoformans has two mating types, α and a, and it can undergo a dimorphic transition from yeast form to a filamentous growth form by mating and monokaryotic fruiting [8]. During mating in C. neoformans, after the fusion of the haploid yeast cells of the opposite mating types, dikaryotic filaments are formed, and a specialized sporulation structure called the basidium is eventually produced at the tip of the filaments. Next, four chains of basidiospores are produced on top of the basidium following the completion of meiosis inside the basidium [9]. Under certain conditions, the haploid cells of the same mating type of C. neoformans, e.g., α cells, can also fuse and undergo monokaryotic fruiting to generate filaments and basidiospores [10]. The major difference between mating and fruiting is that the hyphal cells produced during mating contain two nuclei and are linked by fused clamp connections, whereas those produced during fruiting are mononucleate with unfused clamp connections [10].
As an important human fungal pathogen, C. neoformans has three classical virulence factors: capsule formation, melanin production, and growth at 37 °C [11,12]. Other virulence factors such as urease [13], extracellular phospholipase activity [14], mannitol production [15,16], laccase [17], hyaluronic acid [18], calcineurin [19], and metalloprotease [20,21] also contribute to the infection and the pathogenesis of C. neoformans. However, these studies indicate that fungal virulence is a complex trait, and the determining mechanism of virulence still needs to be further explored.
Oxidoreductases are a class of enzymes known as dehydrogenases or oxidases that catalyze redox reactions, including dehydrogenases, oxygenases, oxidases, and reductases [22]. Redox reactions involve many basic metabolism processes such as glycolysis, the tricarboxylic acid (TCA) cycle, oxidative phosphorylation, and amino acid metabolism, which are essential for life. The dysfunction of oxidoreductases can cause many diseases in humans, such as cancer [23,24,25,26], neurodegenerative disorders [27,28], and cardiovascular disease [29,30]. Oxidoreductases have also been reported to be extremely critical to fungi. In the plant pathogenic fungus Magnaporthe oryzae, dehydrogenase MoSFA1 is required for conidiation and contributes to virulence in the penetration and biotrophic phases [31]. In the entomogenous fungus Beauveria bassiana, fungal benzoquinone oxidoreductase is a host-specific virulence factor and is beneficial for the fungi to resist the benzoquinone exposure [32]. In Candida albicans, oxidoreductase FLPs reduce ubiquinone (coenzyme Q), which can serve as an antioxidant in the membrane to protect Candida albicans from oxidative stress and promote the fungal virulence [33]. In Aspergillus fumigatus, a fungal alcohol dehydrogenase required for the last step of ethanol fermentation in response to hypoxia is critical to pulmonary invasive fungal infection [34]. Moreover, oxidoreductases including alternative oxidase [35], cytochrome c peroxidase [36], mannitol-1-phosphate-5-dehydrogenase [37], thiol peroxidase [38], and thioredoxin reductase [39] already have been confirmed to be extremely significant for the virulence of C. neoformans [40].
In the present study, we have identified and functionally analyzed one oxidoreductase-like protein Olp1 in C. neoformans. Sequence analysis revealed that Olp1 does not contain any currently known domain and had low homology with other non-Cryptococcus proteins. Expression analysis showed that the OLP1 gene was down-regulated during mating and the Olp1 protein was localized in the cytoplasm of cryptococcal cells. Growth under stress conditions showed that the olp1Δ mutants were hypersensitive to LiCl and 5-FC, although the mutants produced normal classical virulence factors. We also found that Olp1 was crucial for sexual reproduction, as the olp1Δ mutants produced short and sparse dikaryotic hyphae and no basidiospores in bilateral mating. Moreover, Olp1 is also required for fungal virulence, as the olp1Δ mutants showed a significant virulence attenuation in the mouse inhalation model. Overall, our results suggest that Olp1 regulates sexual reproduction and fungal virulence in C. neoformans.
2. Materials and Methods
2.1. Ethics Statement
The animal studies conducted at Southwest University were in full compliance with the “Guidelines on Ethical Treatment of Experimental Animals (2006, No. 398)” issued by the Ministry of Science and Technology of China and the “Regulation on the Management of Experimental Animals (2006, No. 195)” issued by Chongqing Municipal People’s Government. The Animal Ethics Committee of Southwest University approved all of the vertebrate studies on 6 March 2019 with a project identification code IACUC-20190306-07.
2.2. Strains, Media, and Growth Conditions
The wild-type Cryptococcus strains and their derivatives used in the present study are shown in Table 1. Yeast extract peptone dextrose (YPD) agar medium was used for the routine cultivation of Cryptococcus strains at 30 °C. Murashige and Skoog medium (MS) and V8 medium used for Cryptococcus mating induction and sporulation assays were prepared as described previously [41]. All other media were prepared as described previously [42].
Table 1.
Strains and plasmids used in this study.
| Strains/Plasmids | Genotype/Properties | Source/Reference |
|---|---|---|
| C. neoformans | ||
| H99 | MATα | [43] |
| KN99a | MAT a | [44] |
| TBL101 | MATα PACTIN-Nop1-mCherry::NAT | [45] |
| TBL102 | MATaPACTIN-Nop1-mCherry::NAT | [45] |
| TBL337 | MATα olp1::NEO | In this study |
| TBL348 | MATaolp1::NEO | In this study |
| TBL349 | MATα olp1::NEO OLP1::NAT | In this study |
| TBL352 | MATa olp1::NEO OLP1:: NAT | In this study |
| TBL353 | MATα olp1Δ::NEO PACT1N-OLP1-mCherry::NAT | In this study |
| TBL354 | MATaolp1Δ::NEO PACT1N-OLP1-mCherry::NAT | In this study |
| TBL371 | MATα olp1Δ::NEO PACTIN-Nop1-mCherry::NAT | In this study |
| TBL372 | MATaolp1Δ::NEO PACTIN-Nop1-mCherry::NAT | In this study |
| TBL373 | MATα olp1Δ::NEO POLP1-OLP1-mCherry::NAT | In this study |
| TBL374 | MATaolp1Δ::NEO POLP1-OLP1-mCherry::NAT | In this study |
| Plasmids | ||
| pJAF1 | Ampr Plasmid harboring NEO marker | [46] |
| pTBL1 | Ampr Plasmid harboring NAT marke | [45] |
| pTBL3 | Ampr Plasmid harboring mCherry-GPD1 terminator and NAT marker | [45] |
| pTBL5 | Ampr Plasmid harboring mCherry-GPD1 terminator and NAT marker under ACTIN promoter | [45] |
| pTBL68 | Ampr Vector for PACTIN-NOP1-mCherry-NAT for nuclear positioning | [45] |
| pTBL85 | Ampr Vector for PACTIN-mCherry-OLP1 for Olp1 localization | In this study |
| pTBL118 | Ampr Vector for POLP1-OLP1-mCherry for Olp1 localization | In this study |
| pTBL205 | Ampr Vector for POLP1-OLP1-NAT for complementation | In this study |
Note: a, a mating type.
2.3. OLP1 Gene Expression Pattern Analysis
To understand the temporal expression pattern of the OLP1 gene and subcellular localization of the Olp1 protein, a HindIII/BamHI genomic DNA fragment containing a 1.5-Kb upstream promoter region, the open reading frame (ORF) of the OLP1 gene without the stop codon, was amplified using primers TL727/TL729 (see Table 2 for primer sequences) and cloned into pTBL3 [45] to generate the POLP1-OLP1-mCherry fusion plasmid pTBL118 (see Table 1 for plasmid information) for Olp1-mCherry fusion proteins. Then, the ScaI linearized pTBL118 was concentrated and biolistically transformed into both α and a mating-type olp1Δ mutant strains (TBL337 and TBL348), respectively. Stable transformants were selected on YPD medium containing nourseothricin sulfate (100 mg/liter). The fluorescence of the transformants was examined using an Olympus inverted confocal laser scanning microscope (Olympus, FV1200).
Table 2.
Primers used in this study.
| Primers | Targeted Genes | Sequence (5′-3′) |
|---|---|---|
| TL17 | M13F | GTAAAACGACGGCCAG |
| TL18 | M13R | CAGGAAACAGCTATGAC |
| TL19 | NEO split F | GGGCGCCCGGTTCTTTTTGTCA |
| TL20 | NEO split R | TTGGTGGTCGAATGGGCAGGTAGC |
| TL59 | NEO R4 | TGTGGATGCTGGCGGAGGATA |
| TL67 | STE20A α F | CCAAAAGCTGATGCTGTGGA |
| TL68 | STE20A α R | AGGACATCTATAGCAGAT |
| TL69 | STE20Aa F | TCCACTGGCAACCCTGCGAG |
| TL70 | STE20Aa R | ATCAGAGACAGAGGAGCAAGAC |
| TL217 | GAPDH qRT-PCR F | TGAGAAGGACCCTGCCAACA |
| TL218 | GAPDH qRT-PCR R | ACTCCGGCTTGTAGGCATCAA |
| TL404 | OLP1 KO F1 | CTCCCCAGACAAGCACATTCC |
| TL405 | OLP1 KO R1 | CTGGCCGTCGTTTTACGACGCGTCTACACCACTCAGCAA |
| TL1253 | OLP1 KO F2 | GTCATAGCTGTTTCCTGCAAGATTCTGTGCGTATGGTGTGC |
| TL1254 | OLP1 KO R2 | GTTTGTTCTTTTGGCGGGTTTGAG |
| TL1255 | OLP1 KO F3 | ATATGAATTGCTGCGTGTGACC |
| TL1256 | OLP1 KO R3 | GCTTATGCTCCTTCTTCCAGTATT |
| TL410 | OLP1 KO F4 | TCCAAAGAAGAAGACAGCAACC |
| TL515 | OLP1 mCherry F | TTAGTAAACTCGCCCAACATGTCTGGATCCATGCCTATTCACACTCTTGCTTC |
| TL516 | OLP1 mCherry R | CTTGCTCACCATTCTAGAACTAGTGGATCCTTTGCCCTCTGGCTTGGTTCTG |
| TL539 | OLP1 QPCR F1 | TACGAGCCTCTCGACGATAC |
| TL540 | OLP1 QPCR R1 | TCTGGCTTGGTTCTGTCTTTAC |
| TL556 | OLP1 Comp F | GATATCGAATTCCTGCAGCCCGGGGGATCCTCCAAAGAAGAAGACAGCAACCTA |
| TL1257 | OLP1 Comp R | CGGTGGCGGCCGCTCTAGAACTAGTGGATCTTTGCCTACAGGATTTTGGTCACT |
| TL727 | OLP1 Pro-OLP1 F1 | TCGACGGTATCGATAAGCTTCGCCGACGACCAAGATACAG |
| TL729 | OLP1 Pro-OLP1 R1 | ATTCTAGAACTAGTGGATCCTTTGCCCTCTGGCTTGGTTCTGTC |
Note: a, a mating type.
2.4. Detection of OLP1 Gene Expression Using qRT-PCR
The expression of OLP1 during mating was also tested by qRT-PCR. Cell cultures preparation, mating mixture collection, RNAs extraction, cDNAs synthesis, and specific methods of Realtime PCRs are the same as previously described [42,45]. The expression levels of the GAPDH gene were used as an internal control. Primers TL217/TL218 and TL539/TL540 were used to amplify the GAPDH gene and OLP1 gene, respectively.
2.5. Generation of OLP1 Gene Knockout, Complementation, and Overexpression Strains
The olp1Δ mutant strains were generated in both H99 and KN99a strain backgrounds using the split marker strategy, as described previously [45,47]. Primers used to amplify the overlapping PCR fragments for OLP1 gene knockout are listed in Table 2. Stable transformants were further confirmed on YPD medium containing antibiotic G418 (200 mg/liter). To screen the olp1Δ mutants, diagnostic PCR was used by analyzing the 5′ junction of the disrupted mutant alleles with positive primers F4/R4 (TL403 and TL59) and negative primers F3/R3 (TL1255 and TL1256). Positive transformants identified by PCR screening were further confirmed by Southern blot analysis.
To generate the olp1Δ mutant complementation strains, a 3.9 Kb genomic DNA fragment that contains the upstream promoter region, the ORF of the OLP1 gene, and its terminator region was amplified with primers TL556/TL1257 and cloned into the pTBL1 [45] vector to generate an OLP1 gene complementation vector pTBL93. The pTBL93 plasmid was linearized by ScaI and biolistically transformed in both α and a mating-type olp1Δ mutants strains. Lithium chloride (LiCl) sensitivity assay was performed to identify transformants that complemented the olp1Δ phenotype.
To construct the OLP1 gene overexpression OLP1OE strains, we amplified a DNA fragment (1.4 Kb) containing the coding region of the OLP1 gene with primers TL515/TL516 and cloned it into an expression vector pTBL5 [45] controlled by the ACTIN promoter to generate an OLP1 gene overexpression vector pTBL85. Then, the resulting vector, pTBL85, was linearized by ScaI and biolistically transformed in both α and a mating-type olp1Δ mutants strains. Stable transformants were selected on YPD medium containing Nourseothricin Sulfate (100 mg/liter). The OLP1OE strains were further confirmed by fluorescence observation and quantitative real-time PCR.
2.6. Generation of Nop1-mCherry Strains
To monitor the nuclear positioning in Cryptococcus, we digested a previously constructed plasmid pTBL68 [45] with SacII and NdeI to generate the NOP1-mCherry-NAT fragments and biolistically transformed the fragments into α and a mating-type strains of the olp1Δ mutants. The native NOP1 gene was replaced with the NOP1-mCherry-NAT cassette by homologous recombination. Positive transformants were further confirmed by PCR to confirm the homologous recombination, and the nuclear signal of the fluorescent mCherry was screened by Olympus inverted confocal laser scanning microscopy (Olympus, FV1200).
2.7. Assays for Melanin, Capsule Production, and Mating
To test the role of Olp1 protein in melanin and capsule production in Cryptococcus, yeast cells of H99, olp1Δ mutants, olp1Δ::OLP1 complementation strains, and OLP1OE overexpression strains were induced on Niger seed agar medium and Dulbecco’s modified Eagle medium (DMEM), respectively. In the mating assay, cell suspensions of opposite mating types (α or a) of each of the above strains were mixed and cocultured on MS or V8 agar medium at 25 °C in the dark. The matings between the α and a Nop1-mCherry strains of wild-type strains (TBL101 × TBL102) or olp1Δ mutants (TBL371 × TBL372) were also induced on MS media, respectively, and their fungal nuclei development were monitored during the whole mating process. The detailed methods were described previously [42,45].
2.8. Virulence Studies
To examine the role of the Olp1 protein in fungal virulence, we washed the overnight cultures of each yeast strain with PBS buffer and resuspended the cultures to a final concentration of 2 × 106 cells/mL. Female C57 BL/6 mice (10 mice of each group) were intranasally infected with 105 cells of each yeast strain, as described previously [13]. Animals that appeared moribund or in pain were sacrificed by CO2 inhalation. Survival data and fungal burden between paired groups were statistically analyzed with PRISM version 8.0 (GraphPad Software, San Diego, CA, USA) (p values of <0.05 were considered significant) as previously described [45].
2.9. Histopathology and Fungal Burdens in Infected Organs
Infected mice were sacrificed at the endpoint of the experiment according to Southwest University-approved animal protocol. The isolation of the infected brains, lungs, and spleens and the preparation of the tissue slides were the same as previously described [45]. Tissue slides were stained with hematoxylin and eosin (H&E) and examined by light microscopy (Olympus, BX53, Tokyo, Japan). The brains, lungs, and spleens of infected mice were dissected and homogenized in PBS buffer. Then, 100 µl of each diluted resuspension was spread on YPD plates containing ampicillin and chloramphenicol and incubated at 30 °C for three days to determine the colonies [45].
3. Results
3.1. Identification of the Oxidoreductases-Like Protein Olp1 in C. neoformans
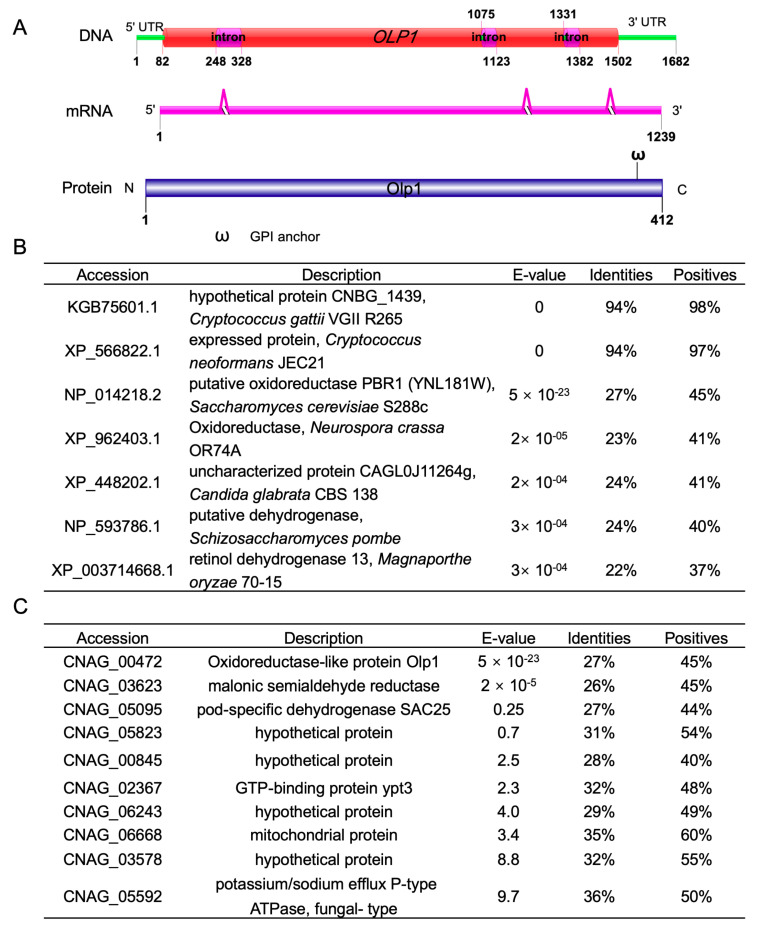
In our previous study on the function of autophagy-related proteins (Atgs), immunoprecipitation (IP) pulldown and liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis were used to identify the interacting proteins of Atgs. From LC-MS/MS data, one protein, CNAG_00472, was found to have potential interactions with Atg5, Atg8, and Atg12, respectively. In this article, we further studied the function of CNAG_00472 in C. neoformans. We first searched the CNAG_00472 gene in the FungiDB database (http://fungiDB.org) [48] and found that the CNAG_00472 gene encodes a protein of 412 amino acids with a predicted molecular mass of 72 KDa. Protein conservative domain analysis showed that this protein did not contain any currently known domain except for a glycosylphosphatidylinositol (GPI) anchor attach site (Figure 1A). Smart blast and phylogenetic analysis revealed that the predicted protein shows high sequence identities (94%) with the species inside the Cryptococcus genus but low sequence identities (22–27%) to several proteins, including putative oxidoreductases in Saccharomyces cerevisiae, Neurospora crassa, and Candida glabrata, dehydrogenases in Schizosaccharomyces pombe and Magnaporthe oryzae (Figure 1B). Therefore, we named this novel protein (CNAG_00472) Olp1 (oxidoreductase-like protein 1). Meanwhile, reciprocal blast analysis using yeast oxidoreductase (YNL181W) as a query was performed against the C. neoformans genome to ensure that the most similar sequence of cryptococcal Olp1 was the same as that of the C. neoformans inquiry gene (Figure 1C). These results suggested that the Olp1 protein might be an oxidoreductases-like protein.
Figure 1.
Sequence analysis of the oxidoreductase-like protein Olp1. (A) Schematic diagram of the genomic DNA, mRNA, and protein of Olp1. (B) The sequences alignment result of Olp1 and its homologs. (C) Reciprocal blast analysis of yeast oxidoreductase (YNL181W) against the C. neoformans genome.
3.2. Expression of the OLP1 Gene in C. neoformans
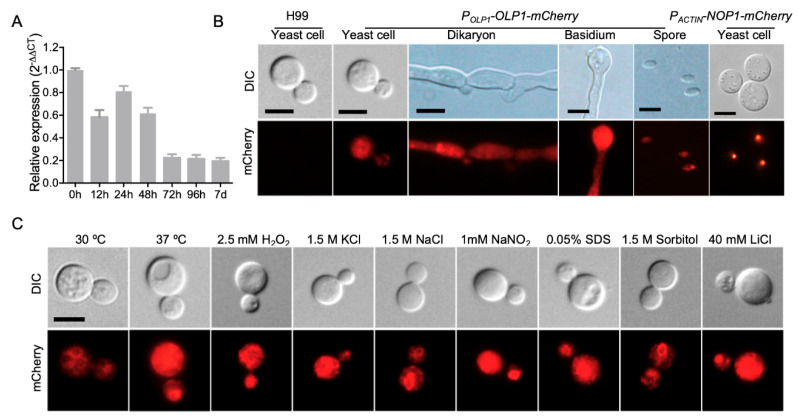
To study the expression pattern of the OLP1 gene in C. neoformans, we first detected the expression levels of the OLP1 gene in different developmental stages using qRT-PCR. Mating mixtures of the wild-type strains (H99 × KN99a) were harvested from V8 plates after incubation for 0, 12, 24, 48, 72 h, and 7 d. RNAs were purified, cDNA was synthesized, and qRT-PCR was performed. The results of qRT-PCR showed that compared to the 0 h time point, the transcription level of the OLP1 gene was down-regulated during mating, especially after 48 h, indicating that Olp1 may play a role in later stages of mating after cell fusion (Figure 2A).
Figure 2.
Expression pattern of the OLP1 gene in C. neoformans. (A) The expression of OLP1 during mating on V8 medium was measured by qRT-PCR. Mating mixtures between H99 and KN99a were harvested from V8 plates after incubation for 0, 12, 24, 48, 72 h, and 7 d. RNAs were purified, cDNA was synthesized, and qRT-PCR was performed. The comparative CT method was used for the relative quantification, and the GAPDH gene was used as an endogenous reference. The error bars show standard deviations of three repeats. (B) Expression and localization of the Olp1-mCherry fusion protein in various development stages of C. neoformans. Representative bright-field and fluorescence images of the yeast cell, dikaryon, basidium, and spores are shown. (C) Subcellular localization of Olp1-mCherry (under control of OLP1 native promoter) in yeast cells under different stress conditions. DIC: differential interference contrast; Bar = 5 μm.
To observe the expression of the OLP1 gene more intuitively in different developmental stages in C. neoformans, we constructed the Cryptococcus strains expressing the Olp1-mCherry fusion proteins (TBL373 and TBL374, see Table 2) under the control of the OLP1 gene native promoter. The strains (TBL373 and TBL374) expressing Olp1-mCherry showed no apparent difference in LiCl resistance than the wild-type strains, which indicated that Olp1-mCherry was fully functional (Figure S1B). The integrity of the Olp1-mCherry fusion proteins was also confirmed by Western blotting (Figure S1A). Mating of the Olp1-mCherry strains (TBL373 and TBL374) was induced on MS medium, and the fluorescence of Olp1-mCherry fusion protein in different developmental stages was observed by confocal microscopy. Fluorescence microscopy showed that the Olp1-mCherry fusion protein was expressed in all different developmental stages of C. neoformans, including yeast cells, dikaryotic hyphae, basidia, and basidiospores, and it was located in the cytoplasm of Cryptococcus cells, indicating that the Olp1 protein may play a role in the sexual reproduction of C. neoformans (Figure 2B).
We also tested the localization of Olp1-mCherry inside the yeast cell under different stress conditions such as high-temperature stress (37 °C), osmotic stress (1.5 M Sorbitol, 1.5 KCl, and 1.5 M NaCl), cell wall stress (0.025% SDS), oxidative stress (2.5 mM H2O2), nitrosative stress (1 mM NaNO2, pH = 4.0), and metal ions (LiCl). As a result, we found no difference between the localizations of Olp1-mCherry under the above stress conditions (Figure 2C).
3.3. Olp1 Plays Roles in Stress Responses
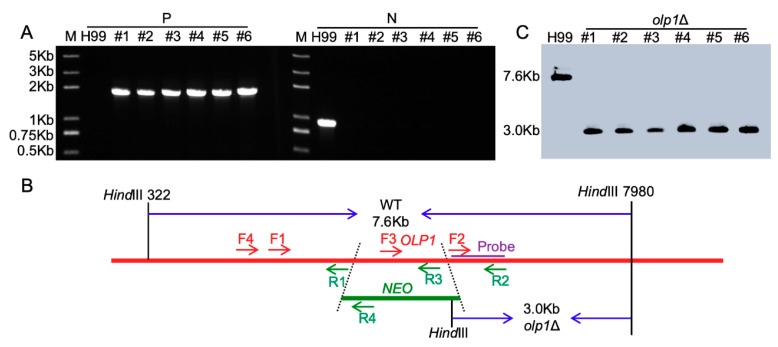
To evaluate the function of the Olp1 protein in C. neoformans, we constructed olp1Δ mutants (TBL337 and BL348) in both mating types of C. neoformans H99 strain backgrounds (Figure 3). The olp1Δ::OLP1 complementation strains (TBL349 and TBL352) and OLP1OE overexpressed strains (TBL353 and TBL354) were also obtained. The overexpression of OLP1 was confirmed by qRT-PCR (Figure 4C).
Figure 3.
Generation of the olp1Δ mutants. (A) PCR verification of OLP1-disrupted transformants; H99: positive control using the wild-type genomic DNA; #1–6: Six G418 resistant transformants used for PCR screening; P: Positive primers (TL410/TL59), F4/R4 in 3B; N: Negative primers (TL1255/TL1256), F3/R3 in 3B; M: 2K Plus DNA Ladder. (B) Restriction enzymes used for digestion of the genomic DNAs for Southern blot. WT: wild-type strain genomic DNA; olp1Δ: olp1Δ mutant genomic DNA; NEO: the G418 resistant marker; The TL1253/TL1254 (F2/R2) PCR products were used as templates to synthesize the probe. The wild-type strain H99 will generate a 7.6-Kb band, while the olp1Δ mutants generate a 3.0-Kb band. (C) Southern blot analysis of the OLP1 disrupted transformants. All genomic DNAs of the six G418 resistant transformants were digested with HindIII, fractionated, and hybridized with a probe located in the downstream flanking sequence of OLP1 shown in Figure 3B. As expected, a 3.0-Kb band was detected in olp1Δ mutants in contrast with a 7.6-Kb band in the wild-type strain H99.
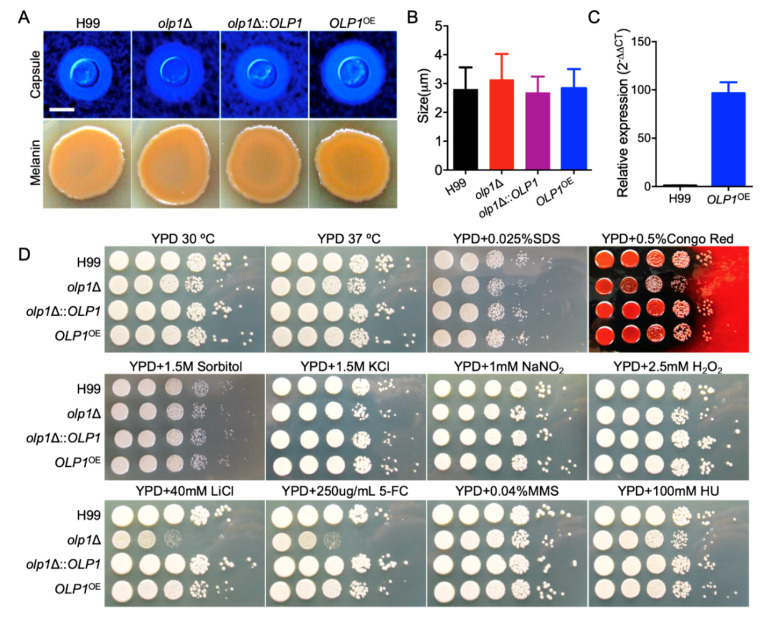
Figure 4.
The olp1Δ mutants are sensitive to 5-fluorocytosine (5-FC) and lithium chloride (LiCl). (A) Capsule formation (top) and melanin production (bottom) of each cryptococcal strain. (B) Statistical analysis of the capsule formation in each Cryptococcus strain. (C) The overexpression of the OLP1 gene was measured by relative qRT-PCR analysis. (D) Growth of each cryptococcal strains on yeast extract peptone dextrose (YPD) or YPD supplemented with various stress reagents. The plates were grown for two days at 30 °C. The name of the strains is indicated on the left and the conditions are indicated at the top.
C. neoformans has three classical virulence factors: capsule formation, melanin production, and the ability to grow at mammalian body temperature. To investigate the role of Olp1 in fungal virulence in C. neoformans, we first examined the development of these virulence factors in the above strains we generated. However, there was no significant difference in the development of the capsule and melanin between wild-type, olp1Δ mutants, OLP1 complemented, or overexpressed strains (Figure 4A,B) except that the olp1Δ mutants showed minor growth defects on YPD pates at 30 or 37 °C (Figure 4D). Notably, the growth of the above strains under stress conditions showed that the olp1Δ mutants were sensitive to 5-FC and LiCl but not sensitive to other tested stress conditions such as hydroxyurea (HU) and methyl methanesulfonate (MMS) (Figure 4D), indicating that the Olp1 protein may play an important role in RNA processing and synthesis or maintaining lithium-ion homeostasis and protecting fungi from lithium-ion toxicity.
3.4. Olp1 is Crucial for Sexual Sporulation
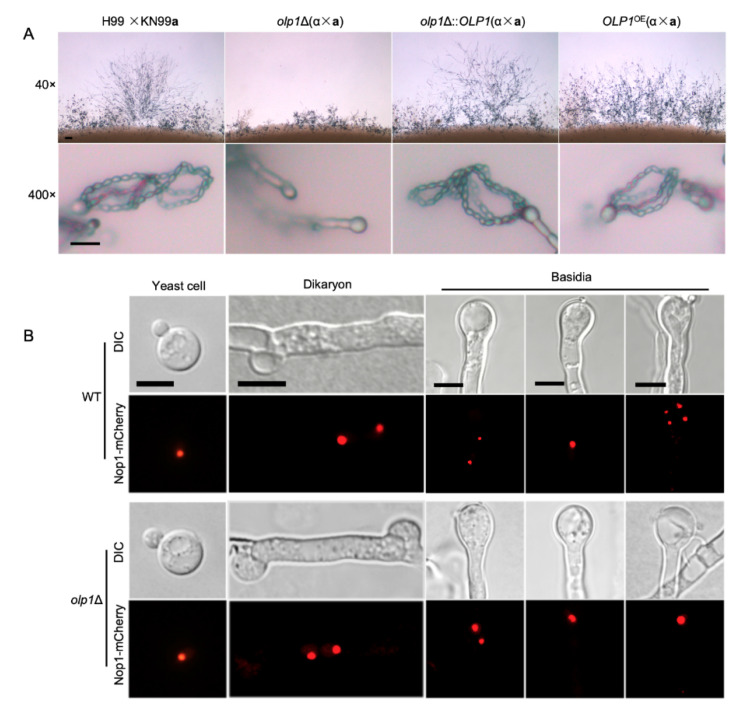
As a basidiomycetes fungus, C. neoformans has two mating types and can undergo sexual reproduction, which involves the fusion of haploid cells of the opposite mating type, α and a, to produce dikaryotic filaments and basidiospores. To investigate whether Olp1 plays a role in mating, we examined the matings of wild-type (H99 × KN99a), olp1Δ mutants (TBL337 × TBL348), olp1Δ::OLP1 complementation strains (TBL349 × TBL352), and OLP1OE overexpression strains (TBL353 × TBL354) on MS media. Interestingly, the bilateral mating between the olp1Δ mutants produced shorter and sparser dikaryotic hyphae and failed to generate basidiospores when compared with wild-type strains, while the OLP1 complemented or overexpressed strains generated normal dikaryotic hyphae and basidiospores (Figure 5A). Therefore, our results demonstrate that the Olp1 protein is crucial for sexual reproduction in C. neoformans.
Figure 5.
Mating hyphae production and sporulation on the wild-type and olp1Δ mutants. (A) Induction of the mating of wild-type (H99 × KN99a), olp1Δ mutants (TBL337 × TBL348), olp1Δ::OLP1 complementation strains (TBL349 × TBL352), and OLP1OE overexpression strains (TBL353 × TBL354) on MS media. Mating structures at 40× magnification (top) and 400× magnification (bottom) were incubated at 25 °C in the dark before being photographed. Bar = 10 μm. (B) The development of fungal nuclei in yeast cells, mating hyphae, and basidia of the wild-type (TBL101 × TBL102, top) and olp1Δ mutants (TBL371 × TBL372, bottom). After incubating on MS medium in the dark for 7 or 14 days, the mating cultures were isolated and visualized by confocal microscopy (Olympus, FV1200). DIC: differential interference contrast; Bars, 5 µm.
3.5. Olp1 Is Involved in Meiosis and Nuclear Division
To further explore why the olp1Δ mutants failed to produce basidiospores during mating, we fused the Nop1 nucleolar protein [49] with the fluorescent mCherry (C-terminus tagged) to monitor the fungal nuclei positioning at different stages of sexual reproduction in C. neoformans. A single nucleus can be observed in each yeast cell of both the wild-type (TBL101 or TBL102) and the olp1Δ mutants (TBL371 × TBL372) cultures (Figure 5B, first panel), and two separated nuclei were observed in each dikaryotic hypha and the young basidia produced from bilateral-mating mixtures of both the wild-type and olp1Δ mutants (Figure 5B, second and third panels). A single fused nucleus could be observed in the young basidium of both the wild-type and olp1Δ mutants, indicating that both strains undergo normal nuclear fusion to produce basidia during mating (Figure 5B, fourth panel). However, the nuclei of the olp1Δ mutants can not undergo meiosis after fusion in the bilateral mating, and only one nucleus could be visualized in each mature basidium after 14 days of incubation, while all basidia from wild-type mating produced four nuclei (Figure 5B, fifth panel). These results indicate that Olp1 plays a crucial role in the meiosis during the sporulation development stage in C. neoformans.
3.6. Olp1 Is Required for Fungal Virulence
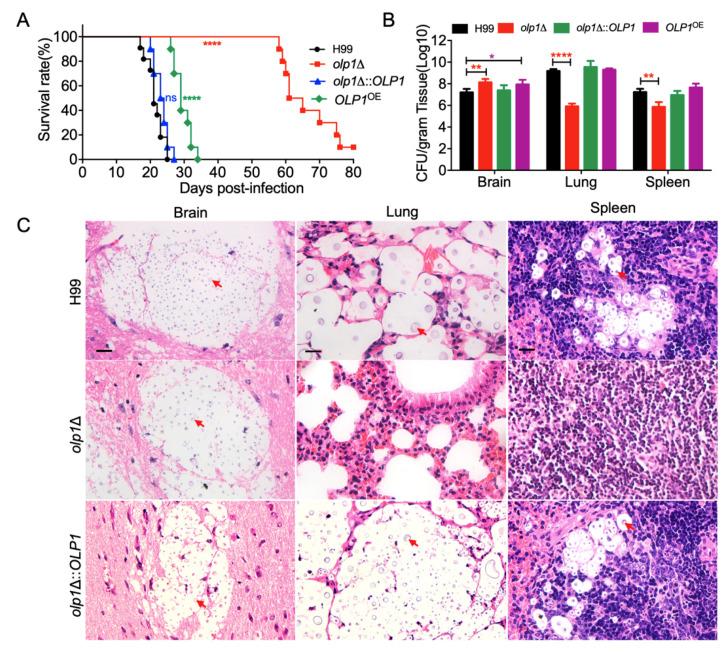
To determine the role of the Olp1 protein in fungal virulence in C. neoformans, we tested the virulence of olp1Δ mutants in a murine inhalation model of cryptococcosis. Female C57BL/6 mice were infected intranasally with 105 yeast cells of the wild-type, olp1Δ mutant, or complemented olp1Δ::OLP1 strain and monitored for signs of morbidity. All mice infected by the wild-type and complemented strains were terminated between 22 and 27 days postinfection (DPI) (p > 0.999), while most of the mice infected by the olp1Δ mutants could survive between 58 and 75 DPI (p < 0.0001). There was still one mouse alive when we terminated the experiment after 80 days postinfection (Figure 6A). Hence, the virulence attenuation observed in the olp1Δ mutant was due to the deletion of the OLP1 gene.
Figure 6.
Olp1 regulates fungal virulence in a mouse systematic infection model. (A) Female C57 BL/6 mice were infected intranasally with 105 cells of the wild-type H99, the olp1Δ mutants (TBL337), olp1Δ::OLP1 complementation strains (TBL349), and OLP1 overexpression strain OLP1OE (TBL353). Both the olp1Δ mutant and OLP1OE strain are less virulent than the wild-type H99 strain. ns, not significant; ****, p ≤ 0.0001 (determined by log-rank [Mantel–Cox] test). (B) Organs from five mice infected with H99, the olp1Δ mutants, olp1Δ::OLP1 complemented strains, and OLP1OE strain were dissected for colony-forming unit (CFU) counting at the end time point of infection. The data shown are the mean ± SD for values from five animals. *, p ≤ 0.05; **, p ≤ 0.01; ****, p ≤ 0.0001 (determined by Mann–Whitney test). (C) Hematoxylin and eosin (H&E)-stained slides were prepared from cross-sections of infected organs at the endpoint of the experiment and visualized by light microscopy. The cryptococcal cells are indicated by arrows. Brain: Bar = 20 μm, Lung: Bar = 20 μm, Spleen: Bar = 20 μm.
To further explore why the olp1Δ mutants have virulence defects, we examined the fungal burdens in the organs of the infected mice at the endpoint of the infection experiments. Brains, lungs, and spleens from five mice infected by each strain were isolated, and the fungal burden of these organs was evaluated as yeast colony-forming units (CFUs) per gram of fresh organ. Mice that were infected by olp1Δ mutants exhibited lower fungal burdens in the lungs and spleens but higher fungal burdens in the brains than those of mice infected with the wild-type strain (Figure 6B) (p < 0.0001, p < 0.01, and p < 0.01, respectively). These data showed that the virulence attenuation in olp1Δ mutants was due to the disruption of the OLP1 gene.
Fungal lesion development in brains, lungs, and spleens was also visualized in H&E-stained slides. Both the wild-type and the complemented olp1Δ::OLP1 strains caused severe lesions in infected brains, lungs, and spleens at 24 DPI (Figure 6C, top, and bottom panels). Meanwhile, a large number of yeast cells with thickened capsules can be easily observed in all of these three organs (Figure 6C, top, and bottom panels). In contrast, the olp1Δ mutants strain only can cause very limited lesions in the infected lungs and spleens, with very few yeast cells observed at the end time point (Figure 6C, middle panel). However, the olp1Δ mutants strain can cause severe lesions in the brain with numerous yeast cells observed at the end time point (Figure 6C), suggesting that the mortality observed in mice infected with the olp1Δ mutants was due to central nervous system (CNS) disease. Taken together, we showed that Olp1 is required for the fungal virulence of C. neoformans.
3.7. Olp1 Is Vital for Fungal Infection Progression
To better understand the olp1Δ mutant–host interaction dynamics during the infection progression, fungal burdens in organs were examined, and fungal lesions development was also visualized in H&E-stained slides at 7, 14, and 21 DPI.
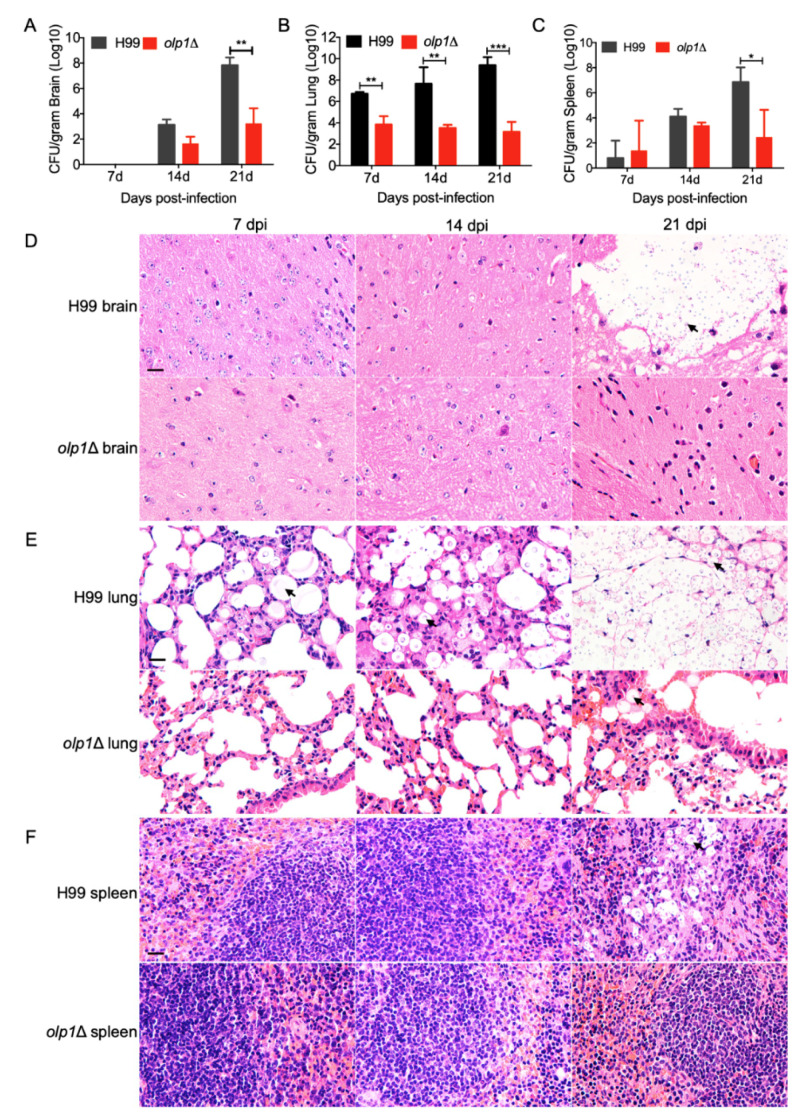
At 7 DPI, there were no cryptococcal yeast cells recovered from the brains of the mice infected with the wild-type or olp1Δ mutants, and the brains remain intact (Figure 7A,D). At 14 DPI, yeast cells can be recovered from the brains of the mice infected with both the wild-type and olp1Δ mutant strains. However, the CFU number recovered from the brains infected by olp1Δ mutants is much less than that recovered from the wild type (Figure 7A). Notably, at 21 DPI, the CFU number recovered from the brains infected by the wild type was significantly higher than that of the olp1Δ mutants (Figure 7A) (p = 0.004). Meanwhile, the mice infected with the wild type had suffered cryptococcosis, and their brains had been severely damaged, while the olp1Δ mutant-infected mice were healthy, and no lesions were found in their brains (Figure 7D). The fungal burdens in the lungs of the mice infected by the wild-type strain are significantly higher in comparison with that of the mice infected by the olp1Δ mutants at 7, 14, and 21 DP1 (Figure 7B) (p = 0.01, p = 0.003, and p = 0.0008, respectively). Interestingly, the fungal burdens (≈103) in the lungs of the mice infected by olp1Δ mutants decreased gradually over time after infection (Figure 7B). The lung damage caused by wild-type infection worsened with the constant proliferation of cryptococcal cells, while the lungs infected by olp1Δ mutant remained relatively intact (Figure 7E). The fungal burdens in the spleens of wild-type infected mice were also higher than that of the olp1Δ mutant-infected mice (Figure 7C) (p = 0.03). Observation of H&E-stained slides also showed the accumulation of cryptococcal cells intensively in the spleen of wild-type infected mice at 21 DPI, while no yeast cell could be observed from the slides of the olp1Δ mutants (Figure 7F).
Figure 7.
Progression of olp1Δ mutants infection in organs of infected animals. (A) Brains from five mice infected with H99 and the olp1Δ mutants were isolated at 7, 14, and 21 days postinfection (DPI), respectively. CFU per gram of fresh organ was measured in brain homogenates. (B) Lungs from five mice infected with H99 and the olp1Δ mutants were isolated at 7, 14, and 21 DPI, respectively. (C) Spleens from five mice infected with H99 and the olp1Δ mutants were isolated at 7, 14, and 21 DPI, respectively. Each data point and the error bar indicate the mean and standard error of the mean for values from five animals. *, p ≤ 0.05, **, p ≤ 0.01, ***, p ≤ 0.001 (determined by Mann–Whitney test). (D–F) H&E-stained slides were prepared from cross-sections of infected brains (D), lungs (E), and spleens (F) at 7, 14, and 21 DPI, respectively, and visualized by light microscopy. Bar, 20 μm. Arrows indicate the cryptococcal cells.
Taken together, our results indicate that the Olp1 protein plays a significant role in the development of meningitis in a murine model.
4. Discussion
Oxidoreductases are a large group of enzymes that play an indispensable role in the primary metabolism of life. In this paper, we identified an oxidoreductase-like protein named Olp1 and proved that it is required for sexual reproduction and fungal virulence in C. neoformans. The olp1Δ mutants produced short and sparse dikaryotic hyphae and failed to produce infectious basidiospores in a bilateral mating assay. Fungi nuclei development assay showed that two nuclei in the basidia of olp1Δ mutants could fuse but were unable to undergo meiosis, which suggested that the Olp1 protein was crucial for regulating meiosis during the sexual reproduction. Notably, the olp1Δ mutants showed significant virulence attenuation in a murine model, implying that the Olp1 protein may play an important role in fungal virulence in C. neoformans.
Virulence factor assay in this study showed that Olp1 was not involved in canonical virulence factors production such as melanin production, capsule formation, and growth at mammalian body temperature. However, the olp1Δ mutants exhibited growth defects on the YPD medium supplemented with LiCl, suggesting that Olp1 is vital to maintain the lithium-ion homeostasis and to protect the cell from the toxicity of lithium-ion. Lithium salts have been used as a mood-stabilizing agent for the treatment of bipolar disorder for a long time, and lithium-ion can have a profound effect on both human behavior and early embryonic development. The mechanism of action of lithium was extraordinarily complex, and some research showed that LiCl had numerous targets such as inositol monophosphatases [50], bisphosphate 3-nucleotidase [51], and glycogen synthase kinase-3 (GSK-3) [52]. There are two identified targets of LiCl in S. cerevisiae: RNA processing enzymes [53] and HAL2 Nucleotidase [54]. In S. cerevisiae, it was reported that the halotolerant protein kinase Hal5p was a high-copy suppressor, which can inhibit nearly one-third of the identified sporulation and meiosis-related lithium-sensitive mutant genes [55]. Our study showed that Olp1 was required for cell growth on the YPD medium supplemented with LiCl, meiosis and fungal sporulation in C. neoformans. Whether Olp1 is under the regulation of Hal5p is an exciting subject that needs to be further investigated.
Our study also showed that the olp1Δ mutants are sensitive to the antifungal drug 5-fluorocytosine (5-FC) (Figure 4C). 5-FC, one of the oldest antifungal agents, can be transported and converted into toxic 5-fluorouracil (5-FU) by cytosine deaminase within the susceptible fungal cell. The metabolites of 5-FU can both inhibit fungal RNA processing and synthesis as well as DNA synthesis by incorporating into them [56]. 5-FC not only inhibits the processing of pre-rRNA into mature rRNA [57] but also disrupts the post-transcriptional modification of tRNAs [58] and the assembly and activity of snRNA/protein complexes, thereby inhibiting the splicing of pre-mRNA [59]. Our result showed that the olp1Δ mutant exhibited susceptibility to 5-FC but not to the DNA-damaging agents such as hydroxyurea (HU) [60] and methyl methanesulfonate (MMS) (Figure 4D) [61]. Thus, we speculated that the Olp1 protein plays an essential role in RNA processing and synthesis. However, further investigation is still needed to determine the exact molecular mechanism of this phenotype.
The putative oxidoreductase encoding gene PBR1 is required for cell viability as the PBR1 deletion strain is inviable in S. cerevisiae, indicating that the oxidoreductase is essential for S. cerevisiae survival [62,63,64]. However, although the olp1Δ mutants showed sexual reproduction defects and significant attenuation in fungal virulence, the olp1Δ mutant cells survived and even appeared healthy under most of the tested growth conditions. Are there any other putative oxidoreductase-like proteins in the C. neoformans genome? To answer this question, we performed a reciprocal blast analysis using yeast oxidoreductase (YNL181W) as a query against the C. neoformans genome, and the reciprocal blast results showed that there are at least nine more oxidoreductase-like proteins except for the Olp1 (Figure 1C). Perhaps due to the functional redundancy caused by the presence of multiple oxidoreductase-like genes in C. neoformans genome, the olp1Δ mutant could still survive and even appear to be healthy in most of the tested growth conditions.
Fungal sporulation is an extremely delicate strategy utilized by fungi to reproduce, disseminate, and survive [65,66]. Surprisingly, our results suggested that the Olp1 protein played an essential role in fungal sporulation, since the mating of the olp1Δ mutants produced shorter and sparser hyphae and failed to produce basidiospores. The fungal nuclei development assay in our study showed that the olp1Δ mutants failed to undergo meiosis after nuclei fusion during mating, which demonstrated that Olp1-mediated homeostasis played an essential role in the regulation of the sporulation and meiosis process. However, why the oxidoreductase-like protein Olp1 is required for the sexual reproduction of C. neoformans, especially the meiotic process of sexual reproduction, is challenging to answer for the time being. So far, there have been reports about oxidase affecting fungal sexual reproduction. In Aspergillus nidulans, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase noxA proved to play an essential role in the sexual fruit body differentiation by affecting the generation of the reactive oxygen species (ROS) [67]. In Neurospora crassa, the NADPH oxidase Nox1 requires the regulatory subunit NOR-1 to control the sexual cell differentiation [68]. The NADPH oxidases regulatory subunit FgNoxR is essential for sexual development in Fusarium graminearum [69]. The above three examples all illustrated the role of reactive oxygen species produced by oxidase in fungal sexual reproduction regulation. As an oxidoreductase-like protein, the Olp1 may also play an essential role in regulating of ROS production and then affect the sexual reproduction process in C. neoformans through disruption of one of the most critical meiosis-specific proteins during the sporulation. However, whether the absence of the Olp1 protein affects the ROS production or meiosis-related proteins involved in sporulation remains unknown and needs to be further studied.
The deletion of the OLP1 gene resulted in significant fungal virulence attenuation in C. neoformans. Fungal burdens at the endpoint showed that the olp1Δ mutants accumulated significantly inside the brains but not in the lungs and spleens. Meanwhile, the olp1Δ mutants need more time (>60 days) than the wild-type strain to reach a similar fungal burden inside the brains at the end of the infection, which might be caused by the different growth rates of the olp1∆ mutants and wild-type strains in the brains. The mammalian brains contain more abundant inositol than that in the plasma [70], which helps Cryptococcus grow inside the brains, since Cryptococcus is the only fungus that can use inositol as a sole carbon source [71,72]. Our previous study showed that brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood–brain barrier [73]. Once in the brain, Cryptococcus can use inositol in the brain for rapid growth. However, in this study, the olp1∆ mutants need more time than the wild-type strain to reach a similar fungal burden at the end of the infection, suggesting that the olp1∆ mutants may grow slower than the wild-type strain inside the brain, which is possibly due to a defect in inositol utilization. The metabolism of inositol involves a variety of oxidoreductase, and Olp1, as an oxidoreductase, might be involved in the metabolism of inositol. Therefore, deletion of the OLP1 gene might affect the metabolism of inositol in Cryptococcus and lead to the slow growth of the olp1∆ mutants in the mouse brain, which results in a longer time for the olp1∆ mutants to reach a similar fungal burden at the end of the infection than the wild-type strain. However, whether the olp1∆ mutants have defects in the utilization of inositol needs to be further investigated.
The growth rate of olp1Δ mutants in mouse lung and spleen tissues was significantly lower than that of wild-type strains. The fungal burdens in the lungs of the olp1Δ mutants-infected mice were at a low level at each time point (≈103). One possible explanation is that the microenvironment of the lung or spleens is more hostile than that of the brain and is not suitable for olp1Δ mutants survival, which also explains that the Olp1 protein is vital for fungal virulence in C. neoformans. Meanwhile, our results also suggested that the disturbance of ion homeostasis and abnormal RNA processing and synthesis may be possible reasons for the virulence defect of olp1Δ mutants in the murine inhalation model of infection. However, further study is still needed to investigate the specific mechanism of fungal virulence attenuation in olp1Δ mutants.
In conclusion, this report describes the identification and characterization of an oxidoreductase-like protein Olp1 in C. neoformans. The functional study of this protein reveals that the Olp1 protein is involved in the regulation of sexual reproduction and virulence in C. neoformans. This protein may be involved in RNA processing and synthesis; it may also play a key role in maintaining lithium-ion homeostasis and protecting fungi from lithium-ion toxicity.
Acknowledgments
We acknowledge use of the C. neoformans genome sequences at the Broad Institute.
Supplementary Materials
The following Material is available online at https://www.mdpi.com/2076-2607/8/11/1730/s1, Figure S1. Verification of Olp1-mCherry strains. (A) Detection of Olp1-mCherry protein by Western blot; M: Easysee protein marker; 1: mCherry protein; 2: Olp1-mCherry fusion protein. (B) Phenotype verification of Olp1-mCherry strains; Olp1-mCherry strains could rescue the LiCl sensitive phenotype of olp1Δ mutants.
Author Contributions
Conceptualization, Q.-K.Y. and T.-B.L.; data curation, Q.-K.Y., L.-T.H. and Y.-J.W.; formal analysis, Q.-K.Y. and T.-B.L.; funding acquisition, T.-B.L.; investigation, Q.-K.Y.; methodology, Q.-K.Y. and T.-B.L.; project administration, Q.-K.Y.; resources, Q.-K.Y. and L.-T.H.; supervision, T.-B.L.; validation, Q.-K.Y.; writing—original draft, Q.-K.Y.; writing—review and editing, T.-B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (31970145, 31400133), Chongqing Research Program of Basic Research and Frontier Technology (cstc2017jcyjBX0034), and the Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2018084).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.May R.C., Stone N.R., Wiesner D.L., Bicanic T., Nielsen K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016;14:106–117. doi: 10.1038/nrmicro.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park B.J., Wannemuehler K.A., Marston B.J., Govender N., Pappas P.G., Chiller T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.Rajasingham R., Smith R.M., Park B.J., Jarvis J.N., Govender N.P., Chiller T.M., Denning D.W., Loyse A., Boulware D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017;17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Vilgalys R., Mitchell T.G. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 2000;9:1471–1481. doi: 10.1046/j.1365-294x.2000.01021.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwon-Chung K.J., Boekhout T., Fell J.W., Diaz M. (1557) Proposal to Conserve the Name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae) Taxon. 2002;51:804–806. doi: 10.2307/1555045. [DOI] [Google Scholar]
- 6.Hagen F., Khayhan K., Theelen B., Kolecka A., Polacheck I., Sionov E., Falk R., Parnmen S., Lumbsch H.T., Boekhout T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Kwon-Chung K.J., Bennett J.E., Wickes B.L., Meyer W., Cuomo C.A., Wollenburg K.R., Bicanic T.A., Castaneda E., Chang Y.C., Chen J.H., et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere. 2017;2 doi: 10.1128/mSphere.00357-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Lin J., Fan Y., Lin X. Life Cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 2019;73:17–42. doi: 10.1146/annurev-micro-020518-120210. [DOI] [PubMed] [Google Scholar]
- 9.Lin X., Heitman J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006;60:69–105. doi: 10.1146/annurev.micro.60.080805.142102. [DOI] [PubMed] [Google Scholar]
- 10.Lin X., Hull C.M., Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- 11.Kozel T.R. Virulence factors of Cryptococcus neoformans. Trends Microbiol. 1995;3:295–299. doi: 10.1016/S0966-842X(00)88957-X. [DOI] [PubMed] [Google Scholar]
- 12.Kronstad J., Jung W.H., Hu G. Beyond the big three: Systematic analysis of virulence factors in Cryptococcus neoformans. Cell Host Microbe. 2008;4:308–310. doi: 10.1016/j.chom.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Cox G.M., Mukherjee J., Cole G.T., Casadevall A., Perfect J.R. Urease as a virulence factor in experimental cryptococcosis. Infect. Immun. 2000;68:443–448. doi: 10.1128/IAI.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox G.M., McDade H.C., Chen S.C., Tucker S.C., Gottfredsson M., Wright L.C., Sorrell T.C., Leidich S.D., Casadevall A., Ghannoum M.A., et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol. Microbiol. 2001;39:166–175. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi V., Wong B., Newman S.L. Oxidative killing of Cryptococcus neoformans by human neutrophils. Evidence that fungal mannitol protects by scavenging reactive oxygen intermediates. J. Immunol. 1996;156:3836–3840. [PubMed] [Google Scholar]
- 16.Chaturvedi V., Flynn T., Niehaus W.G., Wong B. Stress tolerance and pathogenic potential of a mannitol mutant of Cryptococcus neoformans. Pt 4Microbiology. 1996;142:937–943. doi: 10.1099/00221287-142-4-937. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y., Davis M.J., Dayrit J.K., Hadd Z., Meister D.L., Osterholzer J.J., Williamson P.R., Olszewski M.A. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS ONE. 2012;7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jong A., Wu C.H., Gonzales-Gomez I., Kwon-Chung K.J., Chang Y.C., Tseng H.K., Cho W.L., Huang S.H. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J. Biol. Chem. 2012;287:15298–15306. doi: 10.1074/jbc.M112.353375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odom A., Muir S., Lim E., Toffaletti D.L., Perfect J., Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu K., Tham R., Uhrig J.P., Thompson G.R., 3rd, Na Pombejra S., Jamklang M., Bautos J.M., Gelli A. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5:e01101-14. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na Pombejra S., Salemi M., Phinney B.S., Gelli A. The Metalloprotease, Mpr1, Engages AnnexinA2 to Promote the Transcytosis of Fungal Cells across the Blood-Brain Barrier. Front. Cell. Infect. Microbiol. 2017;7:296. doi: 10.3389/fcimb.2017.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May S.W., Padgette S.R. Oxidoreductase Enzymes in Biotechnology: Current Status and Future Potential. Bio/Technology. 1983;1:677. doi: 10.1038/nbt1083-677. [DOI] [Google Scholar]
- 23.King A., Selak M.A., Gottlieb E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb E., Tomlinson I.P. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat. Rev. Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 25.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016;5:546–557. doi: 10.1002/cam4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen J., Barrios R.J., Jaiswal A.K. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010;70:1006–1014. doi: 10.1158/0008-5472.CAN-09-2938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Grunblatt E., Riederer P. Aldehyde dehydrogenase (ALDH) in Alzheimer’s and Parkinson’s disease. J. Neural Transm. (Vienna) 2016;123:83–90. doi: 10.1007/s00702-014-1320-1. [DOI] [PubMed] [Google Scholar]
- 28.Marchitti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 2008;4:697–720. doi: 10.1517/17425255.4.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H., DeLano F.A., Parks D.A., Jamshidi N., Granger D.N., Ishii H., Suematsu M., Zweifach B.W., Schmid-Schonbein G.W. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA. 1998;95:4754–4759. doi: 10.1073/pnas.95.8.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry C.E., Hare J.M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z., Wang J., Chai R., Qiu H., Jiang H., Mao X., Wang Y., Liu F., Sun G. An S-(hydroxymethyl)glutathione dehydrogenase is involved in conidiation and full virulence in the rice blast fungus Magnaporthe oryzae. PLoS ONE. 2015;10:e0120627. doi: 10.1371/journal.pone.0120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedrini N., Ortiz-Urquiza A., Huarte-Bonnet C., Fan Y., Juarez M.P., Keyhani N.O. Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. Proc. Natl. Acad. Sci. USA. 2015;112:E3651–E3660. doi: 10.1073/pnas.1504552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang W., Fa Z., Liao W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet. Biol. 2015;78:7–15. doi: 10.1016/j.fgb.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Grahl N., Puttikamonkul S., Macdonald J.M., Gamcsik M.P., Ngo L.Y., Hohl T.M., Cramer R.A. In Vivo hypoxia and a fungal alcohol dehydrogenase influence the pathogenesis of invasive pulmonary aspergillosis. PLoS Pathog. 2011;7:e1002145. doi: 10.1371/journal.ppat.1002145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhter S., McDade H.C., Gorlach J.M., Heinrich G., Cox G.M., Perfect J.R. Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans. Infect. Immun. 2003;71:5794–5802. doi: 10.1128/IAI.71.10.5794-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giles S.S., Perfect J.R., Cox G.M. Cytochrome c peroxidase contributes to the antioxidant defense of Cryptococcus neoformans. Fungal Genet. Biol. 2005;42:20–29. doi: 10.1016/j.fgb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Suvarna K., Bartiss A., Wong B. Mannitol-1-phosphate dehydrogenase from Cryptococcus neoformans is a zinc-containing long-chain alcohol/polyol dehydrogenase. Pt 10Microbiology. 2000;146:2705–2713. doi: 10.1099/00221287-146-10-2705. [DOI] [PubMed] [Google Scholar]
- 38.Missall T.A., Pusateri M.E., Lodge J.K. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol. Microbiol. 2004;51:1447–1458. doi: 10.1111/j.1365-2958.2004.03921.x. [DOI] [PubMed] [Google Scholar]
- 39.Missall T.A., Lodge J.K. Thioredoxin reductase is essential for viability in the fungal pathogen Cryptococcus neoformans. Eukaryot Cell. 2005;4:487–489. doi: 10.1128/EC.4.2.487-489.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida F., Wolf J.M., Casadevall A. Virulence-Associated Enzymes of Cryptococcus neoformans. Eukaryot Cell. 2015;14:1173–1185. doi: 10.1128/EC.00103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue C.Y., Tada Y., Dong X.N., Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe. 2007;1:263–273. doi: 10.1016/j.chom.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Liu T.B., Wang Y., Stukes S., Chen Q., Casadevall A., Xue C. The F-Box protein Fbp1 regulates sexual reproduction and virulence in Cryptococcus neoformans. Eukaryot Cell. 2011;10:791–802. doi: 10.1128/EC.00004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perfect J.R., Ketabchi N., Cox G.M., Ingram C.W., Beiser C.L. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J. Clin. Microbiol. 1993;31:3305–3309. doi: 10.1128/JCM.31.12.3305-3309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nielsen K., Cox G.M., Wang P., Toffaletti D.L., Perfect J.R., Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect. Immun. 2003;71:4831–4841. doi: 10.1128/IAI.71.9.4831-4841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan C.L., Han L.T., Jiang S.T., Chang A.N., Zhou Z.Y., Liu T.B. The Cys2His2 zinc finger protein Zfp1 regulates sexual reproduction and virulence in Cryptococcus neoformans. Fungal Genet. Biol. 2019;124:59–72. doi: 10.1016/j.fgb.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Fraser J.A., Subaran R.L., Nichols C.B., Heitman J. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: Implications for an outbreak on Vancouver Island, Canada. Eukaryot Cell. 2003;2:1036–1045. doi: 10.1128/EC.2.5.1036-1045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M.S., Kim S.Y., Yoon J.K., Lee Y.W., Bahn Y.S. An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem. Biophys. Res. Commun. 2009;390:983–988. doi: 10.1016/j.bbrc.2009.10.089. [DOI] [PubMed] [Google Scholar]
- 48.Basenko E.Y., Pulman J.A., Shanmugasundram A., Harb O.S., Crouch K., Starns D., Warrenfeltz S., Aurrecoechea C., Stoeckert C.J., Jr., Kissinger J.C., et al. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J. Fungi. 2018;4:39. doi: 10.3390/jof4010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S.C., Heitman J. Function of Cryptococcus neoformans KAR7 (SEC66) in karyogamy during unisexual and opposite-sex mating. Eukaryot Cell. 2012;11:783–794. doi: 10.1128/EC.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berridge M.J., Downes C.P., Hanley M.R. Neural and developmental actions of lithium: A unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 51.Spiegelberg B.D., Dela Cruz J., Law T.H., York J.D. Alteration of lithium pharmacology through manipulation of phosphoadenosine phosphate metabolism. J. Biol. Chem. 2005;280:5400–5405. doi: 10.1074/jbc.M407890200. [DOI] [PubMed] [Google Scholar]
- 52.Stambolic V., Ruel L., Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 53.Dichtl B., Stevens A., Tollervey D. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J. 1997;16:7184–7195. doi: 10.1093/emboj/16.23.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murguia J.R., Belles J.M., Serrano R. The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. J. Biol. Chem. 1996;271:29029–29033. doi: 10.1074/jbc.271.46.29029. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Lin W., Ma X., Lu Q., Ma X., Bian G., Jiang L. The protein kinase Hal5p is the high-copy suppressor of lithium-sensitive mutations of genes involved in the sporulation and meiosis as well as the ergosterol biosynthesis in Saccharomyces cerevisiae. Genomics. 2010;95:290–298. doi: 10.1016/j.ygeno.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 56.Vermes A., Guchelaar H.J., Dankert J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 57.Ghoshal K., Jacob S.T. Specific inhibition of pre-ribosomal RNA processing in extracts from the lymphosarcoma cells treated with 5-fluorouracil. Cancer Res. 1994;54:632–636. [PubMed] [Google Scholar]
- 58.Santi D.V., Hardy L.W. Catalytic mechanism and inhibition of tRNA (uracil-5-)methyltransferase: Evidence for covalent catalysis. Biochemistry. 1987;26:8599–8606. doi: 10.1021/bi00400a016. [DOI] [PubMed] [Google Scholar]
- 59.Patton J.R. Ribonucleoprotein particle assembly and modification of U2 small nuclear RNA containing 5-fluorouridine. Biochemistry. 1993;32:8939–8944. doi: 10.1021/bi00085a027. [DOI] [PubMed] [Google Scholar]
- 60.Yarbro J.W. Mechanism of action of hydroxyurea. Semin. Oncol. 1992;19:1–10. [PubMed] [Google Scholar]
- 61.Lundin C., North M., Erixon K., Walters K., Jenssen D., Goldman A.S., Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 2005;33:3799–3811. doi: 10.1093/nar/gki681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costanzo M., VanderSluis B., Koch E.N., Baryshnikova A., Pons C., Tan G., Wang W., Usaj M., Hanchard J., Lee S.D., et al. A global genetic interaction network maps a wiring diagram of cellular function. Science. 2016;353 doi: 10.1126/science.aaf1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giaever G., Chu A.M., Ni L., Connelly C., Riles L., Veronneau S., Dow S., Lucau-Danila A., Anderson K., Andre B., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez R., Sali A. Large-scale protein structure modeling of the Saccharomyces cerevisiae genome. Proc. Natl. Acad. Sci. USA. 1998;95:13597–13602. doi: 10.1073/pnas.95.23.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahlberg K.R., Etten J.L.V. Physiology and Biochemistry of Fungal Sporulation. Annu. Rev. Phytopathol. 1982;20:281–301. doi: 10.1146/annurev.py.20.090182.001433. [DOI] [Google Scholar]
- 66.Huang M., Hull C.M. Sporulation: How to survive on planet Earth (and beyond) Curr. Genet. 2017;63:831–838. doi: 10.1007/s00294-017-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lara-Ortiz T., Riveros-Rosas H., Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 2003;50:1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- 68.Cano-Dominguez N., Alvarez-Delfin K., Hansberg W., Aguirre J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell. 2008;7:1352–1361. doi: 10.1128/EC.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C., Lin Y., Wang J., Wang Y., Chen M., Norvienyeku J., Li G., Yu W., Wang Z. FgNoxR, a regulatory subunit of NADPH oxidases, is required for female fertility and pathogenicity in Fusarium graminearum. FEMS Microbiol. Lett. 2016;363:fnv223. doi: 10.1093/femsle/fnv223. [DOI] [PubMed] [Google Scholar]
- 70.Fisher S.K., Novak J.E., Agranoff B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002;82:736–754. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 71.Barnett J.A. The utilization of sugars by yeasts. Adv. Carbohydr. Chem. Biochem. 1976;32:125–234. doi: 10.1016/s0065-2318(08)60337-6. [DOI] [PubMed] [Google Scholar]
- 72.Healy M.E., Dillavou C.L., Taylor G.E. Diagnostic medium containing inositol, urea, and caffeic acid for selective growth of Cryptococcus neoformans. J. Clin. Microbiol. 1977;6:387–391. doi: 10.1128/jcm.6.4.387-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu T.B., Kim J.C., Wang Y., Toffaletti D.L., Eugenin E., Perfect J.R., Kim K.J., Xue C. Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog. 2013;9:e1003247. doi: 10.1371/journal.ppat.1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.