Abstract
Background: Emergence agitation (EA) is one of the most common and intractable postoperative complications among children undergoing surgery under general anesthesia. Dexmedetomidine, an α(2)-adrenoceptor agonist, offers an ideal sedation, reduces preoperative anxiety, and facilitates smooth induction of anesthesia, and it is widely used in pediatric surgery. We aimed to evaluate the efficacy of dexmedetomidine for preventing emergence agitation in children after general anesthesia.
Methods: We comprehensively reviewed PubMed, Cochrane Library, EMBASE, and Web of Science databases to search all randomized controlled trials, published before April 22, 2020, investigating the efficacy of dexmedetomidine in preventing the emergence agitation in children after general anesthesia. The meta-analysis was performed using Review Manager 5.3. The primary outcome was the incidence of emergence agitation. Secondary outcomes included the number of patients requiring rescue analgesic, number of patients with postoperative nausea and vomiting, emergence time, extubation time, and time to discharge from the post-anesthesia care unit.
Results: We included a total of 33 studies, comprising 2,549 patients in this meta-analysis. Compared with saline, dexmedetomidine significantly reduced the emergence agitation incidence [risk ratio (RR) 0.29; 95% confidence interval (CI) 0.22–0.37; p < 0.00001], incidence of postoperative nausea and vomiting (RR 0.46; 95% CI 0.3–0.69; p = 0.0002), and the requirement of rescue analgesic (RR 0.29; 95% CI 0.18–0.44; p < 0.00001). Furthermore, children in the dexmedetomidine group experienced a longer emergence time [mean difference (MD) 2.18; 95% CI 0.81–3.56; p = 0.002] and extubation time (MD 0.77; 95% CI 0.22–1.31; p = 0.006) compared with those in the saline group. However, no significant difference was observed in the time to discharge from the post-anesthesia care unit (MD 2.22; 95% CI −2.29–6.74; p = 0.33) between the two groups. No significant differences were observed between the effects of dexmedetomidine and other drugs like midazolam, propofol, fentanyl, tramadol, and clonidine in terms of the emergence agitation incidence and other parameters, except for the requirement of rescue analgesic (RR 0.45; 95% CI 0.33–0.61; p < 0.00001).
Conclusions: Dexmedetomidine can prevent emergence agitation, relieves postoperative pain, decreases the requirement of rescue analgesic, and decreases the postoperative nausea and vomiting events.
Keywords: dexmedetomidine, emergence agitation, children, general anesthesia, meta-analysis
Introduction
Emergence agitation (EA) is a behavioral disturbance during the early post-anesthetic period, characterized by excitement, restlessness, disorientation, and other unusual behaviors, such as crying, shouting, kicking, inconsolability, and non-cooperation. EA incidence in children following sevoflurane anesthesia has been reported to be 10–80% (1, 2). EA is associated with the risk of self-harm, delayed discharge from the post-anesthesia care unit (PACU), extra burden on healthcare workers, increased parent dissatisfaction, and increased overall cost. Although the definition and criteria for EA are not clearly indicated, most children experiencing EA require a drug intervention to mitigate any threat to their safety. Patients with EA may unconsciously remove their endotracheal and stomach tubes, which can result in incision dehiscence, bleeding, urinary retention, and asphyxia. In addition, patients with EA often experience sympathetic excitation and instability of the circulatory system, which is dangerous if the patient is already having cardiovascular and cerebrovascular diseases. Several factors may cause EA, such as preschool age, preoperative anxiety, anesthetic, type of operation, and personal characteristics of the patient (3). Various drugs, including dexmedetomidine, midazolam, propofol, fentanyl, and melatonin have been investigated to prevent the EA incidence; however, the most favorable prophylactic treatment to decrease such incidence remains unknown. Among investigated drugs, dexmedetomidine is known as a highly selective α (2)-adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesic-sparing effects, which causes minimal depression of the respiratory function (4). The efficacy of dexmedetomidine toward EA prevention has been investigated in several clinical trials, using different administration routes and different dosages. We aimed to assess the effect of dexmedetomidine on EA incidence in the present study.
Materials and Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (5). The study was registered on PROSPERO (registration number: CRD42020187711).
Criteria for Study Consideration
The trials selected for this meta-analysis met the following inclusion criteria:
randomized controlled trials;
children aged between 0 and 18 years;
involving comparisons of dexmedetomidine as the intervention drug, delivered via intravenous or intranasal routes, with normal saline and other drugs (such as, midazolam, propofol, and fentanyl);
published in the English language;
involving EA assessment using evaluation scales, namely: five-point scale described by Cole, Aono four-point scale, Watcha four-point scale, a three-point scale, and Riker Sedation–Agitation Scale. Studies involving cardiac surgery were excluded.
Outcome Measures
The primary outcome was EA incidence. Secondary outcomes included the number of patients requiring rescue analgesia, number of patients with postoperative nausea and vomiting (PONV), emergence time, extubation time, and time to discharge from the PACU.
Search Strategy
PubMed, Cochrane Library, EMBASE, and Web of Science databases were comprehensively reviewed to identify randomized controlled clinical trials, published before April 22, 2020, investigating the efficacy of dexmedetomidine in EA prevention among children undergoing surgery with general anesthesia. In addition, the reference list of all the included studies was analyzed for additional potential publications. The detailed search strategies for each database are available in the Appendix.
Data Extraction
Two experienced reviewers (Xiaoli Yang and Zhenyu Hu) independently screened the title and abstract of each literature to verify the suitability of the included trials. Data extraction was conducted independently by two reviewers using a standard data-collection form. Disagreements were resolved through discussion between the two reviewers and the corresponding author (Maohua Wang) to achieve a consensus. The following information was extracted from the included articles: primary author, publication year, country of the study, type of surgery, participant characteristics (age and sex), the administration route and dexmedetomidine dosage, control group's measure, scale, and criteria used for EA assessment.
Risk-of-Bias Assessment
Two reviewers (Xiaoli Yang and Zhenyu Hu) independently assessed the quality of the included trials according to the Cochrane Collaboration tool (Cochrane, London, UK) (6). The included trials were scored as low risk, unclear, or high risk after assessment of bias under the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias. Disagreements were resolved through discussion between the two reviewers and the corresponding author.
Data Analysis
The meta-analysis was conducted using Review Manager (Version 5.3, Nordic Cochrane Center). The Cochran Q test and Higgins I2 statistical tests were used to assess the statistical heterogeneity in the pooled results (7). I2 value was used to determine the level of heterogeneity in results; 0% ≤ I2 < 25% denoted no heterogeneity; 25% ≤ I2 < 50%, denoted low heterogeneity; 50% ≤ I2 < 75% denoted medium heterogeneity; and 75% ≤ I2 ≤ 100% denoted high heterogeneity. Data from all eligible RCTs were combined using the Mantel–Haenszel model to calculate the pooled risk ratio (RR) and 95% confidence interval (CI). Meta-analyses were performed using a random-effects model on account of clinical heterogeneity. This model provides an appropriate estimate of the average treatment effect when studies are statistically heterogeneous, and it typically yields relatively wide CIs resulting in a more conservative statistical claim. Statistical significance was set at a value of p < 0.05.
Results
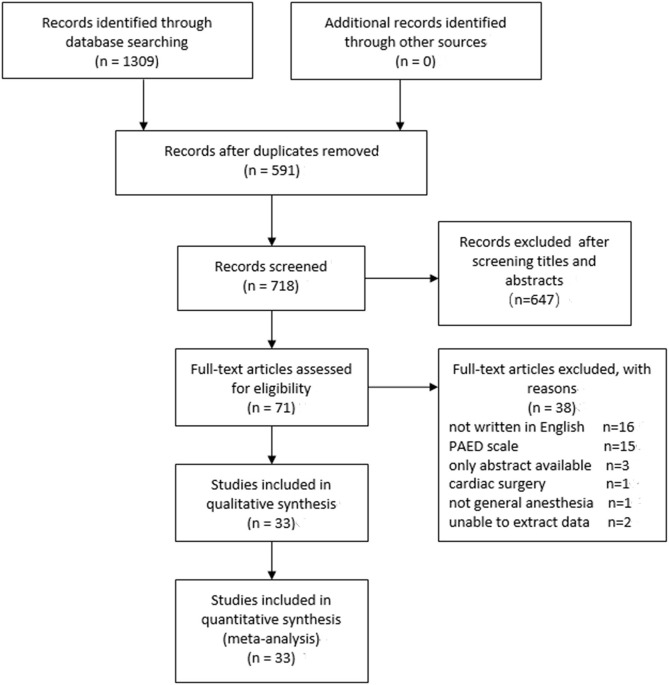
We initially identified 1,309 relevant studies for the analysis, with 591 being excluded for duplication and 647 excluded after screening of title and abstract. Furthermore, 71 potentially eligible articles were reviewed for full texts, of which 36 were excluded for not satisfying the inclusion criteria. Moreover, we could not extract data from two studies. Finally, 33 (8–40) independent studies were included in the meta-analysis. Figure 1 presents the detailed selection process.
Figure 1.
A PRISMA flow diagram of included/excluded studies.
Table 1 summarizes the characteristics of the included trials. All the 33 included trials were published between 2004 and 2020. The sample size in the included trials ranged between 36 and 122, and a total of 2,549 subjects. Age of participants ranged from 3 months to 14 years. Figure 2 presents the risk of bias.
Table 1.
Characteristics of included studies.
| Study ID | Age | Surgery type | Sample size | Anesthesia | Groups | Outcome |
|---|---|---|---|---|---|---|
| Nidhi 2013 (12) | 8–12 Y | Corrective surgery | 36 | Sev, N2O Intubation | DEX (9): 1 ug/kg iv followed by 0.5 ug/kg/h infusion; NS (9): volume-matched iv | ABCDE |
| Bi 2019 (36) | 6–48 M | Fiberoptic bronchoscopy | 40 | Sev LMA | DEX (11):1 ug/kg intranasal; NS (11):0.01 ml/kg intranasal | ADE |
| Sun 2017 (30) | 1–5 Y | Laparoscopic hernia repair | 97 | Sev LMA | DEX1(13):0.25 ug/kg iv; DEX2(14):0.5 ug/kg iv; DEX3(14):1 ug/kg iv; NS (15):2 ml iv | ADEF |
| Boku 2015 (37) | 10–14 M | Palatoplasty | 70 | Sev, N2O Intubation | DEX (17):6 ug/kg/h for 10 min, followed by 0.4 ug/kg/h; NS (17): volume-matched iv | AD |
| Mizrak 2011 (18) | 4.5–11 Y | Strabismus surgery | 60 | Ketamine Intubation | DEX (19):0.5 ug/kg iv; Placebo (19) | AC |
| Erdil 2009 (10) | 2–7 Y | Adenoidectomy | 90 | Sev, N2O Intubation | DEX (19):0.5 ug/kg iv; Fentanyl (19):2.5 ug/kg iv; NS (19): iv | ADE |
| Guler 2005 (35) | 3–7 Y | Adenotonsillectomy | 60 | Sev, N2O Intubation | DEX (19):0.5 ug/kg iv; NS (19): volume-matched iv | ABDE |
| Cho 2020 (13) | 24 M−12 Y | Tonsillectomy | 66 | Sev Intubation | DEX (21):0.3 ug/kg iv; Midazolam (22):0.03 mg/kg iv | ABCDF |
| Li 2016 (23) | 4–6 Y | Tonsillectomy | 80 | Des Intubation | DEX (24):0.2 ug/kg/h; NS (24): volume-matched iv | ABCDF |
| Wei 2015 (9) | 3–24 M | Cleft palate repair | 40 | Sev, Propofol Remifentanil Intubation | DEX (11):0.8 ug/kg/min; NS (11): volume-matched iv | ABDE |
| Bhat 2018 (26) | 1–8 Y | Inguinal hernia | 90 | Sev, N2O LMA | DEX1(19):0.5 ug/kg iv; DEX2(19):1 ug/kg iv; NS (19):5 ml iv | AEF |
| Ahmed 2017 (15) | 3–7 Y | Tonsillectomy and/or Adenoidectomy | 86 | Sev Intubation | DEX (41):1 ug/kg intranasal; NS (41):1 ml intranasal | ABCDEF |
| Song 2016 (22) | 2–6 Y | Strabismus surgery | 103 | Sev, N2O LMA | DEX1(14):0.25 ug/kg iv; DEX2(14):0.5 ug/kg iv; DEX3v (27):1 ug/kg iv; NS (14): iv | ACDF |
| Ali 2016 (11) | 3–6 Y | Orthopedic surgery | 90 | Sev Intubation | DEX (19):0.3 ug/kg iv; Ketofol (19): ketamine 0.25 mg/kg and Propofol 1 mg/kg iv; NS (19):10 ml iv | AD |
| Mukherjee 2015 (19) | 3–7 Y | Day care surgery | 80 | Sev Intubation | DEX (24):1 ug/kg intranasal; Clonidine (24):4 ug/kg intranasal | ABCDEF |
| Liu 2015 (27) | 2–12 Y | Achilles-tendon lengthening | 80 | Sev Intubation | DEX (24):0.5 ug/kg iv; NS (24):10 ml iv | ADEF |
| Sheta 2014 (28) | 3–6 Y | Dental rehabilitation | 72 | Sev, N2O Intubation | DEX (29):1 ug/kg intranasal; Midazolam (29):0.2 mg/kg | ABCE |
| Ali 2013 (8) | 2–6 Y | Adenotonsillectomy | 120 | Sev, N2O Intubation | DEX (24):0.3 ug/kg iv; Propofol (24):1 mg/kg iv; NS (24):10 ml iv | ABDEF |
| Meng 2012 (31) | 5–14 Y | Tonsillectomy | 120 | Sev Intubation | DEX1 (24):0.5 ug/kg iv followed by 0.2 ug/kg/h; DEX2 (24):1 ug/kg followed by 0.4 ug/kg/h; lactated Ringer (24): iv | ADEF |
| Xu 2012 (32) | 3–7 Y | Vitreoretinal surgery | 60 | Sev, Remifentanil Intubation | DEX (19):0.5 ug/kg, iv; NS (19):10 ml iv | ADE |
| Asaad 2011 (33) | 5–10 Y | Elective surgery | 88 | Sev, N2O Intubation | DEX (19):0.15 ug/kg, iv; Fentanyl (27):1 ug/kg; NS (19):10 ml iv | AEF |
| Ibacache 2004 (14) | 1–10 Y | Inguinal hernia repair, orchiopexy, or circumcision | 90 | Sev, N2O LMA | DEX1(19):0.15 ug/kg, iv; DEX2(19):1 ug/kg; NS (19):10 ml iv | AEF |
| Olutoye 2011 (34) | 3–12 Y | Tonsillectomy and adenoidectomy | 109 | Sev, N2O Intubation | DEX1(23):0.75 ug/kg, iv; DEX2(32):1 ug/kg,iv;Morphine1(19):50 ug/kg iv; Morphine2(23):100 ug/kg iv | AB |
| Li 2018 (25) | 2–7 Y | Adenoidectomy with or without tonsillectomy | 90 | Propofol, Remifentanil Intubation | DEX1(19):1 ug/kg, iv; DEX2(19):2 ug/kg, iv; NS (19):1 ml iv | A |
| Kim 2014 (16) | 1–5 Y | Strabismus Surgery | 94 | Des Intubation | DEX (42):0.2 ug/kg/h; NS (42): iv | ACDEF |
| Patel 2010 (20) | 2–10 Y | Tonsillectomy and adenoidectomy | 122 | Sev, N2O Intubation | DEX (61):2 ug/kg followed by 0.7 ug/kg/h; Fentanyl (61):1 ug/kg iv | ACDE |
| Koceroglu 2020 (21) | 2–9 Y | Adenotonsillectomy | 60 | Sev, N2O Intubation | DEX (19):1 ug/kg iv; Tramadol (19):1.5 mg/kg iv | ABDE |
| Akin 2011 (17) | 2–9 Y | Adenotonsillectomy | 90 | Sev, N2O Intubation | DEX (43):1 ug/kg intranasal; Midazolam (43):0.2 mg/kg intranasal | ABCD |
| Kim 2014 (38) | 1–5 Y | Hernioplasty or orchiopexy | 40 | Sev LMA | DEX (11):1 ug/kg followed by 0.1 ug/kg/h; NS (11): same amount IV | AD |
| Shukry 2005 (39) | 1–10 Y | Outpatient surgery | 46 | Sev Intubation | DEX (13):0.2 ug/kg/h infusion; NS (13): volume-matched iv | ADF |
| Surana 2017 (40) | 6 M-12 Y | Cleft palate surgery | 60 | Iso, N2O Intubation | DEX (19):1 ug/kg followed by 0.5 ug/kg/h; Midazolam (19):0.05 mg/kg iv | ACDE |
| Tsiotou 2018 (24) | 3–14 Y | Tonsillectomy | 60 | Propofol, Remifentanil Intubation | DEX (28):1 ug/kg iv; NS (31):50 ml iv | ACD |
| Bhadla 2013 (29) | 5–12 Y | Ophthalmic day-care surgery | 60 | Iso Intubation | DEX (19):0.4 ug/kg iv; Midazolam (19):0.04 mg/kg iv | A |
A, emergence agitation; B, PONV; C, requiring rescue anesthetic; D, extubation time; E, emergence time; F, Time to discharge from the PACU; M, months; Y, years; DEX, dexmedetomidine; NS, normal saline; LMA, laryngeal mask airway; Sev, sevoflurane; Des, desflurane; Iso, isoflurane.
Figure 2.
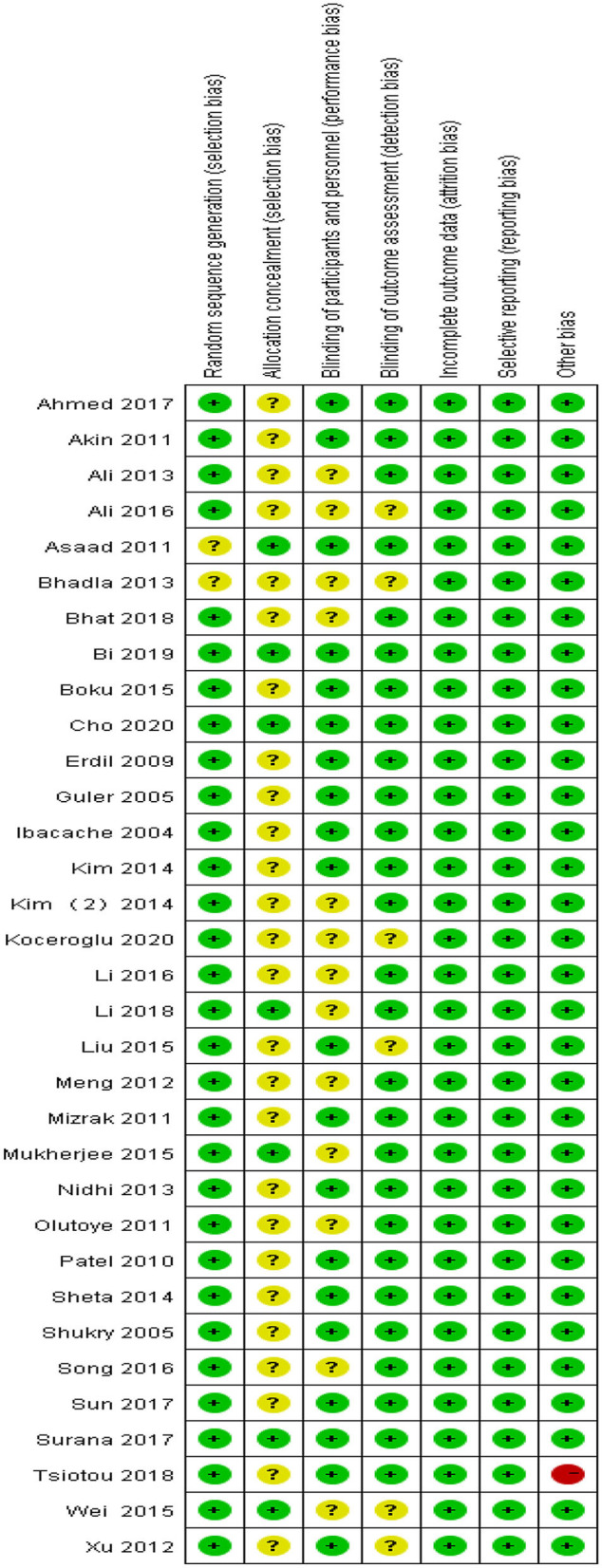
Risk-of-bias summary.
Primary Outcome
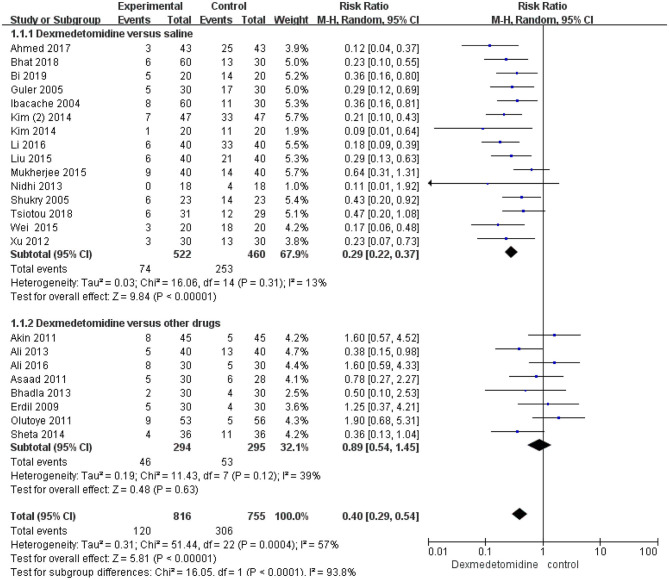
EA incidence was reported in all the included studies. The results of EA in 10 studies could not be pooled. Among the included trials, 15 studies compared the efficacy of dexmedetomidine with that of saline in preventing EA incidence, and the total number of reported events in these trials was 327. The reported EA incidences in the included trials were 14.2% (74 out of 522) and 55% (253 out of 460) in the dexmedetomidine and saline groups, respectively. Dexmedetomidine was associated with a significant reduction in the EA incidence, compared with saline (RR 0.29; 95% CI 0.22–0.37; p < 0.00001) (Figure 3), and heterogeneity was not observed (I2 = 13%). Compared with other anesthetics, dexmedetomidine was not found to significantly reduce the EA incidence (RR 0.89; 95% CI 0.54–1.45; p = 0.63; I2 = 39%) (Figure 3).
Figure 3.
Incidence of emergence agitation (EA): dexmedetomidine vs. saline and other drugs. Forest plot shows that pooled trials were in favor of dexmedetomidine when compared to saline; there was no significant difference between dexmedetomidine and other drugs.
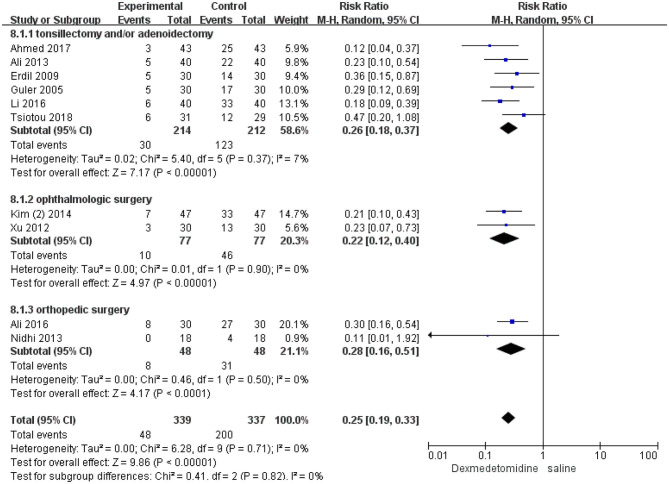
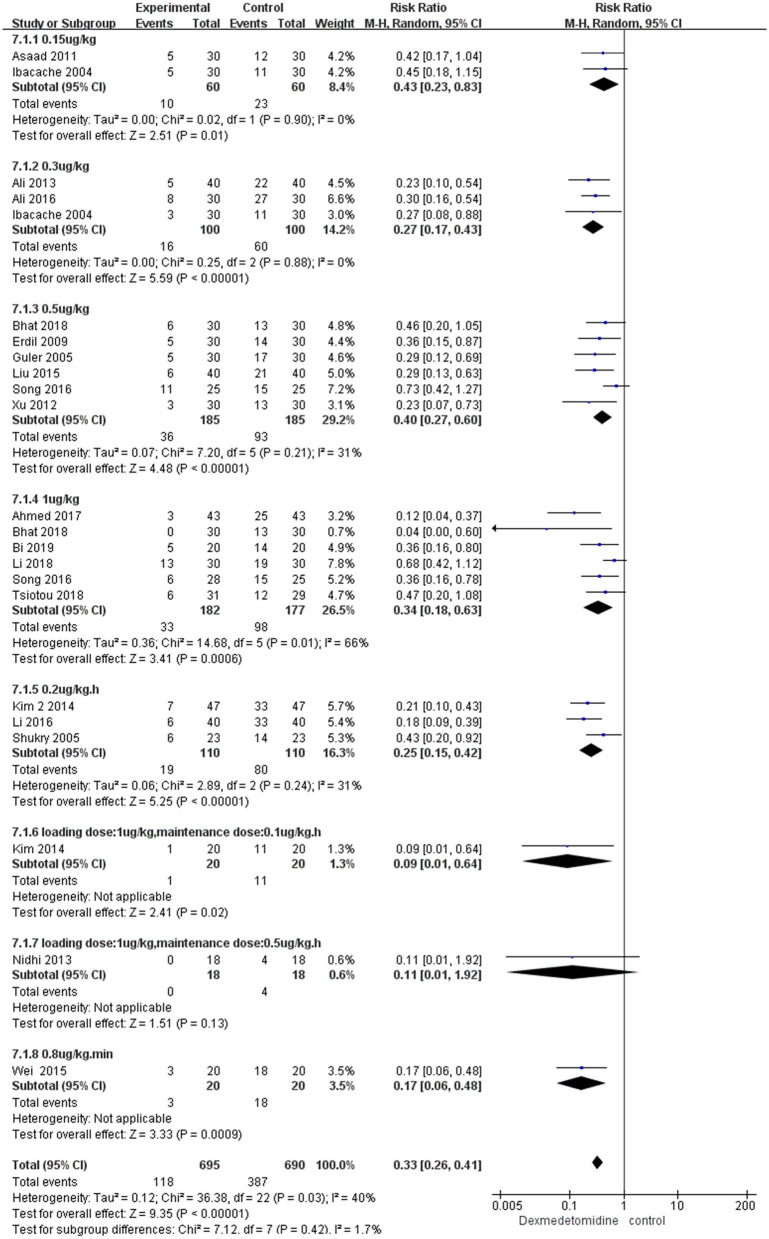
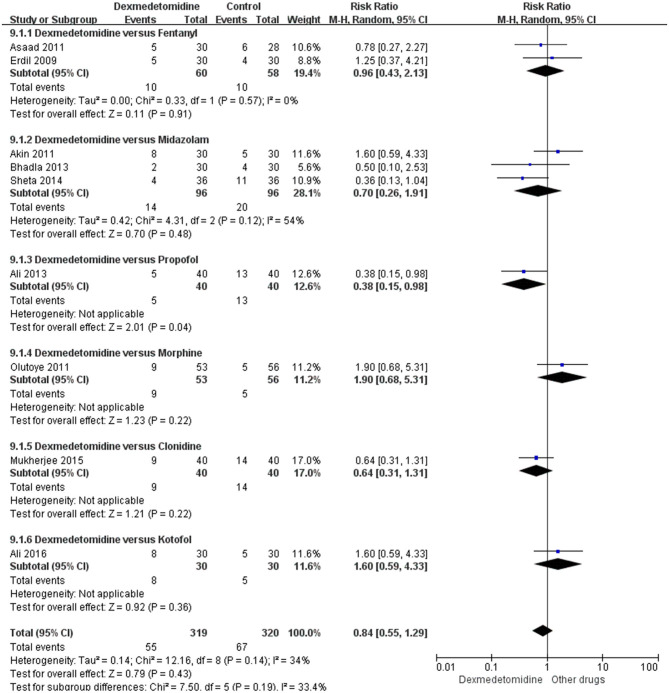
In the subgroup analyses, six studies indicated that dexmedetomidine significantly reduces the EA incidence (RR 0.26; 95% CI 0.18–0.37; p < 0.00001; I2 = 7%) in adenoidectomy with or without tonsillectomy compared with saline. In addition, two studies indicated that dexmedetomidine reduces the EA incidence in ophthalmologic surgery (RR 0.22; 95% CI 0.12–0.4; p < 0.00001; I2 = 0%) and orthopedic surgery (RR 0.28; 95% CI 0.16–0.51; p < 0.0001; I2 = 0%) compared with saline, respectively (Figure 4). Moreover, 16 studies, different dosages of dexmedetomidine with that of saline, indicated that each dosage of dexmedetomidine is effective in preventing EA (Figure 5). Finally, two studies compared dexmedetomidine and fentanyl, three studies compared dexmedetomidine and midazolam, and one study compared dexmedetomidine and propofol; morphine, clonidine, kotofol, respectively, showed no significant differences between them in EA incidence (p > 0.05) (Figure 6).
Figure 4.
Incidence of EA in different surgeries: dexmedetomidine vs. saline.
Figure 5.
Incidence of EA in different dosages of dexmedetomidine vs. saline.
Figure 6.
Forest plot for incidence of EA in dexmedetomidine vs. every other drug.
Secondary Outcomes
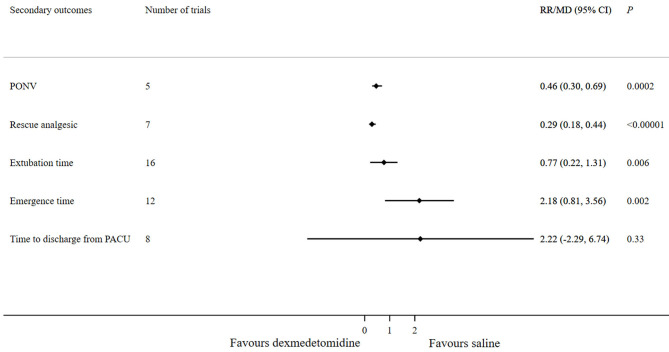
Compared with saline, dexmedetomidine significantly reduced the PONV incidence (RR 0.46; 95% CI 0.3–0.69; p = 0.0002) and the requirement of rescue analgesic (RR 0.29; 95% CI 0.18–0.44; p < 0.00001). Extubation time (MD 0.77; 95% CI 0.22–1.31; p = 0.006) and emergence time (MD 2.18; 95% CI 0.81–3.56; p = 0.002) were longer in the dexmedetomidine group compared with the saline group. Eight studies assessing the time to discharge from the PACU reported no significant difference between the dexmedetomidine and saline groups (MD 2.22; 95% CI −2.29–6.74; p = 0.33) (Figure 7).
Figure 7.
Forest plot for secondary outcomes (dexmedetomidine vs. saline).
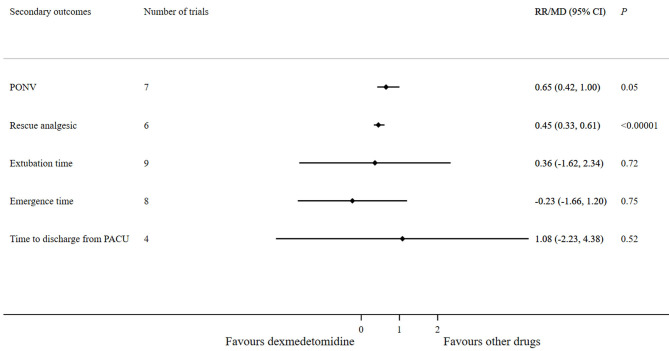
Compared with other anesthetics, dexmedetomidine significantly reduced the requirement of rescue analgesic (RR 0.45; 95% CI 0.33–0.61; p < 0.00001). However, no significant differences were observed between the dexmedetomidine and other anesthetics in terms of PONV incidence (RR 0.65; 95% CI 0.42–1.00; p = 0.05), extubation time (MD 0.36; 95% CI −1.62–2.34; p = 0.72), emergence time (MD −0.23; 95% CI −1.66–1.2; p = 0.75), and time to discharge from the PACU (MD 1.08; 95% CI −2.23–4.38; p = 0.52) (Figure 8).
Figure 8.
Forest plot for secondary outcomes (dexmedetomidine vs. other drugs).
Publication Bias
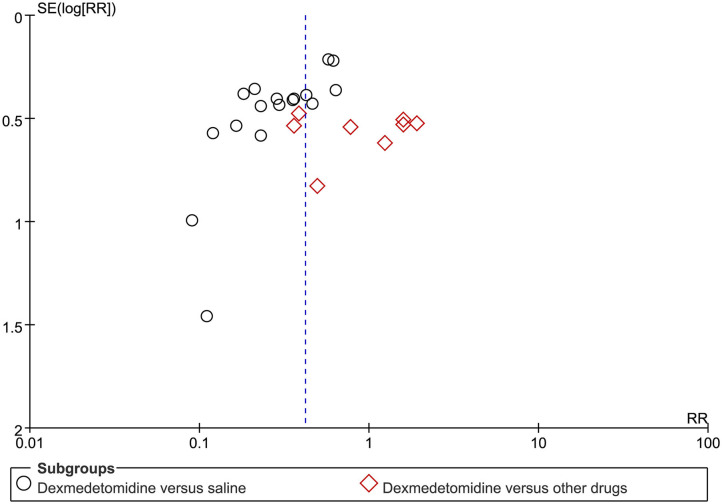
Funnel plots for the primary outcome indicated a slight publication bias (Figure 9).
Figure 9.
Funnel plot for evaluation of potential publication bias.
Discussion
This meta-analysis indicates that dexmedetomidine is efficient in preventing EA, avoiding PONV, and alleviating pain in children under general anesthesia compared with saline, although with prolonged emergence time and extubation time. However, time to discharge from PACU was similar in patients after anesthesia with saline and other anesthetics.
EA is one of the most common postoperative complications in pediatric surgery, following general anesthesia. Although many drugs have been applied to prevent EA, consensus on the most effective drug is lacking.
Several studies comparing the efficacy of dexmedetomidine with that of placebo have been published (41, 44, 45). In line with these studies, this meta-analysis demonstrated that compared with saline, intranasal or intravenous administration of dexmedetomidine significantly reduces the EA incidence. Subgroup analyses with different dosages and different operations indicated that each dosage of dexmedetomidine is efficient in preventing EA, compared with saline. Since the optimal dosage of dexmedetomidine for preventing EA could not be deduced from the present analysis, the lowest dose according to the patients' physical condition and operation type can be considered to avoid the side effects of dexmedetomidine. More prospective studies comparing the effects of different dosages of dexmedetomidine on EA are required to establish the optimal dose. Dexmedetomidine used in tonsillectomy, adenoidectomy, ophthalmologic, and orthopedic surgeries lower the EA incidence compared with saline, which is consistent with the findings of Cho et al. (43), Jiao et al. (46), and Tan et al. (42). Tonsillectomy with or without adenoidectomy is commonly associated with throat pain and discomfort, and the EA risk associated with this procedure is up to 55.88% and may involve “a sense of suffocation” because of edema, difficulty in swallowing, and nausea. Dexmedetomidine, through adequate analgesia and sedation, significantly decreases the EA occurrence, and can be widely used in suitable patients. Dexmedetomidine can reduce EA incidence not only in children receiving general anesthesia but also in children undergoing magnetic resonance imaging, without hemodynamic or respiratory distress that prolong the time to discharge from the hospital (47). This meta-analysis indicates that the PONV event is reduced in children following dexmedetomidine anesthesia compared with that in children under saline administration; however, a meta-analysis published in 2014 could not establish the efficacy of dexmedetomidine in lowering the PONV incidence (45). Dexmedetomidine was also found to significantly reduce the requirement of rescue analgesic, which is consistent with the findings of Cho et al. (43) and Jun et al. (48). Inadequate analgesia is one of the factors contributing to postoperative agitation. Dexmedetomidine activates the α (2)-adrenergic receptor located in the presynaptic and posterior membranes of the spinal cord and inhibits the peripheral nerve fibers A and C which may contribute to the decrease in the demand for a rescue analgesic. Time to emergence and extubation were longer in the dexmedetomidine group compared with saline groups; heterogeneity was observed, may have originated from the study of Yang, wherein children with cerebral palsy were included, and dexmedetomidine reduced the sevoflurane mandate during surgery, thereby decreasing the emergence time and extubation time, which contradict the findings of other studies (27). Unexpectedly, no significant difference was observed in the time to discharge from the PACU between the dexmedetomidine and saline groups; heterogeneity was observed when these studies were pooled owing to the study by Bhat. Overall, we found that dexmedetomidine slightly increases the time to discharge from the PACU by 2.22 min, relative to saline, which is shorter than what was reported in a study by Ni et al. Dexmedetomidine offers favorable analgesia and sedation, and may avoid restlessness, unusual behaviors, such as kicking, shouting, and crying in children, which might account for the reduced stay time in the PACU. Generally, dexmedetomidine is effective in preventing EA, without prolonging the time to discharge from the PACU, and thus, it could decrease the burden on healthcare workers and parents.
Except for the requirement of rescue analgesic, no significant differences were observed between the dexmedetomidine and other anesthetic groups in terms of the EA incidence, PONV event, emergence time, extubation time, and time to discharge from the PACU, which is in line with the findings of Feng et al. (49) and Peng et al. (50). Midazolam, a γ-amino-butyric acid receptor inhibitor, is commonly used for premedication in children, which provides effective sedation, anxiolytic effect, and anterograde amnesia; however, it also produces side effects, such as postoperative behavioral changes, cognitive impairment, paradoxical reactions, and respiratory depression (17, 28, 29). Unlike midazolam, dexmedetomidine exerts its hypnotic action through the activation of central pre- and post-synaptic α(1)-adrenergic receptors in the locus coeruleus, rather than the cerebral cortex, and induces a natural sleep status in which the patients remain easily arousable and cooperative, and therefore, it is increasingly used in children (4). Fentanyl, a short-acting opioid analgesic, also produces sedative effects. All the three drugs act on different sites to exert sedative and analgesic effects. Although we observed no significant difference between the effects of these drugs, we recommend that dexmedetomidine is the most suitable option for EA prophylaxis in children as a premedicant because of fewer adverse effects.
Limitations
This meta-analysis has some limitations. First, studies comparing the efficacy of dexmedetomidine with that of midazolam, fentanyl, clonidine, tramadol, and ketofol are limited; a stronger evidence is required to confirm the effectiveness of dexmedetomidine in preventing EA relative to the above drugs. Second, the patients' age in the included studies was variable, which might have caused discrepancy in the results because pharmacokinetics and pharmacodynamics vary between the age of 3 months and 14 years, which may lead to different results. Third, heterogeneity was observed in some analyses such as in the emergence time, extubation time, and time to discharge from the PACU; however, sensitivity analysis demonstrated that the change in total effects is independent of the inclusion or exclusion of trials.
Conclusions
Compared with saline, dexmedetomidine decreases the EA risk, PONV incidence, and requirement of rescue analgesic in children undergoing surgery under general anesthesia. Overall, dexmedetomidine is an excellent choice to prevent EA, compared with other drugs.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by a grant from the research project of the joint foundation of the Luzhou Government and the Southwest Medical University (2018LZXNYD-ZK02 and 2019LZXNYDJ23), the Sichuan Provincial Science and Technology (2019YJ0692), and the Southwest Medical University research program (2018ZRQN063 and 2019ZQN149).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2020.580226/full#supplementary-material
References
- 1.Welborn L, Hannallah R, Norden J, Ruttimann U, Callan C. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. (1996) 83:917–20. 10.1213/00000539-199611000-00005 [DOI] [PubMed] [Google Scholar]
- 2.Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth. (2000) 10:419–24. 10.1046/j.1460-9592.2000.00560.x [DOI] [PubMed] [Google Scholar]
- 3.Vlajkovic G, Sindjelic R. Emergence delirium in children: many questions, few answers. Anesth Analg. (2007) 104:84–91. 10.1213/01.ane.0000250914.91881.a8 [DOI] [PubMed] [Google Scholar]
- 4.Weerink M, Struys M, Hannivoort L, Barends C, Absalom A, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. (2017) 56:893–913. 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Statist Med. (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 8.Ali M, Abdellatif A. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth. (2013) 7:296–300. 10.4103/1658-354X.115363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng W, Zhang T. Dexmedetomidine decreases the emergence agitation in infant patients undergoing cleft palate repair surgery after general anesthesia. BMC Anesthesiol. (2015) 15:145. 10.1186/s12871-015-0124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdil F, Demirbilek S, Begec Z, Ozturk E, Ulger M, Ersoy M. The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth Intensive Care. (2009) 37:571–6. 10.1177/0310057X0903700405 [DOI] [PubMed] [Google Scholar]
- 11.Ali WA, Mohammed AK, Elshorbagy HM. Dexmedetomidine versus ketofol effect on the incidence of emergence agitation associated with sevoflurane-based anesthesia in children undergoing orthopedic surgery. Egypt J Anaesth. (2016) 32:277–84. 10.1016/j.egja.2016.01.004 [DOI] [Google Scholar]
- 12.Gupta N, Rath G, Prabhakar H, Dash H. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol. (2013) 25:271–8. 10.1097/ANA.0b013e31828cb6c0 [DOI] [PubMed] [Google Scholar]
- 13.Cho E, Cha Y, Shim J, Ahn J, Lee S, Ryu K. Comparison of single minimum dose administration of dexmedetomidine and midazolam for prevention of emergence delirium in children: a randomized controlled trial. J Anesth. (2020) 34:59–65. 10.1007/s00540-019-02705-6 [DOI] [PubMed] [Google Scholar]
- 14.Ibacache M, Muñoz H, Brandes V, Morales A. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. (2004) 98:60–3. 10.1213/01.ANE.0000094947.20838.8E [DOI] [PubMed] [Google Scholar]
- 15.El-Hamid A, Yassin H. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J Anaesth. (2017) 11:137–43. 10.4103/1658-354X.203020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kim S, Lee J, Kang Y, Koo B. Low-dose dexmedetomidine reduces emergence agitation after desflurane anaesthesia in children undergoing strabismus surgery. Yonsei Med J. (2014) 55:508–16. 10.3349/ymj.2014.55.2.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. (2012) 22:871–6. 10.1111/j.1460-9592.2012.03802.x [DOI] [PubMed] [Google Scholar]
- 18.Mizrak A, Erbagci I, Arici T, Avci N, Ganidagli S, Oner U. Dexmedetomidine use during strabismus surgery in agitated children. Med Principles Pract. (2011) 20:427–32. 10.1159/000324554 [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Das A, Basunia S, Chattopadhyay S, Kundu R, Bhattacharyya R. Emergence agitation prevention in paediatric ambulatory surgery: a comparison between intranasal dexmedetomidine and clonidine. J Res Pharm Pract. (2015) 4:24–30. 10.4103/2279-042X.150051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A, Davidson M, Tran M, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. (2010) 111:1004–10. 10.1213/ANE.0b013e3181ee82fa [DOI] [PubMed] [Google Scholar]
- 21.Koceroglu I, Devrim S, Bingol Tanriverdi T, Gura Celik M. The effects of dexmedetomidine and tramadol on post-operative pain and agitation, and extubation quality in paediatric patients undergoing adenotonsillectomy surgery: a randomized trial. J Clin Pharm Ther. (2020) 45:340–6. 10.1111/jcpt.13080 [DOI] [PubMed] [Google Scholar]
- 22.Song I, Seo K, Oh A, Baik J, Kim J, Hwang J, et al. Dexmedetomidine injection during strabismus surgery reduces emergence agitation without increasing the oculocardiac reflex in children: a randomized controlled trial. PLoS ONE. (2016) 11:e0162785. 10.1371/journal.pone.0162785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zhang L, Shi M, Yang S, Li S, Gao S. Impact of dexmedetomidine on pediatric agitation in the postanesthesia care unit. J Perianesth Nurs. (2018) 33:53–7. 10.1016/j.jopan.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Tsiotou A, Malisiova A, Kouptsova E, Mavri M, Anagnostopoulou M, Kalliardou E. Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr Anaesth. (2018) 28:632–8. 10.1111/pan.13397 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Wang C, Xu H, Lu H, Zhang H. Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine. (2018) 97:e12140 10.1097/MD.0000000000012140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat R, Mudukanagoudar M, Shetty S, Kamath S. Study of dose related effects of dexmedetomidine on laryngeal mask airway removal in children - a double blind randomized study. Anaesth Pain Intensive Care. (2018) 22:368–73. [Google Scholar]
- 27.Liu Y, Kang D, Na H, Li B, Xu Y, Ni J, et al. Consequence of dexmedetomidine on emergence delirium following sevoflurane anesthesia in children with cerebral palsy. Int J Clin Exp Med. (2015) 8:16238–44. [PMC free article] [PubMed] [Google Scholar]
- 28.Sheta S, Al-Sarheed M, Abdelhalim A. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: a double-blinded randomized controlled trial. Paediatr Anaesth. (2014) 24:181–9. 10.1111/pan.12287 [DOI] [PubMed] [Google Scholar]
- 29.Bhadla S, Prajapati D, Louis T, Puri G, Panchal S, Bhuva M. Comparison between dexmedetomidine and midazolam premedication in pediatric patients undergoing ophthalmic day-care surgeries. Anesth Essays Res. (2013) 7:248–56. 10.4103/0259-1162.118982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Li Y, Sun Y, Wang X, Ye H, Yuan X. Dexmedetomidine effect on emergence agitation and delirium in children undergoing laparoscopic hernia repair: a preliminary study. J Int Med Res. (2017) 45:973–83. 10.1177/0300060517699467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng Q, Xia Z, Luo T, Wu Y, Tang L, Zhao B, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: a case-control study. Int J Pediatr Otorhinolaryngol. (2012) 76:1036–41. 10.1016/j.ijporl.2012.03.028 [DOI] [PubMed] [Google Scholar]
- 32.Lili X, Jianjun S, Haiyan Z. The application of dexmedetomidine in children undergoing vitreoretinal surgery. J Anesth. (2012) 26:556–61. 10.1007/s00540-012-1354-1 [DOI] [PubMed] [Google Scholar]
- 33.Asaad OM, Hafez M, Mohamed MY, El-mahgoup SS. Comparative study between prophylactic single dose of fentanyl and dexmedetomidine in the management of agitation after sevoflurane anesthesia in children. Egypt J Anaesth. (2011) 27:31–7. 10.1016/j.egja.2010.12.005 [DOI] [Google Scholar]
- 34.Olutoye OA, Glover CD, Diefenderfer JW, McGilberry M, Wyatt MM, Larrier DR, et al. The effect of intraoperative dexmedetomidine on postoperative analgesia and sedation in pediatric patients undergoing tonsillectomy and adenoidectomy. Anesth Analgesia. (2010) 111:490–5. 10.1213/ANE.0b013e3181e33429 [DOI] [PubMed] [Google Scholar]
- 35.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. (2005) 15:762–6. 10.1111/j.1460-9592.2004.01541.x [DOI] [PubMed] [Google Scholar]
- 36.Bi Y, Ma Y, Ni J, Wu L. Efficacy of premedication with intranasal dexmedetomidine for removal of inhaled foreign bodies in children by flexible fiber optic bronchoscopy: a randomized, double-blind, placebo - controlled clinical trial. BMC Anesthesiol. (2019) 19:219. 10.1186/s12871-019-0892-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boku A, Hanamoto H, Oyamaguchi A, Inoue M, Morimoto Y, Niwa H. Effectiveness of dexmedetomidine for emergence agitation in infants undergoing palatoplasty: a randomized controlled trial. Brazil J Anesthesiol. (2016) 66:37–43. 10.1016/j.bjan.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 38.Kim N, Kim S, Yoon H, Kil H. Effect of dexmedetomidine on sevoflurane requirements and emergence agitation in children undergoing ambulatory surgery. Yonsei Med J. (2014) 55:209–15. 10.3349/ymj.2014.55.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukry M, Clyde M, Kalarickal P, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. (2005) 15:1098–104. 10.1111/j.1460-9592.2005.01660.x [DOI] [PubMed] [Google Scholar]
- 40.Surana P, Parikh D, Patkar G, Tendolkar B. A prospective randomized controlled double-blind trial to assess the effects of dexmedetomidine during cleft palate surgery. Korean J Anesthesiol. (2017) 70:633–41. 10.4097/kjae.2017.70.6.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amorim M, Govêia C, Magalhães E, Ladeira L, Moreira L, Miranda D. Effect of dexmedetomidine in children undergoing general anesthesia with sevoflurane: a meta-analysis. Brazil J Anesthesiol. (2017) 67:193–8. 10.1016/j.bjan.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 42.Tan D, Xia H, Sun S, Wang F. Effect of ancillary drugs on sevoflurane related emergence agitation in children undergoing ophthalmic surgery: a Bayesian network meta-analysis. BMC Anesthesiol. (2019) 19:138. 10.1186/s12871-019-0810-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho H, Yoon H, Jin H, Hwang S. Efficacy of dexmedetomidine for perioperative morbidities in pediatric tonsillectomy: a metaanalysis. Laryngoscope. (2018) 128:E184–E93. 10.1002/lary.26888 [DOI] [PubMed] [Google Scholar]
- 44.Ni J, Wei J, Yao Y, Jiang X, Luo L, Luo D. Effect of dexmedetomidine on preventing postoperative agitation in children: a meta-analysis. PLoS ONE. (2015) 10:e0128450. 10.1371/journal.pone.0128450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, Hu J, Liu X, Yan J. Effects of intravenous dexmedetomidine on emergence agitation in children under sevoflurane anesthesia: a meta-analysis of randomized controlled trials. PLoS ONE. (2014) 9:e99718. 10.1371/journal.pone.0099718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao H, Wang H, Jiang Z, Hu J. Comparative efficacy of ancillary drugs in sevoflurane-related emergence agitation after paediatric adenotonsillectomy: a Bayesian network meta-analysis. J Clin Pharm Ther. (2020) 45:1039–49. 10.1111/jcpt.13133 [DOI] [PubMed] [Google Scholar]
- 47.Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth. (2006) 16:748–53. 10.1111/j.1460-9592.2006.01845.x [DOI] [PubMed] [Google Scholar]
- 48.Jun J, Kim K, Kim J, Song S. The effects of intranasal dexmedetomidine premedication in children: a systematic review and meta-analysis. Can J Anaesth. (2017) 64:947–61. 10.1007/s12630-017-0917-x [DOI] [PubMed] [Google Scholar]
- 49.Feng J, Wang X, Lu Y, Pang D, Peng W, Mo J. Effects of dexmedetomidine versus midazolam for premedication in paediatric anaesthesia with sevoflurane: a meta-analysis. J Int Med Res. (2017) 45:912–23. 10.1177/0300060517704595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng K, Wu S, Ji F, Li J. Premedication with dexmedetomidine in pediatric patients: a systematic review and meta-analysis. Clinics. (2014) 69:777–86. 10.6061/clinics/2014(11)12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.