Abstract
Background
Localized splenic histiocytic sarcoma (HS) in dogs is a poorly understood disease, and could have longer survival times than disseminated or hemophagocytic HS. Understanding the clinical behavior of localized splenic HS can refine treatment recommendations.
Objective
To describe the clinical characteristics and outcomes of dogs with localized splenic HS.
Animals
Fourteen client‐owned dogs with histologically confirmed splenic HS that received splenectomy.
Methods
Multi‐institutional retrospective case series—medical records of dogs with splenic HS were reviewed. Dog signalment, clinicopathologic data, primary and adjuvant treatments, and outcomes were obtained. Survival data were calculated using Kaplan‐Meier analysis. Dog variables such as age, weight, platelet counts were reported using descriptive statistics. The Cox proportional hazards regression method was used to determine whether potential risk factors (weight, age, albumin level, hematocrit, and platelet count) were associated with PFI.
Results
Median survival time for the dogs in this study was 427 days. Twelve dogs received adjuvant lomustine‐based chemotherapy. Five dogs (35.7%) were suspected or confirmed to have developed metastatic disease. Eleven dogs died of disease, 1 dog died of unrelated cause, and 2 dogs were alive at final follow‐up.
Conclusions and Clinical Significance
Histiocytic sarcoma in dogs can manifest as a localized form in the spleen. Dogs with localized splenic HS treated with surgery ± chemotherapy can experience survival times over a year.
Keywords: dog, oncology, spleen, splenectomy
Abbreviations
- CCNU
1‐(2‐chloroethyl)‐3‐cyclohexyl‐1‐nitrosourea
- CT
computed tomography
- DHS
disseminated histiocytic sarcoma
- H&E
hematoxylin and eosin
- HHS
hemophagocytic histiocytic sarcoma
- HS
histiocytic sarcoma
- Iba1
ionized calcium‐binding adapter molecule 1.
- IHC
immunohistochemistry
- LHS
localized histiocytic sarcoma
- MST
median survival time
- PAHS
periarticular histiocytic sarcoma
- PFI
progression‐free interval
- VCOG‐CTCAE
veterinary cooperative oncology group‐common terminology for adverse events
1. INTRODUCTION
Histiocytic diseases in dogs include a spectrum of neoplasms ranging from cutaneous histiocytoma to histiocytic sarcoma (HS), encompassing a wide range of biological behaviors and affecting different dog breeds. 1 , 2 , 3 , 4 Histiocytic sarcoma can manifest as multifocal disseminated or localized disease, and it can be classified into 3 distinct forms: hemophagocytic HS (HHS), localized HS (LHS), and disseminated HS (DHS). 2 , 5 Hemophagocytic HS is a unique and extremely aggressive subtype, primarily arising from splenic red pulp macrophages or bone marrow macrophages, and is characterized by organ infiltration of neoplastic macrophages with marked erythrophagocytosis. 5 Localized HS present as discrete lesions in tissues such as the spleen, skin, subcutis, lymph node, articular region, lung and bone marrow. 1 Localized HS, which arises primarily from dendritic cells, is generally considered a subtype with a relatively favorable prognosis. 6 Disseminated HS, previously referred to as malignant histiocytosis, also arises primarily from dendritic cells, and is characterized by multicentric disease at diagnosis, or by the progression of LHS beyond the regional lymph nodes. 1 Disseminated HS carries a poor prognosis, potentially due to DHS being more biologically aggressive. 7 It currently remains unclear whether all cases of DHS represent progression of LHS, or some DHS cases are manifestations of a distinct entity.
Histiocytic sarcomas in dogs are aggressive tumors with metastatic rates as high as 70%‐91%. 8 , 9 However, as we gain understanding of the different manifestations of HS in dogs, the associations between HS subtypes and prognosis also become better delineated. Hemophagocytic HS carries the poorest prognosis of all the HS subtypes, with a median survival of 4 weeks. 5 Dogs with DHS can respond to chemotherapy of either lomustine alone or a combination of lomustine and doxorubicin, with up to a 46%‐58% response rate. 10 , 11 Dogs with LHS such as periarticular HS (PAHS) and primary pulmonary HS can have longer survival times. 6 , 12 Dogs with primary pulmonary HS have significantly longer survival times when treated with surgery and chemotherapy (374 days) compared to dogs that did not receive surgical treatment (131 days). 6 Localized HS can manifest in other anatomic sites including the spleen, but to date, only PAHS and primary pulmonary HS have been characterized in detail. 6 , 12
The spleen is a common site of disease in dogs with HS, as it is frequently involved in cases of disseminated or hemophagocytic HS, with both subtypes associated with poorer prognosis. 4 , 5 , 7 , 9 LHS in dogs can also manifest in the spleen, but it is rarely reported, and thus its biological behavior is poorly understood. 4 With the paucity of prognostic information on splenic LHS in dogs, veterinarians do not have the adequate tools to help owners make informed decisions, potentially resulting in dogs with LHS being euthanized based on survival data gathered from DHS or HHS. Based on the survival data for PAHS and primary pulmonary HS in dogs, we postulate that splenic LHS can yield more favorable outcomes if treated by surgical resection via splenectomy. The objective of this retrospective study was to report disease outcomes, and identify clinical variables associated with survival in dogs with splenic LHS.
2. MATERIALS AND METHODS
A multi‐institutional retrospective study was performed with review of medical records from 3 veterinary academic institutions (Virginia‐Maryland College of Veterinary Medicine, Oregon State University, Auburn University) and 3 private specialty clinics (Vista Veterinary Specialists, Sacramento, CA; Hope Veterinary Specialists, Malvern, PA; MedVet Medical and Cancer Center, Cincinnati, OH). Dogs that underwent abdominal exploratory surgery and splenectomy between the years 2000 and 2019 to resect a splenic tumor that was histologically diagnosed as HS were included. Staging and restaging diagnostics before and after surgery included either thoracic radiographs or computed tomography (CT) scans of the thorax, or both thoracic radiographs and CT scans, abdominal radiographs, either abdominal ultrasound studies or abdominal CT scans, or both abdominal ultrasound and CT scans, fine‐needle aspirates of tissues of interest during staging diagnostics. Medical record data collected included dog characteristics, clinicopathologic data, date of diagnosis, date of surgery, histological and immunohistochemistry (IHC) reports, chemotherapy protocols and associated adverse events, date of disease progression, date of death, cause of death. Adverse events were recorded according to the veterinary cooperative oncology group‐common terminology for adverse events (VCOG‐CTCAE). Follow‐up data were obtained from referring veterinarians or owners.
Age of the dogs was calculated based on date of surgery to resect the splenic HS. Kaplan‐Meier survival analysis was used to estimate median progression‐free interval (PFI) and median survival after surgery. PFI was calculated as the time from surgery to the time of first detectable signs of distant metastasis. Metastases were confirmed either via cytological or histological evaluation of lesions, or were presumed based on suspected lesions noted on physical examination, imaging findings, or both physical examination and imaging findings. Dogs were censored from PFI analysis if no detectable metastasis was noted at last follow‐up or at death. Survival was calculated as time from the date of surgery to date of death due to disease. Dogs with unknown causes of death were presumed to have died from disease. Dogs that were alive or died from causes other than disease were censored from the survival analysis. Dogs that were still alive at time of study had follow‐up times calculated as time from date of surgery to date of last follow‐up.
The D'Agostino‐Pearson test for normality was used. The Log‐Rank test was used to compare the effect of potential risk factors (weight, age, albumin level, hematocrit, and platelet count). The Cox proportional hazards regression method was used to determine whether potential risk factors (weight, age, albumin level, hematocrit, and platelet count) were associated with PFI after treatment. Platelet counts categorized in 3 groups (0‐250 K), (250‐500 K), and (500‐750 K+), based on the quantiles of the actual platelet number on the day of the surgery. Statistical significance was set at P < 0.05. The potential risk factors were entered in the regression model if their P < .05 and removed if P > .1. All reported P‐values were 2‐sided and P‐values <.05 were considered statistically significant. Statistical analyses were performed with standard software (MedCalc Statistical Software version 19.1, MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org; 2019).
3. RESULTS
3.1. Dog characteristics
A total of 14 dogs were identified from the medical record searches as meeting the inclusion criteria for evaluation. Breeds represented included mixed breed dogs (n = 2), golden retriever (n = 2), rottweiler (n = 2), with the remainder of the cases represented by individual distinct breeds—cocker spaniel, Jack Russell terrier, Yorkshire terrier, Bouvier des Flanders, standard poodle, Labrador retriever, miniature daschund, Shih Tzu. There were 8 spayed females, 6 castrated males. The mean age at diagnosis was 11.0 years (SD ± 3.0) and mean weight was 24.1 kg (SD ± 13.8).
3.2. Clinicopathologic and imaging characteristics
Preoperative complete blood counts and serum biochemistry were performed for 14 cases, platelet counts, hematocrit, albumin and cholesterol concentrations were evaluated. Mean values at the time of presentation were 311 214 platelets (SD ± 175 047), albumin 2.8 g/dL (SD ± 0.5), cholesterol 222.0 mg/dL (SD ± 90.0), hematocrit 32.2% (SD ± 10.3). Ten dogs had platelet counts within normal reference range, 1 dog had platelet counts below reference range, 2 dogs had platelet counts above reference range, and platelet count was not available for 1 dog. Eleven dogs had albumin concentrations within normal reference range, and 3 dogs had albumin below reference range. Eight dogs had cholesterol concentrations within normal reference range, 1 dog had a cholesterol level below reference range, 2 dogs had cholesterol concentrations above reference range, and cholesterol concentrations were not available for 3 dogs. Five dogs had hematocrits within normal reference range, and 9 dogs had hematocrits below reference range. Within the 3 platelet groups, there were 4 dogs in the (0‐250 K) group, 4 dogs in the (250‐500 K) group, and 5 dogs in the (500‐750 K+) group.
Before surgery, 14 cases had thoracic radiography performed, of which 3 cases had thoracic CT scans performed in addition to thoracic radiographs. Evidence of a pulmonary mass (3.5 × 4.5 × 2.8 cm) in the accessory lung lobe was noted in 1 case during initial staging. The mass was not sampled for cytologic or histologic evaluation due to the inaccessibility of its location. The dog was euthanized 205 days after splenectomy with no objective increase in the size of the pulmonary mass from original diagnosis or development of new pulmonary lesions. This case was included in the study because the lack of pulmonary disease progression rendered it highly unlikely that the pulmonary mass represented a disseminated form of HS. Before surgery, 13 cases had an abdominal ultrasound performed, 1 case had an abdominal CT scan performed. Abdominal lymphadenopathy was noted in 3 cases, but samples were not obtained for diagnostic evaluation before surgery.
3.3. Treatment and histopathological findings
Surgical removal of the splenic mass via splenectomy was performed in all 14 cases. All resected splenic masses were diagnosed histopathologically with hematoxylin and eosin (H&E) staining as histiocytic sarcoma, with the diagnosis confirmed using either CD18 and/or Iba1 IHC labeling (11 dogs had Iba‐1 labeling, 5 dogs had CD18 labeling performed on their splenic masses), and histopathological evaluation was performed by the home institution's laboratory of choice. Three dogs that had abdominal lymphadenopathy noted during imaging diagnostics for staging had their enlarged abdominal lymph nodes extirpated at the same time as splenectomies were performed, and the lymph nodes underwent histopathological H&E evaluation. One dog was diagnosed with marginal zone (indolent) lymphoma in the periportal lymph node, 2 dogs were diagnosed with nodal HS (1 in the splenic lymph node, location of the lymph node in the second dog was not reported).
Twelve dogs received adjuvant chemotherapy and first line protocols included either a single agent (Lomustine [CCNU], 10 dogs) or an alternating combination of CCNU and doxorubicin (2 dogs). The median number of doses of CCNU as a single agent was 5, and the median dose at first administration was 60 mg/m2. One dog that received alternating CCNU and doxorubicin received 2 doses of each drug, and the other dog received 3 doses of each drug. Doxorubicin was administered at 30 mg/m2 for both dogs, CCNU was administered at 60 mg/m2 for the dog that received 2 doses, and at 69 mg/m2 for dog that received 3 doses. The protocols and dosing of chemotherapeutic drugs were clinician dependent, and the dosing regimen ranged from 4 treatments to indefinite number of treatments. Out of the 12 dogs that received chemotherapy, 4 did not experience any adverse effects with administration of chemotherapy. An average dose reduction of about 10% occurred in 5 dogs due to adverse signs with 2 dogs requiring additional dose reductions due to persistent adverse effects. Adverse effects observed were neutropenia, increase in serum ALT activity, and signs of gastrointestinal disease. One dog had a 2‐week delay in treatment after experiencing adverse effects. One dog had termination of treatment after adverse effects associated with chemotherapy administration. Three dogs received drugs in addition to first‐line chemotherapy including pamidronate (2 dogs), and zoledronate (1 dog). Two dogs developed progressive disease and were treated with second line therapy, including doxorubicin and pamidronate (1 dog), and palliative radiation therapy in combination with vinorelbine and zoledronate (1 dog).
3.4. Dog outcomes
Five out of 14 dogs were suspected or confirmed to have developed metastatic disease. The presence of metastatic disease after splenectomy was suspected based on imaging diagnostics in all 5 dogs: 3 dogs had abdominal ultrasounds performed, 1 dog had thoracic and abdominal CT scans performed, and 1 dog had an abdominal CT scan performed. Metastatic disease was confirmed by cytology in 3 cases, and the sites of metastases were liver (1 dog), liver and hepatic lymph node (1 dog), mesentery (1 dog). One dog had a left adrenal mass and an enlarged left sublumbar lymph node noted on abdominal ultrasound, anemia and thrombocytopenia, the owners declined any further diagnostics and opted for euthanasia. This dog was diagnosed with a pulmonary mass before splenectomy, no progression of pulmonary disease was noted on thoracic radiographs taken just before euthanasia. The adrenal mass and left sublumbar lymphadenopathy were presumed to represent metastatic HS. In the other dog, an abdominal mass was noted incidentally on abdominal palpation during an annual physical examination approximately 5 months after splenectomy, and this mass was observed on a subsequent abdominal CT scan. No diagnostic confirmation of metastatic HS was performed since the owner declined any further diagnostics. This dog with the abdominal mass also was diagnosed with nodal HS in an abdominal lymph node at time of splenectomy, and the subsequent abdominal mass was presumed to represent metastatic HS. The second dog that had a metastatic abdominal (splenic) lymph node diagnosed at time of splenectomy did not develop metastatic disease and died of Evan's Syndrome 557 days after splenectomy.
Overall, 12 dogs were euthanized or died, and 3 dogs were censored from the survival analysis. The overall median survival time (MST) was 427 days, range 115‐1091 days (Figure 1), and the overall median PFI was 205 days, range 134‐514 days. Eleven dogs died of disease, 1 dog died of unrelated cause, and 2 dogs were alive at final follow‐up at 541 and 258 days after surgery. None of the risk factors analyzed had any significant associations with PFI.
FIGURE 1.
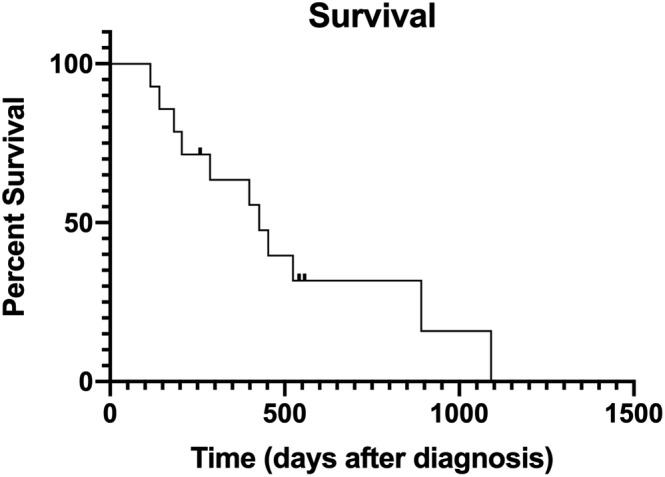
Kaplan–Meier survival curve estimating overall survival in 14 dogs that underwent surgical removal of splenic histiocytic sarcoma. Three dogs were censored from survival analysis. Day 0 designates the day of surgery
4. DISCUSSION
The outcome of localized splenic HS in dogs has not previously been evaluated. Since splenic involvement in dogs with HS is often associated with the disseminated form of the disease, evaluating any potential differences in the biological behavior of localized splenic HS from its disseminated counterpart provides important prognostic information. The MST and PFI for dogs in this study were 427 days and 158 days respectively, which are comparable to that for LHS. 4 , 7 One study reported the DHS form of disease to be associated with an increased risk of death, with a reported MST of 78 days for dogs with DHS compared to 398 days for dogs with LHS. 7 The spleen is a commonly involved organ in DHS, for example, in 1 report featuring DHS in dogs, the spleen was the most frequently affected site, with 16 of 20 dogs having splenic lesions. 4 Given the prevalence of splenic involvement in DHS and the apparent poorer prognosis associated with DHS, determining whether localized splenic HS carries a different prognosis than DHS allows veterinarians to more accurately prognosticate and therefore guide owner decision‐making using data more specific to localized splenic HS rather than assuming that splenic HS is always representative of DHS.
LHS in dogs, especially splenic LHS, has not been extensively characterized. A study evaluating the outcome of 16 dogs with LHS treated with definitive local and adjuvant therapy reported a MST of 568 days, but only 2 of these dogs had splenic HS. The 2 subtypes of LHS that have been specifically evaluated are PAHS and localized primary pulmonary HS. Periarticular HS is a common periarticular tumor in dogs. In the aforementioned study that reported outcome differences between LHS and DHS, the majority of LHS cases (59.6%) were dogs with PAHS. 7 In another report, overall median survival time for dogs with PAHS that received definitive treatment was 391 days, with a significantly longer MST of 980 days for dogs that did not present with metastatic disease compared to the MST of 253 days for dogs with metastasis. 12 Similarly, in localized primary pulmonary HS, dogs treated with definitive therapy experienced a median survival of 374 days, and having metastasis at diagnosis negatively impacted overall survival. 6 There were only 2 dogs in the current study that had confirmed metastatic HS (nodal) at time of surgery compared to the 68% of dogs in the aforementioned study on PAHS, thus a meaningful statistical analysis of potential differences in survival in dogs with and without metastasis was not feasible. 12 The 2 dogs with nodal HS metastasis at time of surgery had variable individual survival times—1 dog survived 286 days before dying of disease, the other survived 557 days before dying of an unrelated cause. The dog with a pulmonary mass diagnosed before surgery was euthanized 205 days after splenectomy due to suspected disease progression (left adrenal mass with left sublumbar lymph node enlargement, thrombocytopenia, anemia), with no progression of the pulmonary lesion, and thus the pulmonary mass was not likely to have represented metastatic HS or DHS. Further evaluation with a larger cohort of dogs with splenic HS can help elucidate whether presenting with metastatic disease is prognostic for survival.
Morphologically, LHS and DHS lesions can have similar, if not identical, histologic features, ranging from large round cells to plump spindle cells and multinucleated giant cells. 4 The immunohistochemical phenotype of LHS and DHS cells is also similar, with expression of markers consistent with a myeloid dendritic antigen presenting cell origin. 4 However, LHS and DHS exhibit different biological behaviors. Thus the question has been raised as to whether DHS represents the advanced form of LHS along the continuum of disease progression, or whether DHS and LHS are separate disease entities. Further studies are needed to elucidate an answer to that question. Since LHS and DHS are not distinguishable histologically and immunohistochemically, the diagnosis of a localized splenic HS currently is mainly a clinical 1, with histologic confirmation of a primary HS lesion confined to the spleen. The variety of morphologic features noted on histologic evaluation of HS emphasizes the utility of immunohistochemical labeling to aid in the diagnosis, as HS can be misdiagnosed as other sarcomas such as soft tissue sarcoma. Immunohistochemical markers for molecules associated with antigen presentation, for example, CD18, MHCII can be used to diagnose HS. 4 , 13 More recently, ionized calcium‐binding adapter molecule 1 (Iba1) IHC labeling has been used to diagnose HS. Iba1 has been shown to be expressed by most subpopulations of the murine macrophage lineage. 14 Iba1 is specific for cells of the macrophage lineage in dogs, and Iba1 has been used to identify HS of the central nervous system in veterinary clinical reports. 15 , 16 , 17 Positive IHC labeling with Iba1 or CD18 was used to confirm the diagnosis of HS in this study.
Dog variables including hematocrit, albumin and cholesterol concentrations, platelet counts, weight and age were evaluated for associations with prognosis. Anemia, thrombocytopenia, hypoalbuminemia, and splenic involvement are associated with decreased survival times, based on univariable analysis in dogs. 10 Thrombocytopenia and hypoalbuminemia were negative predictors of long‐term survival. 10 HS in dogs reported dogs with nonhemophagocytic HS had significantly higher hematocrit and platelet counts than dogs with hemophagocytic HS and hypocholesterolemia is more frequently noted in hemophagocytic HS. 5 Unlike previous reports, this study did not find any significant associations between dog variables (hematocrit, cholesterol, albumin, weight, age) and disease outcome. The small sample size in this study could have led to a type II error.
Limitations of this study include the small study population and its retrospective nature, with its inherent owner recall bias and lack of standardized follow‐up. The small number of dogs in this study does not allow for meaningful statistical and survival analyses and the resultant low statistical power renders our findings of lack of association with survival unproductive. However, increasing awareness of the potential differences in disease characteristics and outcomes of localized splenic HS can facilitate larger‐scale investigations to increase our understanding of this disease subset. None of the owners in this study elected for a necropsy after the death of their pet, thus a cause of death cannot be confirmed. In order to make a conservative estimation of the cause of death, dogs that died of unknown causes were presumed to have died of disease. The participation of multiple clinics in this study contributed to the lack of standardization of staging diagnostics and adjuvant treatment protocols.
Another limitation of this study included the inability to determine whether any of the cases represented the hemophagocytic form of HS. Ideally, CD11d IHC labeling using fresh frozen tissue would be needed to make the diagnosis of hemophagocytic HS, and the lack of fresh frozen tissue was a limitation of this study. However, the relatively favorable MST noted in this study makes it less likely that the dogs had hemophagocytic HS, which has a poor prognosis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Since the period during which the data were acquired ranged from 2000‐2019, we cannot confirm that there was no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
No funding was received for this study. The authors thank Dr Kristin Couto, Dr Christine Mullin, Dr Darren Kelly, and Dr Jeanne Lane for their contribution of cases to the study.
Latifi M, Tuohy JL, Coutermarsh‐Ott SL, Klahn SL, Leeper H, Dervisis N. Clinical outcomes in dogs with localized splenic histiocytic sarcoma treated with splenectomy with or without adjuvant chemotherapy. J Vet Intern Med. 2020;34:2645–2650. 10.1111/jvim.15910
REFERENCES
- 1. Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol. 2014;51:167‐184. [DOI] [PubMed] [Google Scholar]
- 2. Fulmer AK, Mauldin GE. Canine histiocytic neoplasia: an overview. Can Vet J. 2007;48:1041‐1043. 1046‐1050. [PMC free article] [PubMed] [Google Scholar]
- 3. Dobson JM. Breed‐predispositions to cancer in pedigree dogs. ISRN Vet Sci. 2013;2013:941275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74‐83. [DOI] [PubMed] [Google Scholar]
- 5. Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006;43:632‐645. [DOI] [PubMed] [Google Scholar]
- 6. Marlowe KW, Robat CS, Clarke DM, et al. Primary pulmonary histiocytic sarcoma in dogs: a retrospective analysis of 37 cases (2000‐2015). Vet Comp Oncol. 2018;16:658‐663. [DOI] [PubMed] [Google Scholar]
- 7. Dervisis NG, Kiupel M, Qin Q, Cesario L. Clinical prognostic factors in canine histiocytic sarcoma. Vet Comp Oncol. 2017;15:1171‐1180. [DOI] [PubMed] [Google Scholar]
- 8. Craig LE, Julian ME, Ferracone JD. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol. 2002;39:66‐73. [DOI] [PubMed] [Google Scholar]
- 9. Fidel J, Schiller I, Hauser B, et al. Histiocytic sarcomas in flat‐coated retrievers: a summary of 37 cases (November 1998‐March 2005). Vet Comp Oncol. 2006;4:63‐74. [DOI] [PubMed] [Google Scholar]
- 10. Skorupski KA, Clifford CA, Paoloni MC, et al. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Intern Med. 2007;21:121‐126. [DOI] [PubMed] [Google Scholar]
- 11. Cannon C, Borgatti A, Henson M, Husbands B. Evaluation of a combination chemotherapy protocol including lomustine and doxorubicin in canine histiocytic sarcoma. J Small Anim Pract. 2015;56:425‐429. [DOI] [PubMed] [Google Scholar]
- 12. Klahn SL, Kitchell BE, Dervisis NG. Evaluation and comparison of outcomes in dogs with periarticular and nonperiarticular histiocytic sarcoma. J Am Vet Med Assoc. 2011;239:90‐96. [DOI] [PubMed] [Google Scholar]
- 13. Constantino‐Casas F, Mayhew D, Hoather TM, Dobson JM. The clinical presentation and histopathologic‐immunohistochemical classification of histiocytic sarcomas in the Flat Coated Retriever. Vet Pathol. 2011;48:764‐771. [DOI] [PubMed] [Google Scholar]
- 14. Kohler C. Allograft inflammatory factor‐1/Ionized calcium‐binding adapter molecule 1 is specifically expressed by most subpopulations of macrophages and spermatids in testis. Cell Tissue Res. 2007;330:291‐302. [DOI] [PubMed] [Google Scholar]
- 15. Pierezan F, Mansell J, Ambrus A, Hoffmann AR. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J Comp Pathol. 2014;151:347‐351. [DOI] [PubMed] [Google Scholar]
- 16. Ide T, Uchida K, Kagawa Y, Suzuki K, Nakayama H. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. J Vet Diagn Invest. 2011;23:127‐132. [DOI] [PubMed] [Google Scholar]
- 17. Ide T, Uchida K, Tamura S, Nakayama H. Histiocytic sarcoma in the brain of a cat. J Vet Med Sci. 2010;72:99‐102. [DOI] [PubMed] [Google Scholar]


