Abstract
Background: Current guidelines do not suggest in which groups of patients with atrial fibrillation (AF) individual non-vitamin K antagonist oral anticoagulants (NOACs) should be used for the prevention of thromboembolic complications. The aim of this study was to evaluate the frequency of use of apixaban, dabigatran, and rivaroxaban, and attempt to identify factors predisposing their administration. Methods: The Polish Atrial Fibrillation (POL-AF) registry is a prospective, non-interventional study, including consecutive patients with AF hospitalized in ten Polish cardiology centers during the period ranging from January to December 2019. In this study, all patients were treated with NOACs. Results: Among the 2971 patients included in the analysis, 40.4% were treated with rivaroxaban, 32% with apixaban, and 27.6% with dabigatran. The mean age of the total population was 72 ± 11.5 years and 43% were female. A reduced dose of NOAC was used in 35% of patients treated with apixaban, 39.7% of patients treated with dabigatran, and 34.4% of patients treated with rivaroxaban. Independent predictors of the use of apixaban were previous bleeding (OR 2.37, CI 1.67–3.38), GFR < 60 mL/min (OR 1.38, CI 1.25–1.64), heart failure (OR 1.38, CI 1.14–1.67) and age (per 5 years) (OR 1.14, CI 1.09–1.19). GFR < 60 mL/min (OR 0.79, CI 0.66–0.95), female (OR 0.8, CI 0.67–0.96) and age (per 5 years) (OR 0.95, CI 0.91–0.99) diminished the chance of using dabigatran. Previous bleeding (OR 0.43, CI 0.28–0.64), vascular disease (OR 0.84, CI 0.70–0.99), and age (per 5 years) (OR 0.94, CI 0.90–0.97) diminished the chance of choosing rivaroxaban. Conclusions: In hospitalized patients with AF, the most frequently chosen NOAC was rivaroxaban. Apixaban was chosen more often in patients after bleeding, and in those who were advanced in years, with heart failure and impaired renal function. Impaired renal function and female gender were factors that diminished the chance of using dabigatran. Previous bleeding and vascular disease was the factor that diminished the chance of using rivaroxaban. Dabigatran and rivaroxaban have been used less frequently in elderly patients.
Keywords: atrial fibrillation, non-vitamin K antagonist oral anticoagulants, oral anticoagulants, vitamin K antagonists
1. Introduction
The prevention of thromboembolic complications is an important part of the management of patients with atrial fibrillation (AF) [1]. European and American guidelines recommend the use of non-vitamin K antagonist oral anticoagulants (NOACs) over therapy with vitamin K antagonists (VKAs) in most AF patients [2,3]. The number of patients treated with NOACs has increased significantly during the last few years [4,5]. In primary randomized controlled trials leading to their approval, compared to warfarin, NOACs were shown to be either non-inferior or superior for stroke prevention in AF, with similar or reduced rates of bleeding, especially intracranial hemorrhage [6,7,8]. However, no head-to-head comparison between the individual NOACs has been performed. Additionally, there were differences in the study populations of each of the pivotal NOAC trials. In the absence of randomized clinical trials, observational studies utilizing the data from clinical practice may add useful information regarding individual NOACs. The proper use of specific NOACs for stroke prevention in AF patients requires a diligent approach in various settings of daily clinical practice. In the currently binding guidelines referring to the treatment of patients with AF, there is a lack of recommendations concerning the choice of individual NOACs in certain cohorts of patients [4,5]. However, in expert documents, there are guidelines regarding the choice of a specific NOAC in various clinical situations [9,10,11]. With the increased availability of NOACs, the prescription patterns and factors driving treatment may evolve.
The aim of this study was to assess the use frequency of apixaban, dabigatran, and rivaroxaban and the predictors of their prescription in a nationwide cohort of hospitalized patients with AF.
2. Materials and Methods
2.1. Study Design and Study Population
The Polish Atrial Fibrillation (POL-AF) registry is a prospective, observational, multicenter study, whereby consecutive AF patients are enrolled across ten cardiology hospital centers, which represents the Polish cardiology reality well, covering seven academic centers, two district hospitals, and one military hospital. The study was registered in ClinicalTrials.gov: NCT04419012. The aim of the registry was to gain detailed insights into the clinical characteristics and management of patients with AF, especially into the prevention of thromboembolic events. The data were collected from January to December 2019, for two whole weeks out of each month. The registry includes all consecutive patients with AF hospitalized in a participating center during the study period who were hospitalized for urgent and planned reasons. Patients were included if they were at least 18 years of age and had a history of AF documented by electrocardiography or in their medical history. No explicit exclusion criteria were defined to avoid biased selection of patients and to achieve a cohort close to “real life”. Furthermore, consecutive patients were included in each site in order to reduce selection bias. Only patients admitted to hospital to have AF ablation were excluded from the registry because not all of the centers perform catheter ablation. Further, patients undergoing ablation due to AF have a clinical profile that differs from most patients with AF (they are younger and do not have concomitant diseases).
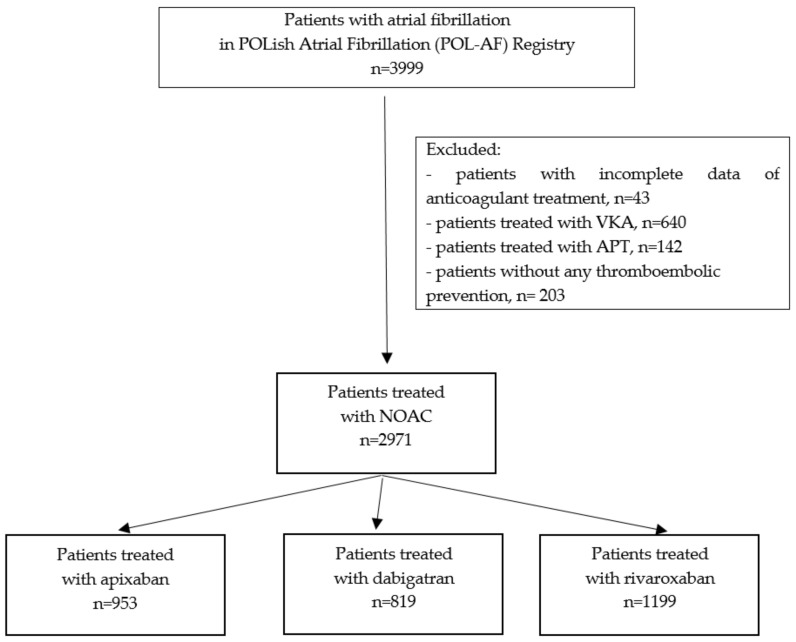
In the presented study, based on the results of the POL-AF registry, patients with AF treated with NOACs were evaluated. Patients receiving VKAs, antiplatelet therapy, and those not being given anticoagulant therapy were excluded from the study. During the study period, 3999 patients with AF were included in the POL-AF registry. After applying the exclusion criteria described above, a total of 2971 patients were included in this study (Figure 1).
Figure 1.
The flow chart of the study. Abbreviations: APT, antiplatelet drug; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
2.2. Covariates
Investigators collected baseline characteristics regarding demographics, medical history, type of AF, diagnostic test results, and pharmacotherapy.
Thromboembolic risk was defined according to a combined congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category (CHA2DS2-VASc) score [12]. Bleeding risk was assessed according to a hypertension, abnormal renal/liver function, stroke, bleeding, labile INR (international normalized ratio), elderly (>65 years), drug/alcohol consumption (HAS-BLED) score [13].
The glomerular filtration rate (GFR), which is used to assess patients’ kidney function, was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.
The study was approved by the Ethics Committee of the Swietokrzyska Medical Chamber in Kielce (104/2018). The Ethics Committee waived the requirement of obtaining informed consent from the patients.
2.3. Statistical Analyses
Continuous data were described by means and standard deviations, whereas categorical data were summarized by frequencies and percentages. Group comparisons were performed using the chi-squared or Fisher’s exact test for categorical variables, while one-way ANOVA was used for continuous variables. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated in logistic regression models. Participating centers were included in multivariable logistic regression models as potential confounders. Two-tailed p-values <0.05 were considered statistically significant. All statistical analyses were performed using the R software package version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Patient Characteristics
Among the 2971 patients included in the analysis, 1199 (40.4%) were treated with rivaroxaban, 953 (32%) with apixaban, and 819 (27.6%) with dabigatran. Apixaban was selected for the highest percentage of patients in 3 centers and rivaroxaban in 7 centers (Supplementary Table S1). The mean age of the total population was 72 ± 11.5 years and 43% were female. Patients on apixaban were older (74.9 ± 11.5 years) compared with patients on dabigatran (70.3 ± 11.1 years) and rivaroxaban (70.8 ± 11.3 years) (p < 0.0001). Patients on apixaban were more likely to be female (47.1%) compared with patients on dabigatran (37.4%) and rivaroxaban (43.5%) (p < 0.0001). Non-permanent AF was diagnosed in 76.1% of patients, most often in patients treated with rivaroxaban (79.4%). Baseline characteristics of the study population are summarized in Table 1. Renal dysfunction, defined as GFR < 60 mL/min, was diagnosed in 50.3% of patients, and most often in patients treated with apixaban (61.5%). Table 2 illustrates the laboratory parameters and echocardiographic measurements in patients treated with a specific NOAC. In the study group, patients were most often hospitalized to have electrical cardioversion (27.5%) and due to heart failure (20%).
Table 1.
Clinical characteristics of patients treated with apixaban, dabigatran, and rivaroxaban.
| Clinical Characteristic |
All n = 2971 |
Apixaban n = 953 |
Dabigatran n = 819 |
Rivaroxaban n = 1199 |
p |
|---|---|---|---|---|---|
| Age Mean (SD), years < 65 65-74 ≥ 75 |
72.0 (11.5) 688 (23.2) 977 (32.9) 1306 (43.9) |
74.9 (11.5) 162 (17.0) 270 (28.3) 521 (54.7) |
70.3 (11.1) 217 (26.5) 296 (36.1) 306 (37.4) |
70.8 (11.3) 309 (25.8) 411 (34.2) 479 (40) |
< 0.0001 < 0.0001 |
| Female, n (%) | 1277 (43.0) | 449 (47.1) | 306 (37.4) | 522 (43.5) | 0.0002 |
| Type of atrial fibrillation | |||||
| Paroxysmal | 1488 (50.1) | 460 (48.3) | 411(50.2) | 617 (51.5) | 0.3384 |
| Persistent | 772 (26.0) | 212 (22.2) | 225 (27.5) | 335 (27.9) | 0.0059 |
| Permanent | 711 (23.9) | 281 (29.5) | 183 (22.3) | 247 (20.6) | < 0.0001 |
| Non-permanent | 2260 (76.1) | 672 (70.5) | 636 (77.7) | 952 (79.4) | < 0.0001 |
| Medical history | |||||
| Hypertension | 2512 (84.6) | 805 (84.5) | 709 (86.6) | 998 (83.2) | 0.1259 |
| Heart failure | 1892 (63.7) | 653 (68.5) | 494 (60.3) | 745 (62.1) | 0.0006 |
| Vascular disease | 1640 (55.2) | 556 (58.3) | 457 (55.8) | 627 (52.3) | 0.0181 |
| Coronary artery disease | 1467 (49.4) | 495 (51.9) | 410 (50.1) | 562 (46.9) | 0.0587 |
| Previous myocardial infarction | 641 (21.6) | 244 (25.6) | 163 (19.9) | 234 (19.5) | 0.0012 |
| Peripheral artery disease | 414 (13.9) | 152 (15.9) | 106 (12.9) | 156 (13) | 0.0929 |
| Previous stroke/transient ischemic attack/peripheral embolism | 488 (16.4) | 146 (15.3) | 148 (18.1) | 194 (16.2) | 0.2842 |
| Diabetes mellitus | 999 (33.6) | 344 (36.1) | 269 (32.8) | 386 (32.2) | 0.1400 |
| Any previous bleeding | 147 (4.9) | 79 (8.3) | 35 (4.3) | 33 (2.8) | < 0.0001 |
| Previous gastric bleeding | 94 (3.2) | 54 (5.7) | 18 (2.2) | 22 (1.8) | < 0.0001 |
| Previous intracranial bleeding | 16 (0.5) | 8 (0.8) | 4 (0.5) | 4 (0.3) | 0.2675 |
| Malignancy | 135 (4.5) | 55 (5.8) | 31 (3.8) | 49 (4.1) | 0.0831 |
| Thromboembolic risk | |||||
| CHA2DS2-VASc score Mean (SD) =0 =1 ≥2 |
4.3 (1.8) 55 (1.9) 145 (4.9) 2771(93.3) |
4.6 (1.7) 5 (0.5) 38 (4.0) 910 (95.5) |
4.2 (1.9) 18 (2.2) 48 (5.9) 753 (91.9) |
4.2 (1.9) 32 (2.7) 59 (4.9) 1108 (92.4) |
< 0.0001 0.0013 |
| Bleeding risk | |||||
| HAS-BLED score Mean (SD) ≥3 |
2.0 (0.9) 705 (23.7) |
2.2 (0.9) 296 (31.1) |
1.9 (0.9) 169 (20.6) |
1.9 (0.8) 240 (20.0) |
< 0.0001 < 0.0001 |
| Reason for hospitalization | |||||
| Electrical cardioversion | 764 (25.7) | 151(15.8) | 237 (28.9) | 376 (31.3) | < 0.0001 |
| Planned coronarography/PCI | 282 (9.5) | 83 (8.7) | 79 (9.6) | 120 (10.0) | 0.5844 |
| Planned CIED implantation/reimplantation | 265 (8.9) | 104 (10.9) | 62 (7.6) | 99 (8.3) | 0.0281 |
| Acute coronary syndrome | 167 (5.6) | 73 (7.7) | 38 (4.6) | 56 (4.7) | 0.0041 |
| Heart failure | 595 (20.0) | 279 (29.3) | 138 (16.8) | 178 (14.8) | < 0.0001 |
| Ablation other than AF | 144 (4.8) | 28 (2.9) | 43 (5.3) | 73 (6.1) | 0.0027 |
| AF without any procedures | 211 (7.1) | 78 (8.2) | 55 (6.7) | 78 (6.5) | 0.2829 |
| Other | 543 (18.3) | 157 (16.5) | 167 (20.4) | 219 (18.3) | 0.1042 |
The numbers are presented as the mean ± standard deviation, or as numbers (percentage) if otherwise mentioned. Abbreviation: AF, atrial fibrillation; CIED, cardiac implantable electronic device; IQR, interquartile range; SD, standard deviation. CHA2DS2-VASc score: congestive heart failure (1 point), hypertension (1 point), age ≥ 75 years (2 points), diabetes mellitus (1 point), stroke/TIA/thromboembolism (2 points), vascular disease (1 point), age 65–74 years (1 point), sex female (1 point). HAS-BLED score: hypertension (1 point), liver disease (1 point), renal disease (1 point), stroke history (1 point), bleeding history (1 point), age >65 years (1 point), and drug (concomitant use of NSAID or antiplatelet agent, 1 point).
Table 2.
Results of the laboratory tests and echocardiographic examinations of patients treated with apixaban, dabigatran, and rivaroxaban. The numbers are presented as the mean ± standard deviation, or numbers (percentage) otherwise mentioned. Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation.
| Clinical Characteristic |
all n = 2971 |
Apixaban n = 953 |
Dabigatran n = 819 |
Rivaroxaban n = 1199 |
p |
|---|---|---|---|---|---|
| Laboratory tests | |||||
| Hemoglobin Mean (SD), g/dL |
13.3 (1.8) n = 2942 | 12.8 (1.9) n = 942 | 13.5 (1.8) n = 809 |
13.5 (1.7) n = 1191 | < 0.0001 |
| White blood cell Mean (SD), K/μL |
7.9 (3.1) n = 2935 | 8.0 (2.9) n = 939 |
7.9 (3.0) n = 807 |
7.9 (3.3) n = 1189 | 0.7752 |
| Platelet Mean (SD), K/μL |
220.1 (72.7) n = 2938 | 214.4 (69.8) n = 940 | 219.5 (66.5) n = 807 | 225 (78.5) n = 1191 | 0.0032 |
| eGFR Mean (SD), ml/min/1.73m2 |
60.3 (20.2) n = 2947 |
54.1 (19.9) n = 945 |
64.1 (19.3) n = 811 |
62.6 (20.0) n = 1191 |
<0.0001 |
| eGFR < 60 mL/min/1.73m2 | 1483 (50.3) n = 2947 |
581 (61.5) n = 945 |
354 (43.6) n = 811 |
548 (46.0) n = 1191 |
<0.0001 |
| Echocardiographic findings | |||||
| Ejection fraction Mean (SD), % |
49.5 (13.3) n = 2343 | 48.0 (14.1) n = 755 | 49.4 (13.0) n = 619 | 50.7 (12.6) n = 969 | 0.0002 |
| Left atrial diameter Mean (SD), mm |
46.5 (6.8) n = 2046 | 46.6 (7.0) n = 671 | 47.0 (6.8) n = 534 |
46.2 (6.6) n = 841 | 0.0796 |
| Left ventricular systolic diameter Mean (SD), mm |
39.4 (8.6) n = 1130 | 39.0 (9.6) n = 373 | 39.5 (8.6) n = 334 |
39.8 (7.6) n = 423 | 0.4639 |
| Left ventricular diastolic diameter Mean (SD), mm |
52.5 (8.2) n = 2230 | 52.3 (8.9) n = 737 | 52.8 (7.9) n = 578 |
52.5 (7.8) n = 915 | 0.4866 |
3.2. Thromboembolic Risk, Bleeding Risk, and Antithrombotic Therapy Use
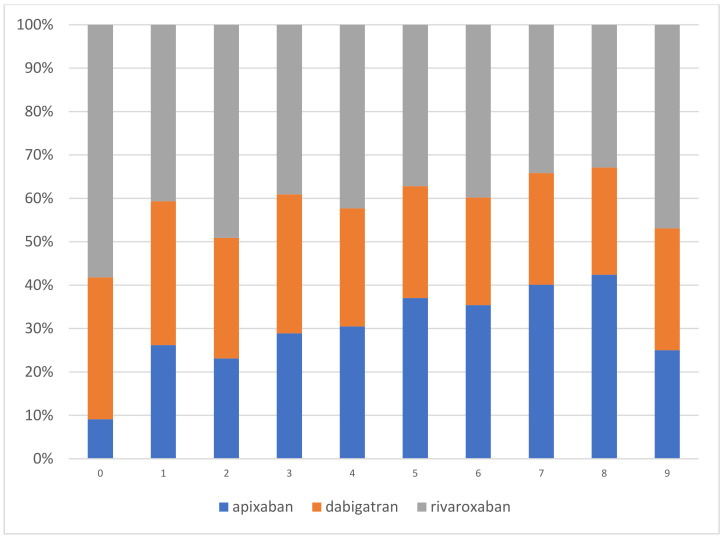
Thromboembolic and bleeding risks according to a specific NOAC treatment are reported in Table 1. Patients treated with apixaban had the highest thromboembolic risk (CHA2DS2-VASc mean± SD 2.7 ± 1.3) and bleeding risk (HAS-BLED mean± SD 2.2 ± 0.9) as compared with patients treated with dabigatran or rivaroxaban (both p < 0.0001). Additionally, they had the highest prevalence of most thromboembolic and bleeding risk factors. Figure 2 shows the prescription patterns for specific NOACs based on their CHA2DS2-VASc scores.
Figure 2.
The prescription patterns for specific NOACs based on their CHA2DS2-VASc scores.
In the study group, 36% of patients were treated with a reduced NOAC dose. Among 1071 patients who were treated with the reduced dose of NOACs, inappropriately reduced doses were observed in 242 patients (22.6%). The reduced dose of NOACs was used in 35% of apixaban patients, 39.7% of dabigatran patients, and 34.4% of rivaroxaban patients (p = 0.037). In most patients (81.8%), the same NOAC as recommended at discharge was used before hospitalization (Table 3).
Table 3.
Detailed data on anticoagulant therapy in the study group. Abbreviations: NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist.
| All n = 2971 |
Apixaban n = 953 |
Dabigatran n = 819 |
Rivaroxaban n = 1199 |
p | |
|---|---|---|---|---|---|
| Reduced dose | 1071 (36.0) | 334 (35) | 325 (39.7) | 412 (34.4) | 0.0372 |
| Antiplatelets with NOAC | 399 (13.4) | 141 (14.8) | 96 (11.7) | 162 (13.5) | 0.1661 |
| Treatment before hospitalization | |||||
| The same NOAC | 2429 (81.8) | 649 (68.1) | 724 (88.4) | 1056 (88.1) | < 0.0001 |
| Another NOAC | 82 (2.8) | 62 (6.5) | 13 (1.6) | 7 (0.6) | < 0.0001 |
| VKA | 81 (2.7) | 48 (5.0) | 14 (1.7) | 19 (1.6) | < 0.0001 |
| Antiplatelets only | 98 (3.3) | 51 (5.4) | 19 (2.3) | 28 (2.3) | < 0.0001 |
| None | 281 (9.5) | 143 (15.0) | 49 (6.0) | 89 (7.4) | < 0.0001 |
3.3. Predictors of the Use of Individual NOACs
In the analysis of individual NOAC selection, logistic regression models were created for apixaban versus dabigatran and rivaroxaban, dabigatran versus apixaban and rivaroxaban, and rivaroxaban versus apixaban and dabigatran.
In the univariate logistic regression analysis, numerous predictors of a specific NOAC choice were found (Supplementary Table S2).
In each of the multivariable models, factors associated with the selection of an individual NOAC versus another NOAC were similar, and included age, heart failure, vascular disease, female, non-permanent AF, malignancy, any previous bleeding, GFR < 60 mL/min, and antiplatelet therapy with NOACs.
Table 4 demonstrates predictors of the use of particular antithrombotic drugs. Independent predictors of the use of apixaban were previous bleeding (OR 2.37, CI 1.67–3.38), GFR < 60 mL/min (OR 1.38, CI 1.25–1.64), heart failure (OR 1.38, CI 1.14–1.67), and age (per 5 years) (OR 1.14, CI 1.09–1.19). GFR < 60 mL/min (OR 0.79, CI 0.66–0.95), female (OR 0.8, CI 0.67–0.96) and age (per 5 years) (OR 0.95, CI 0.91–0.99) diminished the chance of using dabigatran. Previous bleeding (OR 0.43, CI 0.28–0.64), vascular disease (OR 0.84, CI 0.70–0.99), and age (per 5 years) (OR 0.94, CI 0.90–0.97) diminished the chance of choosing rivaroxaban.
Table 4.
Factors associated with the selection of an individual NOAC over another NOAC for stroke prevention in patients with AF, assessed using multivariable logistic regression models (participating centers were included as potential confounders).
| Factors | Apixaban | Dabigatran | Rivaroxaban | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p | OR | 95%CI | p | OR | 95%CI | p | |
| Age (per 5 years) | 1.14 | 1.09–1.19 | <0.0001 | 0.95 | 0.91–0.99 | 0.0112 | 0.94 | 0.90–0.97 | 0.0009 |
| Heart failure | 1.38 | 1.14–1.67 | 0.001 | 0.84 | 0.70–1.03 | 0.0916 | 0.86 | 0.72–1.03 | 0.0958 |
| Vascular disease | 1.03 | 0.86–1.24 | 0.7241 | 1.20 | 0.99–1.45 | 0.0523 | 0.84 | 0.70–0.99 | 0.0464 |
| Female | 1.09 | 0.92–1.29 | 0.3223 | 0.8 | 0.67–0.96 | 0.0139 | 1.11 | 0.94–1.31 | 0.2033 |
| Non-permanent AF | 0.91 | 0.74–1.10 | 0.3319 | 1.03 | 0.83–1.27 | 0.812 | 1.09 | 0.90–1.33 | 0.3706 |
| Malignancy | 1.38 | 0.95–2.00 | 0.09 | 0.83 | 0.54–1.26 | 0.3737 | 0.86 | 0.59–1.26 | 0.4471 |
| Any previous bleeding | 2.37 | 1.67–3.38 | <0.0001 | 0.86 | 0.57–1.28 | 0.4589 | 0.43 | 0.28–0.64 | <0.0001 |
| eGFR < 60 mL/min/1.73m2 | 1.38 | 1.15–1.64 | 0.0004 | 0.79 | 0.66–0.95 | 0.0108 | 0.91 | 0.77–1.08 | 0.2871 |
| Antiplatelets with NOAC | 1.19 | 0.91–1.55 | 0.1904 | 0.76 | 0.57–1.01 | 0.0564 | 1.08 | 0.84–1.39 | 0.5473 |
Abbreviation: AF, atrial fibrillation; CI, confidence interval; eGFR, estimated glomerular filtration rate; NOAC, non-vitamin K antagonist oral anticoagulant; OR, odds ratio.
4. Discussion
The POL-AF registry provides an important view of contemporary antithrombotic therapy in patients with AF. The major findings of the present study are as follows. Firstly, our country-specific registry data showed that the most frequently chosen NOAC was rivaroxaban. Secondly, patients treated with apixaban had the highest CHA2DS2-VASc and HAS-BLED scores. Thirdly, factors predisposing the choice of a particular NOAC were identified.
NOACs have radically changed the management of AF patients, improving both life expectancy and life quality [14]. The frequency of choosing particular NOACs in the prevention of thromboembolic complications in patients with AF depends on the geographical region, clinical characteristics of patients, and doctors’ preferences. It is also possible to observe a change in anticoagulant therapy trends longitudinally, which is connected with the publication of consecutive studies evaluating efficacy and safety of individual NOACs. Dabigatran was the first NOAC available in Poland, rivaroxaban was the second, and apixaban was the third, all of which were available during the whole study period. Edoxaban has been registered in Europe as a drug for preventing thromboembolic complications in patients with AF, however it is not available in Poland. In the present study including hospitalized patients with AF, rivaroxaban was used in 40% of patients treated with NOACs. It was also the most frequently used NOAC in the retrospective population-based cohort of patients with AF [15]. The data from the NCDR PINNACLE registry showed that rivaroxaban was used more commonly compared with dabigatran and apixaban [16]. Additionally, the data from the Eurobservational Research Program on Atrial Fibrillation (EORP-AF) also indicates it as the most often chosen NOAC [17].
The National Danish Patient Registry cohort included patients from the years 2012 to 2015. This study showed an increase in apixaban use since its introduction and a decline in dabigatran use [18]. The highest proportion of patients treated with apixaban was also declared in The Norwegian Patient Registry [19]. In the present study, a reduced NOAC dose was most often administered to patients treated with dabigatran, just as in the Norwegian Patient Registry [20].
In the present study, in the assessment of factors indicating the choice of a particular NOAC, participating centers were included as potential confounders. The influence of a center on the selection of a specific NOAC may be related to local familiarity with these drugs—some sites may have more exposure to and experience with a specific NOAC. When selecting among NOACs, in the absence of robust head-to-head data, it is possible that local site factors are significant. Physicians tend to select those NOACs with which they are most comfortable and knowledgeable.
As the previous study reported, a high percentage of patients with low thromboembolic risk (CHA2DS2-VASc 0 in males or 1 in females) were treated with OACs [20,21,22]. In the present study, 93% of patients were at high risk of thromboembolic complications according to their CHA2DS2-VASc score. By extension, the proportion of low-risk patients receiving NOACs was not high, and most of them were patients with a temporal indication for OACs (before or after ablation or cardioversion).
In the present study, the mean CHA2DS2-VASc scores were 4.6, 4.2, and 4.2 for patients treated with apixaban, dabigatran, and rivaroxaban, respectively. In the XANTUS (Xarelto for Prevention of Stroke in Patients With Atrial Fibrillation) (registry the mean CHA2DS2-VASc score was 3.4 for rivaroxaban-treated patients [23], while in the APAF registry the mean CHA2DS2-VASc score was 3.8 for apixaban-treated patients [24].
In the present study, patients receiving apixaban had the highest CHA2DS2-VASc and HAS-BLED scores in comparison to patients treated with dabigatran and rivaroxaban; this was probably a reflection of the increased risk of heart failure in these patients and their older age.
The same results were obtained in the study by Maura et al. [25] with a group of 127,841 patients with AF who were administered NOACs. In the Norwegian Patient Registry [19], patients taking apixaban had the highest CHA2DS2-VASc scores, whereas the highest HAS-BLED scores were reported in patients receiving rivaroxaban.
In the analysis of factors influencing the choice of particular NOACs, the components of the CHA2DS2-VASc and HAS-BLED scores, and not their results, were taken into account. This is due to the fact that the same results for the aforementioned scores can present in completely different patients. Furthermore, expert documents suggesting the selection of individual NOACs also indicate clinical features, and not results in the scores, as factors relevant to the choice of a particular pharmaceutical.
In the present study, elderly age was a factor predisposing choice of apixaban. In the ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) study, the patients were younger than in the RE-LY (Randomized Evaluation of Long Term Anticoagulant Therapy) and ROCKET-AF (The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation)studies [6,7,8]. However, in the ARISTOTLE study, it was shown that apixaban was equally effective in all age groups [6]. In the subgroup of 88,582 very old ( ≥ 80 y) patients from the ARISTOPHANES (Clinical and Economic Outcomes of Oral Anticoagulants in Non-valvular Atrial Fibrillation) study, apixaban was associated with a lower risk of stroke, systemic embolism, and major bleeding compared with dabigatran and rivaroxaban [26]. Similarly, in a real-world study of 264,479 patients, it was shown that among elderly AF patients, apixaban was associated with significantly lower risks of all-cause, stroke, or systemic embolism-related and major bleeding-related hospitalizations compared with warfarin, dabigatran, and rivaroxaban [27].
As in the POL-AF registry, in the PAROS study of 2027 AF patients, apixaban was more likely than other NOACs to be prescribed in older patients after bleeding and with decreased renal function [28]. In the ORBIT-AF II study, elderly age also predisposed the choice of apixaban vs. rivaroxaban [29].
Previous hemorrhagic complications are a significant factor influencing the withdrawal of OACs in the prevention of thromboembolic complications [30]. In the present study, previous bleeding was a predisposing factor for use of apixaban, which reduced the chance of using rivaroxaban. In a nationwide study of patients with AF in Norway, it was found that dabigatran and apixaban were both associated with a significantly lower risk of major bleeding compared with rivaroxaban [19]. The data from the Danish nationwide registry showed that rivaroxaban was associated with a higher risk of major bleeding compared with apixaban [31].
Decreased renal function is a recognized risk factor for thrombus formation, stroke, systemic embolism, and bleeding events [32,33]. There are also some acknowledged data pointing out that NOACs could reduce the risk of stroke or systemic embolism and major bleeding with respect to different levels of renal function [34,35]. Medicare data showed that apixaban, compared with warfarin, was associated with a decreased risk of stroke or systemic embolism and major bleeding. Risks for both outcomes with rivaroxaban and dabigatran did not differ from risks with warfarin in patients with impaired renal function [36]. In the present study, GFR < 60 mL/min/1.73 m2 was a factor predisposing the choice of apixaban and diminishing the chance of using rivaroxaban or dabigatran.
Although current guidelines make no distinction between non-permanent and permanent AF for stroke prevention, there are clinical data confirming that the type of AF is associated with an increased risk of stroke [37]. Therefore, it is possible that in the future, the type of AF will be taken into consideration in the stratification of thromboembolic risk and in choosing anticoagulant prophylaxis. In the presented study, type of AF was not a predisposing factor for apixaban, dabigatran, or rivaroxaban.
5. Limitations
Several limitations related to the nature of the data used should be underlined. First of all, due to the lack of long-term observation of patients, it was not possible to evaluate the long-term prognosis of patients with AF treated with individual NOACs. Secondly, in the present study, hospitalized patients with AF were assessed; among these, only some had a first-time diagnosis of AF and only in these patients was an anticoagulant therapy initiated. Thus, despite the registry referring to hospitalized patients, anticoagulant therapy for most of them was initiated in ambulatory conditions before hospital admission. In our study, the data related to edoxaban were not presented because this drug is not available in Poland.
Patients admitted to hospital to have AF ablation were excluded from the registry for two reasons. Firstly, not all centers perform catheter ablation. Secondly, it was acknowledged that patients undergoing ablation due to AF have a clinical profile different from most patients with AF (they are younger and do not have concomitant diseases).
Nevertheless, our data present a comprehensive picture of current Polish AF patients and cardiologist practices, which will provide useful and reliable insights into real-world clinical practice.
6. Conclusions
The POL-AF registry shows a full picture of the contemporary use of NOACs in AF patients. In hospitalized patients with AF, the most frequently chosen NOAC was rivaroxaban.
Apixaban was chosen more often in patients after bleeding, and in those who were advanced in years, with heart failure and impaired renal function. Impaired renal function and female gender were factors that diminished the chance of using dabigatran. Previous bleeding and vascular disease was the factor that diminished the chance of using rivaroxaban. Dabigatran and rivaroxaban have been used less frequently in elderly patients. The above results may be of great importance in clinical practice due to the lack of data referring to NOAC application in individual clinical situations.
Acknowledgments
The POL-AF registry was initiated on the scientific platform of “Club 30” of the Polish Cardiac Society. Investigators other than those listed as authors include: Katarzyna Karoń (Warsaw), Paweł Krzesiński (Warsaw), Małgorzata Krzciuk (Ostrowiec Swietokrzyski), Anna Michalska (Kielce), Michał Niedźwiedź (Warsaw), Monika Szewczak (Warsaw), Wiktor Wójcik (Warsaw).
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/11/3565/s1, Table S1: Prescription pattern of apixaban, dabigatran, and rivaroxaban across centers, Table S2: Factors associated with the selection of an individual NOAC over another NOAC for stroke prevention in patients with AF, as assessed using univariable logistic regression models.
Author Contributions
Conceptualization, I.G., O.J., B.U.-Ż., A.K.-C. and B.W.-K.; methodology, I.G., O.J., B.U.-Ż., M.C., A.K.-C., A.T.-K. and B.W.-K.; software, M.C., J.B. (Janusz Bednarski), M.W. (Marcin Wełnicki); validation, I.G., O.J., B.U.-Ż., M.C., M.W. (Maciej Wójcik), R.B., A.S. (Anna Szpotowicz) and B.W.-K.; formal Analysis, I.G., O.J., M.C. and A.M.; investigation, I.G., O.J., B.U.-Ż., A.K.-C., M.B., M.G., T.T., R.R.-S., J.B. (Jacek Bil), M.W. (Michał Wojewódzki), resources, I.G., B.W.-K.; data curation, O.J., E.B.-O., A.S. (Anna Szyszkowska), M.W. (Michał Wojewódzki), M.W. (Marcin Wełnicki); writing—original draft preparation, I.G., O.J., B.U.-Ż., M.C.; writing—review and editing, A.K.-C., A.T.-K. and B.W.-K.; visualization, I.G., O.J., M.M.; supervision, B.W.-K.; funding acquisition, I.G. All authors have read and agreed to the published version of the manuscript.
Funding
Project financed under the program of the Minister of Science and Higher Education called the “Regional Initiative of Excellence” for the years 2019–2022, project no 024/RID/2018/19, with a financing amount of 1,199,900,000 PLN.
Conflicts of Interest
O.J., B.U.-Z., M.Ch., M.M., M.W., R.B., M.B., M.G., T.T., R.R.-S., J.B., M.Wo., A.S., E.B.-A., A.Sz., M.We., M.M.: None. I.G.: research grant from Boehringer-Ingelheim and speaker for Boehringer-Ingelheim and Bayer. A.K.-C.: speaker for Boehringer-Ingelheim, Bayer, Pfizer. J.Be.: speaker for Boehringer-Ingelheim, Bayer, Pfizer. A.T-K.: research grant from Boehringer-Ingelheim, consultant for Boehringer-Ingelheim and Bayer and speaker for Boehringer-Ingelheim. B.W.-K.: speaker for Boehringer-Ingelheim, Bayer, Pfizer.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wolf P.A., Abbott R.D., Kannel W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke. 1991;22:938–983. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart. J. 2020;29:ehaa612. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., Ellinor P.T., Ezekowitz M.D., Field M.E., Furie K.L., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Lopatowska P., Tomaszuk-Kazberuk A., Mlodawska E., Bachorzewska-Gajewska H., Malyszko J., Dobrzycki S., Musial W.J. Do CHA2DS2VASc and HAS-BLED scores influence ‘real-world’ anticoagulation management in atrial fibrillation? 1556 patient registry from the reference cardiology centre. Pharmacoepidemiol. Drug. Saf. 2015;24:1297–1303. doi: 10.1002/pds.3878. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg B.A., Gao H., Shrader P., Pieper K., Thomas L., Camm A.J., Ezekowitz M.D., Fonarow G.C., Gersh B.J., Goldhaber S., et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I and ORBIT-AF II registries. Am. Heart. J. 2017;194:132–140. doi: 10.1016/j.ahj.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Granger C., Alexander J., McMurray J., Lopes R.D., Hylek E.M., Hanna M., Al-Khalidi H.R., Ansell J., Atar D., Avezum A., et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7.Connolly S., Ezekowitz M., Yusuf S., Eikelboom J., Oldgren J., Parekh A., Pogue J., Reilly P.A., Themeles E., Varrone J., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 8.Patel M., Mahaffey K., Garg J., Pan G., Singer D.E., Hacke W., Breithardt G., Halperin J.L., Hankey G.J., Piccini J.P., et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 9.Heidbuchel H., Verhamme P., Alings M., Antz M., Diener H.C., Hacke W., Oldgren J., Sinnaeve P., Camm A.J., Kirchhof P., et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 10.Heidbuchel H., Verhamme P., Alings M., Antz M., Hacke W., Oldgren J., Sinnaeve P., Camm A.J., Kirchhof P. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–651. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 11.Steffel J., Verhamme P., Potpara T.S., Albaladejo P., Antz M., Desteghe L., Haeusler K.G., Oldgren J., Reinecke H., Roldan-Schilling V., et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: Executive summary. Europace. 2018;20:1231–1242. doi: 10.1093/europace/euy054. [DOI] [PubMed] [Google Scholar]
- 12.Lip G.Y.H., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 14.Caturano A., Galiero R., Pafundi P.C. Atrial fibrillation and stroke. A review on the use of vitamin K antagonists and novel oral anticoagulants. Medicina. 2019;55:617. doi: 10.3390/medicina55100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huiart L., Ferdynus C., Renoux C., Beaugrand A., Lafarge S., Bruneau L., Suissa S., Maillard O., Ranouil X. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population-based cross-sectional study in the French health insurance databases. BMJ Open. 2018;8:e018180. doi: 10.1136/bmjopen-2017-018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong C.M., Liu Y., Apruzzese P., Doros G., Cannon C.P., Maddox T.M., Gehi A., Hsu J.C., Lubitz S.A., Virani S., et al. Association of insurance type with receipt of oral anticoagulation in insured patients with atrial fibrillation: A report from the American College of Cardiology NCDR PINNACLE registry. Am. Heart. J. 2018;195:50–59. doi: 10.1016/j.ahj.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Boriani G., Proietti M., Laroche C., Fauchier L., Marin F., Nabauer M., Potpara S., Dan G.A., Kalarus Z., Diemberger I., et al. Contemporary stroke prevention strategies in 11,096 European patients with atrial fibrillation: A report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) long-term general registry. Europace. 2018;20:747–757. doi: 10.1093/europace/eux301. [DOI] [PubMed] [Google Scholar]
- 18.Staerk L., Fosbřl E.L., Gadsbřll K., Sindet-Pedersen C., Pallisgaard J.L., Lamberts M., Lip G.H.Y., Torp-Pedersen C., Gislason G.H., Olesn J.B. Non-vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: Temporal trends 2011–2015 in Denmark. Sci. Rep. 2016;6:31477. doi: 10.1038/srep31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutherford O.W., Jonasson C., Ghanima W., Söderdahl F., Halvorsen S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: A nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2020;6:75–85. doi: 10.1093/ehjcvp/pvz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potpara T.S., Trendafilova E., Dan G.A., Goda A., Zumreta K., Manola S., Music L., Gjini V., Pojskic B., Popescu M.I., et al. The patterns of non-vitamin K antagonist oral anticoagulants (NOACs) use in patients with atrial fibrillation in seven Balkan countries: A Report from the BALKAN-AF Survey. Adv. Ther. 2017;34:2043–2057. doi: 10.1007/s12325-017-0589-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potpara T.S., Dagres N., Mujović N., Vasić D., Ašanin M., Nedeljkovic M., Marin F., Fauchier L., Blomstrom-Lundqvist C., Lip G.H.Y. Decision-Making in clinical practice: Oral anticoagulant therapy in patients with non-valvular atrial fibrillation and a single additional stroke risk factor. Adv. Ther. 2017;34:357–377. doi: 10.1007/s12325-016-0458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazurek M., Huisman M.V., Rothman K.J., Paquette M., Teutsch C., Diener H.C., Dubner S.J., Halperin J.L., Ma C.S., Zint K., et al. Regional differences in antithrombotic treatment for atrial fibrillation: Insights from the GLORIA-AF Phase II Registry. Thromb. Haemost. 2017;117:2376–2388. doi: 10.1160/TH17-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camm A.J., Amarenco P., Haas S., Hess S., Kirchhof P., Kuhls S., van Eickels M., Turpie A.G., XANTUS Investigators XANTUS: A real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur. Heart. J. 2016;37:1145–1153. doi: 10.1093/eurheartj/ehv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeymer U., Lober C., Wolf A., Richard F., Schafer H., Taggeselle J., Kabitz H.J., Prondzinsky R., Süselbeck T., APAF-Investigators Use, persistence, efficacy, and safety of Apixaban in patients with non-valvular atrial fibrillation in unselected patients in Germany. Results of the Prospective Apixaban in Atrial Fibrillation (APAF) Registry. Registry. Cardiol. Ther. 2020;9:467–478. doi: 10.1007/s40119-020-00188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maura G., Billionnet C., Drouin J., Weill A., Neumann A., Pariente A. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of nonvitamin K antagonist oral anticoagulants: Findings from the French healthcare databases, 2011–2016. BMJ Open. 2019;9:e026645. doi: 10.1136/bmjopen-2018-026645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deitelzweig S., Keshishian A., Li X., Kand A., Dhamane A.D., Luo X., Balachander N., Rosenblatt L., Mardekian J., Pan X., et al. Comparisons between oral anticoagulants among older nonvalvular atrial fibrillation patients. J. Am. Geriatr. Soc. 2019;67:1662–1671. doi: 10.1111/jgs.15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin A., Keshishian A., Trocio J., Dina O., Le H., Rosenblatt L., Liu X., Mardekian J., Zhang Q., Baser O., et al. A real-world observational study of hospitalization and health care costs among nonvalvular atrial fibrillation patients prescribed oral anticoagulants in the U.S. Medicare population. J. Manag. Care. Spec. Pharm. 2020;26:639–651. doi: 10.18553/jmcp.2020.26.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falissard B., Picard F., Mahe I., Hanon O., Touzé E., Danchin N., Lamy F.X., Ricci L., Steg P.G. Apixaban for prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation in France: The PAROS cross-sectional study of routine clinical practice. Arch. Cardiovasc. Dis. 2019;112:400–409. doi: 10.1016/j.acvd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg B.A., Shrader P., Thomas L., Ansell J., Fonarow G.C., Gersh B.J., Hylek E., Kowey P.R., Mahaffey K.W., O’Brien E.C., et al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II) Am. Heart. J. 2017;189:40–47. doi: 10.1016/j.ahj.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien E.C., Simon D.N., Allen L.A., Singer D.E., Fonarow G.C., Kowey P.R., Thomas L.E., Ezekowitz M.D., Mahaffey K.W., Chang P., et al. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am. Heart. J. 2014;168:487–494. doi: 10.1016/j.ahj.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Bonde A.N., Martinussen T., Lee C.J., Lip G.Y.H., Staerk L., Bang C.N., Bhattacharya J., Gislason G., Torp-Pedersen C., Olesen J.B., et al. Rivaroxaban versus Apixaban for stroke prevention in atrial fibrillation: An instrumental variable analysis of a nationwide cohort. Circ. Cardiovasc. Qual. Outcomes. 2020;13:e006058. doi: 10.1161/CIRCOUTCOMES.119.006058. [DOI] [PubMed] [Google Scholar]
- 32.Piccini J.P., Stevens S.R., Chang Y., Singer D.E., Lokhnygina Y., Go A.S., Patel M.R., Mahaffey K.W., Halperin J.L., Breithardt G., et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: Validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. doi: 10.1161/CIRCULATIONAHA.112.107128. [DOI] [PubMed] [Google Scholar]
- 33.Kapłon-Cieślicka A., Budnik M., Gawałko M., Peller M., Gorczyca I., Michalska A., Babiarz A., Bodys A., Uliński R., Żochowski M., et al. Atrial fibrillation type and renal dysfunction as important predictors of left atrial thrombus. Heart. 2019;105:1310–1315. doi: 10.1136/heartjnl-2018-314492. [DOI] [PubMed] [Google Scholar]
- 34.Del-Carpio Munoz F., Gharacholou S.M., Munger T.M., Friedman P.A., Asirvatham S.J., Packer D.L., Noseworthy P.A. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am. J. Cardiol. 2016;17:69–75. doi: 10.1016/j.amjcard.2015.09.046. [DOI] [PubMed] [Google Scholar]
- 35.Zou R., Tao J., Shi W., Yang M., Li H., Lin X., Yang S., Hua P. Meta-analysis of safety and efficacy for direct oral anticoagulation treatment of non-valvular atrial fibrillation in relation to renal function. Thromb. Res. 2017;160:41–50. doi: 10.1016/j.thromres.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Wetmore J.B., Roetker N.S., Yan H., Reyes J.L., Herzog C.A. Direct-Acting oral anticoagulants versus Warfarin in Medicare patients with chronic kidney disease and atrial fibrillation. Stroke. 2020;51:2364–2373. doi: 10.1161/STROKEAHA.120.028934. [DOI] [PubMed] [Google Scholar]
- 37.Cho S., Kim J., Kim J.B., Park J., Park J.-K., Kang K.-W., Shim J., Choi E.-K., Lee Y.S., Park H.W., et al. The difference of burden of ectopic beats in different types of atrial fibrillation and the effect of atrial fibrillation type on stroke risk in a prospective cohort of patients with atrial fibrillation (CODE-AF registry) Sci. Rep. 2020;10:6319. doi: 10.1038/s41598-020-63370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.