Abstract
The entire world is currently experiencing difficult times with respect to physical, mental, and socio-economic health. The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since early 2020 caught the world by surprise. While there are promising developments, to date, there is no available drug or vaccine, and researchers are working around the clock to develop a solution. Sadly, all these crucial efforts are being affected and, at times, misguided and derailed by the publication of fake articles by so-called researchers and perhaps the mismanagement by authentic and predatory journals. The problem is that genuine and good quality articles are getting lost in the crowd. More than ever, it is now the time to bring in stricter controls and to follow due diligence before allowing articles into the public domain. At the same time, it has become life-saving to separate the wheat from the chaff so that the genuine studies of first-hand experience of handling and management of COVID-19 patients, and authentic research is not submerged in this flood of unreliable publications.
Keywords: COVID-19, SARS-CoV-2, Fake research
The onset of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) since the end of 2019 has taken the world and the medical fraternity by surprise [1].
At the time of writing, there is no proven drug or vaccine available to work as a cure or protective elixir with an acceptable and reproducible success rate [2]. In this ephemeral state of change, several drugs, like hydroxychloroquine [3], ivermectin [4], and antivirals [5], have been put forward as potential treatments only to be withdrawn later since their benefits do not seem to outweigh potential risks.
Taking advantage of a crisis is nothing new. It is a common trait of human behaviour.
For some years, this has been spreading out into the world of scientific, technical, and medical (STM) publishing [6]. Initially, so-called ‘predatory’ journals [7] were main culprits, but now, it would appear that even the standard benchmark journals are suffering from a deluge of submissions of variable quality. [8]. In the wake of the COVID-19 pandemic and the expedition of related reports and research, it would appear that many have tried to take advantage of a relaxation in publishing norms and controls. The expediting and increased availability of research were originally intended to promote research and dissemination of knowledge. However, this noble goal has been defeated, and some well-established journals have suffered and have had to retract articles [9, 10].
The role and impact of unregulated mainstream and social media networks cannot be overstated in the dissemination of dubious information regarding this pandemic [11]. In addition, a lack of transparent information regarding types of journals and their publication process has been a major cause of this environment of mistrust in pandemic research.
What Are Predatory Journals, and What Is the Spurious and Unwanted Research in Context to COVID-19?
It was Jeffrey Beall, a librarian from the University of Denver, who popularised the term ‘predatory journals’ [6, 12]. The whole purpose of these journals allegedly revolves around the opportunity to make ‘quick’ money by collecting article processing charges (APCs) and by bypassing the process of careful scrutiny and genuine peer review by subject experts. In the absence of proper quality controls, fake articles get published and muddy the waters. There is a danger that such ‘unscientific’ publications may misguide future research resulting in a waste of time, resources, and money.
In recent years, there have been efforts in medical research to develop stricter and more transparent peer-review and publication standards, which has made it more difficult for many so-called ‘researchers’ to thrive in the academic setting.
It is still, however, surprising to see thousands of COVID-19 related articles being published in journals within a very short time span. One of the most intriguing facts, is that the vast majority of authors of these articles may not have actually ever handled COVID-19 cases. Those clinicians and healthcare workers who are actually having to treat and manage patients do not have time to publish at this time.
How to Identify Articles of Questionable Quality?
They have common study questions with plenty of literature already available. There is just an addition of word ‘COVID-19’ or ‘corona,’ ‘time,’ or ‘period’.
Already well-known facts and instructions issued by international healthcare agencies are being republished by all speciality journals be it orthopaedics, general surgery etc. with little or no new/novel information except COVID-19 in the title.
Basic standards of sterility and patient care in, and outside, the operating room which were well expected even before the virus have been highlighted.
Stepping over into the domain of other specialities specially internal and respiratory medicine is apparent.
Most of the articles take the form of online surveys formulated with poor levels of evidence. In the absence of COVID-19, these could never have been published.
What Are the Complications of These Unwanted Publications?
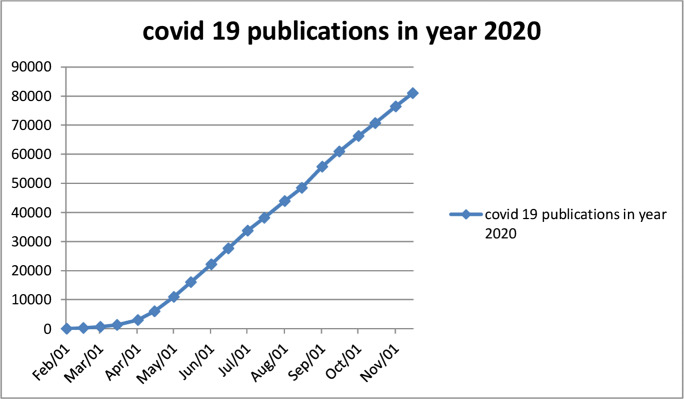
Just over 80,000 articles have been published in the period January 21–November 15, 2020, (dashboard for corona research [13]). This is an absolutely amazing number of articles, and their growth does not look like slowing down (Fig. 1). Such an explosion of research publications is not possible without a loosening of quality controls and scrutiny.
As a result, genuine and good quality articles are lost in the crowd.
Moreover, retractions later on create an extra burden on research resources and create a ‘trust deficit’ within the research ecosystem. Ultimately, genuine research is hampered.
Fig. 1.
Number of COVID-related articles published in recent months [13]
What Do Preprints Actually Mean?
Many valuable contributions to research have been made possible via preprints. They are designed to be the preliminary, initial publication of work which has not yet undergone a rigorous peer-review process. Authors benefit by posting their work in the public domain and from receiving early feedback, critique, and suggestions which may help them develop and improve their paper for later submission and formal publication. Similarly, other investigators gain early access to work which may help them in their own research.
By no means can preprints be justified to be circulated in the general public as established information nor used to formulate or modify public health policies.
The demand to provide ‘latest’ information in hourly news bulletins has lead reporters with none or very limited scientific training to translate preprint information hurriedly into ‘breaking news’.
Popular servers like ‘biorxiv’ and ‘medrxiv’ have already initiated steps in this direction and display disclaimers regarding the inconclusive nature of information of preprints which they host.
It is more important than ever for all academic journals and preprint servers to raise the bar, bring in stricter controls, and maintain standards of due diligence before allowing articles into the public domain. Mainstream media and other outlets should also exercise greater caution before projecting any initial information as a ‘new development’. Preprint and submitted manuscripts should be subjected to scrutiny and analysis to determine whether or not they are really making a contribution to the existing body of COVID-19 research and deserve any form of priority since publications with vested interests may divert attention away from other genuine research in this crucial time?
At the same time, genuine studies of first-hand experience of handling and management of corona virus patients should be dealt with as efficiently as possible.
Author Contributors
1. Dr. Punit Tiwari, MS Orthopaedics.
2. Dr. Harmeet Kaur, MD Radiodiagnosis.
Data Availability
Not applicable.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Punit Tiwari, Email: punit_tiwari28@yahoo.com.
Harmeet Kaur, Email: kaurh28@yahoo.com.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, Rubin EJ. Covid-19 - the search for effective therapy. N Engl J Med. 2020;382(19):1851–1852. doi: 10.1056/NEJMe2005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS. Hydroxychloroquine for the prevention of Covid-19 - searching for evidence [published online ahead of print, 2020 Jun 3]. N Engl J Med. 2020:NEJMe2020388. 10.1056/NEJMe2020388. [DOI] [PMC free article] [PubMed]
- 4.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu R, Wang L, Kuo HD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall J. Dangerous predatory publishers threaten medical research. J Korean Med Sci. 2016;31(10):1511–1513. doi: 10.3346/jkms.2016.31.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss A, Lambert WC, Parish LC. Predatory journals: harmful to patients, the public, and the integrity of scientific research. Skinmed. 2017;15(3):167–168. [PubMed] [Google Scholar]
- 8.Davey M. (2020) Surgisphere: mass audit of papers linked to firm behind hydroxychloroquine Lancet study scandal. The Guardian. Retrieved from http://www.theguardian.com
- 9.Mehra MR, Ruschitzka F, Patel AN. Retraction-hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis [retraction of: Lancet. 2020 May 22;:]. Lancet. 2020;395(10240):1820. 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed]
- 10.Mehra, M. R., Desai, S. S., Kuy, S., Henry, T. D., & Patel, A. N. (2020). Retraction: cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 10.1056/NEJMoa2007621. The New England journal of medicine. 382(26): 2582. 10.1056/NEJMc2021225. [DOI] [PMC free article] [PubMed]
- 11.Tagliabue F, Galassi L, Mariani P. The “pandemic” of disinformation in COVID-19 [published online ahead of print, 2020 Aug 1]. SN Compr Clin Med. 2020:1–3. 10.1007/s42399-020-00439-1. [DOI] [PMC free article] [PubMed]
- 12.Beall J. Predatory publishers are corrupting open access. Nature. 2012;489(7415):179. doi: 10.1038/489179a. [DOI] [PubMed] [Google Scholar]
- 13.Available from https://covid19primer.com/dashboard. Accessed 15 November 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.