Abstract
Background
Chronic kidney disease (CKD) and acute exacerbation of CKD (ACKD) are common in dogs.
Objective
To characterize the etiology, clinical and laboratory findings, and short‐ and long‐term prognosis of dogs with ACKD.
Animals
One hundred dogs with ACKD.
Methods
Medical records of dogs diagnosed with ACKD admitted to a veterinary teaching hospital were retrospectively reviewed.
Results
The most common clinical signs included anorexia (84%), lethargy (77%), vomiting (55%) and diarrhea (37%). Presumptive etiology included inflammatory causes (30%), pyelonephritis (15%), ischemic causes (7%), other (3%), or unknown (45%). Median hospitalization time was 5 days (range, 2‐29 days) and was significantly longer in survivors (6 days; range, 2‐29 days) compared with nonsurvivors (4 days; range, 2‐20 days; P < .001). Mortality rate was 35%. International Renal Interest Society (IRIS) acute kidney injury (AKI) grade at presentation was associated (P = .009) with short‐term survival, but presumptive etiology was not (P = .46). On multivariable analysis; respiratory rate (P = .01), creatine kinase (CK) activity (P = .005) and serum creatinine concentration (SCR; P = .04) at presentation were associated with short‐term outcome. Median survival time of dogs discharged was 105 days (95% confidence interval [CI], 25‐184), with 35 and 8 dogs surviving up to 6 and 12 months, respectively. Presumptive etiology (P = .16) and SCR (P = .59) at discharge were not predictors of long‐term survival.
Conclusion and Clinical Importance
Short‐term outcome of dogs with ACKD is comparable to those with AKI but long‐term prognosis is guarded. The IRIS AKI grade at presentation is a prognostic indicator of short‐term outcome.
Keywords: acute kidney injury, azotemia, canine, outcome, prognosis, renal failure
Abbreviations
- ACKD
acute on chronic kidney disease
- AKI
acute kidney injury
- CK
creatine kinase
- CKD
chronic kidney disease
- DGGR
1,2‐o‐dilautryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester
- GFR
glomerular filtration rate
- IRIS
International Renal Interest Society
- MST
median survival time
- SCR
serum creatinine concentration
1. INTRODUCTION
Chronic kidney disease (CKD) is defined by presence of functional or structural changes in 1 or both kidneys for >3 months. 1 It is the most common kidney disease in small animals, with an estimated prevalence of up to 7% in dogs. 2 , 3 Chronic kidney disease affects dogs of all ages, but more commonly older dogs. 1 It is a progressive disease in dogs, but progression rate is highly variable. 1 , 4 Progression may be gradual and constant or a consequence of sequential acute kidney injury (AKI) episodes of variable magnitude. 5 Recognized markers associated with the progression and outcome of CKD in dogs include presence of anemia, low body condition score, proteinuria, hypertension, hypoalbuminemia, and International Renal Interest Society (IRIS) stage. 1 , 4 , 6 , 7 Chronic kidney disease in dogs may be familial, but more commonly, it is an acquired condition. 3 Multiple causes have been implicated in the pathogenesis of CKD, including glomerular diseases, infections (eg, chronic pyelonephritis), repeated ischemic events, nephrotoxicity, neoplasia, previous AKI or urinary obstruction, but often the etiology is unknown at presentation and remains unidentified throughout the disease course. 3 , 5 , 8 Because of limitations of currently available diagnostic markers, CKD often is diagnosed late in its course, when renal functional impairment exceeds compensatory mechanisms, and irreversible, severe renal parenchymal damage already has occurred.
Acute kidney injury is defined by sudden damage to the renal parenchyma and may be accompanied by an abrupt decrease in glomerular filtration rate (GFR). 9 Although the injury is potentially reversible, the mortality rate of AKI in dogs, cats, and humans is approximately 50%. 9 , 10 , 11 , 12 Several causes have been identified for AKI, including ischemia, infection, nephrotoxicity, and inflammatory processes. 10 , 13 Treatment of AKI is aimed at elimination of the underlying cause so as to minimize further damage, controlling clinical and laboratory abnormalities, preventing and treating complications (eg, hypertension), and providing supportive care until recovery occurs. 10 The prognosis of AKI varies, depending on several factors, including cause (reversible vs irreversible injury), severity of kidney injury, involvement of other body organs, availability of treatment options (eg, hemodialysis), and owners' compliance. 10 , 12 , 13
Animals with stable CKD may experience an acute decrease in kidney function (ie, acute on chronic kidney disease [ACKD]). The pathogenesis, clinical presentation and laboratory abnormalities of ACKD may resemble those of AKI, 5 which occasionally makes differentiation between AKI and ACKD challenging. Although ACKD is common, 8 , 14 , 15 its causes, clinical course and short‐ and long‐term outcomes have yet to be described in dogs. Recently, ACKD was described in cats. 16 Moreover, with the guarded long‐term prognosis of dogs with CKD, 17 information regarding the prognosis of ACKD is essential for both dog owners and veterinarians for proper treatment decision‐making.
The aim of our retrospective study was to characterize the causes, clinical signs, laboratory results, and the short‐ and long‐term prognosis of dogs with ACKD.
2. MATERIALS AND METHODS
2.1. Selection of dogs and definitions
The medical records of dogs diagnosed with ACKD in the Koret School of Veterinary Medicine, The Robert H. Smith Faculty of Agriculture, Food and Environment, Hebrew University of Jerusalem, were retrospectively reviewed (years 2017‐2019). Dogs were included in this study if admitted to the teaching hospital with acute onset of clinical signs compatible with AKI (eg, acute anorexia, lethargy, vomiting) and azotemia (serum creatinine concentration [SCR] >1.6 mg/dL), in accordance with the IRIS guidelines. Additionally, ≥1 of the following criteria had to be fulfilled to establish a diagnosis of CKD: (a) previous diagnosis of CKD based on persistently increased SCR, with concurrent increase in SCR of >25% above its previously documented baseline; (b) dogs with abdominal ultrasound findings compatible with CKD, based on presence of ≥2 of the following: increased renal echogenicity, markedly decreased renal corticomedullary differentiation, decreased kidney size or asymmetry, and renal cysts or irregular renal contour. 18
Survivors were defined as those alive at discharge from the hospital. Nonsurvivors died or were euthanized because of lack of improvement, despite treatment during hospitalization. Dogs were excluded from the study if euthanized within the first 48 hours after admission.
The presumptive causes of ACKD were classified as inflammatory, ischemic, pyelonephritis or other. Inflammatory causes included: pancreatitis, diagnosed based on compatible history, clinical signs and ultrasonographic findings 19 , 20 , 21 or increased serum 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester (DGGR)‐lipase activity (reference interval [RI], <108 U/L); or glomerulopathies, diagnosed based on presence of persistent, high magnitude proteinuria after exclusion of prerenal and postrenal causes of protenuria. Ischemia was defined in dogs with recent history of furosemide (2 dogs) or nonsteroidal anti‐inflammatory drug administration (2 dogs), in dogs that had clinical signs of severe dehydration (2 dogs) or had undergone general anesthesia (1 dog) immediately before occurrence of AKI. Pyelonephritis was diagnosed based on positive urine culture, urine sediment findings (ie, pyuria and bacteruria) and ultrasonograpic findings consistent with pyelonephritis. 22 , 23 “Other cause” included AKI secondary to unilateral ureteral obstruction, hypercalcemia, and type‐1 cardio‐renal syndrome (ie, heart failure in which diuretics were not administered before clinical deterioration and worsening of azotemia). 24 When 2 presumptive causes were present concurrently, the primary cause was used for analysis.
2.2. Collection of samples and laboratory methods
Whole blood samples in potassium‐EDTA tubes for CBC (Advia 120 or 2120i, Siemens, Medical Solutions Diagnostics GmbH, Erlangen,, Germany; Abacus Junior Vet, Diatron, Wien, Austria) and whole blood samples in plain tubes with gel separators for serum biochemistry (Cobas Integra 400 Plus or Cobas 6000, Roche, Mannheim, Germany) were collected at presentation and analyzed within 60 minutes after collection. Urine samples were obtained by cystocentesis for urinalysis including dipstick chemistry (Urilux, Roche, Mannheim Germany) and sediment cytology (SediVue Dx, IDEXX Laboratories Inc, Westbrook, Maine or manual microscopy), as well as for bacterial culture. Urine specific gravity (USG) was measured using a clinical refractometer (Atago, Tokyo, Japan). Pyuria and hematuria were defined as presence of >5 leukocytes or erythrocytes, respectively per high‐power field. Bacteriuria was diagnosed if bacteria were observed in sediment cytology. Proteinuria was defined as a urine dipstick result of ≥1+ (ie, ≥30 mg/dL). Urine protein‐to‐creatinine ratio (Cobas Integra 400 Plus or Cobas 6000, Roche, Mannheim, Germany) was only measured in dogs with severe proteinuria (urine dipstick result +4) and clinical concern that glomerular disease was the inciting cause for ACKD.
2.3. Follow‐up
Long‐term survival of dogs discharged from the hospital was calculated as the number of days from discharge until death or euthanasia. Date of death or euthanasia was obtained from the medical records or by telephone interview. Dogs lost to follow‐up were censored.
To test the association between SCR at discharge and long‐term outcome, dogs were categorized into groups based on the IRIS CKD staging SCR ranges. These are not referred to as CKD stages, because at discharge, these dogs were not considered at steady state and therefore could not have been staged at this time point.
2.4. Statistical analysis
The distribution pattern of continuous variables was assessed using the Shapiro‐Wilk test. Because most data were non‐normally distributed, the Mann‐Whitney's U‐test was used to compare continuous variables between 2 groups. A χ2 or the Fisher's exact test was used to examine the association between 2 qualitative variables. The univariable analysis included the association of the outcome with clinical signs, hematology, biochemistry, and urinalysis variables. Variables significantly (P < .1) associated with death in univariable analysis were subjected to forward multivariable logistic regression analysis to further examine their association with the outcome. Kaplan‐Meier survival curves with log‐rank tests were used to compare median survival times (MST) of dogs categorized based on SCR at presentation and the presumptive cause of AKI as well as SCR categories at discharge. Cox regression analysis was used to test the association of SCR at discharge with long‐term survival. All tests were 2‐tailed, and in all, P < .05 was considered significant. Analyses were performed using a statistical software package (SPSS 22.0 for Windows, IBM Corp., Armonk, New York).
3. RESULTS
3.1. Signalment and survival
A diagnosis of ACKD was recorded in 139 dogs during the study period. Twenty‐eight dogs were excluded because of missing data in their medical records necessary to establish the diagnosis, and 11 were excluded because they had been euthanized within <48 hours of hospitalization. One hundred dogs met the inclusion criteria (males, 49; including 25 castrated [51%]; females, 51; including 40 spayed [78%]) of the following breeds: mixed breed (54), Shih Tzu (6), Cavalier King Charles spaniel, Pekingese and Yorkshire Terrier (4 each) and other breeds represented by <4 dogs per breed (28 dogs).
Sixty‐five dogs (65%) survived and 35 dogs (35%) did not survive, of which 7 dogs (20%) died, and 28 (80%) were euthanized during hospitalization because of lack of improvement or clinical deterioration, despite treatment.
No age difference (P = .35) was found between survivors (median, 155 months; range, 24‐225) and nonsurvivors (median, 132 months; range, 18‐288). No difference was found in body weight (P = .28) between survivors (median, 8.5 kg; range, 1.2‐52.1) and nonsurvivors (median, 6.5 kg; range, 1.4‐40.0).
3.2. Clinical presentation
The most common clinical signs were anorexia, lethargy, vomiting, diarrhea and weight loss (Table 1). Hematochezia, ocular discharge, generalized lymphadenopathy, dyspnea, tenesmus, epistaxis, regurgitation, ataxia, and hematuria were noted in 1 dog each.
TABLE 1.
Clinical findings at presentation in 100 dogs with acute on chronic kidney disease
| Clinical signs | All dogs (n = 100) | Survivors (n = 65) | Nonsurvivors (n = 35) | P value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Anorexia | 84 (84) | 56 (86) | 28 (80) | .30 |
| Lethargy | 77 (77) | 47 (72) | 30 (86) | .10 |
| Vomiting | 55 (55) | 33 (51) | 22 (63) | .17 |
| Diarrhea | 37 (37) | 23 (35) | 14 (40) | .40 |
| Weight loss | 16 (16) | 10 (15) | 6 (17) | .52 |
| Cough | 4 (4) | 2 (3) | 2 (6) | .46 |
| Pain | 4 (4) | 2 (3) | 2 (6) | .46 |
| Urinary incontinence | 3 (3) | 2 (3) | 1 (3) | .48 |
| Circling | 2 (2) | 1 (1.5) | 1 (3) | .33 |
| Seizures | 2 (2) | 0 (0) | 2 (6) | .12 |
Note: Other clinical signs including hematochezia, ocular discharge, generalized lymphadenopathy, dyspnea, tenesmus, epistaxis, regurgitation, ataxia, and hematuria were represented by 1 dog.
The proportions of clinical signs did not differ between the outcome groups (Table 1). Respiratory rate at presentation was lower in nonsurvivors (median, 24 breaths/min; range, 12‐48) compared with survivors (median, 28 breaths/min; range, 20‐80; P = .001). No difference (P = .31) was found in the pulse rate of survivors (120 beats per minute [bpm]; range, 60‐200) compared with nonsurvivors (median, 130 bpm; range, 80‐160). Rectal temperature also did not differ significantly (P = .17) between survivors (median, 38°C; range, 36.5°‐40.5°) and nonsurvivors (median, 38°C; range, 36°‐39.3°).
3.3. Presumptive causes and concurrent diseases
The etiology of ACKD was not determined in 45 dogs (45%). Inflammatory causes were most commonly suspected (30%; Table 2). The proportions of causes did not differ (P = .46) between survivors and nonsurvivors. Pancreatitis was diagnosed in 34 dogs (34%). Other concurrent diseases (n ≥ 4 dogs) included valvular heart disease (12 dogs; 12%), severe periodontal disease and keratoconjunctivitis sicca (4 dogs each, 4%).
TABLE 2.
Suspected causes of acute on chronic kidney disease categorized by the short‐term outcome
| Etiology | All dogs (n = 100) | Survivors (n = 65) | Nonsurvivors (n = 35) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Unknown | 45 (45) | 25 (39) | 20 (57) |
| Inflammatory | 30 (30) | 22 (34) | 8 (23) |
| Ischemic | 7 (7) | 5 (8) | 2 (6) |
| Pyelonephritis | 15 (15) | 10 (15) | 5 (14) |
| Other a | 3 (3) | 3 (5) | 0 (0) |
Unilateral ureteral obstruction (1 dog), hypercalcemia (1 dog), and cardio‐renal syndrome (1 dog).
Median duration of hospitalization of all dogs was 5 days (range, 2‐29) and it was longer (P < .001) in survivors (median, 6 days; range, 2‐29) compared with nonsurvivors (median, 4 days; range, 2‐20). The hospitalization period did not differ (P = .06) among the different presumptive causes (Figure 1).
FIGURE 1.
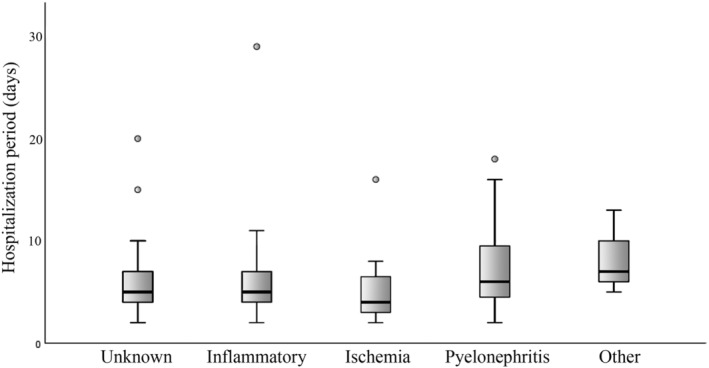
Duration of hospitalization of dogs with ACKD categorized by the presumptive cause of the AKI. The “other” category included AKI secondary to unilateral ureteral obstruction (1 dog), hypercalcemia (1 dog), and type‐1 cardio‐renal syndrome (1 dog). Data are presented as box and whiskers. The box represents the 2nd and 3rd quartiles. The horizontal line within the box represents the median. The whiskers represent the range, and the circles indicate outliers. ACKD, acute on chronic kidney disease; AKI, acute kidney injury
3.4. Hematology and serum biochemistry findings
Anemia (hematocrit, <37.1%) was documented in 51 dogs (51%). The RBC count was lower (P = .01) in nonsurvivors (4.0 × 106/μL; range, 1.64‐7.0 × 106/μL) compared with survivors (5.1 × 106/μL; range, 2.7‐10.2 × 106/μL; Table 3).
TABLE 3.
Complete blood count data at initial presentation of dogs with acute on chronic kidney disease a
| Analyte | RI | All dogs median (range) | Survivors median (range) | Nonsurvivors median (range) | P value |
|---|---|---|---|---|---|
| White blood cells (×103/mm3) | 5.9‐13.9 | 10.5 (1.0‐44.8) | 10.6 (1.0‐38.6) | 10.4 (2.9‐44.8) | .49 |
| Red blood cells (×106/mm3) | 5.7‐8.8 | 4.5 (1.64‐10.2) | 5.1 (2.7‐10.2) | 4.0 (1.64‐7.0) | .01 |
| Hematocrit (%) | 37.1‐57.0 | 31.1 (12.9‐68.3) | 33.0 (12.9‐68.3) | 29.0 (13.5‐48.8) | .04 |
| MCV (fL) | 58.8‐71.2 | 69.9 (56.2‐83.5) | 69.1 (56.2‐78.3) | 71.1 (60.0‐83.5) | .27 |
| MCHC (g/dL) | 31.0‐36.2 | 34 (29.0‐37.8) | 34.0 (29.0‐37.8) | 34.0 (30.7‐37.7) | .87 |
| RDW (%) | 11.9‐14.5 | 14.5 (11.7‐22.1) | 14.6 (11.7‐22.1) | 14.2 (11.9‐20.2) | .63 |
| Platelets (×103/mm3) | 143.3‐400 | 382 (6‐1457) | 382 (18‐896) | 382 (6‐1457) | .73 |
Complete blood count was available in 75 dogs.
Abbreviations: MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RDW, red blood cell distribution width.
Median SCR, urea, and phosphorus concentrations and creatine kinase (CK) activity were significantly higher, whereas serum chloride concentration was significantly lower in nonsurvivors compared with survivors (Table 4). Activity of DGGR lipase was significantly higher (P = .04) in nonsurvivors (median, 1716 U/L; range, 246‐8270) compared with survivors (range, 371 U/L; range, 82‐5182 U/L), but no difference was found in the proportion of dogs diagnosed with pancreatitis between the outcome groups (39% vs 26% in nonsurvivors and survivors respectively; P = .26; Table 4). Venous blood pH and bicarbonate concentration did not differ between outcome groups. There was a significant decrease in survival with increased IRIS AKI grade at presentation (P = .009).
TABLE 4.
Serum biochemistry data at presentation of dogs with acute on chronic kidney disease
| Analyte | RI | All dogs n; median (range) | Survivors n; median (range) | Nonsurvivors n; median (range) | P value |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.3‐1.2 | 100; 5.7 (1.7‐21.8) | 65; 5.0 (1.7‐15.1) | 35; 8.3 (2.7‐21.8) | <.001 |
| CK (U/L) | 51‐399 | 54; 191 (57‐18 715) | 35; 154 (57‐1091) | 19; 269 (101‐18 715) | .009 |
| AST (U/L) | 19‐42 | 56; 32 (17‐418) | 36; 32 (17‐85) | 20; 40 (18‐418) | .32 |
| ALT (U/L) | 19‐67 | 64; 50 (15‐619) | 43; 44 (17‐619) | 21; 54 (15‐548) | .74 |
| ALP (U/L) | 21‐170 | 64; 63 (12‐1879) | 43; 63 (13‐1879) | 21; 69 (12‐714) | .97 |
| GGT (U/L) | 0‐6 | 56; 3 (3‐38) | 36; 3 (3‐24) | 20; 3 (3‐38) | .57 |
| DGGR Lipase (U/L) | 5‐107 | 25; 459 (82‐8270) | 19; 371 (82‐5182) | 6; 1716 (246‐8270) | .04 |
| Amylase (U/L) | 103‐1510 | 65; 1585 (688‐11 487) | 42; 1520 (688‐9190) | 23; 1637 (851‐11 487) | .17 |
| Triglyceride (mg/dL) | 19‐133 | 57; 54 (17‐221) | 27; 56 (18‐221) | 30; 51 (17‐126) | .81 |
| Cholesterol (mg/dL) | 135‐361 | 61; 254 (137‐498) | 39; 235 (137‐498) | 22; 281 (194‐494) | .06 |
| Total bilirubin (mg/dL) | 0.0‐0.2 | 69; 0.15 (0.07‐5.81) | 45; 0.15 (0.07‐5.81) | 24; 0.16 (0.15‐0.38) | .44 |
| Glucose (mg/dL) | 64‐123 | 62; 102 (42‐773) | 42; 98 (41‐773) | 20; 109 (70‐300) | .16 |
| Albumin (g/dL) | 3.0‐4.4 | 90; 3.2 (0.9‐4.5) | 59; 3.2 (1.6‐4.5) | 31; 3.1 (0.9‐4.5) | .91 |
| Total protein (g/dL) | 5.4‐7.6 | 68; 6.3 (4.1‐9.6) | 44; 6.5 (4.1‐9.6) | 24; 6.2(5.1‐7.8) | .29 |
| Urea (mg/dL) | 10‐54 | 97; 287 (62‐628) | 63; 261 (62‐589) | 34; 373 (171‐628) | .001 |
| Phosphate (mg/dL) | 3.0‐6.2 | 83; 12.3 (2.3‐34.4) | 55; 8.7 (2.3‐25.9) | 28; 15.2 (5.3‐34.4) | <.001 |
| Calcium (mg/dL) | 9.7‐11.5 | 67; 10.3 (4.3‐16.6) | 45; 10.5 (4.3‐13.7) | 22; 9.9 (6.3‐16.6) | .94 |
| Sodium (mmol/L) | 140‐154 | 69; 146 (125‐172) | 45; 147 (130‐172) | 24; 145 (125‐156) | .32 |
| Chloride (mmol/L) | 104‐118 | 60; 104 (78‐123) | 39; 106 (78‐123) | 21; 102 (78‐114) | .02 |
| Potassium (mmol/L) | 3.6‐5.3 | 96; 4.9 (3.2‐7.6) | 64; 4.7 (3.2‐6.4) | 32; 5.0 (3.3‐7.6) | .05 |
| Blood pH | 7.35‐7.45 | 88; 7.28 (7.02‐7.46) | 57; 7.25 (7.12‐7.46) | 31; 7.29 (7.02‐7.44) | .14 |
| Bicarbonate (mmol/L) | 20.0‐24.0 | 88; 13.6 (5.7‐34.1) | 57; 13.6 (6.3‐29) | 31; 13.2 (5.7‐34.1) | .47 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; GGT, gamma‐glutamyl transferase; DGGR, 1,2‐o‐dilautryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester.
Median SCR at discharge of surviving dogs was 3.4 mg/dL (range, 0.8‐12.3 mg/dL) and was significantly lower compared with the last SCR measured for nonsurviving dogs (7.7 mg/dL; range, 1.2‐19.8 mg/dL; P < .02).
In the multivariable forward regression model including SCR, RBC count, phosphorus, urea, chloride, CK activity, DGGR lipase, and respiratory rate, only respiratory rate (P = .01), CK activity (P = .005) and SCR (P = .04) remained significantly different between the outcome groups.
3.5. Urinalysis
Urinalysis results were available in 91 dogs. Median USG of all dogs was 1.014 (range, 1.008‐1.040), with no difference (P = .22) between the outcome groups. Abnormalities in urinalysis included proteinuria (urine dipstick protein ≥30 mg/dL; 71/91 dogs; 78%), hematuria (30 dogs; 33%), bacteriuria (28 dogs; 31%), pyuria (24 dogs; 26%), glucosuria (10 dogs; 9%), ketonuria (4 dogs; 3%), bilirubinuria (3 dogs; 3%), and cylindruria (2 dogs; 2%). The occurrence of proteinuria did not differ (P = .16) between the outcome groups. Urine culture (n = 63) was positive in 16/63 dogs (24%) with no difference in the occurrence of a positive urine culture between the outcome groups. The bacterial isolates included Escherichia coli (14 dogs; 88%) and Klebsiella pneumoniae (2 dogs; 12%).
3.6. Follow‐up and long‐term survival
Follow‐up data were available in 61 of the 65 dogs discharged. The MST was 105 days (95% CI, 25‐184), with 35 dogs (57%) and 8 dogs (13%) surviving up to 6 and 12 months, respectively. Three dogs were alive 24 months after presentation with ACKD (Figure 2).
FIGURE 2.
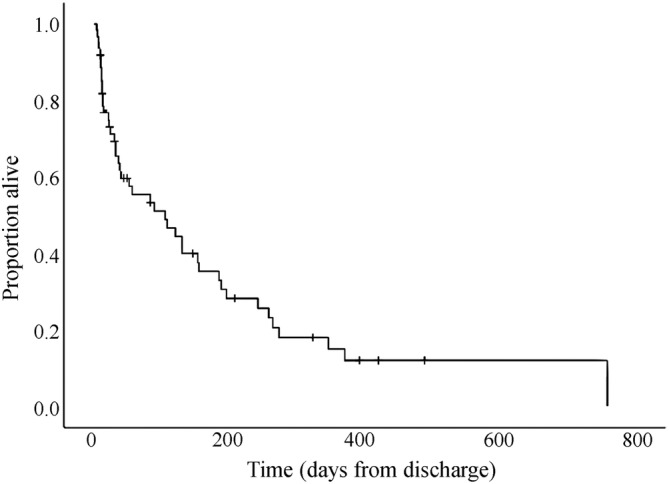
Kaplan‐Meier survival curve of dogs with acute on chronic kidney disease discharged alive from the hospital
The MST of dogs based on SCR categories at discharge was as follows: SCR <1.4 mg/dL, 155 days (3 dogs, 1 censored); SCR between 1.4‐2.8 mg/dL, 130 days (95% CI, 0‐327); SCR between 2.9‐5 mg/dL, 89 days (95% CI, 9‐168) and SCR >5 mg/dL, 32 days (95% CI, 0‐272). No difference (P = .59) was found in MST among categories (Figure 3) or in MST among presumptive causes of AKI (P = .16). No association was found between SCR at presentation and long‐term survival (P = .8).
FIGURE 3.
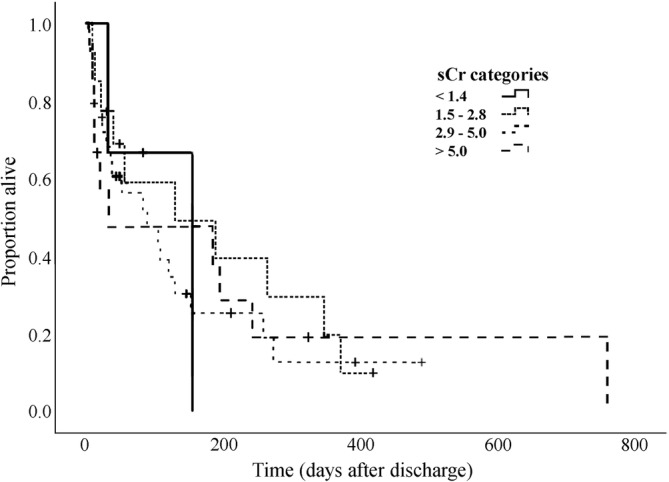
Kaplan‐Meier survival curve of dogs with acute on chronic kidney disease categorized by SCR comparable to IRIS chronic kidney disease stages at discharge from the hospital. IRIS, International Renal Interest Society; SCR, serum creatinine concentration
4. DISCUSSION
Dogs with CKD, as are humans and cats, are at risk for an acute decrease in kidney function, 16 , 25 warranting characterization of the causes and prognostic factors. Better characterization of the disease might aid in preventing and treating this potentially fatal disorder. Moreover, because dogs with ACKD often require prolonged and intensive hospitalization which is asscoiated with high‐cost treatment, tools for assessing short‐ and long‐term prognosis become important in clinical decision‐making.
The clinical presentation of dogs in our study was similar to the reported presentation of dogs with AKI. 5 In both ACKD and AKI, the common clinical signs are nonspecific, and include lethargy, anorexia and vomiting. 13 , 16 This clinical similarity at presentation, with presence of concurrent azotemia in both conditions, can make the differentiation between the 2 conditions challenging. Differentiation is especially difficult when clear history and clinical signs of CKD are absent, recent SCR is not available, and ultrasonographic evidence of CKD is equivocal or absent. Although weight loss often characterizes CKD, 1 it was recorded in only 16% of dogs in our study.
The cause of ACKD was identified in 55% of dogs in our study, and was closely related to AKI's. 9 , 10 The etiology of ACKD in our study, should be regarded as presumptive, because a cause and effect relationship is difficult to prove, especially retrospectively. For example, a definitive diagnosis of ischemia is difficult to make in most clinical settings. Pancreatitis, a common finding in our study, was considered an inflammatory cause in dogs that did not show evidence of other causes of AKI. Pancreatitis can be a cause of AKI, because inflammatory cytokines can lead to decreased renal perfusion and apoptosis of renal tubular cells, 26 , 27 or be a sequel of the disease. Therefore, even when pancreatitis is not the inciting cause, it might perpetuate the injury.
In our study, inflammatory conditions, followed by pyelonephritis and ischemia, were the common presumptive causes, consistent with the causes of ACKD in cats. 16 This observation is in contrast with previous results of dogs with AKI, in which ischemia was the most common cause, followed by nephrotoxicity, a major cause of AKI in dogs, 9 , 10 , 13 which was not documented in our study. It is possible that dogs previously diagnosed with CKD may be less exposed to nephrotoxic substances because of enhanced owner awareness. In addition, dogs with CKD are likely older compared with healthy active dogs and thus potentially less prone to ingestion of nephrotoxins. The cause of AKI was unknown in 45% of the dogs, similar to a previous report. 28 Lack of an identifiable cause of ACKD in our study was not associated with a worse short‐ or long‐term outcome, and should not be considered a negative prognostic factor, in agreement with a recent study of ACKD in cats. 16
In the univariable analysis, concentrations of creatinine, urea and phosphorus, RBC count and CK activity at presentation were associated with outcome. Azotemia and hyperphosphatemia are expected to be associated with the prognosis because they reflect the severity of kidney dysfunction. The association between CK activity and outcome is less clear. Previous studies in ill cats and in humans showed that CK activity is associated with overall disease severity and is a prognostic indicator. 29 , 30 A similar study was not performed in ill dogs, but possibly, CK activity also may be a marker of overall disease severity in dogs. A potential mechanism leading to increased CK activity in dogs with ACKD is hypovolemia and shock, resulting in rhabdomyolysis, as reported in humans, 31 which possibly contributed to mortality. Hypovolemia and shock with resultant muscle injury may have been more common in nonsurvivors during the disease course. Another possible cause for increased CK activity in ACKD is myocardial injury, 32 , 33 which might have had a role in some dogs in our study. Nevertheless, this speculation warrants future prospective studies using specific myocardial injury markers.
In human patients with CKD, the hematocrit is associated with survival. 34 In our study, the lower hematocrit and RBC count documented in nonsurvivors possibly reflected presence of more advanced CKD before the acute insult, compared with survivors. Additionally, more severe kidney injury might have led to gastrointestinal blood loss in nonsurvivors, because of decreased intestinal perfusion and hypergastrinemia. 10
As expected, median USG was low, indicating lack of urinary concentrating ability. One dog however had a USG of 1.040. Although not common, some animals with CKD maintain urine concentrating ability in the early stages of the disease. Indeed, this dog had a SCR of 1.8 mg/dL in the last measurement before acute decompensation.
Pancreatitis occurs commonly in dogs with AKI, in as many as 62% of cases. 9 , 35 Pancreatitis was diagnosed in 34% of dogs in our study, with no difference between outcome groups, but DGGR‐lipase activity was associated with outcome. It is impossible to determine if pancreatitis, being an inflammatory condition, preceded ACKD, possibly triggering the AKI, or if the latter had induced pancreatitis, as a secondary complication. 20 , 36 , 37 Moreover, AKI and pancreatitis share common underlying causes, such as shock, hypovolemia and decreased perfusion. 9 , 35 , 38 Hence, both conditions often coexist, and a cause and effect relationship is difficult to determine. Two explanations are possible for the increased DGGR‐lipase activity in the nonsurvivors despite no difference in the occurrence of pancreatitis. First, pancreatitis was more severe in the nonsurvivors compared with survivors. Second, because lipase is mostly eliminated by glomerular filtration, 39 a more severe decrease in GFR in the nonsurvivors (as reflected by their significantly higher SCR), could have contributed to more prolonged DGGR‐lipase half‐life and hence higher activity in this group.
Median hospitalization duration was 5 days and is comparable to hospitalization duration of dogs with AKI and cats with ACKD. 9 , 16 Hospitalization duration in nonsurvivors was shorter, as expected, because of early death or euthanasia. Hospitalization duration was significantly longer for dogs surviving to discharge, consistent with a previous study indicating that dogs with AKI surviving 5 hospitalization days are more likely to recover. 9 This finding is important because it can guide owners' expectations regarding hospitalization duration.
Previous studies of dogs with AKI reported slightly higher mortality rates (including euthanasia), ranging from 53% to 62%. 9 , 13 , 40 , 41 The mortality rate during hospitalization of dogs with ACKD in our study was lower (35%), which was unexpected, because dogs with CKD have fewer functioning nephrons before the acute insult, and therefore a higher proportion of these nephrons had to recover compared with dogs that had healthy kidneys before AKI. This higher survival rate of dogs with ACKD possibly was related to their particular causes, and the potential reversibility of the injury. The common causes identified in our study, including inflammation, pyelonephritis and ischemia, are considered reversible injuries, whereas nephrotoxicity, commonly reported as a cause for AKI, but not encountered in our study, often is irreversible. 13 , 42 Additionally, because owners are aware of the presence of CKD, they are likely more sensitive to the occurrence of subtle clinical abnormalities, and may seek early medical intervention, which potentially could have improved survival rate. It therefore seems that the short‐term prognosis for dogs with ACKD is at least as favorable as that of those with AKI.
Although short‐term outcome apparently is fair, long‐term prognosis in our study was guarded, with MST of 105 days, and a 1 year survival rate of 13%, which is lower compared with a 33% 1 year survival rate of dogs with severe AKI treated by hemodialysis. 28 However, animals in the aforementioned study likely had normal kidney function before the insult, because dogs with previously diagnosed CKD were excluded. In that study, dogs that did not recover completely had MST of 1913, 1638, and 878 days for IRIS CKD stages 1, 2, and 3 respectively, when staging was done 30 to 90 days after the last dialysis treatment. It appears that dogs with ACKD have poorer long‐term prognosis compared to those with AKI and absence of CKD, consistent with findings in cats with ACKD. 16 The long‐term prognosis of dogs with CKD largely depends on disease stage. In a retrospective study of 107, 214 dogs with CKD, the overall MST was 226 days, with significant differences among IRIS CKD stages. 3 In a study of 21 dogs with CKD with SCR between 2.0 and 3.3 mg/dL, MST was 615 days. 17 In another study, overall MST was 174 days (range, 3‐921), with MST of 220, 180, and 80 days for IRIS CKD stages 2, 3, and 4, respectively. 6 In our study, the MST of dogs with SCR at discharge equivalent to IRIS CKD stages 1, 2, 3, and 4 were 155, 130, 89, and 32 days, respectively, but no significant differences were found among stages. The short MST of the survivors in our study suggests that ACKD adversely affects long‐term prognosis. This finding is supported by studies of AKI in both cats and humans, indicating that in approximately 50% of the patients complete kidney function is not regained after the acute episode. 11 , 12
In patients with ACKD, fractional recovery of kidney function has greater long‐term clinical relevance compared with AKI. It is impossible to assess accurately the extent of recovery in our study, because SCR just before the acute insult was not available in most dogs. Nevertheless, it is possible that SCR just before the acute insult was lower than at discharge, resulting in progression of the CKD to a higher stage by the end of the ACKD episode. Ongoing damage possibly remained at discharge and contributed directly to more rapid progression of the CKD. In our study, SCR at discharge was not associated with long‐term survival, which is in contrast with the findings of a recently published study of cats with ACKD. 16
Our study had several limitations, mostly as a consequence of its retrospective nature. Some of the medical records were incomplete, and in some dogs, several laboratory tests were not performed, thereby weakening the power of some statistical analyses, and possibly precluding identification of some causes of AKI, risk factors and prognostic indicators. Classifying the causes of ACKD was done based on the known causes of AKI, but other possible causes might have been missed. The effect of IRIS CKD stage before the acute decompensation on short‐ and long‐term outcome could not be adequately assessed, because SCR at steady state (just before ACKD) was mostly unavailable. Additionally, treatment of dogs with ACKD was not standardized, which might have had an impact on outcome. In the authors' hospital however, standard treatment guidelines are used for ACKD, which were followed in most cases. Election of euthanasia is based on several factors, and is influenced by owners' financial and emotional constraints, as well as the attending clinician's views. We therefore cannot exclude that the outcome of some of the euthanized dogs was influenced by factors not directly related to their medical condition.
In conclusion, inflammation, pyelonephritis and ischemia are common causes of ACKD in dogs. The short‐term outcome for dogs with ACKD is fair and compares with the AKI survival rate, but long‐term prognosis is less favorable. Respiratory rate, SCR and CK activity are prognostic indicators for survival to discharge but no significant long‐term prognostic factors were identified.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobial.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Dunaevich A, Chen H, Musseri D, et al. Acute on chronic kidney disease in dogs: Etiology, clinical and clinicopathologic findings, prognostic markers, and survival. J Vet Intern Med. 2020;34:2507–2515. 10.1111/jvim.15931
REFERENCES
- 1. Polzin DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract. 2011;41:15‐30. [DOI] [PubMed] [Google Scholar]
- 2. Lund EM, Armstrong PJ, Kirk CA, et al. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336‐1341. [PubMed] [Google Scholar]
- 3. O'Neill DG, Elliott J, Church DB, et al. Chronic kidney disease in dogs in UK veterinary practices: prevalence, risk factors, and survival. J Vet Intern Med. 2013;27:814‐821. [DOI] [PubMed] [Google Scholar]
- 4. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:669‐692. vi. [DOI] [PubMed] [Google Scholar]
- 5. Cowgill LD, Polzin DJ, Elliott J, et al. Is progressive chronic kidney disease a slow acute kidney injury? Vet Clin North Am Small Anim Pract. 2016;46:995‐1013. [DOI] [PubMed] [Google Scholar]
- 6. Parker VJ, Freeman LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med. 2011;25:1306‐1311. [DOI] [PubMed] [Google Scholar]
- 7. Jacob F, Polzin DJ, Osborne CA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. 2005;226:393‐400. [DOI] [PubMed] [Google Scholar]
- 8. Rudinsky AJ, Harjes LM, Byron J, et al. Factors associated with survival in dogs with chronic kidney disease. J Vet Intern Med. 2018;32:1977‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaden SL, Levine J, Breitschwerdt EB. A retrospective case‐control of acute renal failure in 99 dogs. J Vet Intern Med. 1997;11:58‐64. [DOI] [PubMed] [Google Scholar]
- 10. Ross L. Acute kidney injury in dogs and cats. Vet Clin North Am Small Anim Pract. 2011;41:1‐14. [DOI] [PubMed] [Google Scholar]
- 11. Long TE, Sigurdsson MI, Sigurdsson GH, et al. Improved long‐term survival and renal recovery after acute kidney injury in hospitalized patients: a 20 year experience. Nephrol Ther. 2016;21:1027‐1033. [DOI] [PubMed] [Google Scholar]
- 12. Worwag S, Langston CE. Acute intrinsic renal failure in cats: 32 cases (1997‐2004). J Am Vet Med Assoc. 2008;232:728‐732. [DOI] [PubMed] [Google Scholar]
- 13. Segev G, Kass PH, Francey T, et al. A novel clinical scoring system for outcome prediction in dogs with acute kidney injury managed by hemodialysis. J Vet Intern Med. 2008;22:301‐308. [DOI] [PubMed] [Google Scholar]
- 14. Chakrabarti S, Syme HM, Elliott J. Clinicopathological variables predicting progression of azotemia in cats with chronic kidney disease. J Vet Intern Med. 2012;26:275‐281. [DOI] [PubMed] [Google Scholar]
- 15. King JN, Tasker S, Gunn‐Moore DA, et al. Prognostic factors in cats with chronic kidney disease. J Vet Intern Med. 2007;21:906‐916. [PubMed] [Google Scholar]
- 16. Chen H, Dunaevich A, Apfelbaum N, et al. Acute on chronic kidney disease in cats: etiology, clinical and clinicopathologic findings, prognostic markers, and outcome. J Vet Intern Med. 2020;34:1496‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacob F, Polzin DJ, Osborne CA, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J Am Vet Med Assoc. 2002;220:1163‐1170. [DOI] [PubMed] [Google Scholar]
- 18. Bragato N, Borges NC, Fioravanti MCS. B‐mode and Doppler ultrasound of chronic kidney disease in dogs and cats. Vet Res Commun. 2017;41:307‐315. [DOI] [PubMed] [Google Scholar]
- 19. Williams JM, Panciera DL, Larson MM, et al. Ultrasonographic findings of the pancreas in cats with elevated serum pancreatic lipase immunoreactivity. J Vet Intern Med. 2013;27:913‐918. [DOI] [PubMed] [Google Scholar]
- 20. Mansfield C. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J Vet Intern Med. 2012;26:875‐887. [DOI] [PubMed] [Google Scholar]
- 21. Hess RS, Saunders HM, Van Winkle TJ, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986‐1995). J Am Vet Med Assoc. 1998;213:665‐670. [PubMed] [Google Scholar]
- 22. Wettimuny SG. Pyelonephritis in the dog. J Comp Pathol. 1967;77:193‐197. [DOI] [PubMed] [Google Scholar]
- 23. Widmer WR, Biller DS, Adams LG. Ultrasonography of the urinary tract in small animals. J Am Vet Med Assoc. 2004;225:46‐54. [DOI] [PubMed] [Google Scholar]
- 24. Pouchelon J, Atkins C, Bussadori C, et al. Cardiovascular‐renal axis disorders in the domestic dog and cat: a veterinary consensus statement. J Small Anim Pract. 2015;56:537‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu VC, Huang TM, Lai CF, et al. Acute‐on‐chronic kidney injury at hospital discharge is associated with long‐term dialysis and mortality. Kidney Int. 2011;80:1222‐1230. [DOI] [PubMed] [Google Scholar]
- 26. Cuthbertson C, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518‐530. [DOI] [PubMed] [Google Scholar]
- 27. Takeyama Y. Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol. 2005;40:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eatroff AE, Langston CE, Chalhoub S, et al. Long‐term outcome of cats and dogs with acute kidney injury treated with intermittent hemodialysis: 135 cases (1997‐2010). J Am Vet Med Assoc. 2012;241:1471‐1478. [DOI] [PubMed] [Google Scholar]
- 29. Aroch I, Keidar I, Himelstein A, et al. Diagnostic and prognostic value of serum creatine‐kinase activity in ill cats: a retrospective study of 601 cases. J Feline Med Surg. 2010;12:466‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bark CJ. Mitochondrial creatine kinase. A poor prognostic sign. JAMA. 1980;243:2058‐2060. [PubMed] [Google Scholar]
- 31. Poels PJ, Gabreels FJ. Rhabdomyolysis: a review of the literature. Clin Neurol Neurosurg. 1993;95:175‐192. [DOI] [PubMed] [Google Scholar]
- 32. Kidd L, Stepien RL, Amrheiw DP. Clinical findings and coronary artery disease in dogs and cats with acute and subacute myocardial necrosis: 28 cases. J Am Anim Hosp Assoc. 2000;36:199‐208. [DOI] [PubMed] [Google Scholar]
- 33. Gillum RF, Fortmann SP, Prineas RJ, et al. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150‐158. [DOI] [PubMed] [Google Scholar]
- 34. Sato Y, Fujimoto S, Konta T, et al. Anemia as a risk factor for all‐cause mortality: obscure synergic effect of chronic kidney disease. Clin Exp Nephrol. 2018;22:388‐394. [DOI] [PubMed] [Google Scholar]
- 35. Takada K, Palm CA, Epstein SE, et al. Assessment of canine pancreas‐specific lipase and outcomes in dogs with hemodialysis‐dependent acute kidney injury. J Vet Intern Med. 2018;32:722‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishiwaki H, Ko I, Hiura A, et al. Renal microcirculation in experimental acute pancreatitis of dogs. Ren Fail. 1993;15:27‐31. [DOI] [PubMed] [Google Scholar]
- 37. Alfonzo A, Fox J, Imrie C, et al. Acute renal cortical necrosis in a series of young men with severe acute pancreatitis. Clin Nephrol. 2006;66:223–231. [DOI] [PubMed] [Google Scholar]
- 38. Gori E, Lippi I, Guidi G, et al. Acute pancreatitis and acute kidney injury in dogs. Vet J. 2019;245:77‐81. [DOI] [PubMed] [Google Scholar]
- 39. Goodband EL, Serrano G, Constantino‐Casas F, et al. Validation of a commercial 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester lipase assay for diagnosis of canine pancreatitis. Vet Rec Open. 2018;5:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Behrend EN, Grauer GF, Mani I, et al. Hospital‐acquired acute renal failure in dogs: 29 cases (1983‐1992). J Am Vet Med Assoc. 1996;208:537‐541. [PubMed] [Google Scholar]
- 41. Brown N, Segev G, Francey T, et al. Glomerular filtration rate, urine production, and fractional clearance of electrolytes in acute kidney injury in dogs and their association with survival. J Vet Intern Med. 2015;29:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Legatti SAM, El Dib R, Legatti E, et al. Acute kidney injury in cats and dogs: a proportional meta‐analysis of case series studies. PLoS One. 2018;13:e0190772. [DOI] [PMC free article] [PubMed] [Google Scholar]


