Abstract
Cardiovascular calcification is highly prevalent and associated with increased morbidity in chronic kidney disease (CKD). This review examines the impact of uremic toxins, which accumulate in CKD due to a failing kidney function, on cardiovascular calcification. A systematic literature search identified 41 uremic toxins that have been studied in relation to cardiovascular calcification. For 29 substances, a potentially causal role in cardiovascular calcification was addressed in in vitro or animal studies. A calcification-inducing effect was revealed for 16 substances, whereas for three uremic toxins, namely the guanidino compounds asymmetric and symmetric dimethylarginine, as well as guanidinosuccinic acid, a calcification inhibitory effect was identified in vitro. At a mechanistic level, effects of uremic toxins on calcification could be linked to the induction of inflammation or oxidative stress, smooth muscle cell osteogenic transdifferentiation and/or apoptosis, or alkaline phosphatase activity. For all middle molecular weight and protein-bound uremic toxins that were found to affect cardiovascular calcification, an increasing effect on calcification was revealed, supporting the need to focus on an increased removal efficiency of these uremic toxin classes in dialysis. In conclusion, of all uremic toxins studied with respect to calcification regulatory effects to date, more uremic toxins promote rather than reduce cardiovascular calcification processes. Additionally, it highlights that only a relatively small part of uremic toxins has been screened for effects on calcification, supporting further investigation of uremic toxins, as well as of associated post-translational modifications, on cardiovascular calcification processes.
Keywords: cardiovascular calcification, uremic toxins, chronic kidney disease, cardiovascular disease
1. Introduction
Approximately 13% of the population suffers from chronic kidney disease (CKD) globally, and about half of the patients with CKD stages 4-5 also suffer and eventually die from cardiovascular disease (CVD) [1,2]. The presence of cardiovascular calcifications highly increases cardiovascular risk [3,4]. Cardiovascular calcification increases with age in the general population [5], but is massively accelerated [6] and highly frequent in patients with CKD. Reports indicate up to 70% of CKD patients presenting abdominal aortic calcification as detected by plain X-rays [7,8] and even 88% of a cohort of 20–30-year old patients in CKD stage 5D already displayed vascular calcifications [9]. Furthermore, up to 100% of patients in CKD stage 5D displayed calcification in the aortic valve, mitral annulus or mitral valve as imaged by echocardiography [8].
Consequences of cardiovascular calcification include decreased arterial elasticity, an increase in pulse wave velocity, a decrease in coronary artery perfusion, aortic valve stenosis, progression of left ventricular hypertrophy as well as myocardial ischemia and failure [10,11]. These cardiovascular complications are among the main causes of death in CKD patients [2] and cardiovascular calcification heralds a poor prognosis in terms of cardiovascular morbidity in these patients [12,13]. In line, KDIGO (Kidney Disease: Improving Global Outcomes) CKD guidelines suggest to consider patients with advanced CKD (stages 3–5) and cardiovascular calcifications at the highest cardiovascular risk [14].
Cardiovascular calcification is an actively regulated and progressive process that, once initiated, tends to relentlessly increase in severity. The mechanisms underlying cardiovascular calcification involve the accumulation of calcium and phosphate in the extracellular matrix (ECM), the transdifferentiation of vascular smooth muscle cells (VSMC) to osteoblast/chondrocyte-like cells, increased VSMC apoptosis and reduced proliferation [15]. Furthermore, inflammation and oxidative stress have been reported as inducers of VSMC osteogenic transdifferentiation and calcification [16,17]. Additionally, cardiovascular calcification goes along with elastin degradation in the vascular wall, which is mediated by matrix metalloproteinases (MMPs). The enhanced elastin degradation increases the affinity of the ECM for calcium and promotes the growth of hydroxyapatite crystals along the elastic lamina and finally ECM mineralization [18]. Increased cardiovascular calcification in CKD is believed to result from a disturbed balance between inhibitors and inducers of calcification, but the exact mechanisms are not completely understood and are believed to be multifactorial [13].
Uremic toxins are defined as harmful substances that accumulate in the circulation of CKD patients due to the failing function of the kidney [19]. To date, over 140 uremic toxins have been described to be increased in CKD [20,21]. These can be divided into three groups according to their physicochemical characteristics [19]: (i) free, water-soluble, low molecular weight solutes (MW < 500 Da); (ii) water-soluble, middle-sized molecules (MW > 500 Da); (iii) protein-bound solutes. Serum levels of for example p-cresyl sulphate and indoxyl sulphate have been shown to be associated to cardiovascular outcome in CKD [22,23] and positively correlated with vascular calcification and stiffness in CKD [24,25,26]. This has raised the hypothesis that the accumulation of uremic toxins in CKD may contribute to the high prevalence of cardiovascular calcification in these patients. However, despite many comprehensive reviews on cardiovascular calcification in CKD [13,27], there are still many uncertainties about which uremic toxins and associated mechanisms affect cardiovascular calcification. As such information may reveal novel treatment modalities to reduce vascular calcification and could support a future improvement of removal techniques, this systematic review comprehensively analyzes available studies on the effect of uremic toxins on calcification processes.
2. Materials and Methods
2.1. Data Sources and Searches
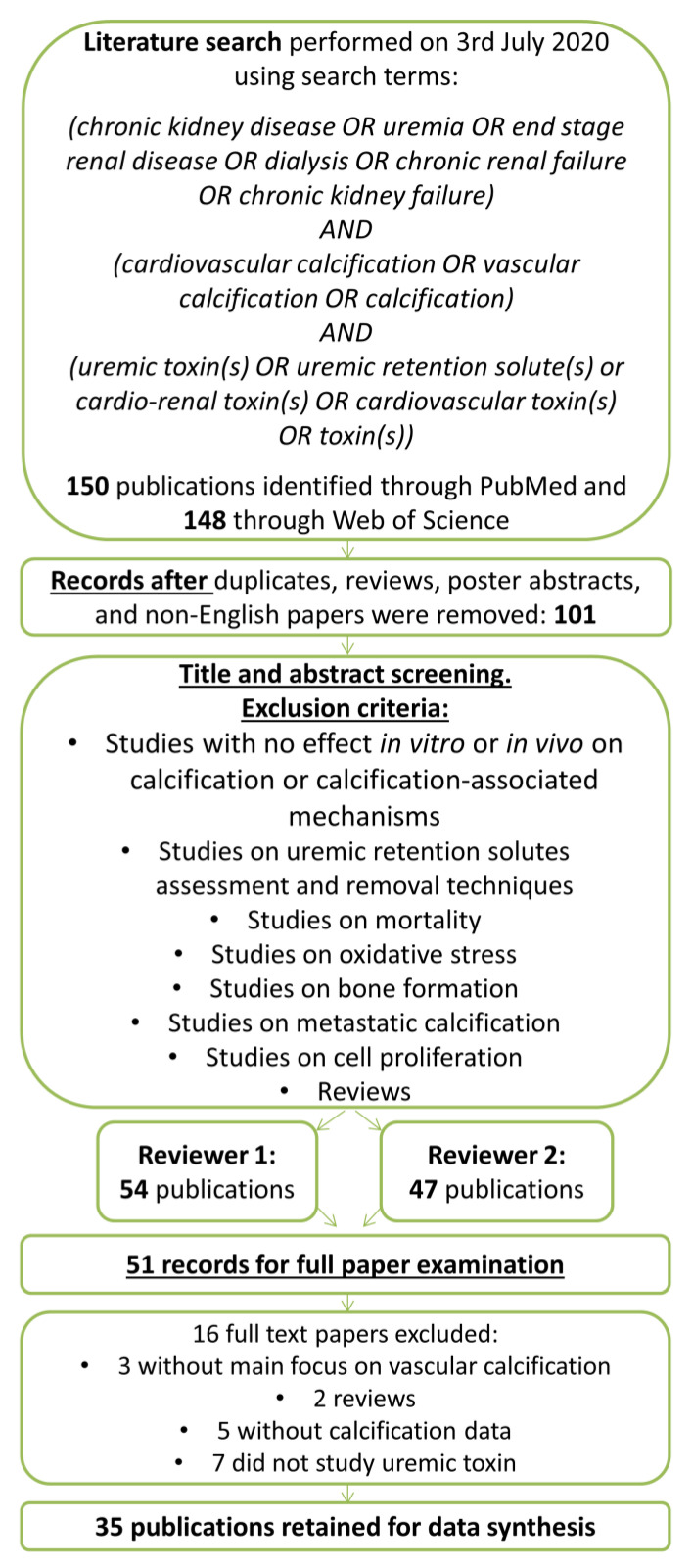
The authors performed an advanced search in both PubMed and Web of Science to identify eligible studies on 3 July 2020. The search strategy is depicted in Figure 1. The following terms and conditions were used for the systematic literature search: chronic kidney disease OR uremia OR end stage renal disease OR dialysis OR chronic renal failure OR chronic kidney failure AND cardiovascular calcification OR vascular calcification OR calcification AND uremic toxin(s) OR uremic retention solute(s) OR cardio-renal toxin(s) OR cardiovascular toxin(s) OR toxin(s). Where indicated, additional literature on identified uremic toxins was added manually after checking the reference lists of the included studies or recent reviews, when required for a correct interpretation of findings. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed while composing and writing the systematic review. The systematic review was registered in the PROSPERO database (CRD42016051604) on 16 November 2016 [28].
Figure 1.
Flow diagram for original paper selection for the systematic review on uremic toxins and cardiovascular calcification.
2.2. Study Selection Criteria
Two screeners independently identified and evaluated papers by the title and the abstract for inclusion or exclusion. The full articles and/or supplemental materials (tables and appendices) were reviewed before the inclusion/exclusion decision. Articles describing the influence of one or more uremic toxin(s) on cardiovascular calcification were included into the analyses. Duplicates, review papers, poster abstracts, and non-English papers were excluded. Papers were also excluded whose main focus was not cardiovascular calcification, which did not study uremic toxins, which described different uremic toxins assessment/removal techniques, or which studied mainly oxidative stress, bone formation, metastatic calcification, or cell proliferation. There were no restrictions on sample size or study duration defined by the authors. A third reviewer was consulted to achieve consensus in case of inconsistencies.
3. Results
3.1. Identification of Uremic Toxins Studied in the Context of Uremia and Cardiovascular Calcification
The search yielded 148 hits in Web of Science and 150 in PubMed. After removing duplicates, review papers, poster abstracts, and non-English papers, the titles, and abstracts of the remaining 101 papers were screened. Out of these, 51 papers were selected for full-text examination and 35 of those papers were identified as relevant for this systematic review. Within these, 41 substances with increased plasma concentration in CKD patients were studied in the context of uremia and cardiovascular calcification. A total of 12 substances were only investigated for a potential association with the extent of cardiovascular calcification in CKD patients (Table S1) and are not further discussed here. For the remaining 29 substances, a potentially causal role in cardiovascular calcification was addressed in in vitro or animal studies. These included 15 low molecular weight uremic toxins (Table 1); 12 middle molecular weight uremic toxins (Table 2) as well as indoxyl sulphate and p-cresyl sulphate as protein-bound uremic toxins (Table 3). Where indicated in Table 1, Table 2 and Table 3, additional studies on these substances were included after screening the reference lists of the included studies or recent reviews.
Table 1.
Low molecular weight substances increased in CKD and examined for causal effects on cardiovascular calcification or associated readouts.
| Effect | Substance | Species | Tissue or Cell Type | Type of Study | Analysis of Calcification or Calcification-Associated Process * by |
Reference | Plasma Level in CKD | Classified as Uremic Toxin ** | |
|---|---|---|---|---|---|---|---|---|---|
| In Vitro | Animal | ||||||||
| INHIBITION | Asymmetric dimethylarginine (ADMA) | Human | VSMC | V $ | Alizarin red staining | Schepers [29] | ↑ [§] | V | |
| Symmetric dimethylarginine (SDMA) | Human | VSMC | V $ | Alizarin red staining | Schepers [29] | ↑ [§] | V | ||
| Guanidinosuccinic acid (GSA) | Human | VSMC | V $ | Alizarin red staining | Schepers [29] | ↑ [§] | V | ||
| INDUCTION | Phosphate (P) | Human | VSMC | V | Calcium content Alizarin red staining |
Bouabdallah [30] | ↑ [31] | ||
| Human | VSMC | V | Alizarin red staining Upregulation of MSX2, SOX9 and OPN Increased apoptosis |
Cazana-Perez [32] | |||||
| Human | VSMC | V | Calcium content Upregulation of CBFA1/RUNX2 and BMP2 |
Guerrero [33] | |||||
| Human | VSMC | V | Alizarin red staining | Schepers [29] | |||||
| Human | VSMC | V $ | High binding affinity of calcium to ECM | Shigematsu [34] 1 | |||||
| Rat | VSMC | V | Von Kossa staining | Shibata [35] | |||||
| Rat | Aorta | V | Calcium content | Guerrero [33] | |||||
| Mouse | Aorta | V 2 | Von Kossa staining | Belmokhtar [36] | |||||
| Mouse | VSMC | V | Alizarin red staining Increased ROS generation Upregulation of PIT-1, BMP2 and CBFA1/RUNX2 |
Belmokhtar [36] | |||||
| Mouse | VSMC | V | Upregulation of BMP2 and OPN | Sage [37] 1 | |||||
| Rat | Aorta | V 3 | Von Kossa staining | Shibata [35] | |||||
| Calcium | Rat | Aorta | V | Calcium content | Azpiazu [38] 1 | ↑ [39] | |||
| Human | VSMC | V | Calcium content | Yang [40] 1 | |||||
| Cytidine | Human | MSC | V $ | ALP activity Calcium content |
Hegner [41] | ↑ [§] | V | ||
| Urea | Human | MSC | V $ | ALP activity Calcium content |
Hegner [41] | ↑ [§] | V | ||
| Threitol | Human | MSC | V $ | ALP activity Calcium content |
Hegner [41] | ↑ [§] | V | ||
| NO EFFECT | Guanidino compounds: | Human | VSMC | V | Alizarin red staining | Schepers [29] | |||
| ADMA | ↑ [§] | V | |||||||
| SDMA | ↑ [§] | V | |||||||
| GSA | ↑ [§] | V | |||||||
| Creatine | ↑ [§] | V | |||||||
| Creatinine | ↑ [§] | V | |||||||
| Guanidine | ↑ [§] | V | |||||||
| Guanidino acetic acid | ↑ [§] | V | |||||||
| Guanidino butyric acid | ↑ [§] | V | |||||||
| Guanidino propionic acid | ↑ [§] | V | |||||||
| Methylguanidine | ↑ [§] | V | |||||||
ALP—alkaline phosphatase; BMP2—bone morphogenetic protein 2; CBFA1/RUNX2—core-binding factor subunit alpha-1/runt-related transcription factor 2; CT—computed tomography; ECM—extracellular matrix; MSC–mesenchymal stromal cells (as progenitors of VSMC); MSX2—msh homeobox 2; OPN—osteopontin; PIT-1—sodium Pi co-transporter-1; ROS—reactive oxygen species; SOX9—SRY-box transcription factor 9; VSMC—vascular smooth muscle cells. * Readouts of calcification-associated processes, such as cell signaling and gene/protein expression, are shown in italic. [§] As described in comprehensive uremic toxins reviews [19,20]. ** As described in comprehensive uremic toxins reviews [19,20,21]. $ In pro-calcifying medium. 1 Literature added manually after checking reference lists of included studies or recent reviews. 2 In vivo mouse model: 5/6 nephrectomy in Apoe−/−mice on a high- phosphorus diet vs. 5/6 nephrectomy in Apoe-/- mice on a control diet for twelve weeks. 3 In vivo rat model: 5/6 nephrectomy in rats on a high- phosphorus and low-calcium diet vs. 5/6 nephrectomy in rats on a control diet for eight weeks; ↑ indicates increased plasma levels in CKD patients.
Table 2.
Middle molecular weight substances increased in CKD and examined for causal effects on cardiovascular calcification or associated readouts.
| Effect | Substance | Species | Tissue or Cell Type | Type of Study | Analysis of Calification or Calcification-Associated Process * by |
Reference | Plasma level in CKD | Classified as Uremic Toxin ** | |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | Animal | ||||||||
| INDUCTION | Bone morphogenetic protein (BMP2) |
Bovine | VSMC | V $ | Calcium content Upregulation of CBFA1/RUNX2 |
Chen [42] | ↑ [42] | ||
| Rat | VSMC | V $ | Upregulation of CBFA1/RUNX2, MSX2 and PIT-1 | Rong [43] 1 | |||||
| Fibroblast growth factor 2 (FGF2) | Human | MSC | V $ | ALP activity Alizarin red staining Calcium content Upregulation of CBFA1/RUNX2, OPN and osterix |
Hegner [41] | ↑ [§] | |||
| Interleukin-1β (IL-1β) |
Human | MSC | V $ | ALP activity Alizarin red staining Calcium content Upregulation of CBFA1/RUNX2, OPN and osterix |
Hegner [41] | ↑ [§] | V | ||
| Interleukin-8 (IL-8) |
Human | VSMC | V $ | Alizarin red staining Calcium content Downregulation of OPN |
Bouabdallah [30] | ↑ [44] | |||
| Parathyroid hormone (PTH) |
Human | MSC | V $ | ALP activity Alizarin red staining Calcium content Upregulation of CBFA1/RUNX2, OPN and osterix |
Hegner [41] | ↑ [§] | V | ||
| S100A12 (RAGE ligand) |
Mouse | VSMC | V | Alizarin red staining Increased ROS generation Upregulation of PIT-1, BMP2 and CBFA1/RUNX2 |
Belmokhtar [36] | ↑ [45] | |||
| Salusin-β | Human | VSMC | V & | Calcium content ALP activity Alizarin red staining Increased ROS generation Upregulation of BMP2 and CBFA1/RUNX2 |
Sun [46] | ↑ [47] | |||
| Rat | Aorta | V & | Calcium content ALP activity Von Kossa staining |
Sun [46] | |||||
| Tumor necrosis factor alpha (TNF-α) |
Human | MSC | V | ALP activity Alizarin red staining Calcium content Upregulation of CBFA1/RUNX2, OPN and osterix |
Hegner [41] | ↑ [§] | V | ||
| Human | VSMC | V $ | Calcium content | Guerrero [33] | |||||
| Human | VSMC | V $ | ALP activity | Zickler [48] | |||||
| Human | VSMC | V $ | Calcium content | Zickler [49] | |||||
| Rat | Aorta | V $ | Calcium content | Guerrero [33] | |||||
| Vascular cell adhesion molecule-1 (VCAM-1) | Human | VSMC | V $ | ALP activity Alizarin red staining |
Zickler [48] | ↑ [50] | |||
| NO EFFECT | Soluble tumor necrosis factor receptor-1 (sTNFR-1) |
Human | VSMC | V $ | ALP activity Alizarin red staining |
Zickler [48] | ↑ [48] | ||
| Soluble tumor necrosis factor receptor-2 (sTNFR-2) |
Human | VSMC | V $ | ALP activity Alizarin red staining |
Zickler [48] | ↑ [48] | |||
| Soluble interleukin-2 receptor (sIL-2R) | Human | VSMC | V $ | ALP activity Alizarin red staining |
Zickler [48] | ↑ [48] | |||
ALP—alkaline phosphatase; BMP2—bone morphogenetic protein 2; CBFA1/RUNX2—core-binding factor subunit alpha-1/runt-related transcription factor 2; MSC—mesenchymal stromal cells (as progenitors of VSMC); OPN—osteopontin; PIT-1—sodium Pi co-transporter-1; ROS—reactive oxygen species; VSMC—vascular smooth muscle cells. * Readouts of calcification-associated processes, such as cell signaling and gene/protein expression, are shown in italic. [§] As described in comprehensive uremic toxins reviews [19,20]. ** As described in comprehensive uremic toxins reviews [19,20,21]. $ In pro-calcifying medium. & In pro as well as non-calcifying media; ↑ indicates increased plasma levels in CKD patients. 1 Literature added manually after checking reference lists of included studies or recent reviews.
Table 3.
Protein-bound substances increased in CKD and examined for causal effects on cardiovascular calcification or associated readouts.
| Effect | Substance | Species | Tissue or Cell Type | Type of Study | Analysis of Calcification or Calcification-Associated Process * by | Reference | Plasma Level in CKD | Classified as Uremic Toxin ** | |
|---|---|---|---|---|---|---|---|---|---|
| In vitro | Animal | ||||||||
| INDUCTION | Indoxyl sulphate (IS) | Human | VSMC | V $ | Calcium content Alizarin red staining Upregulation of CBFA1/RUNX2, ALP, BMP2 and OPN Downregulation of α-SMA and SM22-α |
Bouabdallah [30] | ↑ [§] | V | |
| Human | VSMC | V | Alizarin red staining Upregulation of CBFA1/RUNX2, ALP and OPN Downregulation of α-SMA and SMTN |
Chen [51] | |||||
| Human | VSMC | V | Alizarin red staining Upregulation of CBFA1/RUNX2 and OPN |
Zhang [52] | |||||
| Human | VSMC | V | Alizarin red staining Upregulation of CBFA1/RUNX2 and OPN |
Chen [53] | |||||
| Human | VSMC | V | Alizarin red staining ALP activity Downregulation of α-SMA Upregulation of CBFA1/RUNX2 |
He [54] | |||||
| Human | VSMC | V | Upregulation of p53, p21, and prelamin A | Muteliefu [55] | |||||
| Human | HepG2 | V | Downregulation of fetuin-A | Ochi [56] | |||||
| Rat | Aorta | V $ | Calcium content Alizarin red staining |
Bouabdallah [30] | |||||
| Rat | Aorta | V 1 | Von Kossa staining Upregulation of OPN, CBFA1/RUNX2, ALP and osteocalcin |
Adijiang [57] | |||||
| Rat | Aorta | V 2 | Von Kossa staining | Adijiang [58] | |||||
| Rat | Aorta | V 3 | Alizarin red staining Downregulation of α-SMA and SMTN Upregulation of CBFA1/RUNX2, ALP and OPN |
Chen [51] | |||||
| Rat | Aorta | V 4 | Calcium content Von Kossa staining Activation of inflammation and coagulation pathways |
Opdebeeck [59] | |||||
| Rat | Aorta | V 2 | Upregulation of 8-OHdG and MDA in the calcification area | Muteliefu [55] |
|||||
| p-Cresyl sulphate | Rat | Aorta | V 4 | Calcium content Von Kossa staining Activation of inflammation and coagulation pathways |
Opdebeeck [59] | ↑ [§] | V | ||
ALP—alkaline phosphatase; BMP2—bone morphogenetic protein 2; CBFA1/RUNX2—core-binding factor subunit alpha-1/runt-related transcription factor 2; MDA—malondialdehyde; 8-OHdG—8-hydroxyl-2’-deoxyguanosine; OPN—osteopontin; α-SMA—alpha-smooth muscle actin; SM22-α—smooth muscle protein 22-alpha; SMTN—smoothelin; VSMC—vascular smooth muscle cells. * Readouts of calcification-associated processes, such as cell signaling and gene/protein expression, are shown in italic. [§] As described in comprehensive uremic toxins reviews [19,20]. ** As described in comprehensive uremic toxins reviews [19,20,21]. $ In pro-calcifying medium. 1 In vivo hypertensive rat model: (1) Dahl salt-resistant normotensive rats; (2) Dahl salt-sensitive hypertensive rats; (3) Dahl salt-sensitive hypertensive IS-administered rats for 30 weeks. 2 In vivo hypertensive rat model: (1) Dahl salt-resistant normotensive rats; (2) Dahl salt-resistant normotensive IS-administered rats; (3) Dahl salt-sensitive hypertensive rats; (4) Dahl salt-sensitive hypertensive IS-administered rats for 32 weeks. 3 In vivo uremic rat model: rats subjected to 5/6 nephrectomy were injected with IS at a dosage of 100 mg/kg/48 h for 24 weeks. Control rats with 5/6 nephrectomy received the same volume of phosphate-buffered saline injection every 48 h for 24 weeks. 4 In vivo uremic rat model: Male Wistar rats were exposed to a 10-day adenine sulphate treatment via daily oral gavage (600 mg/kg per day) to induce CKD. CKD rats fed a phosphate-enriched diet (1.2% Pi and 1.06% Ca) were randomly assigned to three treatment groups: (1) vehicle, (2) 150 mg/kg IS, or (3) 150 mg/kg p-cresyl sulphate; ↑ indicates increased plasma levels in CKD patients.
Applied methods for determining calcification extent included histochemical stainings (alizarin red and von Kossa), calcium content measurements using the o-cresolphthalein complexone method and analysis of alkaline phosphatase (ALP) activity. Additionally, to investigate potential underlying mechanisms, studies quantified effects on the expression of calcification inhibitors fetuin-A or osteopontin or analyzed a potential dysregulation of marker proteins and genes previously associated with calcification processes, as explained in more detail below.
3.2. Effect of the Identified Substances on Cardiovascular Calcification
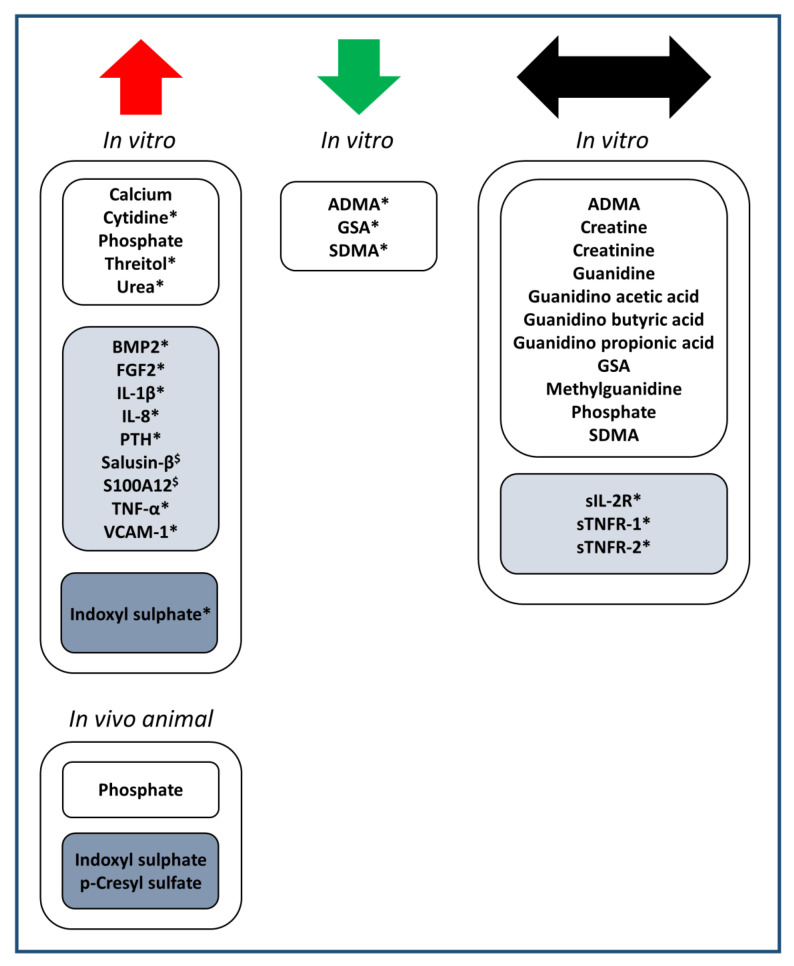
The substances presented in Table 1, Table 2 and Table 3 were categorized according to their effect on cardiovascular calcification, being either inhibiting, inducing, or no effect, as visualized in Figure 2.
Figure 2.
Overview of uremic toxins studied for potentially causal effects on vascular calcification in in vitro or animal studies. The effect on calcification is indicated with arrows in red (increased calcification), green (decreased calcification) or black (no effect). Multiple listing is possible in case of contrasting published findings. Uremic toxins are classified as low molecular weight (white boxes), middle molecular weight (light blue boxes) or protein-bound (dark blue boxes). * in pro-calcifying media; $ in pro- as well as non-calcifying media.
3.2.1. Low Molecular Weight Substances, Increased in Blood in CKD (Table 1)
A high ambient phosphate concentration triggers calcification in a variety of situations, as shown in vitro in human VSMC [29,30,32,33,34], rat and mouse VSMC [35,36], rat aortic rings [33], as well as in aortas of rats subjected to 5/6 nephrectomy [35]. Apo−/− mice with 5/6 nephrectomy displayed aortic valve calcification 12 weeks after surgery both when fed a high phosphate diet as well as a control diet, though with increased calcium deposits detected upon high phosphate diet [36]. Additionally, elevated calcium levels triggered calcification in human VSMC in vitro as well as ex vivo studies with rat aortic rings [38,40].
Calcification of human VSMC could not be induced upon treatment with the guanidino compounds asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), guanidinosuccinic acid (GSA) or seven other guanidino compounds (creatine, creatinine, guanidine, guanidino acetic acid, guanidino butyric acid, guanidino propionic acid, methylguanidine), although ADMA, SDMA, and GSA could reduce human VSMC calcification in high inorganic phosphate media [29]. In contrast, adding the small molecules cytidine, urea, and threitol to human MSC incubated in pro-calcifying medium increased ALP activity and calcium content [41].
3.2.2. Middle Molecular Weight Substances, Increased in Blood in CKD (Table 2)
Addition of bone morphogenetic protein 2 (BMP2) increased the calcium concentration in bovine VSMC cultured in calcifying medium [42]. Additionally, adding fibroblast growth factor 2 (FGF2), interleukin-1β (IL-1β), PTH or tumor necrosis factor alpha (TNF-α) to human MSC in pro-calcifying medium increased ALP activity and the calcium content, and upregulated osteoblast markers such as CBFA1/RUNX2, osteopontin, and osterix [41]. The pro-inflammatory cytokine TNF-α also increased calcium content [33,49] and ALP activity [48] in human VSMC in calcifying medium, as well as increased the calcium content of rat aortic rings in calcifying conditions [33]. Similarly, addition of vascular cell adhesion molecule-1 (VCAM-1) or the pro-inflammatory cytokine interleukin-8 (IL-8) to human VSMC in pro-calcifying medium increased calcification as shown by alizarin red staining and increased ALP activity (for VCAM-1) or calcium content (for IL-8) [30,48]. The RAGE ligand S100A12 induced calcification in mouse VSMC as revealed by alizarin red staining, which was linked to an increased generation of reactive oxygen species (ROS) as well as an increased mRNA expression of the phosphate co-transporter PIT-1 and the osteoblast markers BMP2 and CBFA1/RUNX2 [36]. Overexpression of the peptide salusin-β, shown to be increased in patients undergoing hemodialysis [47], increased calcification of human VSMC and rat aortic rings, not only in calcification media, but also in media without calcification stimulation, as demonstrated by calcium content, alizarin red, or von Kossa staining as well as ALP activity. In parallel, salusin-β increased expression of the osteogenic markers BMP2 and RUNX2, while decreasing expression of contractile SMC markers alpha-smooth muscle actin (α-SMA) and smooth muscle protein 22-alpha (SM22-α) in human VSMC [46]. In contrast, soluble cytokine receptors sTNFR-1 and -2 [48] and sIL-2R [48] did not increase ALP activity nor calcification extent in alizarin red staining when added to human VSMC in pro-calcifying medium.
3.2.3. Protein-Bound Substances, Increased in Blood in CKD (Table 3)
In human VSMC, indoxyl sulphate increased calcium content when added to pro-calcifying incubation medium [30] and induced calcification as shown by alizarin red staining [30,51,52,53,54]. In parallel, it reduced the expression of α-SMA [30,51,54] and SM22-α [30], but upregulated CBFA1/RUNX2 [30,51,52,53,54], osteopontin [30,51,52,53], ALP, and BMP2 [30,51]. Rat aortic rings incubated in high phosphate media in the presence of indoxyl sulphate had increased calcium content and showed enhanced calcification in alizarin red staining [30]. Furthermore, administrating indoxyl sulphate to rats with hypertension [57,58], 5/6 nephrectomy [51] and adenine-induced CKD [59] increased aortic calcium content [59] and calcification as shown by von Kossa [57,58,59] or alizarin red staining [51]. An increased calcium content was also determined in aortas of adenine-induced CKD rats receiving p-cresyl sulphate and confirmed by von Kossa staining [59].
3.3. Mechanisms and Signalling Pathways Associating Uremic Toxins with Calcification
The calcium-phosphate metabolism is one of the key factors for initiation and progression of vascular calcification in CKD [60] and in vitro as well as ex vivo studies showed increased calcification upon increased levels of calcium [38,40] or inorganic phosphate [29,32,33,35]. In late stage CKD (CKD 4–5), most of CKD patients present with hyperphosphatemia [61,62]. On the other hand, in CKD patients, calcium metabolism is complex and the total body calcium levels vary over the different stages of CKD. In this regard, hypocalcemia is quite uncommon in CKD stage 3 and early stage 4, but more often observed in stage 5 as long as patients are not yet on dialysis [63,64].
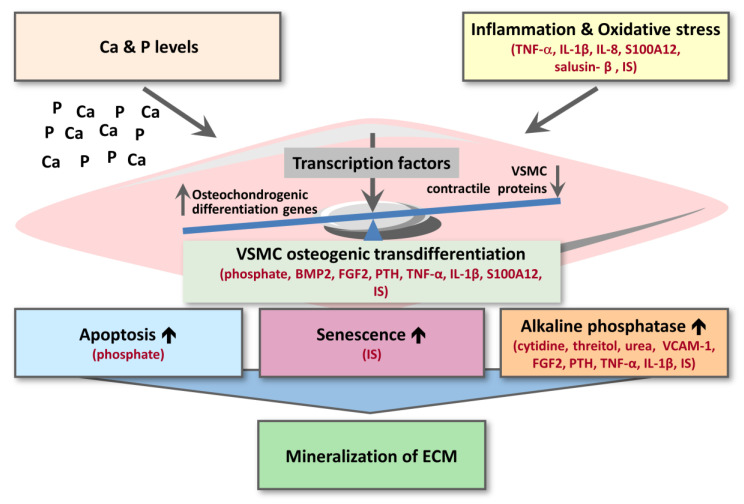
For the other substances from Table 1, Table 2 and Table 3, potential mechanisms underlying their effect on vascular calcification can be subdivided in: (i) inflammation and oxidative stress; (ii) VSMC osteogenic transdifferentiation; (iii) VSMC apoptosis or senescence; (iv) alkaline phosphatase activity (Figure 3). Osteogenic transdifferentiation is driven by the upregulation of transcription factors (e.g., CBFA1/RUNX2, MSX2, SOX9) controlling the expression of osteochondrogenic marker proteins (BMP2, osterix, osteocalcin, ALP, osteopontin) as well as the downregulation of smooth muscle contractile proteins (e.g., SM22-α, α-SMA, SMTN) (Figure 3) [65].
Figure 3.
Scheme of uremic toxins affecting different regulatory steps of cardiovascular calcification. For details, we refer to the text. In red are shown the inducers of cardiovascular calcification. BMP2—bone morphogenetic protein 2; Ca—calcium; ECM—extracellular matrix; FGF2—fibroblast growth factor 2; IL-1β—interleukin-1 beta; IL-8—interleukin-8; IS—indoxyl sulphate; P—phosphate; PTH—parathyroid hormone; TNF-α—tumor necrosis factor-alpha; VCAM-1—vascular cell adhesion molecule-1; VSMC—vascular smooth muscle cell.
3.3.1. Inflammation and Oxidative Stress
On the one hand, cardiovascular calcification seems to accelerate inflammation and oxidative stress [66]. On the other hand, systemic inflammation and oxidative stress have been identified as drivers of cardiovascular calcification in CKD patients. Thus, cardiovascular calcification and inflammation potentially form a vicious circle in CKD. For the substances in Table 1, Table 2 and Table 3, following links with inflammation were identified:
Phosphate (Table 1): Treatment of mouse VSMC with high phosphate medium induced ROS production and ROS-mediated expression of S100 proteins as pro-inflammatory RAGE ligands along with osteogenic marker expression and calcification. The phosphate co-transporter PIT-1, shown to be crucial for phosphate-induced calcification, was upregulated by high phosphate medium through involvement of the RAGE receptor, which could be blocked by anti-oxidant treatment [36].
ADMA, SDMA, and GSA (Table 1): Guanidino compounds constitute a large group of low molecular weight uremic toxins that are generated as a result of protein and amino acid metabolism. They can exert pro-inflammatory as well as anti-inflammatory effects, which may modulate the prevalence of CVD in patients with CKD [67]. Among investigated guanidino compounds, ADMA, SDMA, and GSA inhibited cardiovascular calcification in human VSMC in high inorganic phosphate media [29]. The underlying mechanisms still remain to be clarified, as ADMA, SDMA, and GSA exerted mainly pro-inflammatory effects, at least on leukocytes [29].
TNF-α, interleukin-1β, interleukin-8 and S100A12 (Table 2): Incubation of rat aortic rings as well as human VSMC with phosphate and additionally the pro-inflammatory cytokine TNF-α induced a higher increase in calcium content compared to incubation with high phosphate alone, linking inflammation and vascular calcification [33]. As underlying mechanism, TNF-α-induced VSMC changes towards an osteogenic phenotype were observed [33]. In line with this, incubation of pluripotent undifferentiated MSC with TNF-α as well as with the pro-inflammatory cytokine interleukin-1β (IL-1β) enhanced osteoblastic transdifferentiation and calcification [41], linking pro-inflammatory mediators to vascular calcification over osteogenic transdifferentiation. Additionally, the pro-inflammatory RAGE ligand S100A12 induced expression of osteoblast markers BMP2 and CBFA1/RUNX2 in mouse VSMC along with increased calcification, with ROS-induced PIT-1 expression revealed to be important in S100A12-induced calcification [36]. For the pro-inflammatory cytokine interleukin-8 (IL-8), increased calcification extent in human VSMC in pro-calcifying conditions was mechanistically linked to a downregulation of the calcification inhibitor osteopontin [30].
Salusin-β (Table 2): Overexpressing salusin-β, a small bioactive peptide that is increased in patients undergoing hemodialysis [47], enhanced calcification of human VSMC along with an increased expression of the NAD(P)H oxidase subunits p22 and p47 and enzyme NOX2, which are involved in ROS production. Inhibiting NAD(P)H oxidase or scavenging ROS reduced ALP activity and calcium deposition induced by salusin-β overexpression in VSMC. Additionally, in rat aortas, overexpression of salusin-β increased the expression of the NAD(P)H oxidase subunits and NOX2 as well as intensified the production of malondialdehyde and hydrogen peroxide as oxidative stress markers. Combined, the results suggested that the increase in vascular calcification by salusin-β is due to the activation of the NAD(P)H oxidase-ROS pathway [46].
Indoxyl sulphate and p-cresyl sulphate (Table 3). Treatment of hypertensive rats with the uremic toxin indoxyl sulphate not only induced vascular calcification [57,58], but also increased expression of oxidative stress markers 8-hydroxyl-2′-deoxyguanosine (8-OHdG) and malondialdehyde in aorta [55]. Calcified aortic regions of indoxyl sulphate-treated rats also displayed enhanced expression of p53 (a trigger of apoptosis as well as senescence), the cell cycle inhibitor p21, as well as prelamin A as hallmarks of VSMC senescence [55,58]. In human VSMCs in vitro, these effects were counteracted by anti-oxidant treatment [55]. Based on these findings, a role for indoxyl sulphate-induced oxidative stress in vascular calcification through effects on cell cycle arrest and VSMC senescence was suggested [55,57,58]. Furthermore, in vivo studies of rats with adenine-induced CKD and treated with indoxyl sulphate or p-cresyl sulphate showed, in parallel with increased vascular calcification, activation of inflammation (acute-phase response) and coagulation signaling pathways in the calcified aorta [59].
3.3.2. VSMC Osteogenic Transdifferentiation
A link of increased calcification along with VSMC osteogenic transdifferentiation has been shown in vitro for phosphate (Table 1), BMP2, FGF2, PTH, TNF-α, IL-1β, and S100A12 (Table 2) as well as indoxyl sulphate (Table 3):
Phosphate (Table 1): Along with vascular calcification, a high concentration of phosphate induced a significant increase in CBFA1/RUNX2, BMP2 and osteopontin expression in mouse VSMC [36,37], with an important role for the RAGE receptor and oxidative stress [36]. This latter finding demonstrates a link between inflammation and VSMC osteogenic transdifferentiation induced by high phosphate, with (RAGE receptor-mediated) oxidative stress contributing to increased expression of the phosphate co-transporter PIT-1 as well as RUNX2 upon high-phosphate treatment of VSMC, and with pharmacological inhibition of the phosphate co-transporters PIT-1 and PIT-2 interfering with high phosphate-induced calcification of VSMC [36]. Similarly, high phosphate levels significantly increased MSX2, SOX9, CBFA1/RUNX2, BMP2, and osteopontin in human aortic SMC along with vascular calcification and increased expression of PIT-1 [32,33].
BMP2, FGF2, PTH, TNF-α, IL-1β, and S100A12 (Table 2): In calcifying bovine and rat VSMC, an upregulation of the transcription factors CBFA1/RUNX2 and MSX2 and the phosphate cotransporter PIT-1 was described for BMP2 [42,43]. The pro-inflammatory RAGE ligand S100A12 induced CBFA1/RUNX2 and BMP2 expression in mouse VSMC [36], and an upregulation of the osteoblast markers CBFA1/RUNX2, osterix, and osteopontin was also shown in MSCs stimulated with the uremic toxins FGF2, PTH, TNF-α and IL-1β [41].
Indoxyl sulphate (Table 3): Parallel to inducing vascular calcification, indoxyl sulphate administration to human VSMC [30,51,52,53,54] or rats with hypertension [57] or 5/6 nephrectomy [51] increased the expression of CBFA1/RUNX2, ALP, BMP2 and osteopontin, while a reduction in α-SMA, SM22-α and/or SMTN expression could be observed. Recent in vitro data showed that indoxyl sulphate-induced osteogenic transdifferentiation and calcification of VSMC is mediated by the NF-κB and PI3K/AKT pathways [54].
3.3.3. VSMC Apoptosis and Senescence
Increased apoptosis as well as cellular senescence of VSMC have been suggested as underlying mechanism of cardiovascular calcification. Cellular senescence goes along with increased vascular calcification and increased expression of osteogenic markers (RUNX2, BMP2) [65]. During the apoptosis process, apoptotic bodies are formed that are thought to contribute to vascular calcification through high calcium content and deposition in the extracellular matrix [65]. For the substances in Table 1, Table 2 and Table 3, the following links have been identified:
Phosphate (Table 1): In vitro, high phosphate treatment of human aortic VSMCs induced an increase in cellular apoptosis starting as soon as day 1 of culturing, as revealed by flow cytometry-mediated analysis of annexin V and propidium iodide staining [32].
Indoxyl sulphate (Table 3): As described above, Muteliefu et al. showed that, through increased ROS generation, indoxyl sulphate promotes a VSMC phenotype with increased expression of cell cycle arrest regulators and senescence markers (p53, p21, prelamin A) [55].
3.3.4. Alkaline Phosphatase
Inorganic pyrophosphate (PPi) is a physicochemical inhibitor of hydroxyapatite crystal growth and a potential local as well as circulating inhibitor of cardiovascular calcification [68]. The enzyme ALP is able to hydrolyze PPi into phosphate ions [69]. An increase in ALP activity along with increased calcification in VSMC or MSC was shown for cytidine, threitol and urea [41] (Table 1); VCAM-1 [48], FGF2, PTH, TNF-α, and IL-1β [41] (Table 2), as well as for indoxyl sulphate [54] (Table 3).
4. Discussion
In CKD patients, uremic toxins accumulate due to failing kidney function, but current knowledge of the effect of uremic toxins on cardiovascular calcification is quite limited. Here, we systematically analyzed original research papers on uremic toxins in relation to cardiovascular calcification to examine whether and how uremic toxins may contribute to increased cardiovascular calcification in CKD.
In total, this review identified 29 uremic toxins (15 low molecular weight; 12 middle molecular weight; 2 protein-bound), upregulated in plasma of CKD patients, that have been studied for effects on cardiovascular calcification in in vitro, ex vivo or in vivo animal studies (Table 1, Table 2 and Table 3). For the 16 uremic toxins shown to increase vascular calcification, most could be directly linked to trigger increased VSMC osteogenic transdifferentiation or increased inflammation and oxidative stress (Figure 3). Additionally, the pro-inflammatory cytokines TNF-α and IL-1β as well as the pro-inflammatory S100A12 were shown to drive cellular transdifferentiation into an osteoblastic phenotype along with increasing calcification (Figure 3), directly linking inflammation to calcification over VSMC phenotype switching. Along the same line, indoxyl-sulphate as a protein-bound uremic toxin was shown to promote vascular calcification via inflammation (Figure 3). In addition to effects on VSMC transdifferentiation and inflammation, some substances were also identified to enhance ALP activity along with increasing vascular calcification (Figure 3).
Of the uremic toxins identified to impact on cardiovascular calcification (Table 1, Table 2 and Table 3), more were able to increase calcification (16x) than to decrease it (3x, being the guanidino compounds ADMA, GSA and SDMA) (Figure 2). Overall, this suggests that the uremic milieu in CKD patients shifts the balance to increased cardiovascular calcification. Among all uremic toxins studied, the harmful effect of protein-bound uremic toxin indoxyl sulphate in relation to cardiovascular calcification has been investigated most extensively (Table 3). Of note, for each middle molecular weight uremic toxin and protein-bound uremic toxin that was found to affect cardiovascular calcification, an increasing effect on calcification was revealed. This suggests that an efficient removal of both classes of uremic toxins may contribute to reduce cardiovascular calcification in CKD. However, while removing low molecular weight uremic toxins in CKD stage 5D patients using hemodialysis techniques is rather efficient, the removal of middle molecular weight toxins and especially protein-bound uremic toxins is still limited with current dialysis techniques [70]. For example, removal efficiency for the protein-bound toxins indoxyl sulphate and p-cresyl sulphate during a hemodialysis session was reported to reach only ~35% and 20–29%, respectively, independent of whether low-flux or high-flux membranes were used [71,72]. This is related to the formation of high molecular-weight complexes of these uremic toxins with plasma proteins, which prevents uremic toxin removal through the pores of the dialysis membranes [72]. Furthermore, the hydrophobic nature of these uremic toxins reduces their removal into hydrophilic dialysate. Applying alternative elimination principles in combination with classical filtration and diffusion technologies might be helpful to achieve a more effective removal of protein-bound uremic toxins in the future. This could include the adsorption of protein-bound uremic toxins [73,74,75,76,77], also in combination with fractionated plasma separation [77]. Furthermore, a high-frequency field could enhance the release of hydrophobic uremic toxins from plasma protein complexes and thereby increase their removal from blood during dialysis (patent numbers: WO2013004604A1, EP2729198A1, US20140246367A1). Along the same line, drugs such as ibuprofen have been tested for reducing the binding of protein-bound uremic toxins to plasma proteins, aiming to increase the unbound fraction of these toxins and thereby their removal efficiency during dialysis [78]. Alternative strategies aim at reducing the production of protein-bound uremic toxins in the gut through the use of probiotics, prebiotics, and synbiotics [79] or at reducing their absorption, e.g., by oral administration of the adsorbent AST-120 [80].
Despite the fact that, to date, over 140 uremic toxins have been described to be increased in CKD [20,21], only 29 uremic toxins were studied in relation to calcification in a mechanistic approach. In total, 12 other substances were only investigated for a potential association with the extent of cardiovascular calcification in CKD patients (Table S1). This, for example, also holds true for sclerostin, a regulator of bone metabolism with increased levels in serum and vasculature in CKD [81]. Whereas some studies could not detect a correlation between serum levels of sclerostin and the extent of cardiovascular calcification in patients in CKD stage 5D [82,83], others found a positive or negative correlation with calcification extent in CKD [81]. Sclerostin is a well-known inhibitor of bone formation by blocking the Wnt/beta-catenin pathway and suppressing osteoblast differentiation, but it currently remains unclear whether this sclerostin-Wnt axis also actively reduces vascular calcification [84]. This is not only important in the context of CKD, but also for the aging population, who similarly display increased medial calcification as well as increased sclerostin levels [81]. In addition, insights into a potential inhibitory role of sclerostin in cardiovascular calcification are essential in light of the current development of anti-sclerostin treatment for osteoporosis patients, which conceivably could contribute to increased vascular calcification and cardiovascular risk [81,84]. Thus, this systematic review suggests that investigating the impact of all uremic toxins on cardiovascular calcification systematically and in a standardized way could reveal those substances that would be most interesting for further research and, thereafter, potentially for drug development aiming to prevent or slow down the development and progression of cardiovascular calcification in CKD. Additionally, studying combinatorial and/or synergistic effects of uremic toxins are of importance as well, since previous studies revealed that combining blood levels of low molecular weight uremic toxins may help to improve the estimated survival of CKD stage 5D patients [85]. Preferentially, this should be done by a systematic approach, using standardized models for analyzing cardiovascular calcification in vitro and ex vivo [86].
Finally, in addition to direct effects of uremic toxins on processes impacting on cardiovascular calcification, indirect effects by uremic toxin-mediated post-translational modifications (PTMs) may also affect cardiovascular calcification. PTMs can critically affect protein functions and have been increasingly identified in CKD as well as CVD [87]. As one example, the uremic toxin cyanate as dissociation product of urea triggers carbamylation in CKD [88], with, for example, carbamylation of low-density lipoprotein shown to negatively influence endothelial function in CKD patients [89]. Thus, also uremic toxin-induced PTMs of calcification regulators may act as a link between CKD and increased risk of cardiovascular calcification. This has recently indeed been shown for protein carbamylation, which was revealed to promote calcification of VSMC by impairing mitochondrial function and inducing oxidative stress, thereby suppressing expression of ENNP1 as enzyme involved in PPi production [90]. This supports further research on PTMs in relation to vascular calcification.
Combined, an increased interdisciplinary focus on the mediators driving cardiovascular calcification in CKD as well as methodologies to interfere with calcification inducers is expected to contribute to the battle against increased cardiovascular calcification and mortality in CKD. Nonetheless, many challenges remain to be overcome: in addition to the identification of the main targets (uremic toxins and PTMs), we should focus on therapeutic translation. Here, different uremic toxin removal techniques, as discussed above, may have to be combined to enable a clinically relevant reduction in most protein-bound uremic toxins in CKD patients over extended time periods. Furthermore, although serum levels of, for example, p-cresyl sulphate and indoxyl sulphate have already been shown to positively correlate with vascular calcification and stiffness in CKD patients, [24,25,26] as well as with cardiovascular outcome in CKD [22,23,24,25], clinical studies will need to demonstrate whether reducing levels of such protein-bound uremic toxins indeed affects cardiovascular calcification and mortality in CKD patients.
In summary, this systematic review suggests that the uremic milieu in CKD patients shifts the balance to increased cardiovascular calcification. Of all middle molecular weight and protein-bound uremic toxins that were found to affect cardiovascular calcification, an increasing effect on calcification was revealed, demonstrating the need to focus on increased removal efficiency of these uremic toxin classes in current dialysis methods. Furthermore, this review clearly highlights that only a relatively small part of uremic toxins has been screened for effects on vascular calcification, supporting further efforts into a closer investigation of uremic toxins.
Acknowledgments
The authors are grateful to Claudia Göttsch and D. Fliser for valuable comments on the paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/11/2428/s1, Table S1: Substances studied for correlation with cardiovascular calcification extent in CKD patients.
Author Contributions
J.H., S.O.-A. and J.J. designed the study; S.O-A., J.H. and S.d.l.P.-S. carried out the search; S.O-A., J.H., S.d.l.P-S. and J.J. evaluated papers for inclusion/exclusion; S.O.-A., J.H., S.d.l.P.-S., H.N. and J.J. made the figures and tables and wrote the paper. J.F. contributed in drafting and critically revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Estonian Research Council grant PUTJD66 and by the Estonian Ministry of Education and Research under institutional research financing IUT 19-2 (to J.H.). Furthermore, the study was supported by a grant from the German Research Foundation (DFG Project-ID 322900939 - SFB/TRR219 projects C-01 to J.F., C-04 and S-03 to J.J. and M-05 to H.N.; DFG Project-ID 403224013 - SFB 1382 project A-04 to J.J. and H.N.) and received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 764474 (CaReSyAn).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S., Hobbs F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson S., James M., Wiebe N., Hemmelgarn B., Manns B., Klarenbach S., Tonelli M., Alberta Kidney Disease N. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson P.W., Kauppila L.I., O’Donnell C.J., Kiel D.P., Hannan M., Polak J.M., Cupples L.A. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.CIR.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 4.Wayhs R., Zelinger A., Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J. Am. Coll. Cardiol. 2002;39:225–230. doi: 10.1016/S0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Lind B.K., Kitzman D.W., Gersh B.J., Siscovick D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N. Engl. J. Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 6.Ruderman I., Holt S.G., Hewitson T.D., Smith E.R., Toussaint N.D. Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis. Semin Dial. 2018;31:487–499. doi: 10.1111/sdi.12710. [DOI] [PubMed] [Google Scholar]
- 7.Blacher J., Guerin A.P., Pannier B., Marchais S.J., London G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 8.Kraus M.A., Kalra P.A., Hunter J., Menoyo J., Stankus N. The prevalence of vascular calcification in patients with end-stage renal disease on hemodialysis: A cross-sectional observational study. Adv. Chronic. Dis. 2015;6:84–96. doi: 10.1177/2040622315578654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman W.G., Goldin J., Kuizon B.D., Yoon C., Gales B., Sider D., Wang Y., Chung J., Emerick A., Greaser L., et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 10.Masuda C., Dohi K., Sakurai Y., Bessho Y., Fukuda H., Fujii S., Sugimoto T., Tanabe M., Onishi K., Shiraki K., et al. Impact of chronic kidney disease on the presence and severity of aortic stenosis in patients at high risk for coronary artery disease. Cardiovasc. Ultrasound. 2011;9:31. doi: 10.1186/1476-7120-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizobuchi M., Towler D., Slatopolsky E. Vascular calcification: The killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 12.Raggi P. Cardiovascular disease: Coronary artery calcification predicts risk of CVD in patients with CKD. Nat. Rev. Nephrol. 2017;13:324–326. doi: 10.1038/nrneph.2017.61. [DOI] [PubMed] [Google Scholar]
- 13.Schlieper G., Schurgers L., Brandenburg V., Reutelingsperger C., Floege J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. 2016;31:31–39. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 14.Drueke T.B. The new Kidney Disease: Improving Global Outcomes (KDIGO) guideline for the mineral and bone disorder associated with chronic kidney disease (MBD-CKD) Nephrol. Ther. 2010;6:149–150. doi: 10.1016/j.nephro.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Shioi A., Nishizawa Y. Roles of hyperphosphatemia in vascular calcification. Clin. Calcium. 2009;19:180–185. [PubMed] [Google Scholar]
- 16.Shao J.S., Cheng S.L., Sadhu J., Towler D.A. Inflammation and the osteogenic regulation of vascular calcification: A review and perspective. Hypertension. 2010;55:579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furmanik M., Chatrou M., van Gorp R., Akbulut A., Willems B., Schmidt H., van Eys G., Bochaton-Piallat M.L., Proudfoot D., Biessen E., et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020;127:911–927. doi: 10.1161/CIRCRESAHA.119.316159. [DOI] [PubMed] [Google Scholar]
- 18.Hosaka N., Mizobuchi M., Ogata H., Kumata C., Kondo F., Koiwa F., Kinugasa E., Akizawa T. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif. Tissue Int. 2009;85:523–529. doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 19.Vanholder R., De Smet R., Glorieux G., Argiles A., Baurmeister U., Brunet P., Clark W., Cohen G., De Deyn P.P., Deppisch R., et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 20.Duranton F., Cohen G., De Smet R., Rodriguez M., Jankowski J., Vanholder R., Argiles A., European Uremic Toxin Work G. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanholder R., Pletinck A., Schepers E., Glorieux G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins. 2018;10:33. doi: 10.3390/toxins10010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung S.C., Kuo K.L., Wu C.C., Tarng D.C. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J. Am. Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bammens B., Evenepoel P., Keuleers H., Verbeke K., Vanrenterghem Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 24.Barreto F.C., Barreto D.V., Liabeuf S., Meert N., Glorieux G., Temmar M., Choukroun G., Vanholder R., Massy Z.A., European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liabeuf S., Barreto D.V., Barreto F.C., Meert N., Glorieux G., Schepers E., Temmar M., Choukroun G., Vanholder R., Massy Z.A., et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transpl. 2010;25:1183–1191. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 26.Lai Y.H., Wang C.H., Kuo C.H., Lin Y.L., Tsai J.P., Hsu B.G. Serum P-Cresyl Sulfate Is a Predictor of Central Arterial Stiffness in Patients on Maintenance Hemodialysis. Toxins. 2019;12:10. doi: 10.3390/toxins12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ketteler M., Rothe H., Kruger T., Biggar P.H., Schlieper G. Mechanisms and treatment of extraosseous calcification in chronic kidney disease. Nat. Rev. Nephrol. 2011;7:509–516. doi: 10.1038/nrneph.2011.91. [DOI] [PubMed] [Google Scholar]
- 28. [(accessed on 26 May 2020)]; Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016051604.
- 29.Schepers E., Glorieux G., Dou L., Cerini C., Gayrard N., Louvet L., Maugard C., Preus P., Rodriguez-Ortiz M., Argiles A., et al. Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: An in vitro evaluation. Blood Purif. 2010;30:277–287. doi: 10.1159/000320765. [DOI] [PubMed] [Google Scholar]
- 30.Bouabdallah J., Zibara K., Issa H., Lenglet G., Kchour G., Caus T., Six I., Choukroun G., Kamel S., Bennis Y. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol. Dial. Transpl. 2019;34:1125–1134. doi: 10.1093/ndt/gfy325. [DOI] [PubMed] [Google Scholar]
- 31.Tertti R., Harmoinen A., Leskinen Y., Metsarinne K.P., Saha H. Comparison of calcium phosphate product values using measurement of plasma total calcium and serum ionized calcium. Hemodial. Int. 2007;11:411–416. doi: 10.1111/j.1542-4758.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 32.Cazana-Perez V., Cidad P., Donate-Correa J., Martin-Nunez E., Lopez-Lopez J.R., Perez-Garcia M.T., Giraldez T., Navarro-Gonzalez J.F., Alvarez de la Rosa D. Phenotypic Modulation of Cultured Primary Human Aortic Vascular Smooth Muscle Cells by Uremic Serum. Front. Physiol. 2018;9:89. doi: 10.3389/fphys.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrero F., Montes de Oca A., Aguilera-Tejero E., Zafra R., Rodriguez M., Lopez I. The effect of vitamin D derivatives on vascular calcification associated with inflammation. Nephrol. Dial. Transpl. 2012;27:2206–2212. doi: 10.1093/ndt/gfr555. [DOI] [PubMed] [Google Scholar]
- 34.Shigematsu T., Kono T., Satoh K., Yokoyama K., Yoshida T., Hosoya T., Shirai K. Phosphate overload accelerates vascular calcium deposition in end-stage renal disease patients. Nephrol. Dial. Transpl. 2003;18(Suppl. 3):iii86–iii89. doi: 10.1093/ndt/gfg1022. [DOI] [PubMed] [Google Scholar]
- 35.Shibata M., Shigematsu T., Hatamura I., Saji F., Mune S., Kunimoto K., Hanba Y., Shiizaki K., Sakaguchi T., Negi S. Reduced expression of perlecan in the aorta of secondary hyperparathyroidism model rats with medial calcification. Ren. Fail. 2010;32:214–223. doi: 10.3109/08860220903367544. [DOI] [PubMed] [Google Scholar]
- 36.Belmokhtar K., Ortillon J., Jaisson S., Massy Z.A., Boulagnon Rombi C., Doue M., Maurice P., Fritz G., Gillery P., Schmidt A.M., et al. Receptor for advanced glycation end products: A key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co-transporter PIT-1 expression. Nephrol. Dial. Transpl. 2019;34:2018–2030. doi: 10.1093/ndt/gfz012. [DOI] [PubMed] [Google Scholar]
- 37.Sage A.P., Lu J., Tintut Y., Demer L.L. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azpiazu D., Gonzalez-Parra E., Ortiz A., Egido J., Villa-Bellosta R. Impact of post-dialysis calcium level on ex vivo rat aortic wall calcification. PLoS ONE. 2017;12:e0183730. doi: 10.1371/journal.pone.0183730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moe S.M. Calcium as a cardiovascular toxin in CKD-MBD. Bone. 2017;100:94–99. doi: 10.1016/j.bone.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang H., Curinga G., Giachelli C.M. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 41.Hegner B., Schaub T., Janke D., Zickler D., Lange C., Girndt M., Jankowski J., Schindler R., Dragun D. Targeting proinflammatory cytokines ameliorates calcifying phenotype conversion of vascular progenitors under uremic conditions in vitro. Sci. Rep. 2018;8:12087. doi: 10.1038/s41598-018-30626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen N.X., Duan D., O’Neill K.D., Wolisi G.O., Koczman J.J., Laclair R., Moe S.M. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 43.Rong S., Zhao X., Jin X., Zhang Z., Chen L., Zhu Y., Yuan W. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/beta-catenin pathway. Cell Physiol. Biochem. 2014;34:2049–2060. doi: 10.1159/000366400. [DOI] [PubMed] [Google Scholar]
- 44.Vianna H.R., Soares C.M., Silveira K.D., Elmiro G.S., Mendes P.M., de Sousa Tavares M., Teixeira M.M., Miranda D.M., Simoes E.S.A.C. Cytokines in chronic kidney disease: Potential link of MCP-1 and dyslipidemia in glomerular diseases. Pediatr. Nephrol. 2013;28:463–469. doi: 10.1007/s00467-012-2363-x. [DOI] [PubMed] [Google Scholar]
- 45.Isoyama N., Leurs P., Qureshi A.R., Bruchfeld A., Anderstam B., Heimburger O., Barany P., Stenvinkel P., Lindholm B. Plasma S100A12 and soluble receptor of advanced glycation end product levels and mortality in chronic kidney disease Stage 5 patients. Nephrol. Dial. Transpl. 2015;30:84–91. doi: 10.1093/ndt/gfu259. [DOI] [PubMed] [Google Scholar]
- 46.Sun H., Zhang F., Xu Y., Sun S., Wang H., Du Q., Gu C., Black S.M., Han Y., Tang H. Salusin-beta Promotes Vascular Calcification via Nicotinamide Adenine Dinucleotide Phosphate/Reactive Oxygen Species-Mediated Klotho Downregulation. Antioxid. Redox Signal. 2019;31:1352–1370. doi: 10.1089/ars.2019.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sipahi S., Genc A.B., Acikgoz S.B., Yildirim M., Aksoy Y.E., Vatan M.B., Dheir H., Altindis M. Relationship of salusin-alpha and salusin-beta levels with atherosclerosis in patients undergoing haemodialysis. Singap. Med. J. 2019;60:210–215. doi: 10.11622/smedj.2018123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zickler D., Willy K., Girndt M., Fiedler R., Martus P., Storr M., Schindler R. High cut-off dialysis in chronic haemodialysis patients reduces serum procalcific activity. Nephrol. Dial. Transpl. 2016;31:1706–1712. doi: 10.1093/ndt/gfw293. [DOI] [PubMed] [Google Scholar]
- 49.Zickler D., Luecht C., Willy K., Chen L., Witowski J., Girndt M., Fiedler R., Storr M., Kamhieh-Milz J., Schoon J., et al. Tumour necrosis factor-alpha in uraemic serum promotes osteoblastic transition and calcification of vascular smooth muscle cells via extracellular signal-regulated kinases and activator protein 1/c-FOS-mediated induction of interleukin 6 expression. Nephrol. Dial. Transpl. 2018;33:574–585. doi: 10.1093/ndt/gfx316. [DOI] [PubMed] [Google Scholar]
- 50.Vaccaro F., Mule G., Cottone S., Soresi M., Giannitrapani L., Vadala A., Sparacino V., Calabrese S., Picone F.P., Montalto G., et al. Circulating levels of adhesion molecules in chronic kidney disease correlate with the stage of renal disease and with C-reactive protein. Arch. Med. Res. 2007;38:534–538. doi: 10.1016/j.arcmed.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Chen J., Zhang X., Zhang H., Liu T., Zhang H., Teng J., Ji J., Ding X. Indoxyl Sulfate Enhance the Hypermethylation of Klotho and Promote the Process of Vascular Calcification in Chronic Kidney Disease. Int. J. Biol. Sci. 2016;12:1236–1246. doi: 10.7150/ijbs.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H., Chen J., Shen Z., Gu Y., Xu L., Hu J., Zhang X., Ding X. Indoxyl sulfate accelerates vascular smooth muscle cell calcification via microRNA-29b dependent regulation of Wnt/beta-catenin signaling. Toxicol. Lett. 2018;284:29–36. doi: 10.1016/j.toxlet.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 53.Chen J., Gu Y., Zhang H., Ning Y., Song N., Hu J., Cai J., Shi Y., Ding X., Zhang X. Amelioration of Uremic Toxin Indoxyl Sulfate-Induced Osteoblastic Calcification by SET Domain Containing Lysine Methyltransferase 7/9 Protein. Nephron. 2019;141:287–294. doi: 10.1159/000495885. [DOI] [PubMed] [Google Scholar]
- 54.He X., Jiang H., Gao F., Liang S., Wei M., Chen L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-kappaB signaling pathway. Microsc Res. Tech. 2019;82:2000–2006. doi: 10.1002/jemt.23369. [DOI] [PubMed] [Google Scholar]
- 55.Muteliefu G., Shimizu H., Enomoto A., Nishijima F., Takahashi M., Niwa T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am. J. Physiol. Cell Physiol. 2012;303:C126–C134. doi: 10.1152/ajpcell.00329.2011. [DOI] [PubMed] [Google Scholar]
- 56.Ochi A., Mori K., Nakatani S., Emoto M., Morioka T., Motoyama K., Fukumoto S., Imanishi Y., Shoji T., Ishimura E., et al. Indoxyl sulfate suppresses hepatic fetuin-A expression via the aryl hydrocarbon receptor in HepG2 cells. Nephrol. Dial. Transpl. 2015;30:1683–1692. doi: 10.1093/ndt/gfv250. [DOI] [PubMed] [Google Scholar]
- 57.Adijiang A., Goto S., Uramoto S., Nishijima F., Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transpl. 2008;23:1892–1901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- 58.Adijiang A., Higuchi Y., Nishijima F., Shimizu H., Niwa T. Indoxyl sulfate, a uremic toxin, promotes cell senescence in aorta of hypertensive rats. Biochem. Biophys Res. Commun. 2010;399:637–641. doi: 10.1016/j.bbrc.2010.07.130. [DOI] [PubMed] [Google Scholar]
- 59.Opdebeeck B., Maudsley S., Azmi A., De Mare A., De Leger W., Meijers B., Verhulst A., Evenepoel P., D’Haese P.C., Neven E. Indoxyl Sulfate and p-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019;30:751–766. doi: 10.1681/ASN.2018060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shroff R., Long D.A., Shanahan C. Mechanistic insights into vascular calcification in CKD. J. Am. Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 61.Tentori F., Blayney M.J., Albert J.M., Gillespie B.W., Kerr P.G., Bommer J., Young E.W., Akizawa T., Akiba T., Pisoni R.L., et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am. J. Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Block G.A., Hulbert-Shearon T.E., Levin N.W., Port F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 63.Janmaat C.J., van Diepen M., Gasparini A., Evans M., Qureshi A.R., Arnlov J., Barany P., Elinder C.G., Rotmans J.I., Vervloet M., et al. Lower serum calcium is independently associated with CKD progression. Sci. Rep. 2018;8:5148. doi: 10.1038/s41598-018-23500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Neill W.C. Targeting serum calcium in chronic kidney disease and end-stage renal disease: Is normal too high? Kidney Int. 2016;89:40–45. doi: 10.1016/j.kint.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Durham A.L., Speer M.Y., Scatena M., Giachelli C.M., Shanahan C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018;114:590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joshi F.R., Rajani N.K., Abt M., Woodward M., Bucerius J., Mani V., Tawakol A., Kallend D., Fayad Z.A., Rudd J.H. Does Vascular Calcification Accelerate Inflammation?: A Substudy of the dal-PLAQUE Trial. J. Am. Coll Cardiol. 2016;67:69–78. doi: 10.1016/j.jacc.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 67.Glorieux G.L., Dhondt A.W., Jacobs P., Van Langeraert J., Lameire N.H., De Deyn P.P., Vanholder R.C. In vitro study of the potential role of guanidines in leukocyte functions related to atherogenesis and infection. Kidney Int. 2004;65:2184–2192. doi: 10.1111/j.1523-1755.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 68.Schibler D., Russell R.G., Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin. Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 69.Lomashvili K.A., Garg P., Narisawa S., Millan J.L., O’Neill W.C. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: Potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eloot S., Ledebo I., Ward R.A. Extracorporeal removal of uremic toxins: Can we still do better? Semin Nephrol. 2014;34:209–227. doi: 10.1016/j.semnephrol.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Lesaffer G., De Smet R., Lameire N., Dhondt A., Duym P., Vanholder R. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transpl. 2000;15:50–57. doi: 10.1093/ndt/15.1.50. [DOI] [PubMed] [Google Scholar]
- 72.Martinez A.W., Recht N.S., Hostetter T.H., Meyer T.W. Removal of P-cresol sulfate by hemodialysis. J. Am. Soc. Nephrol. 2005;16:3430–3436. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 73.Sternkopf M., Thoröe-Boveleth S., Beck T., Oleschko K., Erlenkötter A., Tschulena U., Steppan S., Speer T., Goettsch C., Jankowski V., et al. A Bifunctional Adsorber Particle for the Removal of Hydrophobic Uremic Toxins from Whole Blood of Renal Failure Patients. Toxins. 2019;11:389. doi: 10.3390/toxins11070389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato S., Otake K.-I., Chen H., Akpinar I., Buru C.T., Islamoglu T., Snurr R.Q., Farha O.K. Zirconium-Based Metal–Organic Frameworks for the Removal of Protein-Bound Uremic Toxin from Human Serum Albumin. J. Am. Chem. Soc. 2019;141:2568–2576. doi: 10.1021/jacs.8b12525. [DOI] [PubMed] [Google Scholar]
- 75.Pavlenko D., van Geffen E., van Steenbergen M.J., Glorieux G., Vanholder R., Gerritsen K.G., Stamatialis D. New low-flux mixed matrix membranes that offer superior removal of protein-bound toxins from human plasma. Sci. Rep. 2016;6:34429. doi: 10.1038/srep34429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandeman S.R., Zheng Y., Ingavle G.C., Howell C.A., Mikhalovsky S.V., Basnayake K., Boyd O., Davenport A., Beaton N., Davies N. A haemocompatible and scalable nanoporous adsorbent monolith synthesised using a novel lignin binder route to augment the adsorption of poorly removed uraemic toxins in haemodialysis. Biomed. Mater. 2017;12:035001. doi: 10.1088/1748-605X/aa6546. [DOI] [PubMed] [Google Scholar]
- 77.Brettschneider F., Tölle M., von der Giet M., Passlick-Deetjen J., Steppan S., Peter M., Jankowski V., Krause A., Kühne S., Zidek W., et al. Removal of protein-bound, hydrophobic uremic toxins by a combined fractionated plasma separation and adsorption technique. Artif. Organs. 2013;37:409–416. doi: 10.1111/j.1525-1594.2012.01570.x. [DOI] [PubMed] [Google Scholar]
- 78.Madero M., Cano K.B., Campos I., Tao X., Maheshwari V., Brown J., Cornejo B., Handelman G., Thijssen S., Kotanko P. Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin. J. Am. Soc. Nephrol. 2019;14:394–402. doi: 10.2215/CJN.05240418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thongprayoon C., Kaewput W., Hatch S.T., Bathini T., Sharma K., Wijarnpreecha K., Ungprasert P., D’Costa M., Mao M.A., Cheungpasitporn W. Effects of Probiotics on Inflammation and Uremic Toxins Among Patients on Dialysis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2019;64:469–479. doi: 10.1007/s10620-018-5243-9. [DOI] [PubMed] [Google Scholar]
- 80.Schulman G., Vanholder R., Niwa T. AST-120 for the management of progression of chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014;7:49–56. doi: 10.2147/IJNRD.S41339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maré A., D’Haese P.C., Verhulst A. The Role of Sclerostin in Bone and Ectopic Calcification. Int. J. Mol. Sci. 2020;21:3199. doi: 10.3390/ijms21093199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Oliveira R.A., Barreto F.C., Mendes M., dos Reis L.M., Castro J.H., Britto Z.M., Marques I.D., Carvalho A.B., Moyses R.M., Jorgetti V. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 2015;87:1039–1045. doi: 10.1038/ki.2014.372. [DOI] [PubMed] [Google Scholar]
- 83.Desjardins L., Liabeuf S., Oliveira R.B., Louvet L., Kamel S., Lemke H.D., Vanholder R., Choukroun G., Massy Z.A., European Uremic Toxin Work G. Uremic toxicity and sclerostin in chronic kidney disease patients. Nephrol. Ther. 2014;10:463–470. doi: 10.1016/j.nephro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Brandenburg V.M., Verhulst A., Babler A., D’Haese P.C., Evenepoel P., Kaesler N. Sclerostin in chronic kidney disease-mineral bone disorder think first before you block it! Nephrol. Dial. Transpl. 2019;34:408–414. doi: 10.1093/ndt/gfy129. [DOI] [PubMed] [Google Scholar]
- 85.Holmar J.F.I., Uhlin F., Fernstrom A., Luman M. Estimation of dialysis patients’ survival through combined approach of small molecule uremic markers. Proc. Est. Acad. Sci. 2014;63:227–233. doi: 10.3176/proc.2014.3.04. [DOI] [Google Scholar]
- 86.Holmar J., Noels H., Böhm M., Bhargava S., Jankowski J., Orth-Alampour S. Development, establishment and validation of in vitro and ex vivo assays of vascular calcification. Biochem. Biophys Res. Commun. 2020;530:462–470. doi: 10.1016/j.bbrc.2020.05.085. [DOI] [PubMed] [Google Scholar]
- 87.Gajjala P.R., Fliser D., Speer T., Jankowski V., Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol. Dial. Transpl. 2015;30:1814–1824. doi: 10.1093/ndt/gfv048. [DOI] [PubMed] [Google Scholar]
- 88.Kalim S., Karumanchi S.A., Thadhani R.I., Berg H.A. Protein Carbamylation in Kidney Disease: Pathogenesis and Clinical Implications. Am. J. Kidney Dis. 2014;64:793–803. doi: 10.1053/j.ajkd.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Speer T., Owala F.O., Holy E.W., Zewinger S., Frenzel F.L., Stahli B.E., Razavi M., Triem S., Cvija H., Rohrer L., et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart J. 2014;35:3021–3032. doi: 10.1093/eurheartj/ehu111. [DOI] [PubMed] [Google Scholar]
- 90.Mori D., Matsui I., Shimomura A., Hashimoto N., Matsumoto A., Shimada K., Yamaguchi S., Oka T., Kubota K., Yonemoto S., et al. Protein carbamylation exacerbates vascular calcification. Kidney Int. 2018;94:72–90. doi: 10.1016/j.kint.2018.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.