Abstract
Background
Thrombocytopenia in dogs is common in critical care medicine, but availability of fresh platelet concentrates in veterinary medicine can be limiting. Lyophilized platelets have long shelf‐lives and can be easily transported, stored, and administered in various settings.
Objective
To evaluate the efficacy and safety of a novel trehalose‐stabilized canine lyophilized platelet product in thrombocytopenic dogs with clinically‐evident bleeding.
Animals
Eighty‐eight dogs with platelet counts <50 × 103/μL and a standardized bleeding assessment tool (DOGiBAT) score ≥2.
Methods
Multicenter, randomized, non‐blinded, non‐inferiority clinical trial comparing dimethyl sulfoxide (DMSO)‐stabilized cryopreserved platelet concentrates (CPP) with trehalose‐stabilized lyophilized platelets (LP) for control of bleeding in thrombocytopenic dogs. Dogs were randomized to receive 3 × 109 platelets/kg of LP or CPP. Primary outcome measures were change in DOGiBAT score, platelet count, need for additional red cell transfusion and all‐cause mortality.
Results
Fifty dogs received LP and 38 received CPP. Baseline demographics and clinical characteristics of both groups were comparable. At 1‐hour post‐transfusion, LP were superior for change in DOGiBAT score, and non‐inferior at 24‐hours post‐transfusion. The LP were non‐inferior to CPP for change in platelet count, need for additional red blood cell units, and survival to discharge. The LP were superior for change in hematocrit at 1‐hour post‐transfusion, and non‐inferior at 24‐hours. No adverse effects were noted in either group.
Conclusions and Clinical Importance
A novel trehalose‐stabilized canine LP product appears to be logistically superior and is clinically non‐inferior to DMSO‐stabilized canine CPP for management of bleeding in thrombocytopenic dogs.
Keywords: canine, hemorrhage, immune thrombocytopenia, transfusion, trehalose
Abbreviations
- aPTT
activated partial thromboplastin time
- ANOVA
analysis of variance
- CI
confidence interval
- CPP
cryopreserved platelet concentrate
- DMSO
dimethyl sulfoxide
- DOGiBAT
standardized canine bleeding assessment tool
- ETP
endogenous thrombin potential
- G
global clot strength
- IQR
interquartile range
- ITP
immune thrombocytopenia
- K‐time
clot formation time
- LP
lyophilized platelets
- MA
maximum amplitude
- PT
prothrombin time
- RI
reference interval
- R‐time
clot reaction time
- TEG
thromboelastography
- TF
tissue‐factor
- TG
thrombin generation; ANOVA, analysis of variance
1. INTRODUCTION
Thrombocytopenia in dogs is common in critical care medicine with reported prevalence between 5% and 7%, 1 , 2 and is the most frequent indication for platelet transfusion. 3 Immune thrombocytopenia (ITP) is an important cause of severe thrombocytopenia in dogs. 4 Other potential causes of thrombocytopenia in dogs that potentially could benefit from platelet transfusions include disseminated intravascular coagulation, 5 bone marrow disorders, 6 neoplasia, and the rickettsial diseases anaplasmosis and ehrlichiosis. 2 Platelet transfusions can be life‐saving in humans with ITP who are bleeding into critical areas such as the central nervous system and lungs. 7 , 8 In trauma settings in humans, platelet transfusion is recognized as a crucial component of hemostatic resuscitation for major bleeding. 9 , 10 Increased emphasis on providing platelets to patients with traumatic hemorrhage likely decreases mortality, 11 , 12 , 13 but also may increase the need for platelet products. 14 , 15 Lyophilized (freeze‐dried) platelets (LP) 16 , 17 represent a product class that presently is undergoing testing in humans. 18
In humans, current recommendations are to transfuse platelets to patients with platelet counts <50 × 103/μL who are undergoing general surgery or liver biopsy, and to those with active bleeding and platelet counts <30 × 103/μL. 14 In dogs, evidence‐based transfusion triggers are not firmly established, but veterinary recommendations are consistent with those used in human medicine. 19 In dogs, platelets are uncommonly transfused to patients with thrombocytopenia, however, because of a lack of ready availability of appropriate products. 3 , 20 , 21 Additionally, the costs and logistics involved in obtaining and maintaining stores of fresh platelet concentrates are often prohibitive. Canine cryopreserved platelet concentrates (CPP) generated from apheresed platelets stabilized with 6% dimethyl sulfoxide (DMSO) are commercially available in the United States. 22 , 23 , 24 These platelets have demonstrated efficacy in canine thrombocytopenic model systems, 25 and although their efficacy in clinically bleeding dogs is not completely established, 26 they represent a readily available platelet transfusion product for dogs.
A paraformaldehyde‐stabilized canine LP product was tested against fresh platelet concentrates in dogs with clinical bleeding in a randomized clinical trial of 37 dogs. 27 No difference in the need for additional transfusions, bleeding scores, duration of hospitalization, or survival was identified between the 2 groups. However, that product is no longer commercially available. A recently developed trehalose‐stabilized canine LP product offers a novel method to control bleeding in thrombocytopenic dogs. We describe a multicenter, randomized clinical trial aimed at evaluating the safety and efficacy of this product (StablePlate RX) for the control of bleeding in thrombocytopenic dogs. The trial employed a non‐inferiority design comparing conventional canine CPP against the novel LP product. It was hypothesized that the LP product would not be inferior to CPP for reduction in clinical bleeding score, need for additional red blood cell transfusions, or survival to hospital discharge.
2. MATERIALS AND METHODS
2.1. Patient selection and randomization
Ours was a prospective, multicenter, randomized, open‐label (non‐blinded) trial. Dogs with thrombocytopenia and evidence of bleeding presented to 12 study centers (8 private specialist referral practices and 4 university teaching hospitals) were screened. Dogs were eligible for inclusion if they weighed between 2 and 45 kg, had a platelet count <50 × 103/μL, and had a standardized bleeding assessment score (DOGiBAT) of ≥2 (Data S1). This validated scoring system assesses bleeding in 9 anatomical sites and provides grades of 0 (none), 1 (mild‐moderate), or 2 (severe) in each category. Grades are summated to give an individual score, out of a possible maximum score of 18. 28 Before trial initiation, study investigators were trained to use the DOGiBAT score using example cases. 28 Exclusion criteria were any of: severe anemia (hematocrit <20%), surgery within the preceding 48 hours, administration of any blood product within the preceding 72 hours, current treatment for congestive heart failure or primary hypertension, or abnormal prothrombin time (PT) or activated partial thromboplastin time (aPTT). Additional exclusion criteria included administration of tranexamic acid, aminocaproic acid, aspirin, cyclophosphamide, or IV immunoglobulin G within the preceding 72 hours. Concurrent administration of glucocorticoids, azathioprine, cyclosporine, mycophenolate mofetil, or antimicrobial drugs was permitted. Administration of vincristine was permitted 1 hour after platelet product transfusion. After trial initiation, it became necessary to amend these criteria to maintain adequate patient recruitment rates. Fifteen dogs had been enrolled before the amendment. Specifically, the exclusion criteria were amended to allow enrollment of patients with hematocrit values ≥15%, aPTT ≤125% of the upper reference interval (RI) bound, or both. All dogs were enrolled with written, informed client consent, and the study protocol was approved by local clinical studies committees or institutional animal care and use committees. Patient randomization was performed using an online service (https://www.sealedenvelope.com/simple-randomiser/v1) using a predefined randomization list based on the intended sample size of 100 dogs, stratified by center, with each site assigned 10 cases. Investigators were blinded to this randomization list. This sample size was derived from calculations using a non‐inferiority limit of 2 for DOGiBAT score, a SD of 4 with a power of 80% at a 5% significance level.
2.2. Data collection
Dogs were evaluated by physical examination including DOGiBAT scoring and buccal mucosal bleeding time (BMBT) before administration, 1 hour post‐administration, and 24 hours post‐administration. Standardized BMBT tests were performed by study investigators using spring‐loaded lancet devices (Surgicutt Infant) on the mucosa of dogs' upper lips after securing the lip gently with gauze ties. Blood from the incision was blotted with filter paper applied adjacent to (but not touching) the incision. The BMBT was recorded as time from incision to cessation of bleeding. Standardized study forms were used to facilitate consistent scoring and data collection (Data S2). Additionally, at each time point, blood samples were collected for CBC and measurement of PT and aPTT. When available by location, thromboelastography (TEG) and thrombin generation (TG) assays were performed at each time point to evaluate the effect of platelet transfusion on the rate and strength of clot formation and the rate and quantity of TG. Telephone follow‐up with owners was performed at 7 and 14 days post‐enrollment.
2.3. Study products and interventions
Dogs were randomized to receive 3 × 109 platelets/kg of either LP by single IV bolus injection or CPP by slow IV infusion over 1 hour. Dissimilarities in product type, nature, preparation requirements, and administration procedures precluded study blinding. The LP product was made by pooling 18 to 20 apheresis units from a controlled donor colony. Donors were screened according to American College of Veterinary Internal Medicine (ACVIM) guidelines and by blood typing for Dog Erythrocyte Antigen (DEA) 1.1, 4, and 7. 29 All dogs in the pool were evaluated by major and minor crossmatch for compatibility among the pool. Platelet collection was performed by single‐needle centrifugal apheresis (MCS+, Haemonetics, Braintree, Massachusetts). Platelets were collected using a Food and Drug Administration (FDA) qualified apheresis procedure. All collections were leukoreduced using standard techniques approved by the FDA for apheresis with Haemonetics, Terumo, and Fresenius Kabi technologies. All liquid‐stored platelet concentrates utilized for manufacture of the product were evaluated for platelet count, leukocyte count, and red blood cell count before use. Manufacturing involves centrifugation and further separation of leukocytes occurs during this process. Platelet units were allowed to rest for up to 36 hours at room temperature until manufacture. All liquid‐stored platelets were evaluated for pH, lactate, swirl, aggregation, and count before use. All platelets used in the manufacturing process had to meet acceptance criteria consistent with apheresis‐derived platelet concentrate standards for humans published by the American Association of Blood Banks.
For LP manufacture, utilizing a proprietary method, platelets were pooled and combined with trehalose. Briefly, apheresis‐derived leukoreduced platelet concentrates were pooled for manufacture. Approximately 10 to 18 single apheresis units were utilized for each pool. Pooled platelets were centrifuged to remove native plasma and reconstituted with a trehalose buffer. Once reconstituted, platelets were evaluated by count and mean platelet volume (MPV) to determine concentration of the final product. Platelets were transferred to individual vials and lyophilized. Once the lyophilization cycle was complete, the product was exposed to 80°C for 20 to 28 hours as a pathogen inactivation step. Units were provided as vialed dry powder consisting of 1.5 × 1010 platelets in a dehydrated buffer containing 4‐(2‐hydroxyethyl)‐1‐piperazine‐ethanesulfonic acid (HEPES), NaCl, KCl, NaHCO3, dextrose, trehalose, ethanol, and polysucrose. Vialed product was tested using standardized criteria for purity, strength, sterility, and functionality. Vials were reconstituted with 10 mL sterile water immediately before administration.
The CPP were manufactured utilizing donors from the same colony. Pooling 18 to 20 apheresis units, the Valeri method for cryopreservation using 6% DMSO was performed. 30 Units were stored frozen in standard blood banking transfer bags (Terumo BCT, Lakewood, Colorado) providing a dose of 1.5 × 1010 platelets per unit with volumes of 20 to 40 mL per unit.
All products were evaluated for sterility utilizing an FDA‐qualified laboratory (aerobic and anaerobic culture), for endotoxin by limulus amebocyte lysate testing, for count and size by flow cytometry, for residual moisture (LP only), for TG and by flow cytometry (Data S3). Detailed instructions (Data S4) were provided to centers to standardize product administration.
2.4. Primary outcome measures
Our study was a non‐inferiority trial with 4 primary outcomes. We considered H1 proven if: (a) the between‐group difference in the pre‐transfusion to post‐transfusion DOGiBAT score (CPP‐LP) was <2; (b) the between‐group difference in the post‐transfusion to pre‐transfusion platelet count (CPP‐LP) was <35 × 103/μL; (c) the between‐group relative risk for additional red cell transfusions within 24 hours (CPP/LP) was <0.909; and (d) the between‐group relative risk for all‐cause mortality (CPP/LP) was <0.909. Thus, for the between‐group differences, non‐inferiority was deemed established if the lower bound of the 95% confidence interval (CI) of the mean difference in DOGiBAT score was <2 or platelet count was <35 × 103/μL. For relative risk adjudications, non‐inferiority was deemed established if the lower bound of the 95% CI of the relative risk was <0.909. A pre‐planned interim safety and efficacy analysis performed after enrollment of 50% of the planned 100 patients suggested that the trial should continue.
2.5. Secondary outcome measures
Comparisons between the following variables pre‐transfusion and post‐transfusion within each arm of the trial and between the 2 trial arms were performed: leukocyte count, neutrophil count, hematocrit, buccal mucosal bleeding time, PT and aPTT, TEG variables, and TG variables. Duration of hospitalization, rescue platelet product administration (additional platelet product usage within 24 hours) and presence/absence of clinically relevant events at 7 and 14 days post‐enrollment were compared between groups.
2.6. Clinicopathologic and coagulation analyses
Complete blood counts were performed using automated analyzers (ADVIA 2120, Siemens Healthcare Diagnostics, Tarrytown, New York, Element HT5, Heska Corp. Loveland, Colorado). No adjustments were made to these counts to account for minor differences in RIs among centers. Coagulation times were measured using whole blood (CoagDx, IDEXX, Westbrook, ME; VS Pro, Abaxis Inc, Union City, California) or plasma (STA Compact, Diagnostica Stago, Parsippany, New Jersey; ACL Elite, Instrumentation Laboratory, Bedford, Massachusetts). The RIs for these methods were different, precluding comparisons of these results as continuous variables. Thus, results from each center were recorded as low (below RI), normal (within RI), or high (above RI) to enable comparisons.
2.7. TEG and TG
Rotational viscoelastic testing was performed using a computerized instrument (TEG 5000, Haemonetics) with recalcified, non‐activated‐citrated blood (citrate‐native), and recalcified‐citrated blood activated with recombinant human tissue factor (TF), as previously described. 31 , 32 Assays were conducted in accordance with the PROVETS guidelines, with some variations among sites. 33 , 34 Reaction cups warmed to 37°C were loaded with 20 μL of 280 mM CaCl2 and 330 to 340 μL of citrated blood or 330 to 340 μL of citrated blood containing a TF‐phospholipid reagent (Dade Innovin, Siemens Healthcare Diagnostics, Tarrytown, New York) diluted 1 : 3400 or 1 : 50000 in the final (360 μL) reaction mixture. 32 The TEG analyses on non‐activated and TF‐activated blood were performed simultaneously in 2 channels for 60‐minute run times with compilation of the following parameters: reaction time (R), clotting time (K), angle (α), maximal amplitude (MA), and global clot strength (G). 31 As for the coagulation times, results from each center were recorded as low, normal, or high to enable comparisons.
Citrated platelet‐poor plasma aliquots were stored at −80°C pending batch analysis of TG at a single site. Plasma samples were stored for a maximum of 10 months before analysis. Thrombin generation was performed using an integrated spectrofluorimeter/analytic software instrument (Thrombinoscope, Diagnostica Stago) and the manufacturer's TG reagents (PPP‐low, Thrombin calibrator, FLUCa), as previously described. 35 The following values were recorded from the TG assays: Lag time (defined as the time from assay initiation to the beginning of TG); peak (defined as the maximum quantity of thrombin generated during the reaction, nM); and endogenous thrombin potential (ETP, defined by the area under the curve, and representing total thrombin formed over 60 minutes).
2.8. Statistical analysis
Continuous variables were assessed for normality by visual inspection and by using the D'Agostino Pearson normality test. Numerical categorical variables such as DOGiBAT score were assumed to be non‐parametric. Parametric data are reported as mean ± SD, whereas non‐parametric data are reported as median (interquartile range, IQR). Non‐Gaussian data were analyzed using non‐parametric methods. Categorical data were compared by 𝜒2 test. Baseline values for continuous variables between treatment groups were compared using t tests or the Mann‐Whitney U test. Differences in dichotomous variables between the 2 treatment groups were tested using Fisher's exact tests of 2‐way contingency tables. Differences between continuous variables within treatment groups before and after treatment were evaluated by repeated measures 1‐way analysis of variance (ANOVA) and Dunnett's multiple comparisons test or using the Friedman test with Dunn's post hoc multiple comparisons test. Alpha was set at .05. All analyses were 2‐sided and conducted using commercial software (Prism 8.3, GraphPad, La Jolla, CA; SPSS 26, IBM Corp, Armonk, New York). The 95% CIs of differences and relative risks were used to adjudicate non‐inferiority. An online calculator was used to confirm CIs: https://www.socscistatistics.com/confidenceinterval/default4.aspx.
3. RESULTS
3.1. Animals
In total, 88 dogs were enrolled; 50 dogs received LP and 38 dogs received CPP. The dogs had a median age of 7 years (IQR, 4‐10). There were 53 females and 34 males (sex not recorded for 1 dog). The baseline demographics and clinical characteristics in both groups were comparable (Table 1). Final diagnoses were not available for all dogs, but most had primary or secondary immune thrombocytopenia. Other diagnoses included blunt force trauma, babesiosis, metastatic adrenal carcinoma, non‐steroidal anti‐inflammatory‐associated gastrointestinal bleeding, hemangiosarcoma, and sepsis. Transfusion products administered within 24 hours of infusion of LP or CPP included rescue platelet products (n = 3) and packed RBC (PRBC, n = 2). Transfusion products administered during hospitalization (but >24 hours after enrollment) included PRBCs (n = 4), fresh whole blood (n = 2), cryoprecipitate (n = 1) and human albumin (n = 1). A total of 427 drug treatments in 27 distinct classes were administered to the 88 dogs, with a median of 5 treatments (IQR, 3‐6) per dog. Medication classes administered to ≥10% dogs were antimicrobials (n = 104), glucocorticoids (n = 74), other immunosuppressive drugs (n = 48), antiemetics (n = 41), proton pump inhibitors (n = 41), other gastroprotectant drugs (n = 25), vinca alkaloids (n = 24), anxiolytics (n = 15) and analgesics (n = 9). Some drugs were prescribed for pre‐existing conditions. The distribution of medications prescribed was not different between groups (P = .94).
TABLE 1.
Baseline variables in the 2 groups
| Variable | Lyophilized | n | Cryopreserved | n | P‐value | Hypothesis test |
|---|---|---|---|---|---|---|
| Age (y) | 7.1 ± 3.5 | 50 | 7.08 ± 3.55 | 38 | .1 | t test |
| Sex (female/male) | 31/18 | 49 | 22/16 | 38 | .66 | Fisher's exact test |
| Temperature (°F) | 101.8 ± 1.2 | 49 | 101.6 ± 1.4 | 38 | .46 | t test |
| Pulse rate (bpm) | 124 ± 26 | 49 | 125.9 ± 24.9 | 38 | .71 | t test |
| Respiratory rate (rpm) | 31 (28‐36) | 36 | 36 (29‐48) | 29 | .15 | Mann‐Whitney U test |
| DOGiBAT score | 6 (4–6) | 49 | 5 (3–7) | 38 | .45 | Mann‐Whitney U test |
| Platelet count (×103/μL) | 6.0 (0.3‐14.5) | 49 | 8.9 (0.8‐12.0) | 38 | .85 | Mann‐Whitney U test |
| HCT (%) | 36.0 ± 10.9 | 49 | 36.4 ± 10.7 | 38 | .86 | t test |
| Leukocyte count (×103/μL) | 15.3 (9.7‐21.9) | 49 | 14.9 (9.2‐20.6) | 38 | .66 | Mann‐Whitney U test |
| Neutrophil count (×103/μL) | 10.8 (7.9‐17.6) | 44 | 12.5 (6.6‐17.7) | 36 | .8 | Mann‐Whitney U test |
| BMBT (s) | 600 (300‐600) | 11 | 600 (270‐600) | 7 | .68 | Mann‐Whitney U test |
| aPTT (n L/N/H) | 4/36/9 | 50 | 2/25/7 | 34 | .78 | 𝜒2 |
| PT (n L/N/H) | 3/45/2 | 50 | 1/34/1 | 36 | .74 | 𝜒2 |
| R‐time (n L/N/H) | 1/10/3 | 14 | 0/12/5 | 17 | .49 | 𝜒2 |
| K‐time (n L/N/H) | 12/1/2 | 14 | 13/2/1 | 17 | .67 | 𝜒2 |
| Alpha angle (n L/N/H) | 7/6/1 | 14 | 5/11/1 | 17 | .47 | 𝜒2 |
| MA (n L/N/H) | 12/2/0 | 14 | 14/3/0 | 17 | .8 | 𝜒2 |
| G (n L/N/H) | 9/2/0 | 11 | 12/3/0 | 15 | .9 | 𝜒2 |
| Lag time (min) | 2.6 (2.2‐2.8) | 6 | 2.7 (2.7‐2.9) | 9 | .26 | Mann‐Whitney U test |
| Peak thrombin (nM) | 88.5 (77.5‐98.9) | 6 | 91.7 (70.4‐96.9) | 9 | .95 | Mann‐Whitney U test |
| ETP (nM·min) | 422 (294‐442) | 6 | 401 (353‐460) | 9 | .78 | Mann‐Whitney U test |
Note: Parametric data are summarized by mean ± SD, non‐parametric data are summarized by median (interquartile range) and categorical data are displayed as n per category. Differences in the reference intervals for the coagulation variables between centers precluded comparison as continuous variables. Hence coagulation variables were compared as categorized variables (low, normal, or high) based on local reference intervals.
Abbreviations: aPTT, activated partial thromboplastin time; BMBT, buccal mucosal bleeding time; ETP, endogenous thrombin potential; H, above the reference interval; HCT, hematocrit; K‐time, clot formation time; L, Below the reference interval; MA, maximum amplitude; N, within the reference interval; PT, prothrombin time; R‐time, clot reaction time.
3.2. Primary outcomes
For the DOGiBAT scores, there was a greater decrease in pre‐transfusion to post‐transfusion score at the 1‐hour time point in the LP group compared to the CPP group (P = .04), but no difference was found in this metric at the 24‐hour time point (P = .3; Figure 1A). Non‐inferiority analysis suggested that at 1 hour the LP were superior for the change in DOGiBAT score, and non‐inferior at the 24‐hour time point (Figure 2A). Unexpectedly, no differences were found in the change in platelet count pre‐transfusion to post‐transfusion between the 2 groups at either time point (P = .73, P = .59; Figure 1B) and the LP were non‐inferior to the CPP at both time points (Figure 2B). No difference was found in the proportions of additional PRBC transfusions between dogs in the LP group (1/50, 2.0%) and those in the CPP group (1/38, 2.6%), relative risk (CPP/LP) was 1.316 (95% CI, 0.14‐12.35; P = 1). The case fatality rate also was not different between groups. Case fatality rate in the LP group was 24.0% (12/50) and 24.3% (9/37) in the CPP group, relative risk (CPP/LP) was 1.014 (95% CI, 0.479‐2.100; P = 1). Fatality in this context includes both dogs that died naturally and those that were euthanized for disease severity or treatment failure. Analyses suggested that LP were non‐inferior to CPP for additional PRBC transfusion and for survival to discharge.
FIGURE 1.
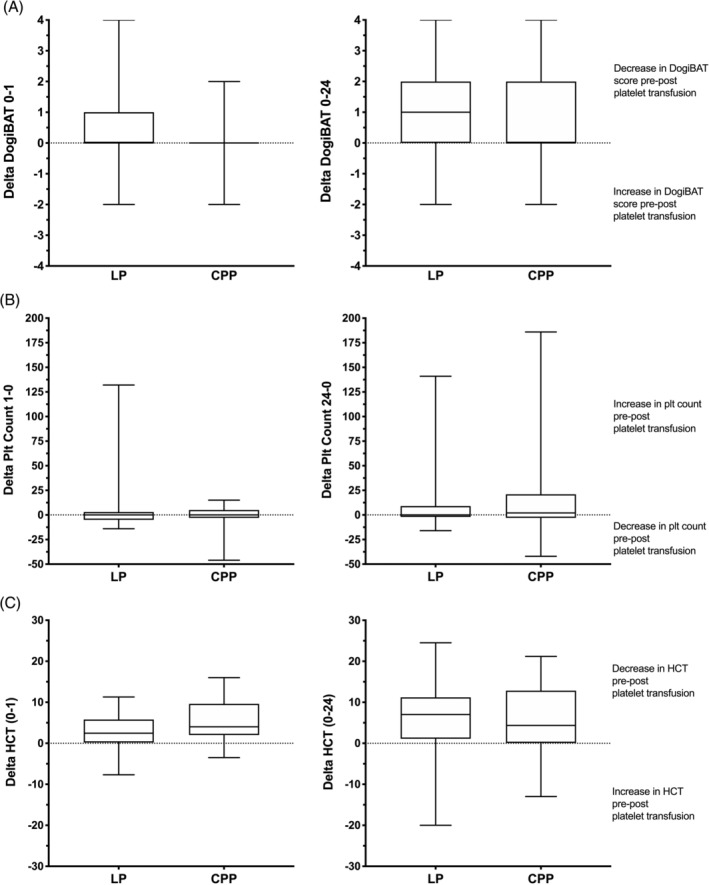
Box and whisker plots of the change in bleeding assessment score, platelet count and hematocrit (Hct) pre‐transfusion to 1 hour post‐transfusion (0‐1) and to 24 hours post‐transfusion (0‐24) for the 2 groups (LP and CPP). The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change and the whiskers represent the minimum and maximum changes in the variables. The horizontal dotted lines represent no change in the variable. Interpretations of the changes in variables above and below these zero change lines are provided. A, The change in (delta) bleeding score was significantly greater in the LP group compared to the CPP group at 1 hour post‐transfusion (ie, the bleeding score reduced more in the LP group than in the CPP group). There was no significant difference in this metric at the 24‐hour time point, however. B, There were no significant differences in the delta platelet count variable between the 2 groups at either the 1‐hour or the 24‐hour time points. In addition, the median delta variables were close to zero in both groups suggesting that on average neither of the products had any effect on the platelet count. There was no significant difference in this metric at the 24‐hour time point. C, The delta hematocrit was significantly greater in the CPP group compared to the LP group at 1‐hour post‐transfusion (ie, hematocrit decreased more in the CPP group), but there was no significant difference in the delta at the 24‐hour time point. CPP, cryopreserved platelets; LP, lyophilized platelets
FIGURE 2.
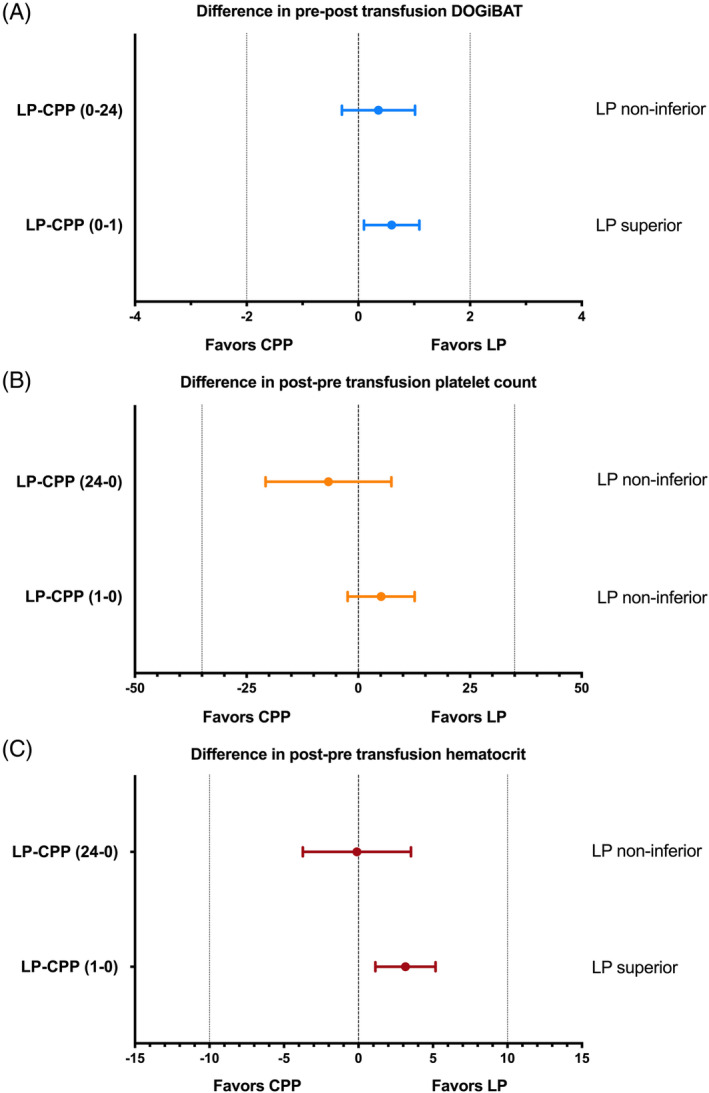
Non‐inferiority analysis plots where dots represent the mean difference between the 2 groups and whiskers represent the 95% confidence intervals of these mean differences. The vertical zero lines indicate no difference between the 2 groups for the variable measured. The additional vertical lines represent the pre‐determined non‐inferiority bounds for each variable. A, Comparison of the change in the canine bleeding assessment tool (DOGiBAT) score after the platelet transfusion compared to before the transfusion for the 2 products. Positive numbers for the mean difference represent a reduction (improvement) in the bleeding score following the transfusion that was larger in the LP group compared to the CPP group. The zero line indicates no change in DOGiBAT following the transfusion, while the lines at −2 and +2 represent the pre‐determined non‐inferiority bounds. At 1‐hour following the transfusion (LP‐CPP (0‐1)) there was a superior effect of the LP product because the lower bound of the confidence interval was above zero. At 24‐hours following the transfusion (LP‐CPP (0‐24) the LP product was non‐inferior to the CPP because the lower bound of the confidence interval was below zero, but above the −2 non‐inferiority bound. (B) Comparison of the change in the platelet count before the platelet transfusion compared to after the transfusion (LP‐CPP (1‐0)). Positive numbers for the mean difference represent an increase in platelet count that was larger in the LP group compared to the CPP group. The LP product was non‐inferior at both 1‐hour and 24‐hours following the transfusion because the lower bound of the confidence interval was above the −35 × 103/μL non‐inferiority bound. C, Comparison of the difference in the hematocrit after the platelet transfusion compared to before. Positive numbers for the mean difference represent a decrease in hematocrit that was smaller in the LP group compared to the CPP group. At 1‐hour following the transfusion (LP‐CPP (1‐0)) the LP product was superior for the change in hematocrit because the lower bound of the confidence interval was above zero. At 24‐hours following the transfusion (LP‐CPP (24‐0)) the LP product was non‐inferior to the CPP because the lower bound of the confidence interval was below zero, but above the −10 cutoff. CPP, cryopreserved platelets; LP, lyophilized platelets
3.3. Secondary outcomes
A smaller decrease in pre‐transfusion to post‐transfusion hematocrit was observed at 1 hour in the LP group compared to the CPP group (P = .01; Figure 1C). No difference was found in this metric at the 24‐hour time point, however (P = .73). Non‐inferiority analysis suggested that at 1 hour LP were superior for change in hematocrit (Figure 2C), and non‐inferior at the 24‐hour time point. No difference was observed in the proportions of rescue platelet product transfusion between dogs in the LP group (2/50, 4.0%) and those in the CPP group (1/38, 2.6%), relative risk (CPP/LP) was 0.658 (95% CI, 0.09‐4.85; P = 1). Duration of hospitalization was longer for dogs in the LP group at 5 days (IQR, 3‐6), compared to dogs in the CPP group at 4 days (IQR, 2‐4.75; P = .01). This difference persisted after dogs that were euthanized were excluded from analyses: 5 days (IQR, 3‐6) vs 4 days (IQR, 2.5‐5; P = .02).
No significant differences in DOGiBAT score were found between groups at any time point. In the LP group, DOGiBAT score significantly decreased between baseline and the 24‐hour time point (Table 2, Figure 3), whereas in the CPP group it decreased significantly between 1 hour and 24 hours. No significant differences in platelet count were observed between groups at any time point or within groups over time (Figure 4). Between groups, no significant differences in hematocrit were identified at any time point, but within groups significant decreases in hematocrit were found between time points (Figure 5). Between groups, no significant differences in leukocyte or neutrophil counts were observed at any time point, but within groups significant increases in leukocyte and neutrophil counts were found between baseline and subsequent time points (Figure S1). Between groups, no significant differences in rectal temperature, heart rate, or respiratory rate were identified at any time point, but within groups several significant changes in these variables were identified over time (Table 2).
TABLE 2.
Summary of serial evaluations of key clinical variables
| T0 | T1 | T24 | ||||
|---|---|---|---|---|---|---|
| LP | CPP | LP | CPP | LP | CPP | |
|
Temperature (°F) |
102 (100.9‐102.8)a |
101.6 (100.9‐102.7) |
101.4 (100.6‐102.1) |
101.3 (100.4‐101.9) |
100.9 (100.2‐101.4)a |
101.1 (100.4‐101.7) |
|
Heart rate (bpm) |
130 (107‐140) |
120 (108‐140)a,b |
120 (91‐131) |
112 (100‐120)a |
110 (100‐130) |
108 (92‐128)b |
|
Respiratory rate (rpm) |
32 (28‐40) |
36 (29–48) |
32 (29‐41) |
32 (28‐40) |
32 (27‐40) |
30 (28‐36) |
|
DOGiBAT score (AU) |
6 (4‐6)a |
5 (3‐7) |
5 (4‐6) |
5 (4‐7)b |
4.5 (3.25‐6)a |
5 (3‐6)b |
|
Hematocrit (%) |
36.0 ± 10.9a | 36.4 ± 10.7b,c | 33.5 ± 10.9a | 29.7 ± 9.7b | 30.1 ± 9.3a | 30.0 ± 9.6c |
|
Platelet count (×103/μL) |
6.0 (0.3‐14.5) |
8.0 (0.8‐12) |
9.0 (0.0‐13.3) |
6.0 (0.0‐15.0) |
9.0 (2.3‐18.0) |
9.0 (1.0‐35.0) |
Note: Data are presented as mean ± SD or median (IQR). Statistically significant serial comparisons within groups are highlighted by superscript letters. Comparisons between LP and CPP groups are not represented in this table. Variables sharing superscript letters were significantly different (P < .05) by repeated measures ANOVA or the Friedman test.
Abbreviations: AU, arbitrary units; CPP, cryopreserved platelets; LP, lyophilized platelets; T0, baseline immediately prior to transfusion; T1, 1‐hour post‐transfusion completion, T24, 24‐hours post‐transfusion completion.
FIGURE 3.
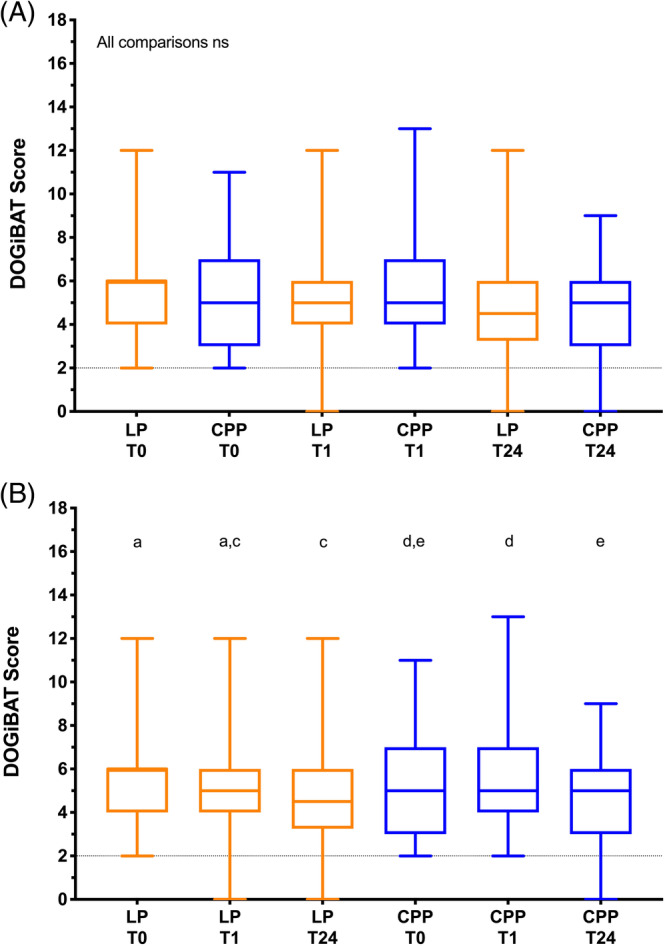
Box and whisker plots of canine bleeding assessment tool scores (DOGiBAT) in the 2 arms of the trial. The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change and the whiskers represent the minimum and maximum changes in the variables. A, Comparisons of DOGiBAT scores between groups at each of the time points baseline (T0), 1 hour after the transfusion (T1) and 24 hours after the transfusion (T24). There were no significant differences between the 2 groups at any of the time points (Kruskal‐Wallis test). B, Comparisons of DOGiBAT scores in each group over time. Plots sharing a letter code were not significantly different from each other. Plots not sharing a letter code were significantly different from each other P < .05 after correction for multiple comparisons (Friedman test). LP represents the lyophilized platelet group; CPP represents the cryopreserved platelet group. The horizontal dotted line indicates the minimum baseline score (2) required for entry into the study
FIGURE 4.
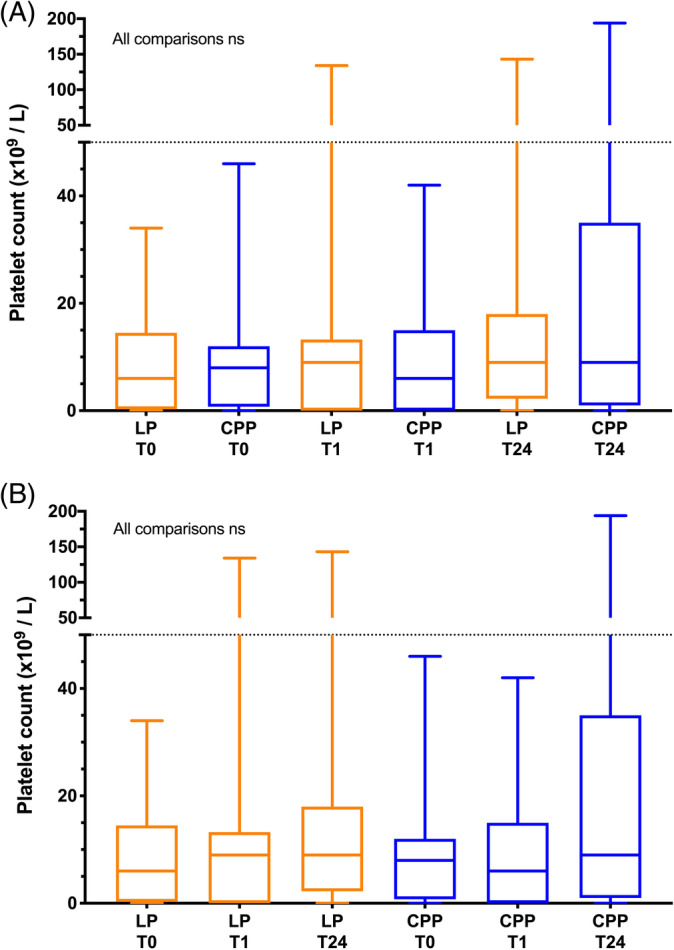
Box and whisker plots of platelet counts (Plt) in the 2 arms of the trial. The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change and the whiskers represent the minimum and maximum changes in the variables. A, Comparisons of platelet counts between groups at each of the time points baseline (T0), 1 hour after the transfusion (T1) and 24 hours after the transfusion (T24). There were no significant differences between the 2 groups at any of the time points (Kruskal‐Wallis test). B, Comparisons of platelet counts in each group over time. There were no significant differences within the groups between any of the time points (Friedman test). LP represents the lyophilized platelet group; CPP represents the cryopreserved platelet group. The horizontal dotted line indicates the maximum baseline platelet count (50 × 103/μL) permissible for entry into the study
FIGURE 5.
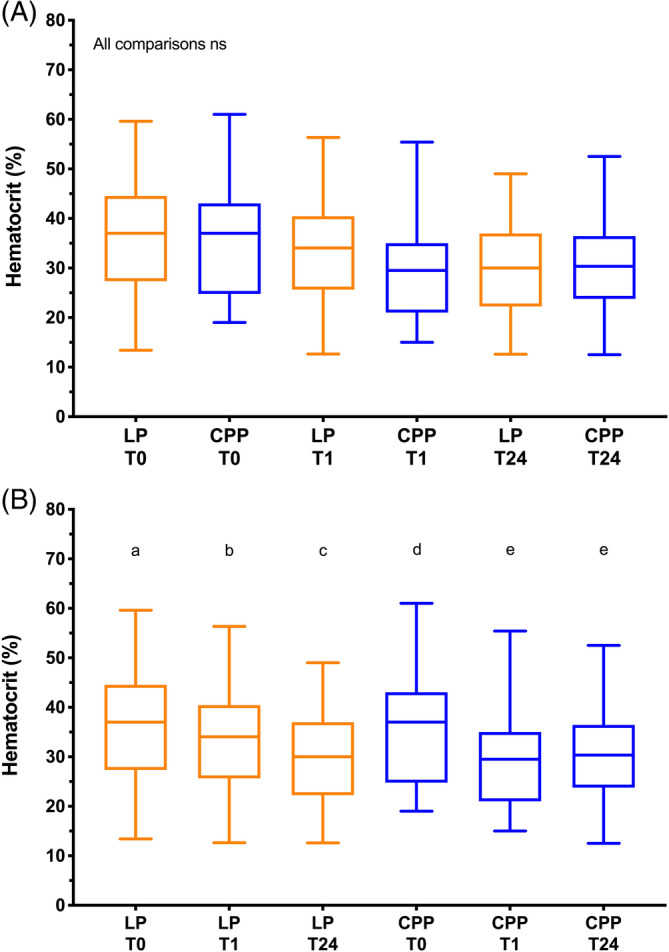
Box and whisker plots of hematocrit (Hct) in the 2 arms of the trial. The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change and the whiskers represent the minimum and maximum changes in the variables. A, Comparisons of hematocrit values between groups at each of the time points baseline (T0), 1 hour after the transfusion (T1) and 24 hours after the transfusion (T24). There were no significant differences between the 2 groups at any of the time points (Kruskal‐Wallis test). B, Comparisons of hematocrit values in each group over time. Plots sharing a letter code were not significantly different from each other. Plots not sharing a letter code were significantly different from each other P < .05 after correction for multiple comparisons (Friedman test). LP represents the lyophilized platelet group; CPP represents the cryopreserved platelet group
3.4. Adverse effects
No clinically relevant adverse effects were noted in either group. All case fatalities (death or euthanasia for disease severity or treatment failure) were evaluated for possible relation to platelet product administration using clinical record review and where necessary follow‐up with study investigators for additional details and clarification; all were found to be unrelated.
3.5. Coagulation analyses
Different RIs for coagulation testing performed at different centers precluded comparisons of these results as continuous variables. After categorization of the results, no significant differences, within or between groups, were found in the frequencies of low, normal, or high values for either PT or aPTT at any time point. Similarly, no significant differences in BMBT were identified within or between groups at any time point, although few patients (n = 18 at baseline) had bleeding times assessed. No significant differences in lag time or peak thrombin were observed within or between groups at any time point, but limited availability of the TG assays resulted in data from only 15 patients. A significant increase in ETP was observed between the 1‐hour and the 24‐hour time points in the CPP group, but no significant differences in ETP were identified between the groups at any time point. No significant differences in any of the TEG parameters were found within or between groups (Table 3).
TABLE 3.
Summary of serial evaluations of coagulation variables
| T0 | T1 | T24 | ||||
|---|---|---|---|---|---|---|
| LP | CPP | LP | CPP | LP | CPP | |
|
BMBT (s) |
600 (300–600) |
600 (270‐600) |
392 (227‐600) |
495 (240‐600) |
563 (262‐600) |
182 (104‐307) |
|
PT (n L/N/H) |
3/45/2 | 1/34/1 | 5/36/1 | 0/30/0 | 0/37/1 | 0/25/4 |
|
aPTT (n L/N/H) |
5/36/9 | 2/25/7 | 2/23/15 | 1/20/7 | 4/24/9 | 2/18/4 |
|
R time (n L/N/H) |
1/11/4 | 0/12/5 | 3/6/5 | 0/6/3 | 1/6/3 | 0/6/4 |
|
K time (n L/N/H) |
13/1/2 | 13/2/1 | 10/3/2 | 7/3/2 | 8/2/0 | 9/2/0 |
|
Alpha angle (n L/N/H) |
8/7/1 | 5/11/1 | 8/5/1 | 2/7/0 | 4/5/1 | 3/7/0 |
|
MA (n L/N/H) |
14/2/0 | 14/3/0 | 13/1/0 | 7/2/0 | 8/2/0 | 6/3/1 |
|
G (n L/N/H) |
9/2/0 | 12/3/0 | 10/1/1 | 6/2/0 | 6/2/0 | 3/5/1 |
|
Lag time (min) |
2.6 (2.2–2.8) |
2.7 (2.7–2.9) |
2.4 (2.2‐3.1) |
2.6 (2.3‐2.9) |
2.0 (2.0‐2.7) |
2.4 (1.8‐2.6) |
|
Peak thrombin (nM) |
88.5 (77.5‐98.9) |
91.7 (70.4‐96.9) |
85.7 (76.9‐92.1) |
76.6 (64.7‐92.6) |
88.0 (82.3‐92.4) |
92.0 (85.9‐105.6) |
|
ETP (nM·min) |
421.6 (293.6‐442.0) |
401.4 (353.4‐460.0) |
409.3 (307.5‐435.1) |
400.8a (321.1‐442.0) |
403.5 (319.4‐424.6) |
441.1a (370.1‐454.3) |
Note: Data are presented as median (IQR) or as number (n) of values below (L), within (N) or above (H) the respective local reference interval. Variables sharing superscript letters were significantly different (P < .05) by repeated measures ANOVA or the Friedman test.
Abbreviations: aPTT, activated partial thromboplastin time; BMBT, buccal mucosal bleeding time; CPP, cryopreserved platelets; ETP, endogenous thrombin potential; K time, clot formation time; LP, lyophilized platelets; MA, maximum amplitude; PT, prothrombin time; R time, reaction time; T0, baseline immediately prior to transfusion; T1, 1‐hour post‐transfusion completion, T24, 24‐hours post‐transfusion completion.
4. DISCUSSION
Identifying effective platelet transfusion products may enhance management of bleeding in thrombocytopenic dogs. Our study was a multicenter, randomized clinical trial designed to assess the efficacy and safety of LP in bleeding dogs with thrombocytopenia. The trial used a non‐inferiority design to benchmark the new product against canine CPP, a readily available active control. Our findings suggest that the tested canine LP product is non‐inferior to CPP based on assessments of bleeding scores, need for additional PRBC transfusions, and survival to hospital discharge. Although the role of platelet transfusion in the management of thrombocytopenia is debated, 36 , 37 our study was conducted on the premise that platelet product transfusion is likely beneficial in dogs with thrombocytopenia and serious or life‐threatening bleeding. This premise reflects current recommendations in veterinary settings, 3 , 19 and aligns with the practice of the study investigators. All of the dogs in our study were markedly thrombocytopenic, and it is likely thrombocytopenia contributed to, or caused, the bleeding observed. However, we cannot exclude the possibility that thrombocytopathia or vasculitis contributed to the bleeding manifestations seen. Assessment of platelet function in dogs with thrombocytopenia often is limited by the number of platelets available for analysis or interference with automated techniques, such as the platelet function analyzer.
Based on serial bleeding assessment scores, hemorrhage decreased in most dogs after LP transfusion. Although on average no decrease in DOGiBAT score occurred within the first 24 hours in the CPP group, no difference in the delta DOGiBAT score was found between the 2 groups at 24 hours. The absolute difference in bleeding score in the LP group at 1 hour was small and its clinical relevance is uncertain. Bleeding scores between groups at each time point were not different, and thus both LP and CPP may have similar efficacy for management of bleeding in thrombocytopenic dogs. Also, all dogs received additional treatments for their underlying disease processes. Most dogs in our study had ITP and hence also were receiving glucocorticoids or mycophenolate mofetil, which have been demonstrated to effectively treat ITP in dogs. 38 , 39 , 40 , 41 Many dogs (27.3%) also received vincristine, which has been demonstrated to hasten platelet count recovery in dogs with ITP and hence might have impacted our findings. 42 , 43 The frequency of administration of vincristine between the 2 groups was not different, however. For ethical reasons, we did not include an inactive placebo arm and hence it is uncertain what would have happened to the bleeding scores in such a control group.
Platelet counts at each time point were not different between groups. Although bleeding scores tended to decrease over time, no significant differences in the platelet counts were observed within either group over time. This discrepancy could be reconciled in several ways. In severe ITP, platelet count and bleeding score correlate poorly. 44 Alternatively, the hemostatic effects of these products might not be completely explained by changes in platelet count. Although some increase in platelet count might be anticipated after administration, infused platelets may form heterotypic aggregates with leukocytes, 45 may be removed from circulation by the reticuloendothelial system, 46 or may be consumed by incorporation into hemostatic thrombi. 47 For CPP, it is probable that some hemostatic effect results from infusion of platelet‐derived microparticles and phosphatidylserine‐expressing membrane fragments that aid hemostasis but are not recognized as platelets by automated hematology analyzers. 22 in vitro analyses of the LP product suggest that microparticle counts are low (IND 17156 Cellphire), but most platelets are activated (phosphatidylserine‐positive), 48 and hence might support hemostasis in vivo after infusion. 48 Thromboelastographic analyses suggest that LP augment final clot strength in vitro, 49 but TEG analyses in our study could not confirm that this effect occurs in dogs in vivo. Analyses of LP under flow conditions also suggest that reconstituted platelets adhere to collagen‐coated microcapillary channels under shear. 48 Analyses conducted in our study suggest that TG potential and clot formation dynamics were not substantially altered by the transfused platelet products. However, these analyses were not available at every center and the data therefore only partially represent the situation.
Hematocrit decreased in both groups over the first 24 hours and mean hematocrit in both groups at 24 hours was 30%. Based on analysis of the delta hematocrit values, a smaller decrease in hematocrit occurred between baseline and 1 hour in the LP group than in the CPP group, suggesting that LP was initially superior to CPP for maintenance of hematocrit. This effect was not sustained, however. The difference in hematocrit at 1 hour post‐transfusion also might have resulted from a dilutional effect associated with the infusion of different volumes of platelet products. The LP product had a consistent concentration and hence each dog received 2 mL/kg. The CPP product varied in volume between 20 and 40 mL and hence each dog received between 4 and 8 mL/kg. In a 20 kg dog, at maximum, this difference would have been 120 mL, which represents 3% of the extracellular fluid volume. Although small, this volume might have been sufficient to explain some of the differences in hematocrit observed. In both groups significant decreases in hematocrit were observed between baseline and 1 hour and between baseline and 24 hours, suggesting the dogs continued to bleed despite therapeutic interventions. Bleeding scores indicate that clinically‐evident bleeding decreased over time, but the decreasing hematocrit is consistent with ongoing blood loss, potentially into the gastrointestinal tract. This bleeding might have been missed by the DOGiBAT score if the bleeding was in the proximal small intestine or if the patient did not defecate because the DOGiBAT is performed on passed feces only.
No significant differences were noted between the frequency of additional red blood cell or additional platelet product transfusion, or in the case fatality rates of the 2 groups. The relative risks for these comparisons include potential benefits of both LP and of CPP and the small numbers of events resulted in wide CI for the relative risk estimates. Post hoc power (α .05, 1‐β .8) calculations suggest that substantially larger trials (n = 1298 for rescue product administration, n = 6752 for additional red cell transfusions, n = 19984 for survival) would be necessary to confirm that the observed differences were statistically significant. These effects might not be identified even with enrollment of more patients and it is therefore reasonable to suggest that non‐inferiority exists. Also, case fatality in our study included dogs euthanized for disease severity or treatment failure. No dogs were euthanized specifically because of financial limitations. This situation may reflect the referral clinic setting of the study, which may have minimized the number of clients with financial constraints. In addition, the study offset some of the costs of care by provision of platelet transfusion products and additional clinicopathologic testing. Including euthanized dogs however, does complicate interpretation of case fatality as an outcome measure.
The duration of hospitalization was 1 day longer for dogs that received LP than for those that received CPP. This difference might have resulted from a shorter duration of hospitalization in dogs that were euthanized compared to dogs that died naturally, but the difference persisted after dogs that were euthanized were excluded from analyses. The cause of the prolonged hospital stays in the dogs randomized to LP is unclear. Bias from the lack of blinding could have led clinicians to be more cautious in discharging dogs that received LP. The finding however might be an epiphenomenon arising from unidentified differences in the disease processes in the 2 groups. The potential that LP administration contributed to disease morbidity or resulted in subclinical adverse reactions that necessitated additional hospitalization cannot be excluded, but no adverse effects were noted during LP administration, during hospitalization or at follow‐up. As such, the cause of the 1 day longer hospitalization is unknown.
Our study was not powered to document a survival benefit of either platelet product. A non‐inferiority design was used for pragmatic reasons of recruitment rates and study costs. In addition, prior data suggested a substantial effect size was unlikely to exist for LP compared to CPP. 27 The LP product offers potential real‐world logistical benefits over CPP, including room temperature storage and ease of shipping. The shelf‐life of LP is also longer than that of CPP (24 months vs 6 months). In the setting of life‐threatening hemorrhage, LP can be obtained, prepared and administered more rapidly than CPP, because they do not require thawing, and the infusion volume is smaller. For a 15 kg dog, administration of LP might take 45 minutes (15 minutes preparation and rehydration, 30 minutes administration), whereas administration of CPP might take 120 to 150 minutes (30 minutes thaw time, 90‐120 minutes administration depending on the volume). These time differences were apparent in our study, but did not translate into a survival benefit, likely because of the overall bleeding severity and the nature of the underlying disease processes. In the context of cerebral or pulmonary hemorrhage, or after exsanguinating trauma, such differences might be more clinically important.
Our study is the largest randomized clinical trial of platelet transfusion products in veterinary medicine. Practical considerations including cost, case accrual rates, and other logistical considerations limited recruitment to 88 cases rather than the intended 100. The study used a non‐inferiority design because there were perceived practical advantages of LP over CPP. The non‐inferiority limits were set based on the clinical judgment of the study investigators and were designed to have real world clinical relevance. These limits were set by consensus, but are inherently subjective because altering the limits would affect interpretation of the results. The trial used clinically relevant outcomes such as transfusion requirements and case fatality, but was likely underpowered to detect small differences.
Our study excluded dogs with severe anemia—initially <20% and then <15% because it was anticipated that dogs with very low hematocrit at presentation likely would have been immediately prescribed transfusion of PRBCs by their attending clinicians. The exclusion criteria for our study therefore were designed to exclude dogs with recent or concurrent transfusion of red blood cell products to maximize the potential for identification of adverse events that specifically could be associated with the novel platelet product. Coadministration with, or recent administration of, other blood products would have confounded our ability to ascribe any adverse events to the platelet product. In addition, there was concern that some PRBC units may contain small numbers of platelets that might have confounded evaluation of the effects of the platelet products.
Our study protocol included use of BMBT as an indicator of hemostatic therapeutic efficacy, as required by the FDA. To minimize risk to patients, study investigators were not required to perform BMBT in patients for which they deemed it was unsafe or for those that had active gingival bleeding. Provisions also were made to allow topical use of the LP product to assist with arresting bleeding after the test. Excessive bleeding was not observed after any of the BMBT tests that were performed. Outside of the scope of this trial, performing BMBT is not recommended in dogs with thrombocytopenia. The BMBT may be a useful test to screen for von Willebrand's disease, thrombocytopathia, or vasculitis, but only in circumstances in which the platelet count is normal.
Study investigators were not blinded to group assignment, although the use of a randomization list did provide allocation concealment for subsequent enrollments (ie, investigators could not predict into which group the next patient would be assigned). Blinding the study was not feasible because 1 product was lyophilized and the other was frozen. Additionally, the CPP had a characteristic odor associated with the DMSO. One primary outcome measure was the DOGiBAT score. This score is categorical and has a series of associated descriptors and images for each category, but score application retains an element of subjective judgment. As such, the lack of blinding might have introduced a bias that could favor LP. The other outcome measures used in the study, including transfusion requirements and survival, were more objective and hence are unlikely to have been influenced by the lack of blinding. Other treatments were not standardized however and group assignment could have influenced clinician decision‐making.
Although study participants were randomized to the 2 treatment groups, the 2 study groups were unequal in size. This difference resulted from stratification of randomization of dogs by center with subsequent unequal enrollment at each site, combined with early cessation of the trial before full enrollment. This difference in the number of dogs allocated to each product might have affected the composition of the dogs in the 2 study groups. Comparison of the baseline variables in the 2 patient groups suggests that any such effects were minimal, but unforeseen and unmeasured effects of these unequal groups cannot be excluded.
The study population was heterogeneous, consisting predominantly of cases of ITP, but including various other conditions, which might have impacted the findings of the study. Randomization of study interventions likely helped control for this heterogeneity, but imbalances in disease states or disease severity could have affected outcome. The stages of disease and therapeutic interventions were not controlled for or standardized and also might have influenced the results. Baseline DOGiBAT scores between groups were similar, but the influence of differences in disease stage or severity might have increased over time. Some variation within and among centers in patient management or prevalence of underlying or concurrent disease processes may have been present. Stratification of cases by center was not feasible because overall sample size was limited and some centers enrolled small numbers of cases. A homogenous study population treated in a standardized fashion might decrease potential sources of bias, but could limit the generalizability of our results.
In summary, our study suggests that LP is not inferior to CPP for the management of bleeding in thrombocytopenic dogs. In the acute time frame (1 hour post‐transfusion), LP may be superior to CPP for reduction in bleeding score and limiting a decrease in hematocrit, but the clinical relevance of the small differences identified is uncertain. Future studies should focus on comparing LP to fresh platelet concentrates or fresh whole blood, on evaluating LP in settings such as trauma and intraoperative bleeding and assessing the potential benefits of combining LP with other hemostatic interventions including antifibrinolytic agents.
CONFLICT OF INTEREST DECLARATION
R. Goggs and B. Brainard have received sponsorship from BodeVet Inc. for separate studies analyzing the properties of the StablePlate RX or related products. J. Kishbaugh is an employee of BodeVet, Inc. A. Hale is an employee and shareholder of BodeVet Inc. All other authors declare they have no conflict of interest relevant to the subject matter of the article. R. Goggs, B. Brainard and D. LeVine wrote the manuscript. A. Hale was consulted for editorial comments on the manuscript only and acted as the industry sponsor liaison.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the following IACUCs: Cornell IACUC 2017‐0011; Iowa State University (19‐075); North Carolina State University (17‐187‐O); University of Georgia (CR‐493). The study protocol was approved by the following clinical studies ethics review committees: BluePearl Science (1‐2017‐K9 BP‐IRB Approval 3.10.16.SP); Friendship Veterinary Hospital (Approved 03‐02‐17). This study was undertaken with written informed client consent.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Fig. S1: Box and whisker plots of leukocyte count (WBC) and neutrophil count in the 2 arms of the trial. The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change, and the whiskers represent the minimum and maximum changes in the variables. (A and C) Comparisons of leukocyte and neutrophil counts between groups at each of the time points baseline (T0), 1 hour after the transfusion (T1) and 24 hours after the transfusion (T24). There were no significant differences between the 2 groups at any of the time points (Kruskal‐Wallis test). (B and D) Comparisons of leukocyte and neutrophil counts in each group over time. Plots sharing a letter code were not significantly different from each other. Plots not sharing a letter code were significantly different from each other P < .05 after correction for multiple comparisons (Friedman test). LP represents the lyophilized platelet group; CPP represents the cryopreserved platelet group.
Data S1: Supporting Information.
Data S2: Supporting Information.
Data S3: Supporting Information.
Data S4: Supporting Information.
ACKNOWLEDGMENTS
Funding provided by BodeVet Inc. through donation of cryopreserved canine platelets and lyophilized platelet units, funding for reagents for laboratory analyses and funding for patient enrollment incentives. This work was presented as an ePoster at the 2020 ACVIM Forum On Demand. The authors thank Dr Jenny Walton for her assistance with medical record review.
Goggs R, Brainard BM, LeVine DN, et al. Lyophilized platelets versus cryopreserved platelets for management of bleeding in thrombocytopenic dogs: A multicenter randomized clinical trial. J Vet Intern Med. 2020;34:2384–2397. 10.1111/jvim.15922
Funding information BodeVet Inc.
REFERENCES
- 1. Grindem CB, Breitschwerdt EB, Corbett WT, et al. Epidemiologic survey of thrombocytopenia in dogs: a report on 987 cases. Vet Clin Pathol. 1991;20:38‐43. [DOI] [PubMed] [Google Scholar]
- 2. Botsch V, Kuchenhoff H, Hartmann K, et al. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec. 2009;164:647‐651. [DOI] [PubMed] [Google Scholar]
- 3. Callan MB, Appleman EH, Sachais BS. Canine platelet transfusions. J Vet Emerg Crit Care. 2009;19:401‐415. [DOI] [PubMed] [Google Scholar]
- 4. Dircks BH, Schuberth HJ, Mischke R. Underlying diseases and clinicopathologic variables of thrombocytopenic dogs with and without platelet‐bound antibodies detected by use of a flow cytometric assay: 83 cases (2004‐2006). J Am Vet Med Assoc. 2009;235:960‐966. [DOI] [PubMed] [Google Scholar]
- 5. Goggs R, Mastrocco A, Brooks MB. Retrospective evaluation of 4 methods for outcome prediction in overt disseminated intravascular coagulation in dogs (2009‐2014): 804 cases. J Vet Emerg Crit Care. 2018;28:541‐550. [DOI] [PubMed] [Google Scholar]
- 6. Miller MD, Lunn KF. Diagnostic use of cytologic examination of bone marrow from dogs with thrombocytopenia: 58 cases (1994‐2004). J Am Vet Med Assoc. 2007;231:1540‐1544. [DOI] [PubMed] [Google Scholar]
- 7. Spahr JE, Rodgers GM. Treatment of immune‐mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol. 2008;83:122‐125. [DOI] [PubMed] [Google Scholar]
- 8. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168‐186. [DOI] [PubMed] [Google Scholar]
- 9. Stensballe J, Henriksen HH, Johansson PI. Early haemorrhage control and management of trauma‐induced coagulopathy: the importance of goal‐directed therapy. Curr Opin Crit Care. 2017;23:503‐510. [DOI] [PubMed] [Google Scholar]
- 10. Cannon JW, Khan MA, Raja AS, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: a practice management guideline from the eastern association for the surgery of trauma. J Trauma Acute Care Surg. 2017;82:605‐617. [DOI] [PubMed] [Google Scholar]
- 11. Perkins JG, Cap AP, Spinella PC, et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J Trauma. 2009;66:S77‐S84. [DOI] [PubMed] [Google Scholar]
- 12. Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493‐500. [DOI] [PubMed] [Google Scholar]
- 13. Cardenas JC, Zhang X, Fox EE, et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized proppr trial. Blood Adv. 2018;2:1696‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365‐394. [DOI] [PubMed] [Google Scholar]
- 15. Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cap AP, Perkins JG. Lyophilized platelets: challenges and opportunities. J Trauma. 2011;70:S59‐S60. [DOI] [PubMed] [Google Scholar]
- 17. Bode AP, Fischer TH. Lyophilized platelets: fifty years in the making. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:125‐133. [DOI] [PubMed] [Google Scholar]
- 18. Barroso J, Osborne B, Teramura G, et al. Safety evaluation of a lyophilized platelet‐derived hemostatic product. Transfusion. 2018;58:2969‐2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abrams‐Ogg AC. Triggers for prophylactic use of platelet transfusions and optimal platelet dosing in thrombocytopenic dogs and cats. Vet Clin North Am Small Anim Pract. 2003;33:1401‐1418. [DOI] [PubMed] [Google Scholar]
- 20. Hux BD, Martin LG. Platelet transfusions: treatment options for hemorrhage secondary to thrombocytopenia. J Vet Emerg Crit Care. 2012;22:73‐80. [DOI] [PubMed] [Google Scholar]
- 21. Callan MB, Marryott K. Platelet products In: Yagi K, Holowaychuk MK, eds. Manual of Veterinary Transfusion Medicine and Blood Banking. 1st ed. Ames, IO: Wiley‐Blackwell; 2016:55‐69. [Google Scholar]
- 22. Guillaumin J, Jandrey KE, Norris JW, et al. Assessment of a dimethyl sulfoxide‐stabilized frozen canine platelet concentrate. Am J Vet Res. 2008;69:1580‐1586. [DOI] [PubMed] [Google Scholar]
- 23. Guillaumin J, Jandrey KE, Norris JW, et al. Analysis of a commercial dimethyl‐sulfoxide‐stabilized frozen canine platelet concentrate by turbidimetric aggregometry. J Vet Emerg Crit Care. 2010;20:571‐577. [DOI] [PubMed] [Google Scholar]
- 24. Appleman EH, Sachais BS, Patel R, et al. Cryopreservation of canine platelets. J Vet Intern Med. 2009;23:138‐145. [DOI] [PubMed] [Google Scholar]
- 25. Valeri CR, Feingold H, Melaragno AJ, et al. Cryopreservation of dog platelets with dimethyl sulfoxide: therapeutic effectiveness of cryopreserved platelets in the treatment of thrombocytopenic dogs, and the effect of platelet storage at −80 degrees c. Cryobiology. 1986;23:387‐394. [DOI] [PubMed] [Google Scholar]
- 26. Ng ZY, Stokes JE, Alvarez L, et al. Cryopreserved platelet concentrate transfusions in 43 dogs: a retrospective study (2007‐2013). J Vet Emerg Crit Care. 2016;26:720‐728. [DOI] [PubMed] [Google Scholar]
- 27. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: a preliminary trial in 37 dogs. J Vet Emerg Crit Care. 2012;22:116‐125. [DOI] [PubMed] [Google Scholar]
- 28. Makielski KM, Brooks MB, Wang C, et al. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med. 2018;32:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wardrop KJ, Birkenheuer A, Blais MC, et al. Update on canine and feline blood donor screening for blood‐borne pathogens. J Vet Intern Med. 2016;30:15‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valeri CR, Ragno G, Khuri S. Freezing human platelets with 6 percent dimethyl sulfoxide with removal of the supernatant solution before freezing and storage at −80 degrees c without postthaw processing. Transfusion. 2005;45:1890‐1898. [DOI] [PubMed] [Google Scholar]
- 31. Goggs R, Wiinberg B, Kjelgaard‐Hansen M, et al. Serial assessment of the coagulation status of dogs with immune‐mediated haemolytic anaemia using thromboelastography. Vet J. 2012;191:347‐353. [DOI] [PubMed] [Google Scholar]
- 32. Wiinberg B, Jensen AL, Rojkjaer R, et al. Validation of human recombinant tissue factor‐activated thromboelastography on citrated whole blood from clinically healthy dogs. Vet Clin Pathol. 2005;34:389‐393. [DOI] [PubMed] [Google Scholar]
- 33. deLaforcade A, Goggs R, Wiinberg B. Systematic evaluation of evidence on veterinary viscoelastic testing part 3: assay activation and test protocol. J Vet Emerg Crit Care. 2014;24:37‐46. [DOI] [PubMed] [Google Scholar]
- 34. Goggs R, Brainard B, de Laforcade AM, et al. Partnership on rotational viscoelastic test standardization (PROVETS): evidence‐based guidelines on rotational viscoelastic assays in veterinary medicine. J Vet Emerg Crit Care. 2014;24:1‐22. [DOI] [PubMed] [Google Scholar]
- 35. Allegret V, Dunn M, Bedard C. Monitoring unfractionated heparin therapy in dogs by measuring thrombin generation. Vet Clin Pathol. 2011;40:24‐31. [DOI] [PubMed] [Google Scholar]
- 36. Kumar A, Mhaskar R, Grossman BJ, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion. 2015;55:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 37. Humbrecht C, Kientz D, Gachet C. Platelet transfusion: current challenges. Transfus Clin Biol. 2018;25:151‐164. [DOI] [PubMed] [Google Scholar]
- 38. Cummings FO, Rizzo SA. Treatment of presumptive primary immune‐mediated thrombocytopenia with mycophenolate mofetil versus cyclosporine in dogs. J Small Anim Pract. 2017;58:96‐102. [DOI] [PubMed] [Google Scholar]
- 39. Scuderi MA, Snead E, Mehain S, et al. Outcome based on treatment protocol in patients with primary canine immune‐mediated thrombocytopenia: 46 cases (2000‐2013). Can Vet J. 2016;57:514‐518. [PMC free article] [PubMed] [Google Scholar]
- 40. Yau VK, Bianco D. Treatment of five haemodynamically stable dogs with immune‐mediated thrombocytopenia using mycophenolate mofetil as single agent. J Small Anim Pract. 2014;55:330‐333. [DOI] [PubMed] [Google Scholar]
- 41. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346‐352. [DOI] [PubMed] [Google Scholar]
- 42. Balog K, Huang AA, Sum SO, et al. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2013;27:536‐541. [DOI] [PubMed] [Google Scholar]
- 43. Rozanski EA, Callan MB, Hughes D, et al. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune‐mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477‐481. [DOI] [PubMed] [Google Scholar]
- 44. Page LK, Psaila B, Provan D, et al. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138:245‐248. [DOI] [PubMed] [Google Scholar]
- 45. Valeri CR, MacGregor H, Barnard MR, et al. Survival of baboon biotin‐x‐n‐hydroxysuccinimide and (111)in‐oxine‐labelled autologous fresh and lyophilized reconstituted platelets. Vox Sang. 2005;88:122‐129. [DOI] [PubMed] [Google Scholar]
- 46. Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood. 2018;131:1512‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Read MS, Reddick RL, Bode AP, et al. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: potential for long‐term storage of dried platelets for transfusion. Proc Natl Acad Sci U S A. 1995;92:397‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishler BC, Hale A, Moskowitz K. Stableplate rx canine promotes in vitro thrombin generation and thrombus formation under high shear. J Vet Intern Med. 2019;33:2483. [Google Scholar]
- 49. Deng X, Wu X, Ge G, et al. Evaluation of the lyophilized platelets function by thromboelastography in an in vitro platelet transfusion model. Clin Lab. 2017;63:507‐513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Box and whisker plots of leukocyte count (WBC) and neutrophil count in the 2 arms of the trial. The middle lines represent the median change, the boxes represent the 25% and 75% percentiles of the change, and the whiskers represent the minimum and maximum changes in the variables. (A and C) Comparisons of leukocyte and neutrophil counts between groups at each of the time points baseline (T0), 1 hour after the transfusion (T1) and 24 hours after the transfusion (T24). There were no significant differences between the 2 groups at any of the time points (Kruskal‐Wallis test). (B and D) Comparisons of leukocyte and neutrophil counts in each group over time. Plots sharing a letter code were not significantly different from each other. Plots not sharing a letter code were significantly different from each other P < .05 after correction for multiple comparisons (Friedman test). LP represents the lyophilized platelet group; CPP represents the cryopreserved platelet group.
Data S1: Supporting Information.
Data S2: Supporting Information.
Data S3: Supporting Information.
Data S4: Supporting Information.


