Abstract
Zika virus (ZIKV) infection in pregnancy is associated with congenital neurological abnormalities. Our understanding of the full clinical spectrum of ZIKV infection is incomplete. Using data from this prospective cohort study consisting of 650 women attending a high-risk pregnancy clinic during the Zika virus outbreak in Brazil, we investigated the extent to which specific symptoms can be utilized to differentiate ZIKV-infected pregnant women from those with other pregnancy-related problems. All were tested for ZIKV in urine by RT–qPCR. Demographic and clinical data including physical symptoms during follow-up were recorded and analyzed with respect to Zika virus exposure status. Forty-eight (7.4%) women were positive for ZIKV by RT–qPCR. The majority (70.8%) were asymptomatic, and only four ZIKV-positive women (8.3%) reported symptoms during pregnancy that met the WHO case definition. Zika-positive and -negative women reported similar frequencies of ZIKV-like symptoms (as per the WHO definition): fever (16.7% vs. 13.6%), arthralgia/arthritis (10.4% vs. 11.3%), rash (4.2% vs. 5.3%), and conjunctivitis (2.1% vs. 3.2%). Most pregnant women positive for ZIKV in urine are asymptomatic and do not deliver a baby with microcephaly. Physical symptoms alone did not differentiate between high-risk pregnant women positive or negative for ZIKV.
Keywords: zika, congenital infections
1. Introduction
More than three years after the appearance of the Zika virus (ZIKV) in the Americas [1], our understanding of the clinical presentation and consequences of ZIKV infection in pregnant women is still incomplete. The proportion of ZIKV-infected pregnant women who display symptoms, identification of the most common symptoms of ZIKV in pregnancy and their sensitivity and specificity, and whether symptomatic ZIKV-infected pregnant women are at greater risk for delivering an infant with congenital abnormalities remain to be definitively determined.
The incidence of asymptomatic ZIKV infections in pregnant women is widely quoted to be 80% based on estimates from household survey data from Yap Island, Micronesia, 2007 [2]. Systematic reviews to update this figure have used subsequently published epidemiological studies; however, this has proved difficult due to the paucity of studies looking specifically at the clinical spectrum of the ZIKV infection in pregnancy, marked heterogeneity in the reported asymptomatic rates of disease, and small sample sizes [3]. For example, in a recent WHO systematic review looking at the proportion of asymptomatic ZIKV infections in a number of population sub-groups, the proportion of asymptomatic infections in pregnant women varied from 10% to 83% [3]. Data from a countrywide pregnancy cohort study in French Guiana [4] showed striking intra-study heterogeneity in the asymptomatic infection rate of ZIKV-infected pregnant women, related to a number of socio-demographic factors. For example, although 77% of pregnant women overall were asymptomatic, those living in the urbanized coastal areas reported significantly more symptoms than those living in the remote interior (35% versus 17%, p = 0.001), and women over 30 were also more likely to report symptoms compared to younger women (28% versus 20%, p = 0.03).
The frequency of individual symptoms reported by pregnant women with confirmed ZIKV infection also varies according to different reports and is hard to assess, because entry criteria for many cohort studies are defined around the presence of being positive for certain symptoms at recruitment, for example, rash or fever [5,6,7,8]. Furthermore, areas that are endemic for ZIKV are also endemic for other flaviviruses; co-infection is possible and complicates the clinical picture [9,10]. The current standard clinical case definition for the ZIKV infection proposed by the World Health Organization (WHO) [11] was last updated in 2016. No ZIKV studies to date have evaluated its sensitivity or specificity in the detection of the clinical ZIKV infection during pregnancy.
There is also no consensus on whether newborns are more likely to exhibit negative sequelae at birth if their ZIKV-infected mothers are symptomatic during pregnancy. A systematic review of 9 case series performed in Brazil and Colombia and 3 cohort studies from the USA concluded that the ratio of symptomatic versus asymptomatic maternal ZIKV infections that resulted in adverse fetal outcomes was 1:1 [12]. However, other publications have hypothesized that women with a symptomatic infection may have a higher viral load than do women with asymptomatic infections and this may translate to a higher probability of birth defects in their offspring [4,13,14].
The aims of the present study are to determine in a region with active ZIKV transmission the relative percentage of ZIKV-positive pregnant women who are asymptomatic and whether symptoms of ZIKV in RT–PCR-positive pregnant women can be differentiated from symptoms present in women with high-risk pregnancies who are ZIKV-negative.
2. Materials and Methods
2.1. Study Design and Participants
The data reported in this prospective cohort study originated from the Jundiaí Zika Cohort initiated in March 2016 at Jundiaí University Hospital in São Paulo State, Brazil. During the recruitment period (01 March 2016–23 August 2017), all the women attending a high-risk pregnancy clinic, due to the presence of the risk factors threatening the life or health of the pregnant woman or her fetus [15] at any stage of pregnancy, were considered eligible and offered the opportunity to participate in the study. The only exclusion criteria were women with life-threatening conditions or who could not provide informed consent. Research nurses who interviewed the women at enrolment and reviewed their antenatal records gathered detailed demographic, medical, and antenatal information. In addition, all participants were specifically asked if they had experienced the following symptoms during their pregnancy: fever, rash, conjunctivitis, joint pain or swelling, headache, vomiting, lymphadenopathy, bleeding, myalgia, or pruritus. Women who were symptomatic at recruitment, had experienced symptoms earlier in the pregnancy, and/or who developed symptoms consistent with the WHO definition of suspected ZIKV infection (rash and/or fever and at least one of the following symptoms: arthralgia, arthritis, or non-purulent conjunctivitis (see Table 1)) [11] were noted.
Table 1.
WHO 2016 interim case definitions for confirmed and suspected Zika virus infection.
| Case Definition | Description |
|---|---|
| Confirmed case | A person with laboratory confirmation of recent Zika virus infection:
|
| Suspected case | A person presenting with rash and/or fever and at least one of the following signs or symptoms:
|
Research nurses collected blood, saliva, and urine from all subjects at the time of enrolment, 2–3 weeks after recruitment and subsequently on a 2–3 monthly basis during routine check-ups. Trained volunteers carried out pre-arranged weekly follow-up telephone interviews and inquired whether they had experienced any new symptoms consistent with the ZIKV infection. If symptoms were reported, the women were advised to attend the hospital for clinical review and blood, saliva, and urine were collected at this time as well.
Of note, the methods above describe the recruitment, enrolment, and follow-up of all women in the cohort. At the analysis stage, some individuals or dyads had to be excluded from the analysis, mainly due to missing information pertaining to risk factors or outcomes of interest. This will be described in detail later.
2.2. Laboratory Procedures
All laboratory procedures were performed on de-identified samples. It was opted to prioritize the detection of the ZIKV in urine samples by reverse transcriptase–polymerase chain reaction (RT–PCR) and to store blood and saliva for future analysis. Studies have shown that the ZIKV RNA is unlikely to be detected in serum after the first week of illness, whereas it can be detected in urine for at least 2 weeks after the symptom onset [16,17,18].
Total RNA was extracted from urine by a commercial QIAamp Viral RNA Kit (Qiagen®, Hilden, Germany) following the manufacturer’s instructions and stored at −80 °C until used. Reverse transcription (RT) and qPCR were performed with a GoTaq® 1-Step RT–qPCR System (Promega®, Wisconsin, USA) on an ABI Prism 7500 SDS Real-Time cycler (Applied Biosystems). The primers and probes designed by Lanciotti and colleagues [16] are complementary to the nonstructural 5 protein (polymerase). The RT cycle consisted of a 10 min cycle at 50 °C and a 15 min cycle at 95 °C. The PCR consisted of forty cycles of 15 s at 95 °C and a 1 min cycle at 60 °C. Three positive controls (RNA extracted from positive ZIKV samples) and two negative controls (H2O) were included. We considered positive those samples that presented with a threshold cycle (Ct) less than 38.5, as per Lanciotti and colleagues [19]. In the cases where the results were inconclusive, repetitions were performed with serial dilutions.
2.3. Neonatal Categorisation
Sonographers specialized in fetal medicine performed antenatal ultrasound scanning at months 3, 5, 7, and 8 in asymptomatic women and monthly in symptomatic women at the São Paulo Radiology Centre using Voluson 730 Expert/Voluson E6, GE equipment. Anthropometric measures at birth (i.e., neonatal weight, length, and head circumference) were obtained for all live-born infants, and the equipment used was consistent. The weight was assessed using digital scales, length—using a recumbent baby length scale, and head circumference—using a standardized non-elastic tape measure. Z-scores for weight, length, and head circumference were determined using the online Intergrowth calculator, which takes into account gestational age and sex [20,21,22]. Gestational age was estimated using the first trimester ultrasound (USS) when available and by the last menstrual period (LMP) when USS was unavailable. Microcephaly was defined as a head circumference z-score of less than −2, determined using the online Intergrowth-21st calculator which takes into account gestational age and sex [21].
2.4. Statistical Analysis
The sample size for the cohort was calculated using an estimated prevalence of cases of microcephaly among neonates of ZIKV RT–PCR-positive pregnant women of 2%. A final analytical cohort size of n = 531 would give us 80% power to detect a crude relative risk of 2 with a probability of type I error (α) of 5%. Categorical variables were compared among women with and without symptoms, and symptoms were compared between different case definitions using the chi-squared test, except where there were less than 5 in any cell, in which case the Fisher’s exact test was used. For the calculation of sensitivity and specificity of the standard clinical case definition, ZIKV RT–PCR was used as the “gold standard” diagnostic test. All statistical analyses were carried out using the STATA™ version 15.1 software.
2.5. Sensitivity and Specificity
The sensitivity and specificity of the current WHO case definition were calculated using the standard formulas, using only the women that had at least one symptom during pregnancy. Asymptomatic individuals were removed for the calculation of sensitivity and specificity. In other words, true positives were defined as symptomatic ZIKV RT–PCR-positive women correctly identified by the WHO case definition and false negatives were defined as symptomatic ZIKV RT–PCR-positive women that did not meet this case definition. True negatives were defined as ZIKV RT–PCR-negative women who were symptomatic but did not meet the full WHO symptomatic case criteria and false positives were defined as symptomatic ZIKV RT–PCR-negative women that met the WHO suspected clinical case criteria. With one caveat, that the current most optimal diagnostic test in areas with active ZIKV transmission is ZIKV RT–PCR and this test has a narrow window for detection of the virus, we cannot assume that any women were truly negative and therefore the number of false positives may be overestimated and the number of true positives underestimated.
3. Results
3.1. Participant Characteristics
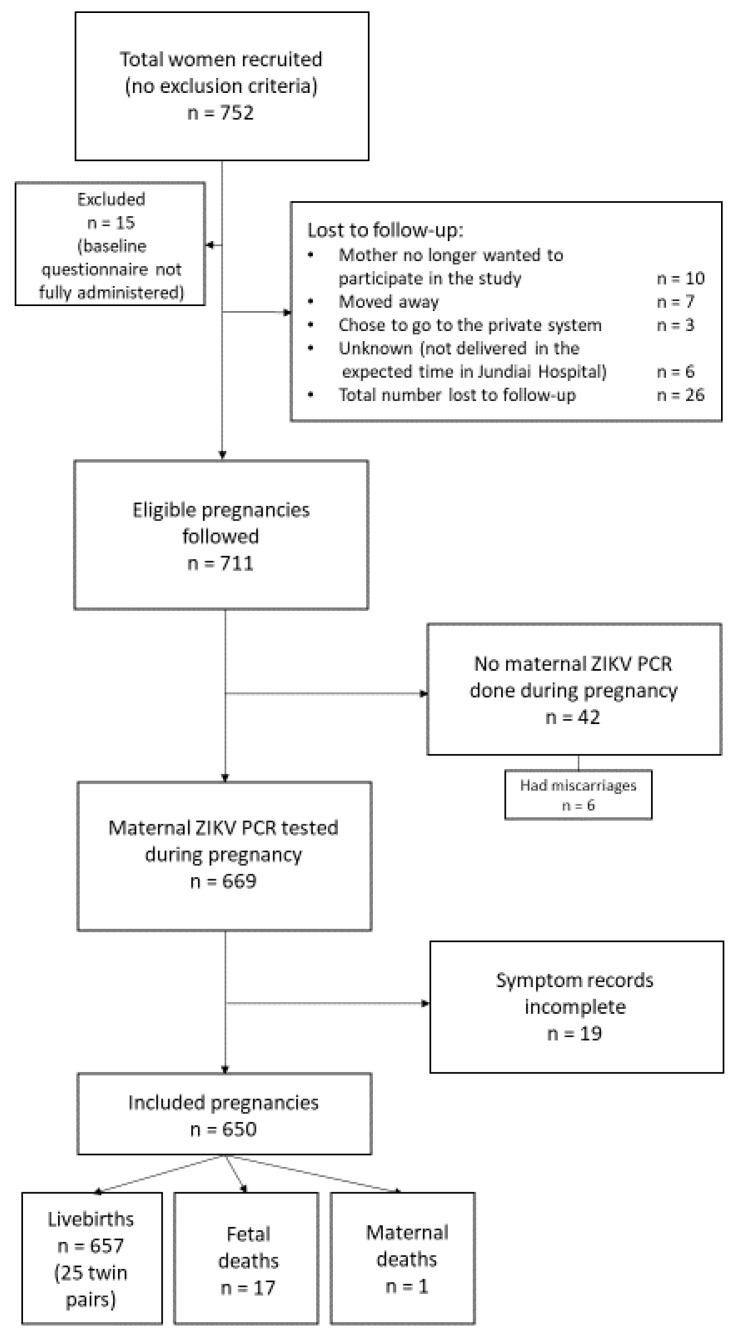
A flow diagram of study participants is shown in Figure 1. A total of 752 women were initially enrolled in the study between March 2016 and August 2017; 26 were eventually lost to follow-up. The women with missing data pertaining to risk factors and outcomes of interest were also excluded. Forty two women did not have a ZIKV RT–PCR sample taken during pregnancy. Additionally, 19 women did not have information on symptoms during pregnancy. Among the remaining 650 women, there were 675 live births (including 25 twin pairs), 17 fetal deaths, and one maternal death.
Figure 1.
Flow diagram for the selection of study participants.
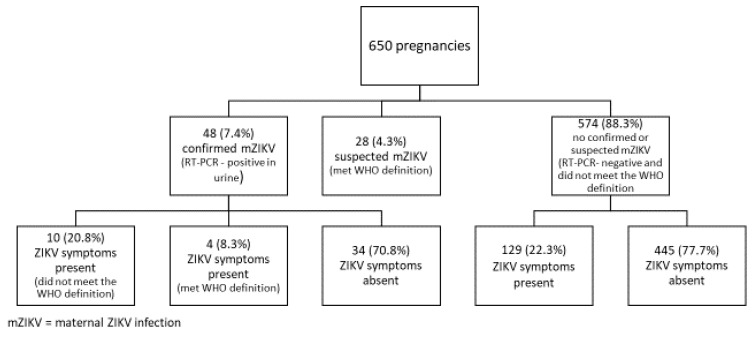
Of the 650 pregnant women in the final cohort, 32 (4.9%) were recruited in the first trimester, 228 (35.1%) in the second trimester, and 378 (58.1%) in the third trimester. In addition, 48 (7.4%) were ZIKV-positive according to the RT–PCR, 28 (4.3%) were positive by the WHO criteria but were ZIKV-negative, and 574 (88.3%) were negative according to both the RT–PCR and symptoms (Figure 2).
Figure 2.
Distribution of pregnant women according to the WHO ZIKV case definitions.
Only 4 (8.3%) of the 48 ZIKV-positive women met the WHO definition of a symptomatic case, 10 (20.8%) had at least one ZIKV-like symptom during pregnancy but did not meet the WHO definition, and 34 (70.8%) did not report any symptoms compatible with the ZIKV infection during pregnancy. Of the 574 pregnant women who did not meet the definition for either a confirmed or a suspected case, 129 (22.3%) had at least one symptom that was consistent with the ZIKV infection and 445 (77.7%) never reported any symptoms during pregnancy.
Socio-demographic characteristics of the study population are shown in Table 2. There were no differences in maternal age, level of education, race, co-habitation, and delivery by cesarean section between symptomatic and asymptomatic women. The time of symptom initiation was known for 13 of the 14 women with the confirmed symptomatic ZIKV infection. Six (46.2%) had symptoms in the first trimester, 4 (30.8%)—in the second trimester, and 3 (23.1%)—in the third trimester. In 25 women who met the WHO case definition but who were ZIKV RT–PCR-negative, 11 (39.3%) had symptoms in the first trimester, 11—in the second trimester and 3 (21.4%) had symptoms in their third trimester. Of note, the mean number of separate urine RT–PCR results available for each woman throughout their pregnancy was 2.02 among ZIKV-positive women and 1.95 among ZIKV-negative women.
Table 2.
Maternal characteristics of participants in the Jundiaí Zika Cohort.
| Variable | ZIKV Symptoms Present * (n = 171) | ZIKV Symptoms Absent (n = 479) | p-Value |
|---|---|---|---|
| Age | |||
| 13–19 years | 32 (18.7%) | 65 (13.6%) | 0.07 |
| 20–34 years | 112 (65.5%) | 305 (63.7%) | |
| 35–46 years | 27 (15.8%) | 109 (22.8%) | |
| Missing | 0 | 0 | |
| Education | |||
| ≤8 years | 30 (17.8%) | 78 (16.6%) | 0.96 |
| 9–11 years | 40 (23.7%) | 106 (22.6%) | |
| 12 years | 74 (43.8%) | 210 (44.8%) | |
| >12 years | 25 (14.8%) | 75 (16.0%) | |
| Missing | 2 (1.2%) | 9 (1.9%) | |
| Ethnicity/race | |||
| White | 94 (56.3%) | 246 (52.5%) | 0.86 $ |
| Mixed race | 55 (32.9%) | 165 (35.2%) | |
| Black | 15 (9.0%) | 49 (10.5%) | |
| Other (Asian/Indigenous) | 3 (1.8%) | 9 (1.9%) | |
| Missing | 4 (2.3%) | 10 (2.1%) | |
| Relationship with partner | |||
| Married/co-habiting | 129 (76.3%) | 361 (76.7%) | 0.93 |
| Single/divorced/widowed | 40 (23.7%) | 111 (23.4%) | |
| Missing | 2 (1.2%) | 8 (1.7%) | |
| Type of delivery | |||
| Vaginal/forceps | 75 (51.4%) | 200 (50.4%) | 0.84 |
| C-section | 71 (48.6%) | 197 (49.6%) | |
| Missing | 2 (1.4%) | 1 (0.25%) | |
| ZIKV RT–PCR status | |||
| Positive in urine | 14 (8.2%) | 34 (7.1%) | 0.64 |
| Negative in urine | 157 (91.8%) | 445 (92.9) |
* At least one symptom compatible with ZIKV infection as per the current WHO standard clinical case definition. Note: percentages for all categories were calculated with exclusion of those with missing data from the denominator. $ All the p-values calculated using the chi-squared test [2] except for those labeled with $, which were calculated using the Fisher’s exact test. The “missing” category was not included as a category when the p-value was estimated.
Of the 48 women who were ZIKV RT–PCR-positive, 4 (8.3%) reported symptoms during pregnancy that met the WHO definition of a clinical case. Twenty eight (4.3%) ZIKV-negative women also had symptoms consistent with the WHO definition. Among the ZIKV-positive cases, 25 (52.1%) complained of a headache, 8 (16.7%) reported fever, 5 (10.4%) had arthralgia/arthritis, and 2 (4.2%) had a rash. Among the Zika-negative women, 320 (53.2%) complained of headache, 82 (13.6%) had fever, 68 (11.3%) had arthralgia/arthritis, and 32 (5.3%) had a rash during pregnancy. The results are summarized in Table 3. None of these differences reached statistical significance. Of note, the lag time between symptoms and the ZIKV RT–PCR testing ranged from 1 to 177 days (median, 58 days) among the 28 women who had WHO symptoms but tested negative and ranged from 22 to 171 days (median, 53.5 days) among the 4 women who had WHO symptoms and tested RT–PCR-positive.
Table 3.
Symptoms in women with and without PCR-confirmed ZIKV infection.
| No. (%) Positive | ||||
|---|---|---|---|---|
| Signs/Symptoms | PCR-Positive n = 48 |
PCR-Negative n = 602 |
p-Value ** | |
| WHO criteria | Fever | 8 (16.7) | 82 (13.6) | 0.55 |
| Arthralgia/arthritis | 5 (10.4) | 68 (11.3) | 0.85 | |
| Rash | 2 (4.2) | 32 (5.3) | 0.73 | |
| Conjunctivitis | 1 (2.1) | 19 (3.2) | 0.68 | |
| Other symptoms | Myalgia | 4 (8.3) | 74 (12.3) | 0.42 |
| Headache | 25 (52.1) | 320 (53.2) | 0.89 | |
| Lymphadenopathy | 1 (2.1) | 33 (5.5) | 0.31 | |
| Total symptomatic | Fulfilled required WHO criteria * | 4 (8.3) | 28 (4.7) | 0.20 |
| Did not fulfill WHO criteria Total symptomatic |
10 (20.8) 14 (29.2) |
129 (21.4) 157 (26.1) |
0.20 0.69 |
|
| No symptoms | 34 (70.8) | 445 (73.9) | 0.64 | |
*A person presenting with rash and/or fever and at least one of the following signs or symptoms: arthralgia, arthritis, or conjunctivitis (non-purulent/hyperemic). ** The chi-squared test was used unless there was < 5 in any cell, in which case the Fisher’s exact test was used.
In our cohort of pregnant women, the sensitivity of the current WHO ZIKV clinical case definition was 28.6% and the specificity was 84.9% (Table 4).
Table 4.
Sensitivity, specificity, positive and negative predictive values of the WHO standard clinical case definition for ZIKV infection applied to the symptomatic pregnant women in the Jundiai Zika Cohort.
| Symptomatic Women with the ZIKV Infection | ||||
|---|---|---|---|---|
| ZIKV RT–PCR-Positive | ZIKV RT–PCR-Negative | |||
| Have symptoms that fulfill the WHO standard case definition | Yes | 4 (TP) | 28 (FP) | PPV = TP / (TP + FP) = 12.5% |
| No | 10 (FN) | 157 (TN) | NPV = TN / (TN + FN) = 94.0% |
|
| Sensitivity = TP / (TP + FN) = 28.6% |
Specificity = TN / (FP + TN) = 84.9% |
|||
TP = true positive; TN = true negative; FP = false positive; FN = false negative; PPV = positive predictive value; NPV = negative predictive value.
3.2. Additional Findings
Although not part of the main objectives of this study, we studied the head circumference of neonates in the cohort at birth as well as the presence of microcephaly as markers of adverse neurological outcomes. Head circumference z-scores of the cohort of newborns at birth were analyzed and stratified by the maternal case definition. The infants of suspected cases, as per the WHO standard case definition, had head circumference z-scores that ranged from −1.05 to 2.71 with a mean of 0.63; and infants of the RT–PCR-confirmed cases had head circumference z-scores that ranged from −2.77 to 2.58 with a mean of 0.51 (p = 0.34 using the two-sample t-test). Of the two women with RT–PCR-confirmed ZIKV infection who had newborns with microcephaly (head circumference z-score < −2) at birth, one was asymptomatic and the other reported having had a rash in the first trimester of pregnancy.
4. Discussion
In a population of high-risk pregnant women, 7.4% of whom were RT–PCR-positive for ZIKV during pregnancy, most of those positive for the ZIKV infection in urine (70.8%) were asymptomatic. In addition, ZIKV-positive women could not be differentiated from ZIKV-negative women on the basis of symptoms. Lastly, the WHO case definition of suspected ZIKV-positive cases had low sensitivity (28.6%) for detecting RT–PCR confirmed ZIKV cases, but a high specificity (84.9%). Fever was the most frequently reported symptom in ZIKV-infected women (16.7%), but was also reported by 13.6% of women without suspected or confirmed ZIKV infection. The lack of specificity of fever as a symptom for the ZIKV infection has also been reported by others [4,5].
Our results are consistent with a prior report from French Guiana where only 2.4% of Zika-positive women had symptoms that met the WHO standard clinical case definition for the ZIKV disease [4]. The prevalence of asymptomatic ZIKV infections in our cohort (73.9%) is also comparable to reports from Yap Island, Micronesia, during the outbreak in 2007 [16] and French Guiana in 2016 [4] where 81% and 77% of the cohorts were asymptomatic, respectively. In our cohort, rash was infrequently reported in the ZIKV-positive women (4.2%). This is in stark contrast to the high prevalence of rash (90%) among the 31 patients with confirmed ZIKV during the Yap Island outbreak in 2007 [16]. The differences between these two groups include the fact that, in our study, women were pregnant and, therefore, perhaps, differing immunological responses may have led to distinct clinical manifestations [23]. Another factor which has been discussed previously [4] is the difference in skin pigmentation between various study populations which may cause rash to manifest in different ways or be more or less likely to be reported. Underreporting may have also played a part, particularly during the start of the outbreak, as the phenotype of the rash was not well recognized by patients or health workers. Arthritis and arthralgia were present in 10.4% of our confirmed ZIKV-positive women and 11.3% of the women negative for ZIKV infection. This manifestation appears at the top of the list of symptoms in many ZIKV studies [2,4,5,7,24], as does headache [2,4,5,7,8], which, of note, also forms part of the Centers for Disease Control (CDC) ZIKV clinical case definition [25]. In our study, headache was frequently reported by women with confirmed ZIKV-positive infection (52.1%), as well as by ZIKV RT–qPCR-negative women (53.2%). Thus, it may be especially difficult to differentiate between Zika-positive and -negative women on the basis of symptoms when analyzing high-risk pregnancy populations.
Given the low sensitivity of the current WHO clinical case definition for the detection of the clinical ZIKV infection in pregnant women, we propose incorporating arthritis/arthralgia into the major symptoms and adding headache to the minor symptoms. This change would increase the sensitivity to 50% in our study population. Although still far from ideal (as we would still be misclassifying 50% of true positives as false negatives), these additions may be of particular value in pregnant women in regions with active ZIKV transmission where even a presentation with fever and headache (which is otherwise non-specific) would be sufficient to alert a healthcare worker to test for the ZIKV.
Our study has several limitations. It cannot be ruled out that an unknown proportion of women in our cohort who were ZIKV-negative in their urine may, nevertheless, have been positive for this virus. The concomitant use of serology, not available in the present investigation, may have provided additional validation of maternal exposure to this virus, although serological tests for the ZIKV antibody also have significant inherent problems, notably, cross-reactivity with other flaviviruses [26] and the fact that individuals previously exposed to Dengue Virus (DENV) do not mount a ZIKV IgM response [27]. In this study, most women were recruited in the 2nd or 3rd trimester of pregnancy due to time lags, including time to diagnosis of high-risk pregnancy and appointment waiting time. Therefore, women who were infected with the ZIKV in the first trimester of pregnancy might have been missed by the RT–PCR done at recruitment. It is also possible that some women entered the study having previously been exposed to the ZIKV and that some women who tested ZIKV RT–PCR-positive on recruitment were in fact infected prior to pregnancy but still shedding virus in the urine. If this was the case, these women may not have reported symptoms occurring prior to pregnancy and therefore the symptom status may have been incorrectly classified. As prolonged shedding of the ZIKV in the urine is known to occur, there was no limitation set between the onset of symptoms and the ZIKV RT–PCR-positivity. In other words, a woman who was symptomatic but tested RT–PCR-positive in the urine several weeks later was considered to be symptomatic with confirmed ZIKV infection.
As adverse fetal outcomes are fortunately rare following the ZIKV infection, due to small case numbers, it was not possible to make meaningful associations between symptomatology and fetal pathology in this study. This question will have to be revisited in future meta-analyses. Birth outcomes in this cohort were further explored in detail in a separate study [28]. The longitudinal monitoring and developmental follow-up of children in our cohort congenitally exposed to the ZIKV continues. Outcomes of these assessments will be reported in a separate study.
In conclusion, we suggest that institutions revisit clinical case definitions proposed at the start of the ZIKV outbreak and, using available evidence, come to a consensus regarding the most appropriate definition. A more sensitive case definition that includes arthralgia/arthritis as a major symptom and headache as a minor symptom should be considered for pregnant women living in areas with active ZIKV transmission.
Acknowledgments
Members of the Jundiai Zika Cohort Group include Alexandra Siqueira Melo, Ana Paula Antunes Pascalicchio Bertozzi, Anderson Pereira Soares, André Prado Grion, Andrea Cristina Botelho Silva, Antônio Fernandes Moron, Clóvis Antônio Lopes Pinto, Cristiane Martins, Danila Soares Tambalo, Danila Vedovello, Diego Lima, Dora Fix Ventura, Eduardo Massad, Eduardo Roberto Bagni, Fabiana Martins Soares de Souza, Fernanda Cangerana, Fernanda Guerra Velasco, Fernando N. Arita, Francisco del Moral Hernandez, Geovanne Ribeiro dos Santos, Hérbene José Figuinha Milani, Heydi Segundo Tabares, Juliana Paula Gomes de Almeida, Kallene Vidal, Luiz Baran, Magda Maria Sales Carneiro Sampaio, Mayana Zatz, Marcelo Costa, Márcia Borges Machado, Margareth Martha Arilha Silva, Maria de Fátima Rizzo, Maria Manoela Duarte Rodrigues, Marielton P. Cunha, Max Damico, Mirella Barboni, Mirella Nayane B. Leite, Patricia Carvalho Loiola, Paolo Marinho de Andrade Zanotto, Rafael Izbicki, Renata Chrystina Bianchi de Barros, Rita de Cássia Aguirre B. Dezena, Renato Pereira de Souza, Rosa Estela Gazeta, Sandra Helena Alves Bonon, Shahab Zaki Pour, Sergio Rosemberg, Sergio Vranjec, Silvia Maria Ribeiro Oyama, Stéphanno Gomes Pereira Sarmento, Tânia Mendes Quintella, Tânia Ritti Ferraretto, Tathiana Ghisi de Souza, Thamirys Cosmo Gillo Fajardo, Valtenice França, Waldinei Merces Rodrigues.
Author Contributions
N.S.C.: Conceptualization, data curation, formal analysis, methodology, investigation, validation, visualization, writing -original draft and review & editing. E.B.B.: Methodology, resources, supervision, validation, visualization, writing- review & editing. M.F.d.A.: Formal analysis, funding acquisition, methodology, resources, supervision, validation, visualization, writing- review & editing. S.S.W.: Visualization, writing- review & editing. S.D.P.: Conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation. All authors have read and agreed to the published version of the manuscript.
Funding
N.S.C. was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES–Brazil). EBB was partially funded by the Wellcome Trust (205377/Z/16/Z), the United Kingdom’s Department for International Development, and the European Union’s Horizon 2020 research and innovation program under ZikaPLAN grant agreement No. 734584. Jundiaí Zika Cohort received funding from the São Paulo Research Foundation (FAPESP) (grant number 2016/08578-0) and the London School of Hygiene and Tropical Medicine (grant number ER1605).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ECDC . Rapid Risk Assessment. Zika Virus Epidemic in the Americas: Potential Association with Microcephaly and Guillain-Barre Syndrome. ECDC; Stockholm, Sweden: 2015. [Google Scholar]
- 2.Duffy M.R., Chen T.-H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S., Pretrick M., Marfel M., Holzbauer S., DuBray C., et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Haby M.M., Pinart M., Elias V., Reveiz L. Prevalence of asymptomatic Zika virus infection: A systematic review. Bull. World Heal. Organ. 2018;96:402–413D. doi: 10.2471/BLT.17.201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flamand C., Fritzell C., Matheus S., Dueymes M., Carles G., Favre A., Enfissi A., Adde A., Demar M., Kazanji M., et al. The proportion of asymptomatic infections and spectrum of disease among pregnant women infected by Zika virus: Systematic monitoring in French Guiana, 2016. Eurosurveillance. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.44.17-00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brasil P., Pereira J.P., Jr., Moreira M.E., Ribeiro Nogueira R.M., Damasceno L., Wakimoto M., Rabello R.S., Valderramos S.G., Halai U.-A., Salles T.S., et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Eng. J. Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daudens-Vaysse E., Ledrans M., Gay N., Ardillon V., Cassadou S., Najioullah F., Leparc-Goffart I., Rousset D., Herrmann C., Cesaire R., et al. Zika emergence in the French Territories of America and description of first confirmed cases of Zika virus infection on Martinique, November 2015 to February 2016. Eurosurveillance. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.28.30285. [DOI] [PubMed] [Google Scholar]
- 7.Hoen B., Schaub B., Funk A.L., Ardillon V., Boullard M., Cabié A., Callier C., Carles G., Cassadou S., Césaire R., et al. Pregnancy outcomes after ZIKV infection in French Territories in the Americas. N. Eng. J. Med. 2018;378:985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira M., Júnior N.N., Estofolete C., Terzian A.B., Guimarães G., Zini N., Da Silva R.A., Silva G.D., Franco L.J., Rahal P., et al. Adverse birth outcomes associated with Zika virus exposure during pregnancy in São José do Rio Preto, Brazil. Clin. Microbiol. Infect. 2018;24:646–652. doi: 10.1016/j.cmi.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Sardi S.I., Somasekar S., Naccache S.N., Bandeira A.C., Tauro L.B., Campos G.S., Chiu C.Y. Coinfections of Zika and Chikungunya Viruses in Bahia, Brazil, identified by metagenomic next-generation sequencing. J. Clin. Microbiol. 2016;54:2348–2353. doi: 10.1128/JCM.00877-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lobkowicz L., Ramond A., Clemente N.S., Ximenes R.A.D.A., Miranda-Filho D.D.B., Montarroyos U.R., Martelli C.M.T., De Araújo T.V.B., Brickley E.B. The frequency and clinical presentation of Zika virus coinfections: A systematic review. BMJ Glob. Heal. 2020;5:e002350. doi: 10.1136/bmjgh-2020-002350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Zika Virus Disease—Interim Case Definition. [(accessed on 27 March 2018)]; Available online: http://apps.who.int/iris/handle/10665/204381.
- 12.Paixao E., Leong W.-Y., Rodrigues L.C., Wilder-Smith A. Asymptomatic prenatal Zika virus infection and Congenital Zika Syndrome. Open Forum Infect. Dis. 2018;5 doi: 10.1093/ofid/ofy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freitas A.R.R., Napimoga M.H., Donalisio M.R. Could clinical symptoms be a predictor of complications in Zika virus infection? Lancet. 2016;388:338. doi: 10.1016/S0140-6736(16)31104-7. [DOI] [PubMed] [Google Scholar]
- 14.Cauchemez S., Besnard M., Garel C., Fontanet A., Mallet H.-P. Could clinical symptoms be a predictor of complications in Zika virus infection?—Authors’ reply. Lancet. 2016;388:338–339. doi: 10.1016/S0140-6736(16)31017-0. [DOI] [PubMed] [Google Scholar]
- 15.NIH - Eunice Kennedy Shriver National Institute for Child Health and Human Development High Risk Pregnancy. [(accessed on 27 March 2018)]; Available online: https://www.nichd.nih.gov/health/topics/high-risk.
- 16.Lanciotti R.S., Kosoy O.L., Laven J.J., Velez J.O., Lambert A.J., Johnson A.J., Stanfield S.M., Duffy M.R. Genetic and serologic properties of Zika Virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingham A.M. Comparison of test results for Zika Virus RNA in Urine, Serum, and Saliva specimens from persons with travel-associated Zika virus disease—Florida, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016;65:475–478. doi: 10.15585/mmwr.mm6518e2. [DOI] [PubMed] [Google Scholar]
- 18.Paz-Bailey G., Rosenberg E.S., Doyle K., Muñoz-Jordán J.L., Santiago G.A., Klein L., Perez-Padilla J., Medina F.A., Waterman S.H., Gubern C.G., et al. Persistence of Zika virus in body fluids—Preliminary report. N. Eng. J. Med. 2017;379:1234–1243. doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanciotti R.S., Lambert A.J., Holodniy M., Saavedra S., Signor L.D.C.C. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016;22:933–935. doi: 10.3201/eid2205.160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intergrowth-21st INTERGROWTH-21st Newborn Size at Birth Chart. [(accessed on 7 October 2016)]; Available online: https://intergrowth21.tghn.org/articles/intergrowth-21st-newborn-size-birth-chart/
- 21.Intergrowth-21st Newborn biometry by Intergrowth-21st standards/references. Online calculator. [(accessed on 1 July 2018)]; Available online: http://intergrowth21.ndog.ox.ac.uk.
- 22.Villar J., Ismail L.C., Victora C.G., Ohuma E.O., Bertino E., Altman D.G., Lambert A., Papageorghiou A.T., Carvalho M., Jaffer Y.A., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 23.Mor G., Cardenas I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wæhre T., Maagard A., Tappe D., Cadar D., Schmidt-Chanasit J. Zika virus infection after travel to Tahiti, December 2013. Emerg. Infect. Dis. 2014;20:1412–1414. doi: 10.3201/eid2008.140302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC Zika Virus. Symptoms, Testing and Treatment. [(accessed on 10 October 2018)]; Available online: https://www.cdc.gov/zika/symptoms/symptoms.html.
- 26.Stettler K., Beltramello M., Espinosa D.A., Graham V., Cassotta A., Bianchi S., Vanzetta F., Minola A., Jaconi S., Mele F., et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 27.Petridou C., Simpson A., Charlett A., Lyall H., Dhesi Z., Aarons E. Zika virus infection in travellers returning to the United Kingdom during the period of the outbreak in the Americas (2016-17): A retrospective analysis. Travel Med. Infect. Dis. 2019;29:21–27. doi: 10.1016/j.tmaid.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Clemente N.S., Brickley E.B., Paixão E.S., De Almeida M.F., Gazeta R.E., Vedovello D., Rodrigues L.C., Witkin S.S., Passos S.D. Zika virus infection in pregnancy and adverse fetal outcomes in São Paulo State, Brazil: A prospective cohort study. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-69235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]