Abstract
Childhood obesity contributes to many diseases, including asthma. There is literature to suggest that asthma developing as a consequence of obesity has a non-allergic or non-T2 phenotype. In this review, we use obesity-related asthma as a prototype of non-T2 asthma in children to discuss non-allergic mechanisms underlying severe childhood asthma. Obese asthmatic children have evidence of systemic T helper (Th)1 polarization with monocyte activation, which are mediated by insulin resistance and dyslipidemia, the common metabolic abnormalities associated with obesity, and are associated with pulmonary function deficits found in obese asthmatics. In addition to pleiotropy, or common genetic influence between obesity and asthma, DNA methylation, with hypomethylation of promoters of genes associated with non-T2 immune responses has been associated with pediatric obesity-related asthma. A transcriptomic approach to investigate the pathways underlying non-T2 inflammation in asthma has identified upregulation of genes in the CDC42 pathway. CDC42 is a RhoGTPase that plays a key role in Th cell physiology, including preferential Th cell differentiation to Th1 cells, and cytokine production and exocytosis. Until further investigation of the CDC42 pathway and other novel pathways is conducted to identify targeted therapies for obesity-related asthma, dietary interventions, including diet modification, rather than caloric restriction alone, may be considered to decrease the disease burden. Carotenoids are protective against metabolic abnormalities and both carotenoids and 25-OH cholecalciferol, a vitamin D metabolite, positively correlate with pulmonary function indices suggesting that diet rich in these micronutrients may be beneficial for obese children with asthma.
Keywords: Asthma, Early Wheeze, Obesity, Immune Response
Obesity and asthma- a causal association
Increase in the prevalence of childhood obesity over the past several decades has identified its myriad effects and contribution to chronic pediatric diseases, including asthma. While initial cross-sectional studies identified an association between obesity and asthma, prospective studies, and more recently, meta-analyses, have consistently found obesity to be an independent predictor of asthma1. Overweight/ obese children have a relative risk of 1.2–1.8 for incident asthma1–6. This association varies by sex7,8. Moreover, the association between asthma and obesity appears to be bidirectional, since asthma increases the relative risk for obesity by 1.5 to 1.7 fold9,10. Although few studies have investigated the underlying biologic mechanisms, there is epidemiologic evidence to suggest that asthma developing as a consequence of obesity is non-allergic4,11.
In this review, I use obesity-related asthma as a prototype of non-T2 asthma in children to discuss the non-allergic mechanisms by which obesity contributes to asthma. The review includes discussion on the influence of genetic predisposition, and that of acquired abnormalities, including obesity-mediated systemic immune alterations and metabolic abnormalities, since these two aspects of obesity have been best investigated thus far, are associated with higher disease severity with poor medication responsiveness and disease control, and are distinct from mechanisms that underlie allergic childhood asthma12–14. In addition, the association of dietary micronutrients with disease burden is discussed to highlight dietary modification as a potential therapeutic approach for childhood obesity-related asthma. These mechanisms are summarized in Figure 1.
Figure 1.
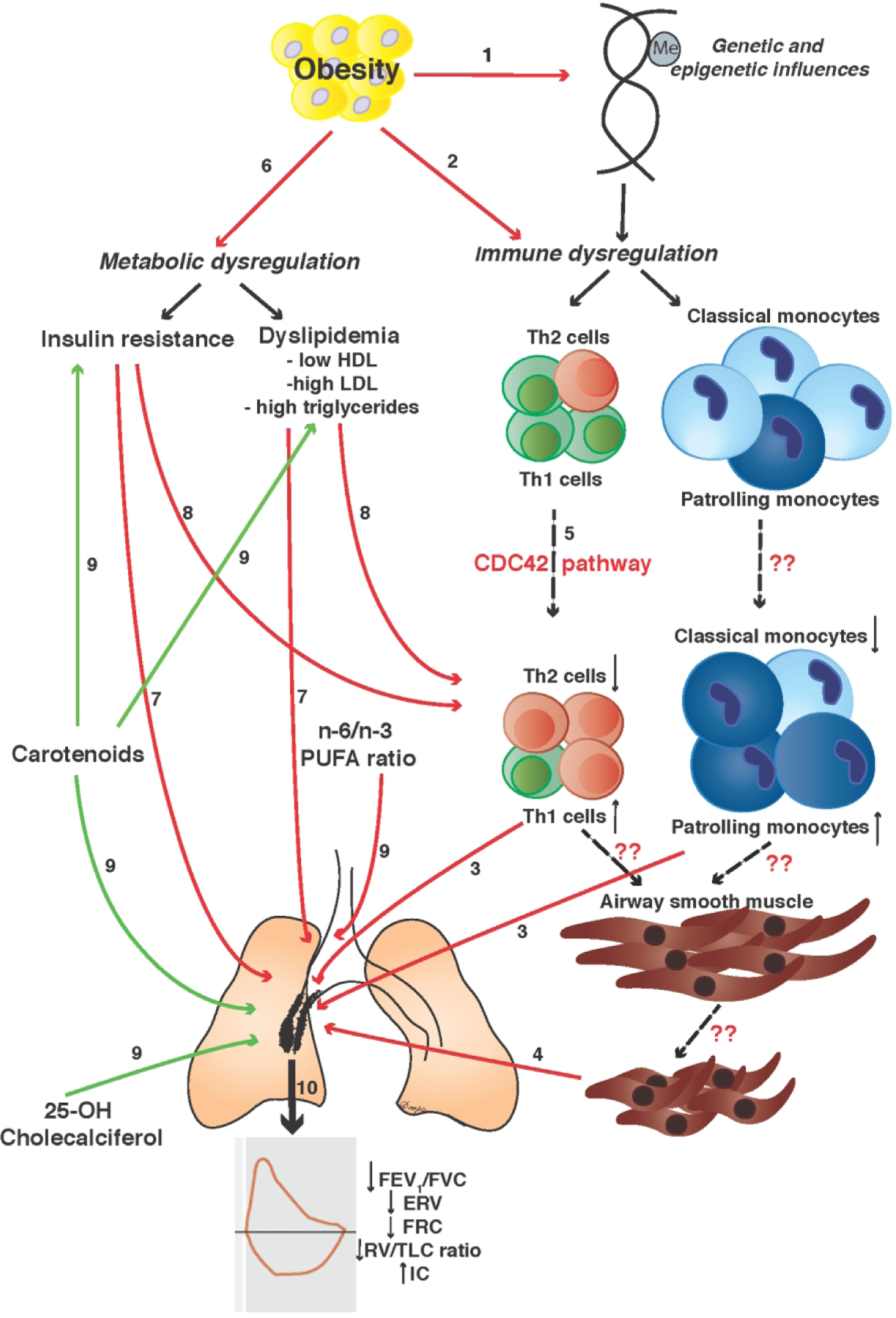
This figure summarizes the various mechanisms by which obesity is associated with asthma. 1) Obesity shares genetic influences with asthma and is also associated with DNA hypomethylation of promoters of genes associated with non-T2 immune responses. 2) Obesity causes immune dysregulation with higher Th1/Th2 ratio or Th1 polarization and increased activation of monocytes with more patrolling and fewer classical monocytes in systemic circulation. 3) Th1 polarization and monocyte activation are inversely associated with pulmonary function deficits found in obese children with asthma. 4) Whether obesity-mediated immune dysregulation links with pulmonary function through increased airway smooth muscle contractility is not known. 5) Although the mechanisms that underlie Th1 polarization and monocyte activation are not well understood (dashed arrows), upregulation of genes in CDC42 pathway have been reported; CDC42 causes preferential differentiation of naïve Th cells to Th1 cells and cytokine production and exocytosis. 6) Insulin resistance and dyslipidemia are the most common obesity-mediated metabolic abnormalities. Insulin resistance is directly associated and HDL is inversely associated with 7) pulmonary function deficits and 8) Th1 polarization found in obese children with asthma. Insulin resistance mediates the association of Th1 polarization with pulmonary function deficits. 9) Micronutrients are associated with pulmonary function deficits in obese children with asthma; while carotenoids and 25-hydroxy cholecalciferol are protective, n-6/n-3 polyunsaturated fatty acid (PUFA) ratio is associated with lower pulmonary function. Carotenoids are also protective against insulin resistance and dyslipidemia. 10) Together, these mechanisms are associated with pulmonary function deficits associated with obesity-related asthma.
Genetics and epigenetics of pediatric obesity-related asthma.
There is evidence of pleiotropy, or common genetic influence, between obesity and asthma15,16. In a predominantly Caucasian cohort of same-sex twins, there was high heritability for both asthma (53%) and obesity (77%)17. Moreover, higher between-twin correlation for asthma and obesity among monozygotic as compared to dizygotic twins suggests a genetic contribution to the association between asthma and obesity17. Specific genes have also been associated with asthma and body mass index. In a study including a predominantly Caucasian cohort and a Costa Rican cohort, several single nucleotide polymorphisms (SNPs) in the Protein Kinase C Alpha (PRKCA) gene linked asthma with body mass index15,16. More recently, Li et.al. took a unique approach of investigating the risk conferred by known SNPs associated with obesity to incident asthma and risk conferred by known SNPs associated with asthma to incident obesity. They found that genetic predisposition to obesity was associated with significantly higher odds of developing asthma, while the contribution of genetic susceptibility for asthma to developing incident obesity was lower and not significant18. Although these studies have laid the foundation for investigation of genetic contribution to obesity-related asthma, there is much detail that still needs to be elucidated, particularly investigation of genetic susceptibility in minority populations, who have a higher burden of both asthma and obesity19,20.
In addition, it is known that gene by environment interactions play a role in multifactorial diseases like asthma and obesity21,22. DNA methylation is one of the better investigated epigenetic mechanisms that may explain the gene by environment interaction for these multifactorial diseases23,24. We and others have reported on DNA methylation patterns in peripheral blood mononuclear cells that are distinct in obese children with asthma as compared to obese children without asthma or healthy-weight children with asthma [Figure 1]25. We found DNA hypomethylation of several gene promoters, including CCL5, IL27, IL2RA, STAT1, IFNG, and TBX21, which are associated with Th1 polarization and monocyte activation, and hypermethylation of FCER2, associated with IgE mediated immune responses, and SOCS2, SOCS3, and TGFB1, which diminish Th cell pro-inflammatory responses. In addition, several genes associated with immunometabolic responses including PPARG, PIK3R1 and PIK3AP1 were hypomethylated while ALOX15 was hypermethylated in peripheral blood mononuclear cells from obese children with asthma25. Together, this pilot study highlighted that epigenetics may identify differentially activated immunometabolic pathways in obese asthmatic children. A recent study conducted on Swiss adults with non-atopic asthma to investigate the modifying effect of obesity revealed differential DNA methylation in several genes that overlapped with the ones that our group had found among minority children with obesity-related asthma26. These studies suggest a contribution of DNA methylation to the obese asthma phenotype. As these patterns are better understood, they will reveal genetic mechanisms that may predispose to obese asthma and may be modifiable to therapeutic intervention.
Immune profile of pediatric obesity-related asthma
Obesity is a state of low-grade systemic inflammation initiated by the relative hypoxic environment of rapidly proliferating adipose tissue27 and sustained by leptin, a pro-inflammatory adipokine28. As understood thus far, the hypoxic adipocytes release monocyte chemotactic protein (MCP-1) in response to which monocytes are recruited to adipose tissue where they differentiate into M1 macrophages29–31. These macrophages orchestrate local and systemic inflammatory responses, with preferential recruitment and activation of pro-inflammatory T helper (Th)1 cells relative to anti-inflammatory T regulatory cells, augmenting systemic pro-inflammatory response32. Traditionally, Th1 cell activation is inversely associated with Th2 cell activation33. This association is pertinent in the context of obesity-related asthma since obesity is associated with Th1 activation with neutrophilic inflammation34, while classic childhood asthma35 has a Th2 phenotype with eosinophilic inflammation33. Given these two distinct patterns of systemic inflammation in obesity and asthma, my research group and others have investigated the inflammatory phenotype in obesity-related asthma, when both obesity and asthma co-exist. As compared to healthy-weight asthmatic children and obese non-asthmatic controls, obese girls had evidence of non-eosinophilic asthma36. Among predominantly African American and Hispanic pre-adolescent and adolescent cohorts, both groups had evidence of systemic Th1 polarization, with an elevated Th1/Th2 ratio, relative to their healthy-weight counterparts, findings that did not differ by sex37,38. Furthermore, Th1 polarization correlated with leptin, and IL-6, a cytokine downstream in the leptin-mediated immune pathway, suggesting that obesity was driving the Th1 systemic inflammation in obese asthmatic children [Figure 1]37.
Investigation of monocyte activation patterns in the adolescent cohort further validated the role of obesity-mediated inflammation in obesity-related asthma. Obese asthmatic adolescents had evidence of monocyte activation, with fewer classical monocytes, and more patrolling monocytes as compared to healthy-weight controls [Figure 1]38. Although the proportion of classical and patrolling monocytes did not differ between obese asthmatic and obese non-asthmatic children, Th1/Th2 ratio inversely correlated with classical monocytes and directly correlated with patrolling monocytes only in obese asthmatic children, suggesting that obesity-mediated inflammation was more robust in obese children with asthma as compared to the obese non-asthmatic controls38. These immune patterns provide the framework for this review on obesity-related asthma as a prototype of non-T2 asthma in children.
Pulmonary function deficits in obesity-related asthma and their association with the systemic immune profile
Pulmonary function deficits in obese asthmatic children have been extensively described and recently summarized in a meta-analysis39. As compared to air trapping observed in the context of airflow obstruction and increased airway resistance in healthy-weight asthma40, lower FEV1/FVC ratio in obese children with asthma co-occurs with lower lung volumes including lower functional residual capacity (FRC), expiratory reserve volume (ERV), and residual volume (RV) relative to total lung capacity (TLC)37,38,41,42. The overlap in these pulmonary function patterns between children and adults39 suggests that the impact of obesity on pulmonary physiology starts early in life. Moreover, this constellation of pulmonary function abnormalities suggests that alteration in airway caliber, potentially due to altered diaphragmatic movement, rather than inherent airway hyper-responsiveness, underlies the obese asthma phenotype43–46. This speculation is supported by greater contribution of truncal adiposity as compared to general adiposity to disease burden and pulmonary function abnormalities in obese asthmatics47,48. Associations between systemic non-atopic Th1 polarized immune patterns with pulmonary function deficits have been reported in obese children with asthma. While FEV1/FVC ratio inversely correlated with circulating biomarkers of Th1 inflammation, including interferon-gamma (IFNy) and interferon-gamma induced protein-10 (IP-10), in obese asthmatic pre-adolescents37, systemic Th1 polarization inversely correlated with RV, RV/TLC ratio and FRC in adolescents [Figure 1]38.
In addition to Th1 polarization, monocyte activation is associated with disease burden and low lung volumes in obese children with asthma [Figure 1]38. While the proportion of classical monocytes directly correlated with better asthma control, proportion of patrolling monocytes correlated with asthma severity38. CCR2 expression, a marker of monocyte differentiation that decreases as classical monocytes differentiate into patrolling monocytes49, directly correlated with lung volumes supporting an inverse association between monocyte activation and lung volumes38. These relationships were not observed in healthy-weight children with asthma. Together, these studies suggest that obesity mediated systemic non-atopic inflammation is associated with asthma disease burden and pulmonary function deficits.
Metabolic dysregulation is associated with obesity-related asthma.
In addition to influencing the systemic immune profile, childhood obesity is associated with metabolic abnormalities50. Obesity-mediated inflammation likely underlies development of insulin resistance and dyslipidemia51, the two metabolic abnormalities commonly found in obese children. Several studies have reported on higher prevalence of insulin resistance, its surrogate marker, acanthosis nigricans, and dyslipidemia (decreased HDL, in context of increased LDL, total cholesterol levels, and triglycerides) in asthmatic children as compared to non-asthmatic children52–54. A linear relationship between insulin resistance quantified using homeostatic measurement of insulin resistance (HOMA-IR) and asthma prevalence has been described in children52. Insulin resistance and dyslipidemia are inversely related to pulmonary function [Figure 1]55,56. Specifically, higher levels of insulin resistance and lower levels of HDL are associated with lower FEV1/FVC ratio and insulin resistance is an independent predictor of ERV, when adjusted for general and truncal adiposity55. These findings support a role of metabolic abnormalities in altered pulmonary function in obese asthmatics, independent of truncal adiposity55.
The pathophysiologic mechanisms by which metabolic abnormalities impact pulmonary physiology are poorly understood. It is known that airway smooth muscle cells express insulin receptors and develop a pro-contractile phenotype when exposed to insulin57,58. However, a recent investigation of the obese asthmatic airway smooth muscle (ASM) phenotype found that although obese asthmatic ASM cells from adult donors had higher calcium influx in response to muscarinic stimulation and were more contractile than healthy-weight ASM cells, the higher calcium influx was independent of insulin pre-treatment [Figure 1]59. Based on these findings, and the association of insulin resistance with pulmonary function, it may be speculated that insulin influences pulmonary physiology through altered immune responses rather than directly inducing increased ASM contractility. Mechanisms that explain the association of lipids with the obese asthma phenotype and interaction between dyslipidemia and insulin resistance are not known60.
Metabolic dysregulation mediates the association of systemic inflammation and pulmonary function among obese asthmatic children
In keeping with the literature on the pro-inflammatory role of insulin resistance and dyslipidemia61, frequently referred to as metabolic syndrome, we have reported an association between markers of metabolic dysregulation and immune responses in the obese children with asthma. Insulin resistance directly correlated with Th1/Th2 ratio38, while serum HDL was negatively associated with Th1/Th2 ratio and patrolling monocytes, which are elevated in obesity [Figure 1]38. Given the association of metabolic dysregulation as well as non-T2 inflammation with decreased pulmonary function, we investigated whether the association of Th1 polarization with pulmonary function was mediated by metabolic abnormalities associated with the obesity-mediated immune responses. We indeed found that insulin resistance mediated the association of Th1 polarization with pulmonary function indices. Although HDL was inversely associated with Th1 polarization and also with FEV1/FVC ratio, the association of Th1 polarization and of monocytes with FEV1/FVC ratio was not mediated by HDL levels38. Together, these relationships suggest that there is a complex relationship between metabolic dysregulation, obesity-mediated immune responses, and their association with pulmonary function in obese children with asthma62.
Novel pathways underlying non-allergic immune responses in obesity-related asthma
In light of the findings detailed above, it is evident that obesity-mediated non-T2 immune responses and metabolic dysregulation are associated with obesity-related asthma which has high disease burden that is not responsive to currently available medications. These observations are further supported by the presence of an endotype of low-atopy in conjunction with obesity among participants of the Severe Asthma Research Program63. Although the mechanisms that underlie T2 asthma have been extensively investigated, leading to the development of targeted therapies like omalizumab, an anti-IgE antibody64, there is a dearth of information on the biology of non-T2 asthma. To address this knowledge gap, taking an unbiased transcriptomic approach, gene expression in CD4+ T cells (Th cells) from obese children was compared to that that in Th cells from healthy-weight children with asthma65. There was upregulation of several genes in the CDC42 pathway, among which transcripts of two genes, CDC42EP4 and DOCK5 inversely correlated with FEV1/FVC ratio, uniquely among obese asthmatic children [Figure 1]65. CDC42 is a RhoGTPase that plays a key role in several aspects of Th cell physiology, including Th cell differentiation, preferentially to Th1 cells66, and in cytokine production and exocytosis67. Based on these studies, it may be hypothesize that CDC42 pathway is one potential mechanism that underlies the non-T2 responses in obesity-related asthma. Intriguingly, CDC42 was found to play a role in c-Jun N-terminal kinase (JNK) signaling pathway activation in fatty liver disease, suggesting that signaling through small Rho-GTPases may be a common mechanism underlying diseases occurring due to obesity-mediated inflammation68. Given the ubiquitous role of CDC42 in cellular function69, future studies are needed to investigate the specific downstream pathways that are upregulated in obese asthmatic Th cells and the mechanisms by which these systemic inflammatory patterns contribute to the obese asthma phenotype.
Management of obesity-related asthma: Need for a different approach
Putting together the different obesity-mediated mechanisms that explain the pathophysiology of obesity-related non-T2 asthma, it is evident that the current approach for childhood asthma management may not be effective in obese children with asthma. Indeed, obese children with asthma are poorly responsive/ non-responsive to currently available asthma controller and reliever medications13,14. There have been a few investigations on the use of inhibitors of non-T2 immune responses, such as anti-TNF therapy, for asthma70. Although some, but not all, studies found an improvement in asthma symptoms among individuals with severe asthma71, the occurrence of severe side-effects, including secondary infections72 has prevented further clinical trials and inclusion of these medications in mainstream asthma management. Thus, until there is development of effective and safe targeted therapies for non-T2 immune responses for asthma, we need to take a different approach for the management of obesity-related asthma.
One of the approaches to manage obesity-related asthma is to address body weight. Weight loss studies conducted in children have shown an improvement in asthma disease burden, including improvement in pulmonary function, although there was no change in systemic inflammation73. These findings differed from pre-post studies on adult bariatric surgery patients where in addition to improvement in airway responsiveness74 and lung volumes75, there was improvement in systemic inflammatory measures which correlated with improvement in pulmonary function, supporting a role of systemic immune responses in the obese asthma phenotype74–76. In light of conflicting data between adults and children, there remains a need for mechanistic studies to elucidate the impact of differences in extent, timing, and modality of weight loss/ weight control during different stages of life, to decrease in disease burden in obesity-related asthma.
The contribution of dietary factors to obesity-related asthma
It is known that weight loss among children, particularly among those of minority ethnicity, is difficult77. Furthermore, since very early weight gain among children is associated with incident asthma1,78,79, weight loss may not be a very effective strategy for management of obesity-related asthma in children. Instead, diet modification with inclusion of healthy dietary choices may address both the asthma disease burden as well as obesity-mediated complications including immune abnormalities and metabolic dysregulation. Thus, diet modification may offer a more feasible approach to manage obesity-related asthma.
Carotenoids, vitamin D, and fatty acids
Several studies have linked dietary intake to asthma disease burden, starting as early as dietary choices made by the mother during the antenatal period80. A higher proportion of processed food relative to fruit and vegetable intake has been associated with higher asthma incidence and disease burden81. Although vitamin A and D supplementation decreases the disease burden80, few studies have quantified differences in these micronutrients in obese as compared to healthy-weight children with asthma. My lab has previously reported in 25-OH cholecalciferol levels and recently published on the differences in circulating carotenoids as an objective measure of fruit and vegetable intake in obese and healthy-weight children with asthma [Figure 1]82,83. Low 25-hydroxy cholecalciferol levels were associated with lower pulmonary function in obese, but not healthy weight children with asthma. Similarly, while carotenoid levels were associated with higher FEV1/FVC ratio as well as improved metabolic profile, with decreased insulin resistance and higher HDL levels, only among obese, but not healthy-weight children with asthma82,83. Together, these studies support the approach of modified dietary intake for pediatric obesity-related asthma. Whether it will be more effective than weight loss approaches in decreasing disease burden needs to be investigated.
Conclusion
In summary, I have discussed childhood obesity-related asthma as a prototype of pediatric severe non-T2 asthma. Several distinct mechanisms that are associated with disease burden and pulmonary function deficits have been summarized, including the association of Th1 polarization with lower FEV1/FVC ratio and lower ERV and FRC, an association that is mediated by insulin resistance. Until there is development of targeted therapies for non-T2 asthma, there is evidence to suggest that diet modification may be a potential approach for disease management. More importantly, this review highlights the dearth of literature on non-T2 asthma in children, specifically focusing on pediatric obesity-related asthma, the prevalence of which is on the increase, given the increase in obesity prevalence in children. The review also highlights several areas of future research, including the need to elucidate mechanisms that underlie non-T2 asthma, with the goal to identify therapeutic targets, that will be specifically developed for non-T2 systemic immune responses, and will be safe as well as effective.
References:
- 1.Lang JE, Bunnell HT, Hossain MJ, et al. Being Overweight or Obese and the Development of Asthma. Pediatrics. 2018;142(6). [DOI] [PubMed] [Google Scholar]
- 2.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003;36(6):514–521. [DOI] [PubMed] [Google Scholar]
- 3.Rzehak P, Wijga AH, Keil T, et al. Body mass index trajectory classes and incident asthma in childhood: results from 8 European Birth Cohorts--a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013;131(6):1528–1536. [DOI] [PubMed] [Google Scholar]
- 4.Gilliland FD, Berhane K, Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158(5):406–415. [DOI] [PubMed] [Google Scholar]
- 5.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14(3):222–231. [DOI] [PubMed] [Google Scholar]
- 6.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu KD, Billimek J, Bar-Yoseph R, Radom-Aizik S, Cooper DM, Anton-Culver H. Sex Differences in the Relationship between Fitness and Obesity on Risk for Asthma in Adolescents. J Pediatr. 2016;176:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maltz L, Matz EL, Gordish-Dressman H, et al. Sex differences in the association between neck circumference and asthma. Pediatr Pulmonol. 2016;51(9):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras ZA, Chen Z, Roumeliotaki T, et al. Does early onset asthma increase childhood obesity risk? A pooled analysis of 16 European cohorts. Eur Respir J. 2018;52(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Salam MT, Alderete TL, et al. Effects of Childhood Asthma on the Development of Obesity among School-aged Children. Am J Respir Crit Care Med. 2017;195(9):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinmayr G, Forastiere F, Büchele G, et al. Overweight/Obesity and Respiratory and Allergic Disease in Children: International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. PloS One. 2014;9(12):e113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borrell LN, Nguyen EA, Roth LA, et al. Childhood obesity and asthma control in the GALA II and SAGE II studies. Am J Respir Crit Care Med. 2013;187(7):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGarry ME, Castellanos E, Thakur N, et al. Obesity and bronchodilator response in black and Hispanic children and adolescents with asthma. Chest. 2015;147(6):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forno E, Lescher R, Strunk R, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melen E, Himes BE, Brehm JM, et al. Analyses of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010;126(3):631–637 e631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy A, Tantisira KG, Soto-Quiros ME, et al. PRKCA: a positional candidate gene for body mass index and asthma. Am J Hum Genet. 2009;85(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallstrand TS, Fischer ME, Wurfel MM, Afari N, Buchwald D, Goldberg J. Genetic pleiotropy between asthma and obesity in a community-based sample of twins. J Allergy Clin Immunol. 2005;116(6):1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu S, Gilliland FD, Conti DV. Elucidation of causal direction between asthma and obesity: a bi-directional Mendelian randomization study. Int J Epidemiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief. 2017(288):1–8. [PubMed] [Google Scholar]
- 20.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Mutius E Gene-environment interactions in asthma. J Aller Clin Immunol. 2009;123(1):3–11. [DOI] [PubMed] [Google Scholar]
- 22.Huang T, Hu FB. Gene-environment interactions and obesity: recent developments and future directions. BMC Med Genomics. 2015;8 Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Nahm S, Mendez MA, Benjamin-Neelon SE, et al. DNA methylation of imprinted genes at birth is associated with child weight status at birth, 1 year, and 3 years. Clin Epigenetics. 2018;10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese SE, Xu CJ, den Dekker HT, et al. Epigenome-wide meta-analysis of DNA methylation and childhood asthma. J Allergy Clin Immunol. 2019;143(6):2062–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastogi D, Suzuki M, Greally JM. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci Rep. 2013;3:2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong A, Imboden M, Ghantous A, et al. DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non-Atopic Asthma. Int J Environ Res Public Health. 2019;16(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007;31(9):1420–1428. [DOI] [PubMed] [Google Scholar]
- 28.Fantuzzi G Adipose tissue, adipokines, and inflammation. J Allergy Clin Immmunol. 2005;115(5):911–919. [DOI] [PubMed] [Google Scholar]
- 29.Ferrante AW Jr. Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262(4):408–414. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante AW Jr. The immune cells in adipose tissue. Diabetes Obes Metab. 2013;15 (Suppl. 3):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalmas E, Clément K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32(7):307–314. [DOI] [PubMed] [Google Scholar]
- 32.Matarese G, Procaccini C, De Rosa V, Horvath TL, La Cava A. Regulatory T cells in obesity: the leptin connection. Trends Mol Med. 2010;16(6):247–256. [DOI] [PubMed] [Google Scholar]
- 33.Kay AB. Allergy and Allergic Diseases. N Engl J Med. 2001;344(1):30–37. [DOI] [PubMed] [Google Scholar]
- 34.Tateda K, Moore TA, Deng JC, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol. 2001;66(5):3355–3361. [DOI] [PubMed] [Google Scholar]
- 35.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001;344(5):350–362. [DOI] [PubMed] [Google Scholar]
- 36.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42(4):1012–1019. [DOI] [PubMed] [Google Scholar]
- 37.Rastogi D, Canfield S, Andrade A, et al. Obesity-associated asthma in children: A distinct entity. Chest. 2012;141(4):895–905. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi D, Fraser S, Oh J, et al. Inflammation, Metabolic Dysregulation and Pulmonary Function Among Obese Asthmatic Urban Adolescents. Am J Resp Crit Care Med. 2015;191(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forno E, Han Y-Y, Mullen J, Celedón JC. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. The Journal of Allergy and Clinical Immunology In Practice. 2018;6(2):570–581.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jain NCR, Gleason MC, Newell JD Jr, Gelfand EW, Spahn JD. Quantitative computed tomography detects peripheral airway disease in asthmatic children. Pediatr Pulmonol. 2005;40(3):211–218. [DOI] [PubMed] [Google Scholar]
- 41.Davidson WJ, Mackenzie-Rife KA, Witmans MB, et al. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. 2014;49(10):1003–1010. [DOI] [PubMed] [Google Scholar]
- 42.Gibson N, Johnston K, Bear N, Stick S, Logie K, Hall G. Expiratory flow limitation and breathing strategies in overweight adolescents during submaximal exercise. Int J Obes. 2013;38(1):22–26. [DOI] [PubMed] [Google Scholar]
- 43.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98(2):512–517. [DOI] [PubMed] [Google Scholar]
- 44.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108(1):206–211. [DOI] [PubMed] [Google Scholar]
- 45.Salome CM, Munoz PA, Berend N, Thorpe CW, Schachter LM, King GG. Effect of obesity on breathlessness and airway responsiveness to methacholine in non-asthmatic subjects. Int J Obes. 2008;32(3):502–509. [DOI] [PubMed] [Google Scholar]
- 46.King GG, Brown NJ, Diba C, et al. The effects of body weight on airway calibre. Eur Resp J. 2005;25(5):896–901. [Google Scholar]
- 47.Papoutsakis C, Chondronikola M, Antonogeorgos G, et al. Associations between central obesity and asthma in children and adolescents: a case-control study. J Asthma. 2015;52(2):128–134. [DOI] [PubMed] [Google Scholar]
- 48.Forno E, Acosta-Pérez E, Brehm JM, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immmunol. 2014;133(5):1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94(3):875–883. [PubMed] [Google Scholar]
- 50.Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):21–29. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez DA, Puglisi MJ, Hasty AH. Impact of increased adipose tissue mass on inflammation, insulin resistance, and dyslipidemia. Curr Diab Rep. 2009;9(1):26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma. 2007;44(6):469–473. [DOI] [PubMed] [Google Scholar]
- 53.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2011;183(4):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arshi M, Cardinal J, Hill RJ, Davies PS, Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15(5):779–784. [DOI] [PubMed] [Google Scholar]
- 55.Rastogi D, Bhalani K, Hall CB, Isasi CR. Association of pulmonary function with adiposity and metabolic abnormalities in urban minority adolescents. Ann Amer Thor Soc. 2014;11(5):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immmunol. 2015;136(2):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaafsma D, Gosens R, Ris JM, Zaagsma J, Meurs H, Nelemans SA. Insulin induces airway smooth muscle contraction. Br J Pharmacol. 2007;150(2):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaafsma D, McNeill KD, Stelmack GL, et al. Insulin increases the expression of contractile phenotypic markers in airway smooth muscle. Am J Physiol Cell Physiol. 2007;293(1):C429–439. [DOI] [PubMed] [Google Scholar]
- 59.Orfanos S, Jude J, Deeney BT, et al. Obesity increases airway smooth muscle responses to contractile agonists. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L673–L681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation, and asthma. J Allergy Clin Immmunol. 2014;133(5):1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rastogi D, Holguin F. Metabolic Dysregulation, Systemic Inflammation, and Pediatric Obesity-related Asthma. Ann Am Thorac Soc. 2017;14(Supplement_5):S363–S367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Busse WW. Biological treatments for severe asthma: where do we stand? Curr Opin Allergy Clin Immunol. 2018;18(6):509–518. [DOI] [PubMed] [Google Scholar]
- 65.Rastogi D, Nico J, Johnson AD, et al. CDC42-related genes are upregulated in T helper cells from obese asthmatic children. J Allergy Clin Immunol. 2018;141(2):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Panhuys N TCR Signal Strength Alters T-DC Activation and Interaction Times and Directs the Outcome of Differentiation. Front Immunol. 2016;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chemin K, Bohineust A, Dogniaux S, et al. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189(5):2159–2168. [DOI] [PubMed] [Google Scholar]
- 68.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. 2012;56(1):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melendez J, Grogg M, Zheng Y. Signaling role of Cdc42 in regulating mammalian physiology. J Biol Chem. 2011;286(4):2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63(7):584–591. [DOI] [PubMed] [Google Scholar]
- 71.Howarth PH, Babu KS, Arshad HS, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005;60(12):1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rennard SI, Fogarty C, Kelsen S, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2007;175(9):926–934. [DOI] [PubMed] [Google Scholar]
- 73.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43(7):775–784. [DOI] [PubMed] [Google Scholar]
- 74.Dixon AE, Pratley RE, Forgione PM, et al. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128(3):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106(5):651–660. [DOI] [PubMed] [Google Scholar]
- 76.Sideleva O, Black K, Dixon AE. Effects of obesity and weight loss on airway physiology and inflammation in asthma. Pulm Pharmacol Ther. 2013;26(4):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan UI, Rieder J, Cohen HW, Coupey SM, Wildman RP. Effect of modest changes in BMI on cardiovascular disease risk markers in severely obese, minority adolescents. Obes Res Clin Pract. 2010;4(3):e163–246. [DOI] [PubMed] [Google Scholar]
- 78.Matos SMA, Jesus SR, Saldiva SRDM, et al. Weight gain in the first two years of life, asthma and atopy: the SCAALA cohort study. Public Health Nutr. 2014;17(11):2537–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sonnenschein-van der Voort AMM, Howe LD, Granell R, et al. Influence of childhood growth on asthma and lung function in adolescence. J Allergy Clin Immunol. 2015;135(6):1435–1443.e1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vijayakanthi N, Greally JM, Rastogi D. Pediatric Obesity-Related Asthma: The Role of Metabolic Dysregulation. Pediatrics. 2016;137(5):e20150812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han YY, Forno E, Alvarez M, et al. Diet, Lung Function, and Asthma Exacerbations in Puerto Rican Children. Pediatr Allergy Immunol Pulmonol. 2017;30(4):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lautenbacher LA, Jariwala SP, Markowitz ME, Rastogi D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr Pulmonol. 2016;51(12):1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tobias TAM, Wood LG, Rastogi D. Carotenoids, fatty acids and disease burden in obese minority adolescents with asthma. Clin Exp Allergy. 2019;49(6):838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]


