As of October 2020, coronavirus disease 2019 (COVID-19) has caused over 43 million infections and over 1.1 million deaths worldwide. Remdesivir (GS-5734), a nucleotide prodrug that inhibits RNA-dependent RNA polymerase, accelerates recovery in patients with moderate to severe COVID-19 and was approved by emergency use authorization in May 2020.1,2 However, all studies of remdesivir have excluded patients with kidney impairment using estimated glomerular filtration rate (eGFR) cutoffs of 30 or 50 mL/min per 1.73 m2 because of theoretical concerns about the accumulation of remdesivir’s active metabolite or its sulfobutylether-beta-cyclodextrin carrier.3
Acute kidney injury (AKI) occurs at a rate of 30% to 40% in hospitalized patients with COVID-19.4 Chronic kidney disease and end-stage renal disease are also common comorbidities in patients who develop severe COVID-19. There are currently no available data on the use of remdesivir in patients with eGFR <30 ml/min per 1.73 m2, and antiviral strategies are desperately needed in this population. We conducted a multicenter, observational, retrospective case series of adults with COVID-19 between May 7, 2020, and July 15, 2020, to describe changes in alanine aminotransferase (ALT) and serum creatinine during remdesivir therapy and to report adverse events (AEs) attributed to remdesivir in hospitalized patients with COVID-19 who had eGFR <30 mL/min per 1.73 m2 or were receiving renal replacement therapy (RRT) at the time of starting remdesivir.
Results
A total of 18 patients with eGFR <30 ml/min per 1.73 m2 at the time of remdesivir initiation were included; baseline characteristics are shown in Table 1. The median age was 68 years (interquartile range, 55–76), 50% were female, and 78% were from racial/ethnic minority groups. Medical comorbidities were common, and 16 (89%) had documented chronic kidney disease (eGFR <60 ml/min per 1.73 m2) before the index hospitalization for COVID-19. Eleven patients were in intensive care, and 9 were mechanically ventilated at the time of remdesivir initiation. At the time of remdesivir initiation, 5 patients were receiving RRT (2 had ESRD and 3 had AKI). Among patients with eGFR <30 ml/min per 1.73 m2 who were not on RRT at the time of starting remdesivir, 5 met the criteria for AKI, and 8 had stable chronic kidney disease.
Table 1.
Baseline characteristics of patients with estimated glomerular filtration rate (eGFR) <30 ml/min per 1.73 m2 or on renal replacement therapy (RRT) treated with remdesivir
| Baseline characteristics, n = 18 patients | n (%) or median (IQR) |
|---|---|
| Age, yr, median (IQR) | 68 (55–76) |
| Female, n (%) | 9 (50) |
| Race, n (%) | |
| White, Hispanic | 9 (50) |
| Black, non-Hispanic | 5 (28) |
| White, non-Hispanic | 4 (22) |
| Kidney function group, n (%) | |
| Stable CKD with eGFR <30 | 8 (44) |
| AKI, not on dialysisa | 5 (28) |
| AKI on RRT | 3 (17) |
| ESRD | 2 (11) |
| Creatinine at initiation of RDV, mg/dl, median (IQR) | |
| Stable CKD with eGFR <30 | 2.30 (1.79–2.81) |
| AKI, not on dialysis | 2.55 (1.79–2.92) |
| Comorbidities, n (%) | |
| CKD (eGFR <60 ml/min per 1.73 m2) | 16 (89) |
| ESRD | 2 (11) |
| Hypertension | 15 (83) |
| Diabetes mellitus | 9 (50) |
| Coronary artery disease | 5 (28) |
| Transplant recipient | 3 (17) |
| Chronic obstructive pulmonary disease | 2 (11) |
| Liver disease/cirrhosis | 1 (6) |
| BMI, median (IQR) | 28.6 (24.9–31) |
| Baseline medications, n (%) | |
| ACE inhibitors | 2 (11) |
| Angiotensin II receptor blockers | 2 (11) |
| Immunosuppression | 4 (22) |
| Diuretics | 5 (28) |
| Statins | 7 (39) |
| RDV dosing regimen prescribed, n (%)b | |
| 5-day course | 16 (89) |
| 10-day course | 2 (11) |
| RDV doses actually administered, n (%) | |
| 1 dose | 2 (11) |
| 3 doses | 2 (11) |
| 4 doses | 2 (11) |
| 5 doses | 11 (61) |
| 10 doses | 1 (6) |
| Days from symptom onset to RDV start, median (IQR) | 7 (3–10.5) |
| Adjuvant COVID-19 therapies used, n (%) | |
| Tocilizumab | 3 (17) |
| Canakinumab | 2 (11) |
| Dexamethasone | 4 (22) |
| Methylprednisolone/prednisone | 5 (28) |
| Convalescent plasma | 1 (6) |
| Labs at admission, median (IQR) | |
| Hemoglobin, g/dl | 11.0 (9.9–12.1) |
| D-dimer, ng/ml | 2196 (1145–3750) |
| Albumin, g/dl | 3.4 (2.7–3.8) |
| C-reactive protein, mg/l | 133.1 (59.3–221.0) |
| Location of admission, n (%) | |
| Intensive care unit | 11 (61) |
| Hospital floor | 7 (39) |
| Oxygen support required at baseline, n (%) | |
| Mechanical ventilation | 9 (50) |
| High-flow nasal cannula or nonrebreather | 3 (17) |
| Nasal cannula, ≤4 L | 6 (33) |
| On vasopressors at RDV start, n (%) | 6 (33) |
| On ECMO at RDV start, n (%) | 1 (6) |
ACE, angiotensin-converting enzyme; AKI, acute kidney injury; BMI, body mass index; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ESRD, end-stage renal disease; IQR, interquartile range; RDV, remdesivir.
Among the 8 patients meeting the definition of AKI, 6 also had baseline CKD.
All patients receive 200 mg i.v. bolus on day 1 followed by 100 mg/d. Remdesivir solution was used in 16 cases, and the powder formulation was used in 2 cases.
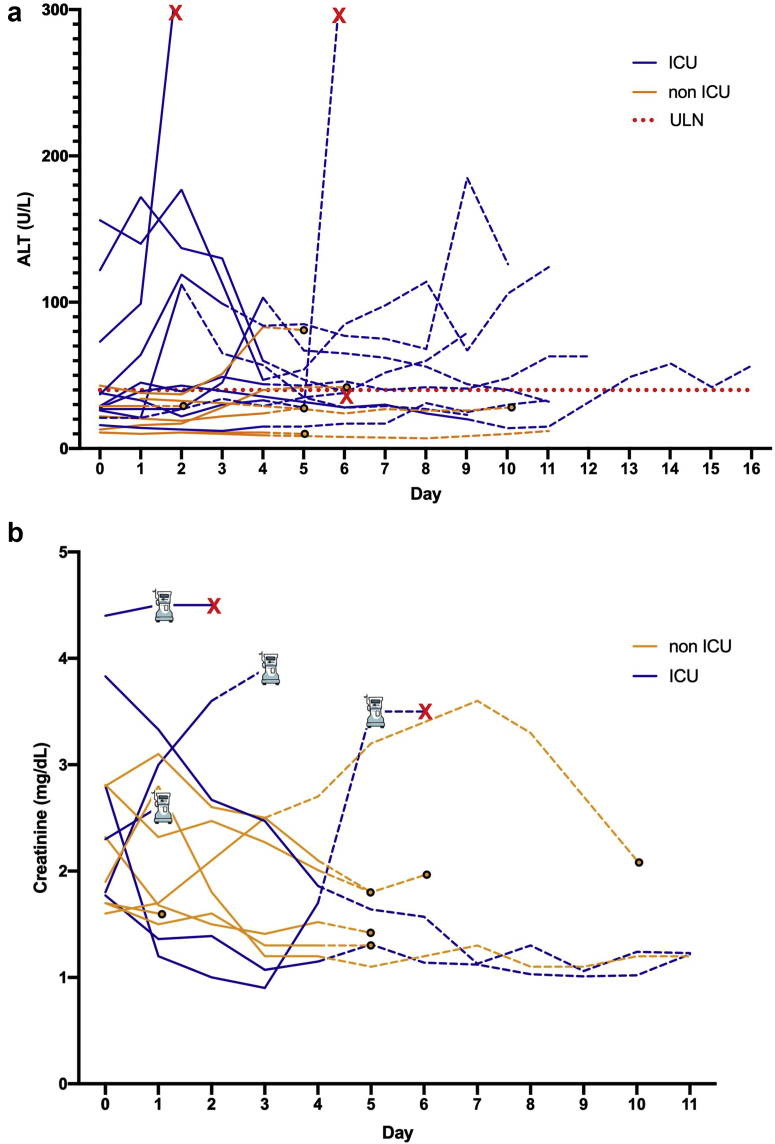
Two patients developed ALT >5 times the upper limit of normal (Figure 1a), both attributed to shock liver and not remdesivir. Other ALT abnormalities occurred, predominantly in critically ill patients, including 3 cases possibly or likely attributed to remdesivir use per study investigators (2 grade 1 and 1 grade 2, Figure 1a). Eight of 13 patients not requiring RRT at the time of remdesivir initiation experienced improved creatinine during remdesivir, whereas 5 worsened, including 4 who progressed to require RRT (Figure 1b). In 1 case, study investigators determined the worsening creatinine was likely attributed to remdesivir.
Figure 1.
Alanine aminotransferase (ALT) and serum creatinine trends during and after remdesivir. (a) ALT values for all 18 patients. Serum creatinine trends for the 13 patients not requiring renal replacement therapy (RRT) before starting remdesivir are shown in Figure 1b. Patients with end-stage kidney disease (2) or acute kidney injury on RRT (3) at the time of starting remdesivir are not shown in Figure 1b. Patients who initiated remdesivir in the intensive care unit (ICU) are shown in blue. Non-ICU patients (yellow) were receiving care on a medical floor or step-down unit. ALT and creatinine values recorded while on remdesivir treatment are shown with solid lines, and the following 7 days are shown with dashed lines. The date of death is shown with a red X, and hospital discharge is annotated by a solid circle. One patient died while taking remdesivir due to cardiac arrest and shock that was not attributed to remdesivir by the site investigator.
Overall, treatment was well tolerated, with few other AEs attributed to remdesivir, including 2 cases of hyperglycemia (each grade 2) each on day 4 of treatment, 1 grade 3 anemia on day 3 of treatment, and burning at the infusion site on the final fifth dose in 1 case. Five patients discontinued remdesivir early, only 2 of them due to AEs attributed to remdesivir (burning at i.v. site during the final dose and worsening kidney function); the remainder stopped due to improved clinical status (n = 2) or patient preference (n = 1). Overall, 28-day mortality was 44% (8/18). Among patients requiring intensive care at the time of remdesivir initiation, 8 of 11 died. All 7 patients who were not requiring intensive care at baseline survived to 28 days.
Discussion
In this multicenter case series, among 18 patients receiving remdesivir despite eGFR <30 ml/min per 1.73 m2, none had high-grade ALT elevations attributed to remdesivir. The majority of patients had improving kidney function, although in 1 case worsening creatinine was attributed as likely related to remdesivir by a study investigator. The overall 28-day mortality of 44% in this cohort is similar to that reported for inpatients with COVID-19 and stage 3 AKI (54%–60%) or baseline ESRD (31%).4, 5, 6, 7 All 7 patients not requiring intensive care at the time of remdesivir initiation survived to 28 days, consistent with subgroup analyses of clinical trials showing that the greatest benefit for remdesivir is in hospitalized patients requiring supplemental oxygen but not mechanical ventilation.1 A recent report by Thakare et al.8 of remdesivir use in patients with severe AKI (n = 30) and ESRD (n = 16) from India also demonstrated very few patients had clinically significant ALT abnormalities and noted very few treatment-related AEs.
The exclusion of patients with eGFR <30 ml/min per 1.73 m2 from clinical trials has created an important gap in the knowledge of safety data for remdesivir because up to 1 in 3 critically ill patients with COVID-19 may have eGFR <30 ml/min per 1.73 m2.5 Concerns about accumulation of the sulfobutylether-beta-cyclodextrin carrier should be allayed by the available safety data in patients with kidney failure treated with voriconazole, which uses the same carrier.3 The lyophilized powder formulation of remdesivir, which contains 3 g sulfobutylether-beta-cyclodextrin per 100-mg dose compared with 6 g in the liquid formulation, is preferred in patients with eGFR <30 ml/min per 1.73 m2.9 In this series, 16 of 18 patients received the liquid formulation due to product availability; yet, even this preparation still contains significantly less than the maximum recommended safety threshold of 250 mg/kg/d sulfobutylether-beta-cyclodextrin.3
Our study has several important limitations. First, it is limited by the small number of cases; however, it includes a racially and ethnically diverse population. Second, the lack of a comparison group and the retrospective ascertainment of AE relatedness are important limitations to our ability to draw conclusions about safety or efficacy. To avoid selection bias that we felt could be biased in favor of remdesivir, we did not include a contemporary or historic control group because safety and efficacy can only be determined by a prospective placebo-controlled trial. Finally, although calculating eGFR in patients who have unstable creatinine is problematic, we included anyone whose creatinine most proximal to remdesivir initiation corresponded to an eGFR <30 ml/min per 1.73 m2 because, in practice, these are patients for whom treating clinicians will have to make the difficult decision about whether or not to initiate remdesivir.
This multicenter report including consecutive patients from 4 hospital systems is the first case series of remdesivir use in patients with eGFR <30 ml/min per 1.73 m2 in the United States and highlights the urgent need for prospective studies in this common and high-risk population.
Disclosure
MES has received research grants to institution and served on scientific advisory boards for Gilead Sciences in the area of hepatitis C virus infection.
Acknowledgments
MES is supported by National Institutes of Health (NIH) K23 DK 117014 and the Claflin Distinguished Scholars Award. The NIH had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author Contributions
CE, MES, and KDJ designed the study. CES, IAS, ZM, JSH, RW, PGG, SLD-P, JCP, DWK, ARL, KDJ, and MES contributed to data entry. CES, IAS, RB, KDJ, and MES performed data analysis. IAS made the figure items. CE, RB, and MES drafted the first draft; all authors provided critical review and approved the final manuscript.
Footnotes
Supplementary Methods.
Contributor Information
Kenar D. Jhaveri, Email: kjhaveri@northwell.edu.
Meghan E. Sise, Email: msise@partners.org.
Supplementary Material
Supplementary Methods.
References
- 1.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 2.Spinner C.D., Gottlieb R.L., Criner G.J. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamsick M.L., Gandhi R.G., Bidell M.R. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31:1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Hayek S.S., Wang W. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng J.H., Hirsch J.S., Wanchoo R. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakare S., Gandhi C., Modi T. Safety of Remdesivir in Patients With Acute Kidney Injury or CKD. Kidney Int Rep. 2021;6:206–210. doi: 10.1016/j.ekir.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humeniuk R., Mathias A., Cao H. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.