Highlights
-
•
SARS-CoV-2 was found in 14.3 % of healthcare workers in emergency department.
-
•
Rapid serological tests for SARS-CoV-2 can increase the diagnostic yield.
-
•
Screening of asymptomatic healthcare workers for SARS-CoV-2 is advised.
-
•
Infection control among transportation and cleaning staff should be stressed.
Abbreviations: COVID-19, Coronavirus disease 2019; EDs, Emergency departments; HCWs, Heathcare workers; NPS, Nasopharyngeal swab; PPE, Personal protective equipment; RST, Rapid serological test; RT-PCR, Reverse transcription polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome Coronavirus-2; UTM, Universal Transport Media
Keywords: COVID-19, SARS-CoV-2, HCWs, Emergency department
Abstract
Background
Healthcare workers (HCWs) represent a high-risk category during the coronavirus disease 2019 (COVID-19) pandemic crisis, with frontline HCWs at emergency departments (EDs) may be at an even higher risk. Determining the spread of infection among HCWs may have implications for infection control policies in hospitals. This study aimed to detect severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among asymptomatic HCWs of the ED of a large tertiary center in Cairo, Egypt.
Methods
The study was conducted from June 1st to June 14th, 2020. All the recommended national and international indications on infection control measures were followed. Two hundred and three HCWs were included in the study and tested by nasopharyngeal swab (NPS) and rapid serological test (RST). Descriptive statistical analyses were used to summarize the data.
Results
Of the 203 HCWs, 29 (14.3 %) tested positive by real-time reverse transcription polymerase chain reaction (RT-PCR). Thirty-seven (18.2 %) HCWs tested positive with RST: 20 with both IgM and IgG; 14 with IgM only, and 3 with IgG only. Age, gender, and/or occupation were not risk factors for SARS-CoV-2 infection.
Conclusions
Point prevalence of COVID-19 in asymptomatic HCWs in ED of tertiary care facility is 14.3 % by RT-PCR. This illustrates the importance of screening all HCWs regardless of symptoms, and the need for strict measures in securing HCWs to reduce transmission from healthcare facilities to the community during the current pandemic.
1. Background
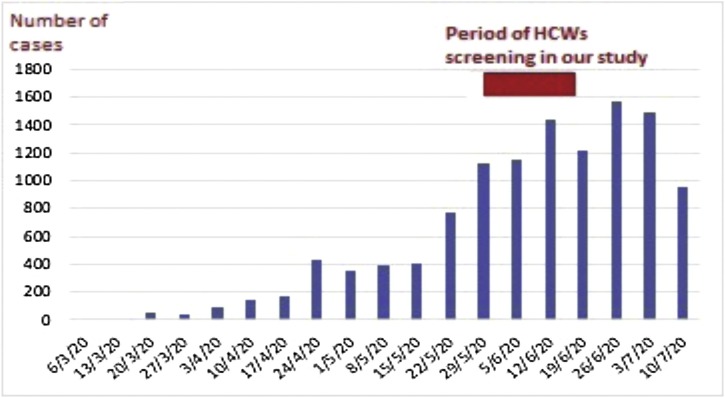
The COVID-19 pandemic [1], caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, poses an immense global threat that continues to affect lives every day [2]. In Egypt, the first confirmed case was officially announced on February 14th, 2020 [3]. Cases gradually increased, reaching 26,384 by June 1st, 2020 and 90,000 cases by July 23rd, 2020 [4,5] (Fig. 1 ).
Fig. 1.
Officially reported COVID-19 cases by day at weekly intervals according to Ministry of Health and Population, Egypt.
Abbreviations: HCWs, healthcare workers.
Hospital-associated transmission has been recognized as an important route in spreading the infection [6,7]. Healthcare workers (HCWs), particularly those at the frontlines, represent a high-risk category [6,8] in whom SARS-CoV-2 infection resulted in substantial morbidity and mortality [2]. Worldwide reports have confirmed that HCWs are disproportionately affected by SARS-CoV-2, as they represented 9 % of the total cases in early reports from Italy [9]. Currently, very few data exist on the number of asymptomatic HCWs and their role in disease transmission. Since recent data suggest that the transmission of SARS-CoV-2 mostly occurs before the onset of symptoms [10], determining the rate of infection among HCWs, including asymptomatic cases, is a key to reduce the rate of nosocomial spread [11].
Kasr Al-Aini University Hospital, a tertiary care referral center, along with its affiliated hospitals represent the largest university hospital complex in Egypt. It comprises 5600 beds, an outpatient clinic, and a large emergency department (ED) delivering health services in all specialties. Suspected cases of COVID-19 are served in a special zone apart from the ED.
In this study, we report on SARS-CoV-2 infection as confirmed by real-time reverse transcription polymerase chain reaction (RT-PCR) among asymptomatic frontline HCWs at the ED of Kasr Al-Aini University Hospital in the first half of June 2020, at the height of the spread of infection in Egypt.
2. Patients and methods
2.1. Study population
Ethical committee approval was obtained for the study. During the period from June 1st to June 14th, 2020; a total of 575 HCWs were scheduled to work in the ED at Kasr Al-Aini University Hospital, Cairo University. These HCWs included physicians, nurses, workers, technicians, and clerks. Twenty-five subjects were excluded from work as they had symptoms suggestive of COVID-19, and/or tested positive for SARS-CoV-2 by RT-PCR. The remaining 550 HCWs were invited to participate in the study. 203 subjects (37 %) agreed to participate and each signed an informed consent. All participants completed a questionnaire which included demographic data, occupation, past medical history, implementation of infection control measures and use of personal protective equipment (PPE), and exposure to suspected or confirmed COVID-19 cases in addition to presence of symptoms compatible with COVID-19.
2.2. Molecular detection of SARS-CoV-2 by RT-PCR testing
Nasopharyngeal swabs (NPS) were collected at designated sites and transferred with Universal Transport Media (UTM). SARS-CoV-2 RNA was extracted from NPS by QIAGEN EXTRACTION KIT. The extracted RNA was reverse transcribed into cDNA and amplified in one step using TaqPath™ COVID-19 CE-IVD RT-PCR ComboKit from Thermofisher SCIENTIFIC, Revision D.0 (Catalog Number A48067). 5 μl of RNA extract was added to a whole reaction volume of 25 μl according to the manufacturer’s instructions. Fast Dx Applied Biosystems 7500 real-time PCR instruments was used for the amplification. In the process, probes were annealed to three target sequences specific to SARS-CoV-2: ORF1ab, Nucleocapsid (N) and Spike (S) primers/probes for bacteriophage MS2. Two of the three genes and the MS2 (positive control) must be positive or the result was considered inconclusive.
2.3. SARS-CoV-2 rapid IgG–IgM serological test (RST)
Simultaneous with the NPS collection, serum immunoglobulins were detected by COVID-19 IgM/IgG antibody rapid diagnostic test (Artron Laboratories, Burnaby, Canada). It is a qualitative lateral flow immunochromatographic assay for the rapid determination of presence or absence of both anti-SARS-CoV-2-IgM and anti-SARS-CoV-2-IgG in human specimens with a sensitivity of 93.4 % and specificity of 97.7 %, as reported by the manufacturer. 10 μL serum was added and was incubated for 20–30 s. Subsequently, 2 drops of buffer were added, and the results were detected visually after 15–20 min. The presence of both the control line and IgM or IgG line indicated a positive result for IgM or IgG antibody, respectively.
All subjects with a positive RST result and negative RT-PCR NPS were invited for re-testing for RT-PCR a week after the initial NPS.
2.4. Statistical analysis
Continuous variables were described with medians and ranges. Categorical variables were described as frequency and percentages. The Pearson Chi-Square test was used as appropriate. A 2-sided P<0.05 was considered statistically significant.
3. Results
From June 1st to June 14th, 2020, a total of 203 frontline ED HCWs participated in the study (104 men and 99 women, with a mean age of 31.9 ± 6.6 years), representing 37 % of the eligible working force (n = 550). Of the enrolled participants, 74 (36.5 %) were physicians, 89 (43.8 %), were nurses, 24 (11.8 %) were cleaning and patients’ transportation personnel, and 14 (6.9 %) were administrative. None of them reported a household contact with an infected person. However, approximately 86 % reported occupational contact with suspected or confirmed COVID-19. More than 90 % of the participants confirmed adherence to the recommendations of PPE use at the workplace, as well as proper hand hygiene practice (Table 1 ).
Table 1.
Characteristics of the 203 healthcare workers screened for SARS-CoV-2, stratified according to RT-PCR results.
| Overall screened HCWs(n = 203) | SARS-CoV-2 Negative PCR (n = 174) | SARS-CoV-2 Positive PCR (n = 29) | P-value* | |
|---|---|---|---|---|
| Age in years: mean ± SD | 31.9 ± 6.6 | 31.9 ± 6.6 | 31.7 ± 5.7 | P = 0.86 |
| Gender: n (%) | P = 0.60 | |||
|
99 (48.8 %) | 83 (47.7 %) | 16 (55.2 %) | |
|
104 (51.2 %) | 91 (52.3 %) | 13 (44.8 %) | |
| Occupation: n (%) | P = 0.49 | |||
|
89 (43.8 %) | 74 (42.5 %) | 15 (51.7 %) | |
|
74 (36.5 %) | 67 (38.5 %) | 7 (24.1 %) | |
|
24 (11.8 %) | 19 (10.9 %) | 5 (17.2 %) | |
|
2 (0.99 %) | 2 (1.2 %) | 0 (0 %) | |
|
14 (6.9 %) | 12 (6.9 %) | 2 (6.9 %) | |
| Contact with suspected or confirmed COVID-19 | 176 (86.7 %) | 154 (88.5 %) | 22 (75.8 %) | P = 0.14 |
| Proper hand hygiene practice | 192 (94.6 %) | 165 (94.8 %) | 27 (93.1 %) | P = 0.42 |
| Use of personal protective equipment as recommended | 189 (93.1 %) | 162 (93.1 %) | 27 (93.1 %) | P = 0.98 |
| Co-morbidities: n (%) | 25 (12.3 %) | 23 (13.2 %) | 2 (6.9 %)** | P = 0.41 |
Abbreviations: HCWs, healthcare workers; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription polymerase chain reaction; SD, standard deviation; COVID-19, coronavirus disease 2019.
P-values calculated by chi-square test.
One subject had diabetes and the other had hypertension.
At the beginning of the study, RT-PCR for SARS-CoV-2 RNA in NPS was positive in 27 subjects, while RST was positive in 37 subjects, 26 of whom had a negative RT-PCR, and 11 showed a positive RT-PCR. Among the 26 subjects with a positive RST and an initially negative RT-PCR, two subjects tested positive during RT-PCR re-testing which was done one week later for this group of subjects. Thus, the overall point prevalence of positive RT-PCR was 29/203 (14.3 %). There was no significant difference between HCWs testing positive or negative for SARS-CoV-2 regarding age, sex, occupation, presence of co-morbidities, contact with COVID-19 cases, use of PPE as recommended or proper hand hygiene practice.
Regarding RST, twenty six out of the 174 HCWs with negative RT-PCR (15 %) had positive immunoglobulins (7 IgM, 2 IgG, 17 both IgM and IgG), whereas, in those with positive RT-PCR, immunoglobulins were positive in 11/29 (38 %) (7 IgM, 1 IgG, 3 both IgM and IgG).
4. Discussion
With the spread of SARS-CoV-2 infection in Egypt, several reports surfaced regarding COVID-19 related morbidities and mortalities among HCWs in hospital settings, particularly those at the frontlines. In April 2020, 2.37 % of the officially reported COVID-19 cases were HCWs, with a presumed case fatality rate of 6.5 % [12].
In this context, after designation of several hospitals in Egypt (including their EDs) as isolation hospitals exclusively for COVID-19 patients, EDs in other tertiary referral centers as that of Cairo University Hospitals were subjected to exceptionally larger numbers of re-directed patients seeking emergency services and their companions, exposing HCWs to a greater risk of SARS-CoV-2 infection.
In the present study, we found a 14.3 % overall point prevalence of SARS-CoV-2 in the HCWs in ED using RT-PCR. To our knowledge, this is the first study to report the prevalence of COVID-19 among asymptomatic frontline HCWs in an ED setting in Egypt.
It has been established that HCWs are at greater risk of testing positive for SARS-CoV-2, with some studies reporting a 12-fold increased risk compared to the general population [13]. A report from Papoutsi et al., stated that the median HCWs infection percentage among total cases retrieved from data of 41 countries was 10.04 % (range 0 %–24 %) [12]. However, the prevalence rate among HCWs varied among studies from different countries. High prevalence rates in HCWs were reported in studies from the Netherlands and the UK using molecular testing (9 % and 18 %, respectively) [14,15]. On the other hand, studies from University Hospitals Birmingham NHS, England and D. Cotugno Hospital in Naples, Italy showed point prevalence of 2.39 % and 1.7 %, respectively using molecular testing [16,11]. In addition to different screening methods, the interpretation of the variability in prevalence rates among HCWs in these studies should also take into consideration the time of screening in relation to the pandemic and the group of HCWs being screened.
The high prevalence rate encountered in our study conducted in June 2020 among asymptomatic HCWs denotes the importance of periodic screening of HCWs for SARS−COV-2 irrespective of the presence of symptoms as they are at continuous risk of exposure during the pandemic. Although most public health strategies rely on early detection of the disease to limit its spread, it is clear that asymptomatic transmission plays an important role in the spread of SARS−COV-2 infection worldwide [17]. The rapid and easy transmission from person to person can be explained by the fact that SARS-CoV-2 can already be heavily shedding in the upper respiratory tract even in presymptomatic cases [18].
Although NPS is currently used to obtain samples for molecular testing for SARS-CoV-2, the sensitivity of RT-PCR in clinical practice varies depending on the site and quality of sampling, reaching 63 % in one study, which may underestimate the extent of infection [19,20]. To increase the detection rate of more cases infected with COVID-19, we added RST to complement molecular testing. In 26 HCWs (12.8 %), antibodies could be detected by RST while initial NPS results were negative. This raised the cumulative prevalence among HCWs with at least one positive test (RT-PCR and/or RST) to 27.1 % of screened HCWs, which could represent either currently asymptomatic/presymptomatic cases or previous infection. In a similar study in a referral hospital in Belgium, 41/326 HCWs were confirmed positive by RT-PCR and/or serology, representing an overall infection rate of 12.6 % [21]. Results of RST should be interpreted cautiously and need further validation to determine their accuracy and reliability [22].
A potential limitation of the use of SARS-CoV-2 serological tests, is the possible cross-reactivity with other human coronaviruses. However, in another study evaluating SARS-CoV-2 immunoassays, the RST used in our study had a 100 % specificity with no cross-reactivity with control serum samples from patients with acute viral respiratory tract infections caused by other coronaviruses [23]. In our study, for HCWs with a positive RST and negative RT-PCR, a repeat NPS was done after one week to increase the diagnostic yield. Two new positive cases were diagnosed from the second NPS (one of them had isolated IgM, and the other had both IgM and IgG).
It is worth mentioning that in the current study, frequency of SARS-CoV-2 infection was higher in staff dealing with patients’ transportation and cleaning (20.8 %, 5/24 screened), in nurses (16.9 %, 15/89 screened), and in administrative employees (14.3 %, 2/14 screened) than in physicians (9.5 %, 7/74 screened). This emphasizes the need for providing more stringent infection control measures, education, and supervision among these groups of HCWs.
In conclusion, the point prevalence of COVID-19 among frontline HCWs in the ED using RT-PCR is 14.3 %. Control measures are necessary to protect HCWs from COVID-19 and to reduce transmission from infected HCWs to the community. An effective measure could be regular HCWs screening in the hospital setting through molecular testing, even in absence of symptoms.
Funding source
This work was funded by a grant from the Ideation Fund of the Academy of Scientific Research and Technology, Egypt [grant number 7177].
CRediT authorship contribution statement
Reham Abdelmoniem: Investigation, Writing - original draft. Rabab Fouad: Supervision. Shereen Shawky: Investigation, Resources. Khaled Amer: Investigation. Tarek Elnagdy: Conceptualization, Investigation. Wael A. Hassan: Investigation, Formal analysis. Ahmed M. Ali: Investigation. Moushira Ezzelarab: Investigation. Yasmine Gaber: Investigation. Hedy A. Badary: Investigation. Sherief Musa: Conceptualization, Writing - review & editing. Hala Talaat: Supervision. Abdel Meguid Kassem: Conceptualization, Funding acquisition, Writing - review & editing. Omnia Tantawi: Investigation, Writing - original draft.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.World Health Organization . 2020. WHO Director-general’s Opening Remarks at the Media Briefing on COVID-19. 11 March. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (Accessed 12 March 2020. [Google Scholar]
- 2.Mani N.S., Budak J.Z., Lan K.F., Bryson-Cahn C., Zelikoff A., Barker G.E.C., Grant C.W. Prevalence of COVID-19 infection and outcomes among symptomatic healthcare workers in seattle, Washington. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa761. ciaa761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report–26.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200215-sitrep-26-covid-19.pdf?sfvrsn=a4cc6787_2 Accessed 15 February. [Google Scholar]
- 4.2020. Daily Report on COVID-19 by Egyptian Ministry of Health and Population.https://www.facebook.com/egypt.mohp/photos/a.123442675873020/177239867159967 Accessed 1 June. [Google Scholar]
- 5.2020. Daily Report on COVID-19 by Egyptian Ministry of Health and Population.https://www.facebook.com/egypt.mohp/photos/a.122315979319023/202247777992509 Accessed 24 July. [Google Scholar]
- 6.Zhou P., Huang Z., Xiao Y., Huang X., Fan X.G. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect. Control Hosp. Epidemiol. 2020;(41):745e6. doi: 10.1017/ice.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh D. Occupational risks for COVID-19 infection. J. Occup. Med. Toxicol. 2020;70 doi: 10.1093/occmed/kqaa036. 3e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;(323):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 10.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(672) doi: 10.1038/s41591-020-0869-5. e5. [DOI] [PubMed] [Google Scholar]
- 11.Fusco F.M., Pisaturo M., Iodice V., Bellopede R., Tambaro O., Parrellaet G. COVID-19 among healthcare workers in a specialist infectious diseases setting in Naples, Southern Italy: results of a cross-sectional surveillance study. J. Hosp. Infect. 2020;105:596–600. doi: 10.1016/j.jhin.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papoutsi E., Giannakoulis V.G., Ntella V., Pappa S., Katsaounou P. Global burden of COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020;6(April) doi: 10.1183/23120541.00195-2020. 00195-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tostmann A., Bradley J., Bousema T., Yiek W.K., Holwerda M., Bleeker-Rovers C. Strong associations and moderate predictive value of early symptoms for SARSCoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000508. pii = 2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley A.J., Evans C., Colton H., Ankcorn M., Cope A., State A. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS foundation trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14) doi: 10.2807/1560-7917.ES.2020.25.14.2000433. pii = 2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shields A.M., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E.L., Allen J.D., Al-Taei S., Backhouse C., Bosworth A., Dunbar L., Ebanks D. 2020. SARS-CoV-2 Seroconversion in Health Care Workers. medRxiv. Jan 1. [DOI] [Google Scholar]
- 17.Arons M.M., Hatfield K.M., Reddy S.C. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson J., Whiting P.F., Brush J.E. Interpreting a COVID-19 test result. BMJ. 2020;369:m1808. doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 21.Martin C., Montesinos I., Dauby N. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Y.W., Schmitz J.E., Persing D.H. The laboratory diagnosis of covid-19 infection: current issues and challenges. J. Clin. Microbiol. 2020;58(May (6)):e00512–00520. doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C., Fomsgaard A., Krogfelt K.A. 2020. Evaluation of Nine Commercial SARS-CoV-2 Immunoassays. MedRxiv. (2020.04.09.20056325) [DOI] [Google Scholar]