Abstract
Current surgical approaches to radical prostatectomy are associated with high rates of erectile dysfunction and incontinence. These complications occur secondary to the disruption of surrounding healthy tissue, which is required to expose the prostate. The urethra offers the least invasive access to the prostate, and feasibility has been demonstrated of enucleating the prostate with an endoscope using Holmium laser, which can itself be aimed by concentric tube robots. However, the transurethral approach to radical prostatectomy has thus far been limited by the lack of a suitable means to perform an anastomosis of the urethra to the bladder after prostate removal. Only a few intraluminal anastomotic devices currently exist, and none are small enough to pass through the urethra. In this paper we describe a new way to perform an anastomosis in the small luminal space of the urethra, harnessing the dexterity and customizability of concentric tube manipulators. We demonstrate a successful initial proof-of-concept anastomosis in an anthropomorphic phantom of the urethra and bladder.
Keywords: Transurethral Suturing, Concentric Tube Robots, Minimally-Invasive Surgery, Natural Orifice Surgery
I. Introduction
PROSTATE cancer is the most prevalent cancer in men. 1 in every 9 men will develop prostate cancer in their life time, with over 190,000 new diagnoses per year in the USA alone [1]. Many of these patients will undergo radical prostatectomy, which is often performed using the da Vinci robot through the abdomen. Transabdominal radical prostatectomy requires mobilizing the prostate via extensive dissection/retraction of surrounding anatomical support structures, nerves, and blood vessels. After prostate removal, an anastomosis is performed to re-connect the urethra to the bladder, a task where the da Vinci’s wrist dexterity and stereo vision capabilities are particularly useful. Complications including incontinence and erectile dysfunction are known to occur following surgery, and have been reported to be as high as 70% for erectile dysfunction and 21% for urinary incontinence [2]. Although outcomes related to specific hospitals and specific surgeons vary widely, some level of these complications is intrinsic to the procedure as currently performed.
It is generally agreed by surgeons that the required dissection and retraction of nerves and other structures is largely responsible for these complications. The goal of minimizing this disruption of surrounding structures has motivated exploration of transurethral approaches, which have been attempted with straight, rigid endoscopes [3]–[5]. In these procedures, the prostate is removed with a fiber optic laser delivered through an endoscope, a procedure that is similar to Holmium Laser Enucleation of the Prostate, with the additional removal of the prostate capsule. The main conclusion of these studies was that resection was possible with standard techniques, but that more dexterous tools at the endoscope tip are needed, to enable high-quality anastomotic suturing [3], [6], [7]. In this paper, we propose the use of needle-size, precurved concentric tube manipulators [8], [9] delivered through an endoscope to enable anastomotic suturing after radical prostatectomy. The feasibility of tissue removal with such a system [10], as well as tendon-based sheaths [11] has been shown in the context of transurethral enucleation for benign prostatic hyperplasia (BPH), and similar systems have been used in neurosurgery applications [12], [15]. However, the reconstruction of the bladder to the urethra after prostate removal remains a fundamental challenge. In this work, we demonstrate the feasibility of performing a transurethral anastomosis on a urethra-bladder phantom, using two concentric tube manipulators to perform suturing.
II. Material and Methods
Our suturing approach uses two concentric tube manipulators delivered through a rigid endoscope. The needle arm (the left arm in Fig. 1) pierces tissue in Fig. 1A, grasps the suture in an end effector conceptually similar to a kidney stone retrieval basket (Fig. 1B), and then pulls the suture through tissue (Fig. 1C). The other concentric tube arm, which functions as a grasper with a forceps at its tip, then manipulates the suture back into the bladder in preparation for the needle arm’s next pass (Fig. 1D). The result of several such sutures is a complete anastomosis as shown in Fig. 1E.
Fig. 1.
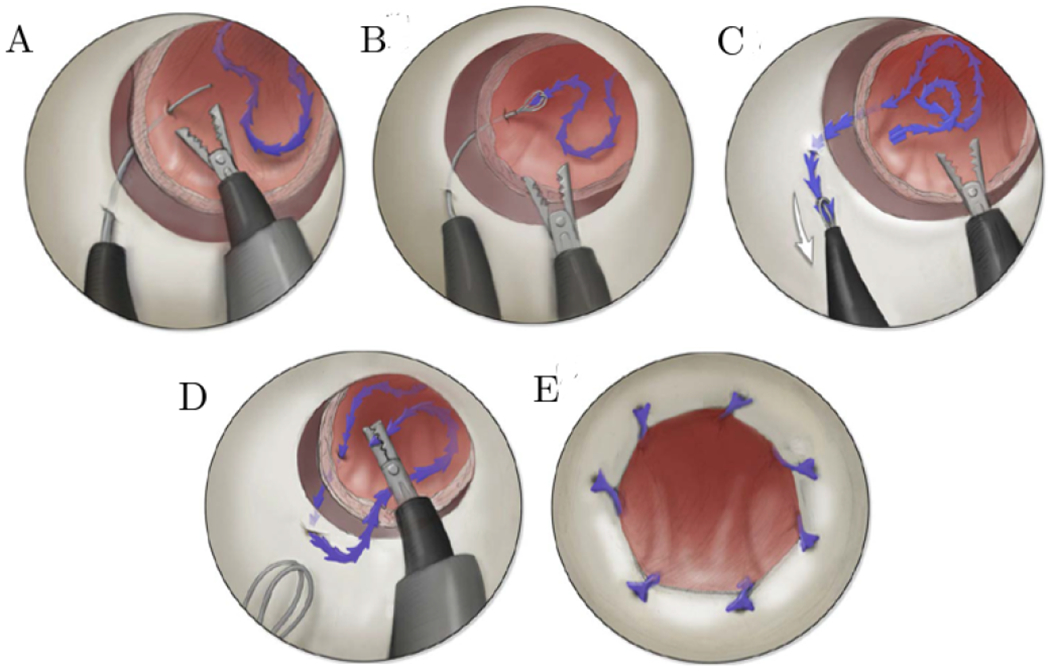
An illustration of the steps in our transurethral suturing concept. (A) Initially the needle arm pierces through the urethra and bladder, before (B) grasping the suture using a basket and then (C) pulling the suture through the bladder and then urethra walls. After this, (D) the manipulator arm grasps the suture and delivers it back into the bladder, in preparation for the next stitch. (E) An illustration of the final anastomosis from the endoscope’s point of view.
A. Concentric Tube Manipulator and Needle
The tube parameters summarized in Fig. 2, top describe the manipulator arm (top) and needle arm (bottom). The needle arm consists of one curved tube with the curvature of kN = 20 m−1 and curved section length of Lc,N = 50 mm. The tube tip is manually ground into a beveled point, so that it can easily pierce tissue. To grasp suture, a modified kidney stone retrieval basket is deployed through the needle arm (Zero Tip™, Boston Scientific, Marlborough, MA, USA). The cross-section of the needle tube is elliptic to lock the axial rotation [13], which prevents elastic instability while enabling follow-the-leader insertion [14] in which the needle shaft follows its tip exactly.
Fig. 2.
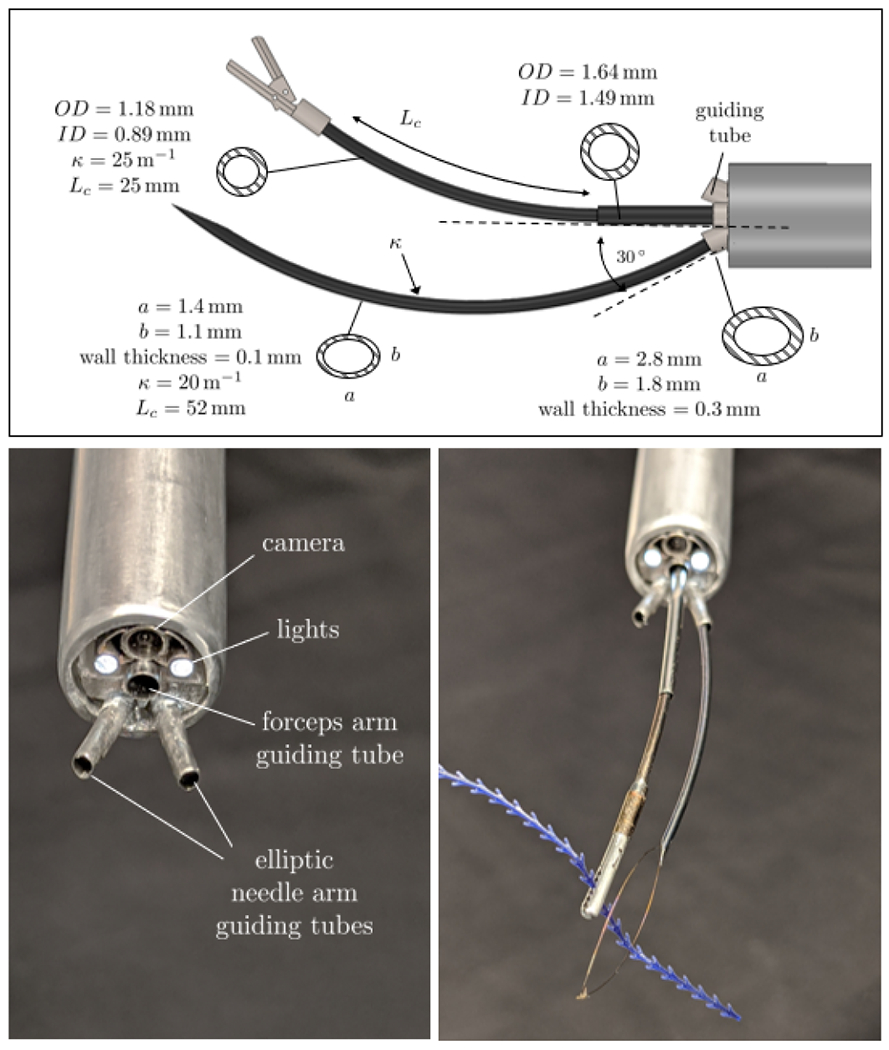
Top: Tube parameters for the robot arms used in the experiment; Bottom left: Close-up of the endoscope tip; Bottom right: Concentric tube robots prototype deployed through the endoscope.
The manipulator arm consists of two tubes. The outer tube is straight and inner is curved with tube curvature kM 1 = 25 m−1 and curved length of Lc,M = 25mm. The forceps fixed at the tip of the inner tube was adapted from the 27023 FE Grasping Forceps (Karl Storz, Tuttlingen, Germany). Fig 2, bottom right shows the prototype of the concentric tube arms deployed through the endoscope. A 26 Fr resectoscope (27050 SC) with a wide angle lens (27292 AMA Hopkins®, Karl Storz, Tuttlingen, Germany) nested inside an aluminum sheath was used to access the surgical site transurethrally (Fig. 3, bottom left). The lens has a 6 ° angled view downwards.
Fig. 3.
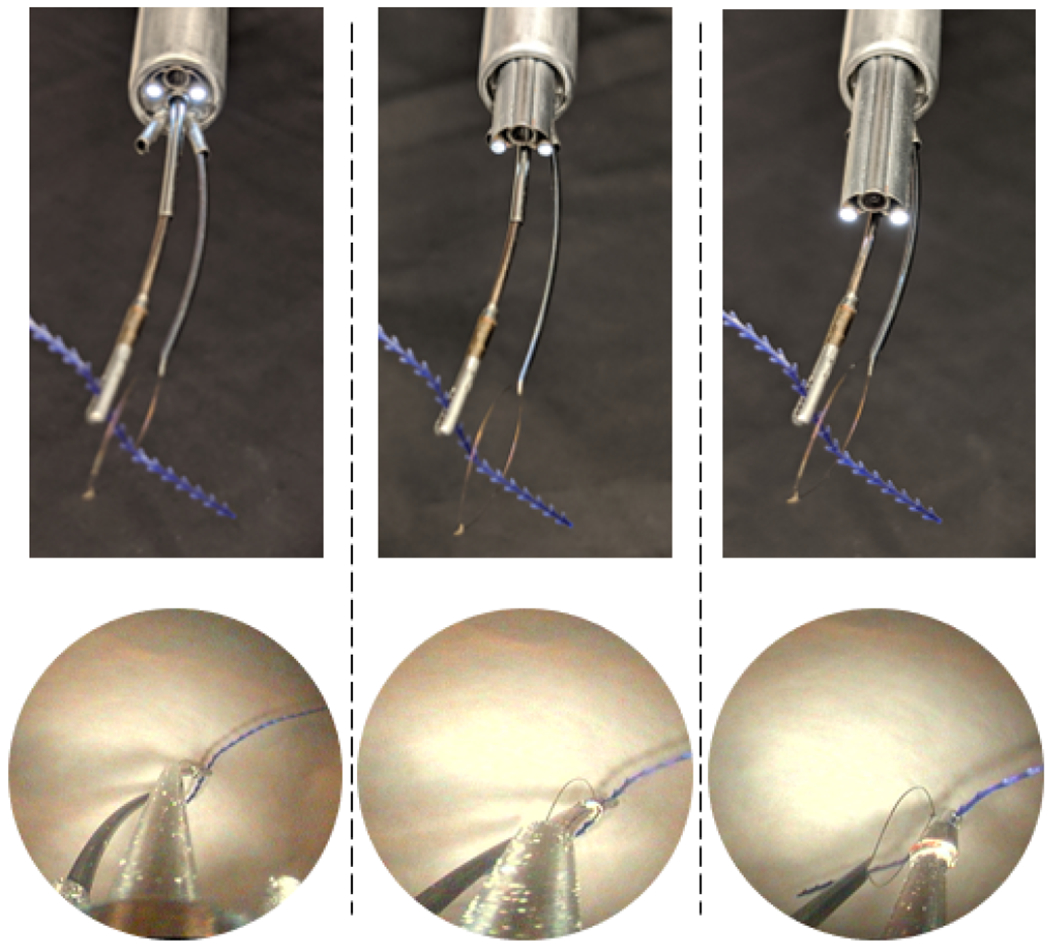
Lens translation demonstration with corresponding endoscopic view (bottom row). The lens is translated toward the end effectors form left to right.
Three customized stainless steel guide tubes were delivered through the 5mm channel in the resectoscope. Two of them were made with an elliptic cross-section and enable delivery of the needle arm (only one of these oval guides was used at a time for the needle arm). The elliptic cross-section guide tubes point away from the centerline helping to aim the needle arm toward the urethra wall. The third guide tube (in the center) has a round cross-section and facilitates insertion of the manipulator arm. All three guide tubes are glued at the tip of the aluminum sheath via a customized 3D-printed adaptor.
B. Experimental Setup
We demonstrated our transurethral anastomosis concept in an anatomical phantom model consisting of a silicone bladder and urethra (Shore A30), mounted on rubber bands and foam (see Fig. 4, right). The bladder neck diameter is 14mm and the wall thickness is approximately 2.5 mm. These anatomical dimensions were extracted manually from pelvic MRI images. The urethral inner diameter was set to 12mm, which is slightly larger than a human urethra, in order to accommodate our aluminum outer sheath, which has an inside diameter comparable to a clinical sheath, but a larger (12.5mm) outside diameter. We note that the aluminum outer sheath was used simply for convenience in this work and will be replaced in the near future with a stainless steel sheath that matches clinical equipment dimensions exactly. The fact that our experiments were successful through an accurate inside diameter provides an indication that this replacement should be straightforward. To improve visualization of the manipulators during suturing, we added a translational degree of freedom for the resectoscope lens, enabling it to linearly extend from the tip of the outer sheath (see Fig. 3). The translation of the lens in our system was performed manually by sliding the lens forward within a 3D-printed housing as shown in Fig. 4, right (orange arrow). The robot arms are moved by a motorized actuation unit based on the differential drive mechanism of Rox et al. [15] providing 2 DoF for each concentric tube, enabling translation and rotation. This actuation unit was used previously by Gafford et al. for bronchoscopy in the central airway [16]. The actuation unit was mounted in a ball bearing frame providing manual (i.e. non-motorized) pitch, roll, and yaw, and additional translation of the whole actuation unit along the endoscope axis. The setup is shown in Fig. 4.
Fig. 4.
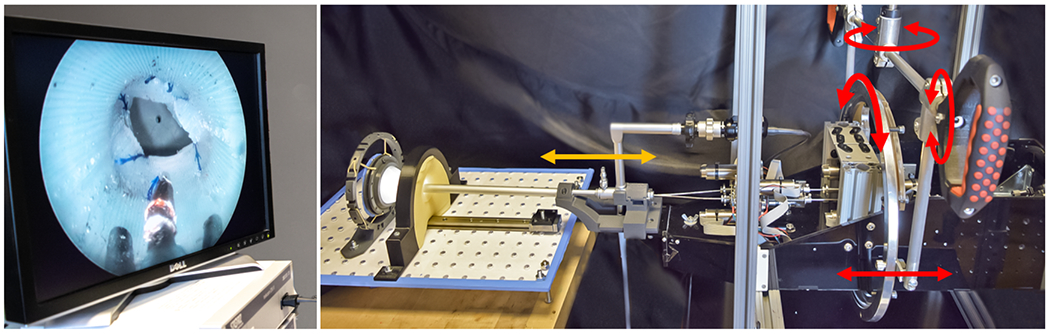
Experimental setup. Left: Endoscope view available for the surgeon; Right: Actuation unit carrying the endoscope inserted into the anatomical phantom model. Red arrows indicate manual actuated DoF for actuation unit motion. Orange arrow indicates the manual lens translation DoF.
C. Experimental Evaluation
We investigated the feasibility of our proposed suturing approach using the phantom and the prototype described previously. The bladder and the urethra were separated by approximately 25 mm, representing the surgeon operator’s estimate of the anatomical configuration during surgery after the prostate removal and immediately prior to anastomosis. A urologic surgeon used a graphical user interface to move the tubes in the concentric tube robot, and an assistant positioned the robot itself and actuated the end effectors based on instructions from the surgeon. Only visual feedback from the endoscope was used to perform the experiments. Several prior initial anastomosis were performed to discover potential challenges and experiment with different suturing approaches. Two barbed sutures, each 100mm long, (Stratafix™ Symmetric PDS™ 30, Ethicon, Bridgewater, NJ, USA) were used. The suture has an anchor on one end, with uni-directional barbs that prevent it from sliding backward through tissue, without the need to tie knots. This suture was placed in the bladder manually. The forceps was used to grasp and hold the bladder wall to stabilize it and facilitate needle passage (Fig. 5A). The forceps was then used to manipulate the suture and place it inside the basket (Fig. 5B1–B2). After the suture was pulled through the bladder and then urethra wall by the needle arm (Fig. 5C), the forceps was used to move it inside the urethra and back into the bladder in preparation for grasping after the next needle pass (Fig. 5D). To evenly distribute the suture circumferentially, the actuation unit was rotated inside the bearing frame (i.e. about the endoscope axis) and the needle arm was switched between the two elliptical guide tubes as needed.
Fig. 5.
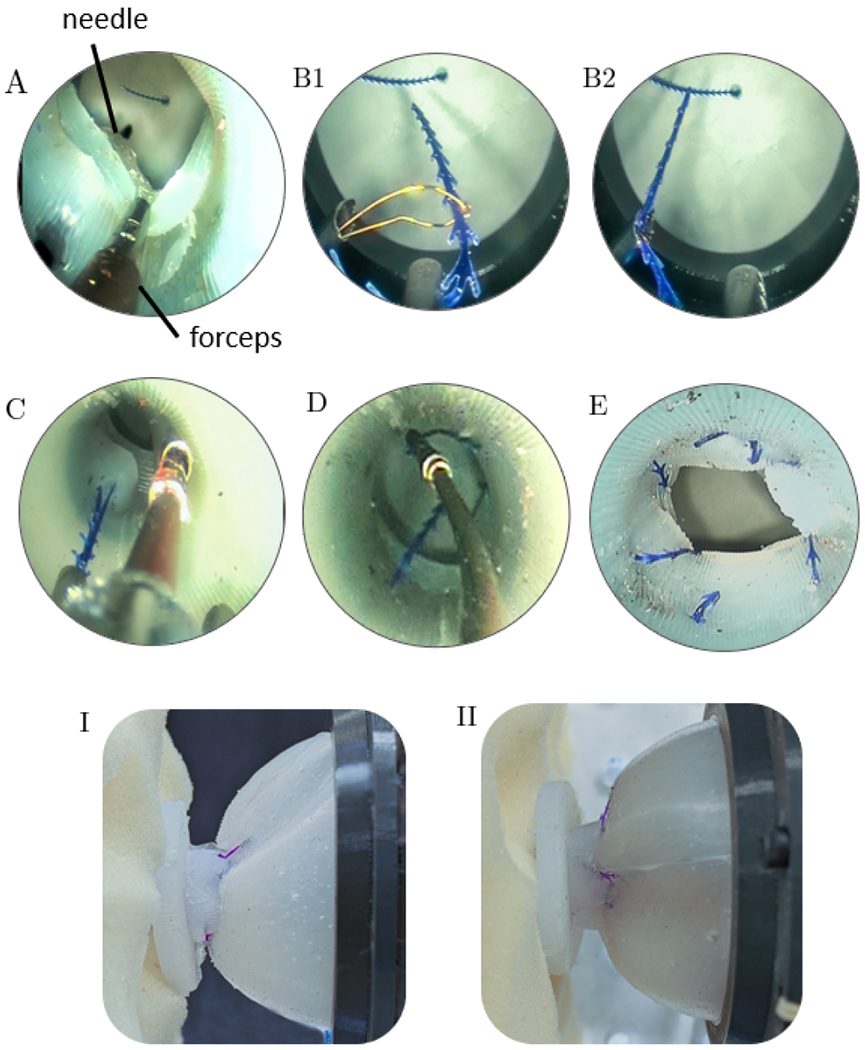
Suturing Results. (A) The needle arm has pierced the urethra and bladder. (B1) The basket deployed from the needle and (B2) grasps the suture. (C) The suture is pulled through the wall of the bladder and then of the urethra. (D) The manipulator arm grasps the suture and returns its end to the bladder, in preparation for the next suture. (E) The final result. A complete anastomosis, seen from the endoscope’s viewpoint. (I-II) Views of the anastomosis from outside the bladder and urethra, showing the two attached to one another.
III. Results
The anastomosis was performed with 7 stitches using two running sutures and one interrupted suture. As shown in Fig. 5E,I,II, the urethra and bladder were successfully anastomosed. Sutures were first started at antipodal points to fix the radial orientation of the bladder and urethra to each other. Afterwards, the suture was alternated following every 2 needle passes to ensure even circumferential suture spacing and prevent anastamotic gaps. Tightening the sutures occurred after placement of the final stitches, to minimize individual suture tension and enable the endoscope to view the space outside the urethra during needle passes, as needed. The single interrupted suture was placed to close a residual gap in the anastamosis left by the two running sutures at the 12 o’clock position, as is conventionally performed from outside the anastamosis for remaining gaps in da Vinci-assisted radical prostatectomy. Such a gap is typically the result of the opening in the bladder being larger than the urethra, tissue distortion after each suture is placed and tightened, or unequal spacing of the stitches, and occurs routinely in da Vinci anastomoses today. Endoscope translation relative to the sheath (Fig. 3, top row) was used frequently to visualize the needle and suture as needed. The steps in the suturing process are showing in Fig. 5, which follows the illustration presented previously in Fig. 1. Results from an external view of the bladder and urethra after anastomosis are shown in Fig. 5I,II.
IV. Conclusion
This paper proposes a new approach to suturing within a lumen using transendoscopic concentric tube manipulators, and demonstrates its feasibility in a phantom model. Novel features of our system include a new axial endoscope lens degree of freedom, and the use of specialized needle and manipulator arms. The needle arm uses ellipsoidal cross-sections to enable follow-the-leader deployment, and the manipulator arm features round cross-sections for more general movements. In future work, we plan to perform suturing studies comparable to those described in this paper in a cadaver model. We note that the current work is simply an initial feasibility study on a new concept for passing sutures to perform anastomosis from within the lumen. Future work is needed to address repeatability, time efficiency, and test the resulting suture lines for leaks. If these experiments are successful, the system will provide exactly the type of new and improved instrumentation with enhanced dexterity that has limited clinical deployment of transurethral radical prostatectomy in the past. More broadly, our new suturing approach provides a means of performing suturing at the tip of a thin endoscope, enabling suturing in much more highly constrained areas of the body than has previously been possible.
Acknowledgments
This work was funded by the NIH under R01 EB026901. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- [1].Siegel RL, Miller KD, and Jemal A, ”Cancer statistics 2020”, CA: A Cancer Journal for Clinicians, vol. 70, no. 1, pp. 7–30, 2020. [DOI] [PubMed] [Google Scholar]
- [2].Haglind E, Carlsson S, Stranne J, Wallerstedt A, Wilderäng U, Thorsteinsdottir T, Lagerkvist M, Damber J, Bjartell A, Hugosson J, Wiklund P, and Steineck G, “Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective, controlled, nonrandomised trial”, European Urology, vol. 68, no. 2, pp. 216–225, 2015. [DOI] [PubMed] [Google Scholar]
- [3].Humphreys MR, Sauer JS, Ryan AR, Leslie KO, Castle EP, Lingeman JE, and Andrews PE. ‘Natural orifice transluminal endoscopic radical prostatectomy: initial perioperative and pathologic results”, Urology, vol. 78, no. 6, pp. 1211–1217, 2011. [DOI] [PubMed] [Google Scholar]
- [4].Humphreys M, Krambeck A, Andrews P, Castle E, and Lingeman J. “Natural orifice translumenal endoscopic surgical radical prostatectomy: Proof of concept”, Journal of Endourology, vol. 23, no. 4, pp. 669–675, 2009. [DOI] [PubMed] [Google Scholar]
- [5].Krambeck AE, Humphreys MR, Andrews PE, and Lingeman JE. ‘Natural orifice translumenal endoscopic surgery: radical prostatectomy in the canine model”, Journal of endourology, vol. 24, no. 9, pp. 1493–1496, 2010. [DOI] [PubMed] [Google Scholar]
- [6].Tyson MD, and Humphreys MR, ”Urological applications of natural orifice transluminal endoscopic surgery (NOTES)”, Nature Reviews Urology, vol. 11, pp. 324–332, 2014. [DOI] [PubMed] [Google Scholar]
- [7].Autorino R, Yakoubi R, White WM, Gettman M, De Sio M, Quattrone C, Di Palma C, Izzo A, Correia-Pinto J, Kaouk JH, and Lima E, ”Natural orifice transluminal endoscopic surgery (NOTES): where are we going? Bibliometric assessment of notes”, BJU International, vol. 111, pp. 11–16, 2013. [DOI] [PubMed] [Google Scholar]
- [8].Rucker DC, Jones BA, and Webster RJ III. ”A geometrically exact model for externally loaded concentric tube continuum robots”. IEEE Transactions on Robotics, vol. 26, no. 5, pp. 769–780, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dupont PE, Lock J, Itkowitz B, and Butler E. “Design and Control of Concentric-Tube Robots”. IEEE Transactions on Robotics, vol. 26, no. 2, pp. 209–225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hendrick RJ, Mitchell CR, Herrell SD III, and Webster RJ III, ”Hand-Held Transendoscopic Robotic Manipulators: A Transurethral Laser Prostate Surgery Case Study”, International Journal of Robotics Research, vol. 34, no. 15, pp. 1559–1572, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Russo S, Dario P, and Menciassi A, “A novel robotic platform for laser-assisted transurethral surgery of the prostate”, IEEE transactions on Bio-Medical Engineering, vol. 62, no. 2, pp. 489–500. [DOI] [PubMed] [Google Scholar]
- [12].Bodani V, Azimian H, Looi T, and Drake JM, ‘Design and evaluation of a concentric tube robot for minimally-invasive endoscopic paediatric neurosurgery”, Hamlyn Symposium Medical Robotics, 2014. [Google Scholar]
- [13].Swaney PJ, Gilbert HB, Hendrick RJ, Commichau O, Alterovitz R, and Wester RJ III. ”Transoral steerable needles in the lung: How non-annular concentric tube robots can improve targeting”, Hamlyn Symposium Medical Robotics, 2015. [Google Scholar]
- [14].Gilbert HB, Neimat J, and Webster RJ III, “Concentric Tube Robots as Steerable Needles: Achieving Follow-The-Leader Deployment”, IEEE Transactions on Robotics, vol. 31, no. 2, pp. 246–258, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rox MF, Ropella DS, Hendrick RJ, Blum E, Naftel RP, Bow HC, Herrel SD III, Weaver KD, Chambless LB, and Webster RJ III, ”Mechatronic Design of a Two-Arm Concentric Tube Robot System for Rigid Neuroendoscopy”, IEEE/ASME Transactions on Mechatronics, vol. 25, no. 3, pp. 1432–1443, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gafford JB, Webster S, Dillon N, Blum E, Hendrick RJ, Maldonado F, , Gillaspie EA, Rickman OB, Herrell SD III, and Webster RJ III, ”A Concentric Tube Robot System for Rigid Bronchoscopy: A Feasibility Study on Central Airway Obstruction Removal”, Annals of Biomedical Engineering, vol. 48, no. 1, pp. 181–191, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


