Abstract
Background
Little epidemiological evaluation of recurrent seizure disorders in cats currently exists in veterinary literature.
Objectives
To report the prevalence and risk factors for recurrent seizure disorders (RSD) and epilepsy in cats presented to primary care veterinary practices in the United Kingdom (UK).
Animals
A total of 285 547 cats under veterinary care during 2013 presenting to 282 primary care clinics in the UK.
Methods
Cohort study using multivariable logistic regression modeling for risk factor analysis.
Results
There were 458 confirmed RSD cases, giving a 1‐year period prevalence of 0.16% (95% confidence interval [CI], 0.15‐0.18). A subset of 114 (24.89%) cases was recorded as having epilepsy, giving a 1‐year period prevalence of 0.04% (95% CI, 0.03‐0.5). Increasing age was significantly associated with increasing odds of RSD. Breed, sex, neuter status, and body weight were not associated with RSD. Epilepsy was most frequently diagnosed in adult to middle‐aged cats. Cats aged 3.0 to <6.0 years had 3.32 times higher odds of epilepsy diagnosis compared to cats <3.0 years of age. Insured cats were more likely to be diagnosed with epilepsy compared to noninsured cats.
Conclusions and Clinical Importance
Although less common than in dogs, RSD and epilepsy still comprise an important disorder group in the UK cat population. Aging is a significant risk factor for these disorders in cats.
Keywords: feline, first opinion, general practice, pedigree, purebred, VetCompass
Abbreviations
- ASD
antiseizure drug
- CI
confidence intervals
- CSF
cerebrospinal fluid
- EPR
electronic patient record
- IQR
interquartile range
- MRI
magnetic resonance imaging
- OR
odds ratio
- ROC
receiver operating characteristic
- RSD
recurrent seizure disorders; UK, United Kingdom
1. INTRODUCTION
Neurological disorders are common in cats and are among the leading causes of mortality in the pet feline population. 1 , 2 Of these neurological disorders, seizures are a common reason for cats to be presented to a veterinary neurologist, with the prevalence of seizures in referral populations of cats reported as 2.1% in Germany 3 and 3.5% in Austria. 4 Cats with seizures represent an important and challenging presentation for the general veterinarian, and these conditions have the potential to cause great concern and emotional distress for owners. 5 Recurrent seizure disorders (RSD) including epilepsy have been the topic of extensive veterinary research in companion animal species over the last 75 years, but almost exclusively based on caseloads from referral populations and teaching hospitals. However, the reliability of generalization from these secondary and tertiary care subsets to the wider cat population has been questioned. 6 Research on RSD in dogs utilizing datasets from primary practice electronic patient records (EPR) have reported a 1‐year period prevalence for seizure occurrence in dogs of 0.82% and for suspected idiopathic epilepsy of 0.62% to 0.75%. 7 , 8 However, to date, no similar studies have described RSD in cats.
Recurrent seizure disorders in cats represent a diagnostic challenge for several reasons. Seizure type in cats is often atypical. 9 , 10 , 11 Cats frequently present with focal epileptic seizures, 12 , 13 which can be misleading for both owners and practitioners and may be mistaken for other paroxysmal events. 14 , 15 Second, debate about the true prevalence and clinical relevance of spontaneous genetic epilepsy in cats is ongoing. Traditionally, genetic epilepsy was believed to be rare in cats, 12 , 16 but a genetic basis since has been identified in a laboratory cat colony and now is considered an important differential diagnosis in cats. 11 , 17 , 18
Confusion about diagnosis of epilepsy in both cats and dogs is exacerbated by inconsistent use of the diagnostic terms “epilepsy” and “idiopathic epilepsy” in the veterinary literature. 16 , 19 The International Veterinary Epilepsy Task Force (IVETF) addressed these issues for dogs, but not for cats. 5 Fluidity of these definitions has led some authors to adopt the term “epilepsy of unknown cause” to describe RSD with a clinically unremarkable diagnostic evaluation, with the aim of maintaining a neutral stance on whether these disorders are truly “idiopathic” or if the diagnostic techniques available in veterinary medicine are currently unable to elucidate the etiology. 20 Although concise definitions that can be universally agreed on undoubtedly will aid future research efforts by use of precise terms to differentiate specific etiologies, overzealous definitions should not detract from insight that is currently available through more generalized use. 5 Defining disorders based on their phenotypic signature (eg, RSD) rather than reliance on uncertain biomedical terms (eg, epilepsy) applied by clinicians may offer increased reliability for research into disorder frequency. 21 , 22
Evidence relevant to the general population of animals under primary veterinary care should be derived from the general population of animals under primary veterinary care. 23 Consequently, several large projects are now underway that aim to merge anonymized clinical data from primary care veterinary clinics into single databases for research. 24 , 25 , 26 , 27 Research using primary care veterinary clinical records benefits from contemporaneous recording at the time of the clinical events by veterinary professionals across the spectrum of species and disorders recorded during their care. 28 We aimed to estimate the prevalence of RSD and epilepsy in the wider cat population under primary veterinary care in the United Kingdom (UK) and to evaluate demographic risk factors for their occurrence. A secondary aim was to explore risk factors associated with diagnosis of epilepsy among the subset of cats with RSD. This information could promote understanding of the clinical rationale applied by clinicians when assigning biomedical diagnostic terms to neurological clinical cases in cats.
2. METHODS
The VetCompass Programme collates deidentified EPR data from primary care veterinary practices in the UK for epidemiological research. 26 VetCompass collects information fields that include clinic attended, species, breed, date of birth, sex, neuter status, insurance status, body weight, and clinical information from free‐form text clinical notes and summary diagnosis terms (VeNom codes 29 ), as well as treatment and deceased status with relevant dates.
A cohort study of cats attending VetCompass practices was used to estimate the prevalence and risk factors for RSD and epilepsy. The study population included all cats under veterinary care within the VetCompass database from January 1, 2013 to December 31, 2013. Recurrent seizure disorder cases required evidence that they met the case definition during 2013. “Under veterinary care” was defined as having at least 1 EPR recorded from January 1st to December 31st 2013 or at least one EPR both before and after 2013. With no prior reported prevalence values for RSD in cats available, sample size estimation was explored using both 0.25% and 0.10% expected prevalence as conservative estimates, substantially lower than the 0.82% reported in dogs in the UK. 8 Sample size calculations estimated that 38 176 cats were required to estimate the prevalence of a disorder with a 0.25% expected prevalence and 15 321 cats were required to estimate the prevalence of a disorder with a 0.10% to a precision of 0.05% at a 95% confidence level from a UK cat population of 8 million cats. 30 , 31 Ethical approval was granted by the Royal Veterinary College Ethics and Welfare Committee (2016/BSc 20172).
Given an a priori awareness of the likely inconsistency of clinical diagnoses applied to seizure disorders in cats, 16 , 19 we applied a phenotypic signature approach to searching and assigning the RSD cases that were included in the analysis. 22 Case inclusion criteria for RSD required that at least 1 of the following criteria applied during 2013: at least 2 episodes of seizure events with a minimum of 24 hours between the first and final events, a final diagnosis of epilepsy or synonym (eg, epileptic) was recorded in the EPR, or was prescribed an antiseizure drug (ASD) to manage a seizure‐related disorder (based on an assumption that ASD treatment was unlikely to be prescribed for a solitary seizure event). Cats with only extracranial reactive seizures (ie, seizures secondary to a primary condition external to the brain, such as hepatic encephalopathy, electrolyte imbalances, toxicity, or agonal seizuring) were excluded. The case definition for epilepsy required that a final diagnosis of epilepsy or synonym (eg, epileptic) was recorded in the EPR by the primary care veterinary team. Case finding involved initial screening of all EPR for candidate RSD cases using a bank of search terms including epil*, seiz*, seizure~2, had 1 fit, had 2 fits, phenob*, epiphen~2, anti‐epil*, anti‐convuls*, potassium bromide, KBr, levetira*, keppra, zonisam*, 2 fits, short fit, lyrica, pregabalin, and gabapentin. Candidate cases were randomized and the clinical notes of all candidates were reviewed to evaluate for case inclusion. Additional data were extracted on confirmed cases to describe the date of the first recorded seizure for the RSD overall, and whether the case was diagnosed with epilepsy by the first opinion practitioner. Confirmed RSD cases were grouped as “RSD cases” and all remaining study cats were grouped as “noncases.”
A “purebred” variable categorized all cats of recognizable breeds as “purebred” and the remaining cats as “crossbred.” A “breed” variable included individual breeds that had at least 1 RSD case or that were represented by at least 1000 study animals, a grouped category of all remaining purebreds, and a general grouping of crossbred cats. This approach was taken to allow focus on commonly affected breeds and on common breeds, and to facilitate statistical power for the individual breed analyses. 32 A “neuter” variable described the status of the cat (neutered or intact) recorded at the final EPR. An “insurance” variable described whether a cat was insured at any point during the study period. An “age” variable categorized age (years): <3.0, 3.0‐<6.0, 6.0‐<9.0, 9.0‐<12.0, 12.0‐<15.0, ≥15.0, not recorded. Age (years) was calculated for RSD cases at the first recorded seizure event and for noncase cats at December 31st, 2013 (the latest date the cat was known to be seizure‐free). This approach was taken so that the age results would reflect the odds of “becoming” a case rather than the odds of “being” a case. An “adult body weight” variable categorized adult body weight: <3.0 kg, 3.0‐<4.0 kg, 4.0‐<5.0 kg, 5.0‐<6.0 kg, ≥6.0 kg, not available. Adult body weight described the maximum body weight recorded during the study period for cats >6 months of age. “Dominant color” defined a categorical variable that included all colors that were recorded as comprising some or all of the coloration for at least 5000 study animals along with a grouped category of all remaining less‐common colors. The dominant color ascribed for individual cats was determined by the first color term used to describe the cat. “Self‐color” defined a binary variable describing whether the cat was recorded as self‐colored (ie, with only a single solid color) or not. 33
After data checking and cleaning in Excel (Microsoft Office Excel 2013, Microsoft Corp.), analyses were conducted using Stata Version 13 (Stata Corporation). The 1‐year period prevalence describes the proportion of all study animals recorded with the disorder during a specified 12‐month period, which was 2013 in the current study. 34 The 95% confidence interval (CI) estimates were derived from standard errors, based on approximation to the binomial distribution. 35 Descriptive statistics characterized the purebred status, breed, sex, color, neuter status, insurance, age and adult body weight for the RSD cases and noncases. The chi squared test was used for statistical comparison between categorical variables. 35
2.1. Risk factors for RSD in the overall population of cats
Binary logistic regression modeling was used to evaluate univariable associations between risk factors (purebred, breed, adult body weight, age, sex, neuter, insurance, dominant color, self‐color), and RSD. Risk factors with liberal associations in univariable modeling (P < .2) were taken forward for multivariable evaluation. Model development used manual backwards stepwise elimination. Pair‐wise interaction effects were evaluated for the final model variables. Confounding effects from dropped variables were assessed by individual reintroduction to the final model. The likelihood ratio test was used to compare a random effects model with clinic entered as a random effect against the nonrandom effects model with P < .05 cut‐off used for selection of the random effects model. The Hosmer‐Lemeshow test statistic 36 and the area under the receiver operating characteristic (ROC) curve were used to evaluate model fit. 34 Statistical significance was set at P < .05.
2.2. Risk factors for epilepsy in the overall population of cats
These methods were repeated to evaluate risk factors associated with epilepsy in the overall population after dropping RSD cases that were not classified as epilepsy.
2.3. Risk factors for epilepsy among cats with RSD
These methods also were applied to explore risk factors associated with diagnosis of epilepsy among the subset of cats that were recorded with RSD.
3. RESULTS
The study population consisted of 285 547 cats under veterinary care during 2013 attending 282 primary care clinics in the UK. There were 1497 cats identified as candidate RSD cases. All candidate cases were checked to confirm 458 (30.59% of the candidates) as RSD cases, giving an overall 1‐year period prevalence for RSD in cats in the UK of 0.16% (95% CI, 0.15‐0.18). Breeds with the highest RSD prevalence were Foreign (1.89% of the breed affected; 95% CI, 0.05‐10.07) and Burmilla (0.84%; 95% CI, 0.02‐4.59). The prevalence of RSD did not differ between crossbred cats (0.17%; 95% CI, 0.15‐0.18) and purebred cats (0.13%; 95% CI, 0.09‐0.18, P = .165; Table 1).
TABLE 1.
Prevalence of recurrent seizure disorders (RSDs) in common cat breeds under primary veterinary care in the UK
| Breed | No. cats in study | No. cases | Prevalence % | 95% CI |
|---|---|---|---|---|
| Foreign | 53 | 1 | 1.89 | 0.05‐10.07 |
| Burmilla | 119 | 1 | 0.84 | 0.02‐4.59 |
| Exotic | 450 | 2 | 0.44 | 0.05‐1.60 |
| Birman | 1150 | 5 | 0.43 | 0.14‐1.01 |
| Siberian | 236 | 1 | 0.42 | 0.01‐2.34 |
| Burmese | 1500 | 4 | 0.27 | 0.07‐0.68 |
| Russian | 507 | 1 | 0.20 | 0.01‐1.09 |
| Norwegian Forest | 568 | 1 | 0.18 | 0.00‐0.98 |
| Crossbred | 252 349 | 417 | 0.17 | 0.15‐0.18 |
| British Short Hair | 5283 | 8 | 0.15 | 0.07‐0.30 |
| Persian | 3314 | 5 | 0.15 | 0.05‐0.35 |
| Bengal | 3344 | 4 | 0.12 | 0.03‐0.31 |
| Maine Coon | 1969 | 2 | 0.10 | 0.01‐0.37 |
| Ragdoll | 2905 | 2 | 0.07 | 0.01‐0.25 |
| Siamese | 2503 | 1 | 0.04 | 0.00‐0.22 |
| British Blue | 1507 | 0 | 0.00 | 0.00‐0.24 |
| Other purebreds | 3664 | 0 | 0.00 | 0.00‐0.10 |
| Breed not recorded | 4123 | 3 | 0.07 | 0.02‐0.21 |
| Overall | 285 547 | 458 | 0.16 | 0.15‐0.18 |
Abbreviation: CI, confidence interval.
Of the 458 RSD cases, 114 (24.89%) were recorded with epilepsy by the first opinion veterinary practitioners, giving an overall 1‐year period prevalence for first opinion‐classified epilepsy in cats in the UK of 0.04% (95% CI, 0.03‐0.05). In addition, the possibility of epilepsy was discussed in the clinical records of an additional 69 RSD cases without confirmation recorded in the notes.
Of the RSD cases with complete data available for that variable, 38/455 (8.4%) were purebred, 240/456 (52.6%) were female, 285/324 (88.0%) were neutered, 88/161 (54.7%) were insured, and 235/448 (52.5%) were self‐colored. The most common colors of RSD cases (color information available on 448 cats) were black (n = 182, 40.6%) and tabby (n = 94, 21.0%; Table 2). The median adult body weight for RSD cats was 4.4 kg (interquartile range [IQR], 3.6‐5.5; range, 2.2‐9.6). The median age at first recorded seizure event for RSD cases overall was 8.9 years (IQR, 3.5‐14.2; range, 0.2‐25.1). The median age at the first recorded seizure event for the subset of RSD cases recorded with epilepsy was 6.4 years (IQR, 3.5‐11.3; range, 0.2‐18.0) (Figure 1).
TABLE 2.
Descriptive and univariable logistic regression results for risk factors associated with diagnosis of recurrent seizure disorders (RSD) in cats under primary veterinary care in the UK
| Variable | Category | Case no. (%) | Noncase no. (%) | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|---|---|
| Purebred status | Crossbred | 417 (91.1) | 251 932 (88.4) | Base | .1 | ||
| Purebred | 38 (8.3) | 29 034 (10.2) | 0.79 | 0.57‐1.10 | .2 | ||
| Unrecorded | 3 (0.7) | 4123 (1.5) | 0.44 | 0.14‐1.37 | .2 | ||
| Common breeds | Crossbred | 417 (91.1) | 251 932 (88.4) | Base | .2 | ||
| Bengal | 4 (0.9) | 3340 (1.2) | 0.72 | 0.27‐1.94 | .5 | ||
| Birman | 5 (1.1) | 1145 (0.4) | 2.64 | 1.09‐6.38 | .03 | ||
| British Blue | 0 (0.0) | 1507 (0.5) | … | ||||
| British Short Hair | 8 (1.8) | 5275 (1.9) | 0.92 | 0.45‐1.85 | .8 | ||
| Burmese | 4 (0.9) | 1496 (0.5) | 1.62 | 0.60‐4.33 | .3 | ||
| Burmilla | 1 (0.2) | 118 (0.0) | 5.12 | 0.71‐36.74 | .1 | ||
| Exotic | 2 (0.4) | 448 (0.2) | 2.70 | 0.67‐10.85 | .2 | ||
| Foreign | 1 (0.2) | 52 (0.0) | 11.62 | 1.60‐84.24 | .02 | ||
| Maine Coon | 2 (0.4) | 1967 (0.7) | 0.61 | 0.15‐2.47 | .5 | ||
| Norwegian Forest | 1 (0.2) | 567 (0.2) | 1.07 | 0.15‐7.60 | 1 | ||
| Other purebreds | 0 (0.00) | 3664 (1.3) | … | ||||
| Persian | 5 (1.1) | 3309 (1.2) | 0.91 | 0.38‐2.21 | .8 | ||
| Ragdoll | 2 (0.4) | 2903 (1.0) | 0.42 | 0.10‐1.67 | .2 | ||
| Russian | 1 (0.2) | 506 (0.2) | 1.19 | 0.17‐8.51 | .9 | ||
| Siamese | 1 (0.2) | 2502 (0.9) | 0.24 | 0.03‐1.72 | .2 | ||
| Siberian | 1 (0.2) | 235 (0.1) | 2.57 | 0.36‐18.37 | .4 | ||
| Unrecorded | 3 (0.7) | 4123 (1.5) | 0.44 | 0.14‐1.37 | .2 | ||
| Self‐color | Single | 235 (51.3) | 137 752 (48.3) | Base | .08 | ||
| Multiple | 213 (46.5) | 136 251 (47.8) | 0.92 | 0.76‐1.10 | .4 | ||
| Unrecorded | 10 (2.2) | 11 082 (3.9) | 0.53 | 0.28‐1.00 | .05 | ||
| Dominant color | Black | 182 (39.7) | 108 551 (38.1) | Base | .43 | ||
| Tabby | 94 (20.5) | 52 152 (18.3) | 1.08 | 0.84‐1.38 | .6 | ||
| Ginger | 35 (7.6) | 25 902 (9.1) | 0.81 | 0.56‐1.16 | .24 | ||
| Tortoiseshell | 40 (8.7) | 24 508 (8.6) | 0.97 | 0.69‐1.37 | .9 | ||
| Gray | 24 (5.2) | 16 182 (5.7) | 0.88 | 0.58‐1.35 | .6 | ||
| White | 28 (6.1) | 15 357 (5.4) | 1.09 | 0.73‐1.62 | .7 | ||
| Blue | 10 (2.2) | 6219 (2.2) | 0.96 | 0.51‐1.82 | .9 | ||
| Other color | 35 (7.6) | 25 132 (8.8) | 0.83 | 0.58‐1.19 | .32 | ||
| Unrecorded | 10 (2.2) | 11 086 (3.9) | 0.54 | 0.28‐1.02 | .06 | ||
| Age category (years) | <3.0 | 97 (21.2) | 105 557 (37.0) | Base | <.001 | ||
| 3.0‐<6.0 | 66 (14.4) | 59 813 (21.0) | 1.20 | 0.88‐1.64 | .2 | ||
| 6.0‐<9.0 | 65 (14.2) | 38 117 (13.4) | 1.86 | 1.36‐2.54 | <.001 | ||
| 9.0‐<12.0 | 63 (13.8) | 26 999 (9.5) | 2.54 | 1.85‐3.49 | <.001 | ||
| 12.0‐<15.0 | 64 (14.0) | 22 536 (7.9) | 3.09 | 2.25‐4.24 | <.001 | ||
| ≥15.0 | 97 (21.2) | 22 239 (7.8) | 4.75 | 3.58‐6.29 | <.001 | ||
| Unrecorded | 6 (1.3) | 9808 (3.4) | 0.67 | 0.29‐1.52 | .33 | ||
| Adult body weight (kg) (>6 months) | <3.0 | 16 (3.5) | 13 023 (4.6) | Base | .0008 | ||
| 3.0‐<4.0 | 59 (12.9) | 43 179 (15.2) | 1.11 | 0.64‐1.93 | .71 | ||
| 4.0‐<5.0 | 68 (14.9) | 53 374 (18.7) | 1.04 | 0.60‐1.79 | .9 | ||
| 5.0‐<6.0 | 36 (7.9) | 31 580 (11.1) | 0.93 | 0.51‐1.67 | .803 | ||
| ≥6.0 | 34 (7.4) | 16 660 (5.8) | 1.66 | 0.92‐3.01 | .09 | ||
| Unrecorded | 245 (53.5) | 127 273 (44.6) | 1.57 | 0.94‐2.60 | .08 | ||
| Sex | Female | 240 (52.4) | 145 668 (51.1) | Base | .5 | ||
| Male | 216 (47.2) | 136 860 (48.0) | 0.96 | 0.80‐1.15 | .7 | ||
| Unrecorded | 2 (0.4) | 2561 (0.9) | 0.47 | 0.12‐1.91 | .3 | ||
| Neuter status | Entire | 39 (8.5) | 40 927 (14.4) | Base | <.001 | ||
| Neutered | 285 (62.2) | 183 783 (64.5) | 1.63 | 1.16‐2.27 | .004 | ||
| Unrecorded | 134 (29.3) | 60 379 (21.2) | 2.33 | 1.63‐3.33 | <.001 | ||
| Insurance | Noninsured | 73 (15.9) | 28 652 (10.1) | Base | <.001 | ||
| Insured | 88 (19.2) | 26 636 (9.3) | 1.30 | 0.95‐1.77 | .1 | ||
| Unrecorded | 297 (64.9) | 229 801 (80.6) | 0.51 | 0.39‐0.66 | <.001 |
Notes: Column percentages shown in brackets. N = 285 547.
Abbreviation: CI, confidence interval.
FIGURE 1.
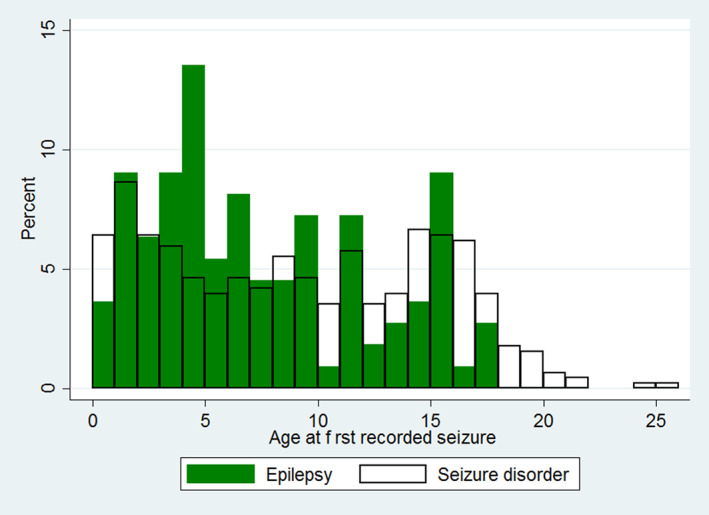
Age (years) at the first recorded seizure event for cats under primary veterinary care in the UK diagnosed with recurrent seizure disorders (RSD) (N = 458) and epilepsy (N = 114)
Of the noncase cats with complete data, 29 034/280 966 (10.3%) were purebred, 145 668/282 528 (51.6%) were female, 183 783/224 710 (81.8%) were neutered, 26 636/55 288 (48.2%) were insured and 137 752/274 003 (50.3%) were self‐colored. The most common colors of noncase cats (color information available on 274 003 cats) were black (n = 108 551; 39.6%) and tabby (n = 52 152; 19.0%). The median adult body weight for noncase cats was 4.4 kg (IQR, 3.7‐5.2; range: 1.0‐19.8). The median age for noncase cats on December 31, 2013 was 4.4 years (IQR, 1.7‐9.3; range, 0.0‐28.7; Table 2). Data completeness varied for the variables assessed: sex, 99.1%; breed, 98.6%; age, 96.6%; color, 96.1%; neuter status; 78.8%, body weight; 59.6%, insurance; 19.4%.
3.1. Risk factors for RSD in the overall population of cats
Univariable logistic regression modeling identified 7 variables liberally associated with RSD that were further evaluated in multivariable logistic regression modeling: purebred, breed, self‐color, age, adult body weight, neuter status and insurance. The final multivariable model retained 2 risk factors: age, and insurance. No biologically significant interactions were identified in the final model. The final model was not improved by inclusion of the clinic attended as a random effect (rho, 0.008; P = .26). The final model showed acceptable model fit (Hosmer‐Lemeshow test statistic, P = .14) and discrimination (area under the ROC curve, 0.681). The odds of RSD increased progressively as cats aged. Compared with cats aged <3.0 years, cats aged 9.0 to <12.0 years had 2.23 times the odds (95% CI, 1.62‐3.07; P < .001) and cats aged >15.0 years had 4.27 times the odds (95% CI, 3.21‐5.69; P < .001). Insured cats had 1.49 (95% CI, 1.09‐2.04; P = .01) times the odds of RSD compared with uninsured cats (Table 3).
TABLE 3.
Final multivariable logistic regression model for risk factors associated with diagnosis of recurrent seizure disorders (RSDs) in cats under primary veterinary care in the UK
| Variable | Category | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|
| Age category (years) | <3.0 | Base | <.001 | ||
| 3.0‐<6.0 | 1.08 | 0.79‐1.48 | .64 | ||
| 6.0‐<9.0 | 1.64 | 1.20‐2.26 | .002 | ||
| 9.0‐<12.0 | 2.23 | 1.62‐3.07 | <.001 | ||
| 12.0‐<15.0 | 2.72 | 1.98‐3.75 | <.001 | ||
| ≥15.0 | 4.27 | 3.21‐5.69 | <.001 | ||
| Unrecorded | 0.71 | 0.31‐1.61 | .41 | ||
| Insurance | Noninsured | Base | <.001 | ||
| Insured | 1.49 | 1.09‐2.04 | .012 | ||
| Unrecorded | 0.66 | 0.51‐0.86 | .002 |
Notes: N = 285 547.
Abbreviation: CI, confidence interval.
3.2. Risk factors for epilepsy in the overall population of cats
Univariable logistic regression modeling identified 5 variables that were liberally associated with epilepsy and were further evaluated in multivariable logistic regression modeling: breed, age, adult body weight, neuter status and insurance (Table 4). The final multivariable model retained 2 risk factors: age, and insurance. No biologically significant interactions were identified in the final model. The final model was not improved by inclusion of the clinic attended as a random effect (rho, 0.008; P < .44). The final model showed acceptable model fit (Hosmer‐Lemeshow test statistic, P = .16) and discrimination (area under the ROC curve, 0.700). Compared with cats aged <3.0 years, the odds of epilepsy were higher in all older age groups except for cats aged 12.0 to <15.0 years. Insured cats had 2.38 (95% CI, 1.31‐4.33; P = .004) times the odds of epilepsy compared with uninsured cats (Table 5).
TABLE 4.
Descriptive and univariable logistic regression results for risk factors associated with diagnosis of epilepsy by primary care practitioners in cats under primary veterinary care in the UK
| Variable | Category | Case no. (%) | Noncase no. (%) | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|---|---|
| Purebred status | Crossbred | 103 (90.4) | 251 932 (88.4) | Base | .81 | ||
| Purebred | 11 (9.6) | 29 034 (10.2) | 0.93 | 0.50‐1.73 | .81 | ||
| Unrecorded | 0 (0.0) | 4123 (1.5) | … | ||||
| Common breeds | Crossbred | 103 (90.4) | 251 932 (88.4) | Base | .2 | ||
| Bengal | 1 (0.9) | 3340 (1.2) | 0.73 | 0.10‐5.25 | .8 | ||
| Birman | 1 (0.9) | 1145 (0.4) | 2.14 | 0.30‐15.32 | .5 | ||
| British Blue | 0 (0.0) | 1507 (0.5) | … | ||||
| British Short Hair | 4 (3.5) | 5275 (1.9) | 1.85 | 0.68‐5.04 | .23 | ||
| Burmese | 2 (1.8) | 1496 (0.5) | 3.27 | 0.81‐13.26 | .1 | ||
| Burmilla | 0 (0.0) | 118 (0.0) | … | ||||
| Exotic | 2 (1.8) | 448 (0.2) | 10.92 | 2.69‐44.38 | .001 | ||
| Foreign | 0 (0.0) | 52 (0.0) | … | ||||
| Maine Coon | 0 (0.0) | 1967 (0.7) | … | ||||
| Norwegian Forest | 0 (0.0) | 567 (0.2) | … | ||||
| Other purebreds | 0 (0.0) | 3664 (1.3) | … | ||||
| Persian | 0 (0.0) | 3309 (1.2) | … | ||||
| Ragdoll | 0 (0.0) | 2903 (1.0) | … | ||||
| Russian | 0 (0.0) | 506 (0.2) | … | ||||
| Siamese | 1 (0.9) | 2502 (0.9) | 0.98 | 0.14‐7.01 | 1 | ||
| Siberian | 0 (0.0) | 235 (0.1) | … | ||||
| Unrecorded | 0 (0.0) | 4123 (1.5) | … | ||||
| Self‐color | Single | 55 (48.3) | 137 752 (48.3) | Base | .41 | ||
| Multiple | 57 (50.0) | 136 251 (47.8) | 1.05 | 0.72‐1.52 | .81 | ||
| Unrecorded | 2 (1.7) | 11 082 (3.9) | 0.45 | 0.11‐1.85 | .27 | ||
| Dominant color | Black | 53 (46.5) | 108 551 (38.1) | Base | .34 | ||
| Tabby | 19 (16.7) | 52 152 (18.3) | 0.75 | 0.44‐1.26 | .3 | ||
| Ginger | 9 (7.9) | 25 902 (9.1) | 0.71 | 0.35‐1.44 | .4 | ||
| Tortoiseshell | 11 (9.7) | 24 508 (8.6) | 0.92 | 0.48‐1.76 | .8 | ||
| Gray | 2 (1.8) | 16 182 (5.7) | 0.25 | 0.06‐1.04 | .1 | ||
| White | 7 (6.1) | 15 357 (5.4) | 0.93 | 0.42‐2.05 | .9 | ||
| Blue | 3 (2.6) | 6219 (2.2) | 0.99 | 0.31‐3.16 | 1 | ||
| Other color | 8 (7.0) | 25 132 (8.8) | 0.65 | 0.31‐1.37 | .3 | ||
| Unrecorded | 2 (1.8) | 11 086 (3.9) | 0.37 | 0.09‐1.52 | .2 | ||
| Age category (years) | <3.0 | 21 (18.4) | 105 557 (37.0) | Base | .001 | ||
| 3.0‐<6.0 | 31 (27.2) | 59 813 (21.0) | 2.61 | 1.50‐4.53 | .001 | ||
| 6.0‐<9.0 | 19 (16.7) | 38 117 (13.4) | 2.51 | 1.35‐4.66 | .004 | ||
| 9.0‐<12.0 | 17 (14.9) | 26 999 (9.5) | 3.17 | 1.67‐6.00 | <.001 | ||
| 12.0‐<15.0 | 9 (7.9) | 22 536 (7.9) | 2.01 | 0.92‐4.38 | .1 | ||
| ≥15.0 | 14 (12.3) | 22 239 (7.8) | 3.16 | 1.61‐6.22 | .001 | ||
| Unrecorded | 3 (2.6) | 9808 (3.4) | 1.54 | 0.46‐5.16 | .5 | ||
| Adult body weight (kg) (>6 months) | <3.0 | 1 (0.9) | 13 023 (4.6) | 0.26 | 0.03‐1.95 | .2 | .02 |
| 3.0‐<4.0 | 13 (11.4) | 43 179 (15.2) | Base | ||||
| 4.0‐<5.0 | 13 (11.4) | 53 374 (18.7) | 0.81 | 0.37‐1.75 | .6 | ||
| 5.0‐<6.0 | 15 (13.2) | 31 580 (11.1) | 1.58 | 0.75‐3.32 | .23 | ||
| ≥6.0 | 9 (7.9) | 16 660 (5.8) | 1.79 | 0.77‐4.20 | .2 | ||
| Unrecorded | 63 (55.3) | 127 273 (44.6) | 1.64 | 0.90‐2.99 | .103 | ||
| Sex | Female | 62 (54.4) | 145 668 (51.1) | Base | .8 | ||
| Male | 51 (44.7) | 136 860 (48.0) | 0.88 | 0.60‐1.27 | .5 | ||
| Unrecorded | 1 (0.9) | 2561 (0.9) | 0.92 | 0.13‐6.62 | .93 | ||
| Neuter status | Entire | 6 (5.3) | 40 927 (14.4) | Base | .001 | ||
| Neutered | 72 (63.2) | 183 783 (64.5) | 2.67 | 1.16‐6.15 | .021 | ||
| Unrecorded | 36 (31.6) | 60 379 (21.2) | 4.07 | 1.71‐9.65 | .001 | ||
| Insurance | Noninsured | 16 (14.0) | 28 652 (10.1) | Base | <.001 | ||
| Insured | 34 (29.8) | 26 636 (9.3) | 2.29 | 1.26‐4.14 | .006 | ||
| Unrecorded | 64 (56.1) | 229 801 (80.6) | 0.49 | 0.29‐0.86 | .01 |
Notes: Column percentages shown in brackets. N = 285 203.
Abbreviation: CI, confidence interval.
TABLE 5.
Final multivariable logistic regression model for risk factors associated with diagnosis of epilepsy by primary‐care practitioners in cats under primary veterinary care in the UK
| Variable | Category | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|
| Age category (years) | <3.0 | Base | .04 | ||
| 3.0‐<6.0 | 2.15 | 1.22‐3.76 | .008 | ||
| 6.0‐<9.0 | 2.01 | 1.07‐3.76 | .03 | ||
| 9.0‐<12.0 | 2.50 | 1.31‐4.79 | .006 | ||
| 12.0‐<15.0 | 1.61 | 0.73‐3.55 | .234 | ||
| ≥15.0 | 2.69 | 1.36‐5.34 | .005 | ||
| Unrecorded | 1.74 | 0.52‐5.85 | .4 | ||
| Insurance | Noninsured | Base | <.001 | ||
| Insured | 2.38 | 1.31‐4.33 | .004 | ||
| Unrecorded | 0.59 | 0.34‐1.04 | .07 |
Notes: N = 285 203.
Abbreviation: CI, confidence interval.
3.3. Risk factors for epilepsy among cats with RSD
Multivariable logistic regression modeling identified age as the only factor associated with classification of epilepsy (114 epilepsy cases) among the subset of 458 cats with RSD. Cats aged 3.0 to <6.0 years had 3.32 (95% CI, 1.66‐6.67; P = .001) times the odds of RSD compared with cats aged <3.0 years. Insurance was retained as a confounder in the final model (Table 6).
TABLE 6.
Final multivariable logistic regression model for risk factors associated with classification as epilepsy by primary‐care practitioners among cats diagnosed with recurrent seizure disorders (RSDs) under primary veterinary care in the UK
| Variable | Category | Odds ratio | 95% CI | Category P‐value | Variable P‐value |
|---|---|---|---|---|---|
| Age category (years) | <3.0 | Base | .005 | ||
| 3.0‐<6.0 | 3.32 | 1.66‐6.67 | .001 | ||
| 6.0‐<9.0 | 1.53 | 0.74‐3.18 | .2 | ||
| 9.0‐<12.0 | 1.38 | 0.65‐2.91 | .4 | ||
| 12.0‐<15.0 | 0.61 | 0.26‐1.45 | .3 | ||
| ≥15.0 | 0.61 | 0.28‐1.45 | .3 | ||
| Unrecorded | 4.72 | 0.87‐25.41 | .1 | ||
| Insurance | Noninsured | Base | <.001 | ||
| Insured | 1.88 | 0.91‐3.90 | .1 | ||
| Unrecorded | 0.77 | 0.40‐1.47 | .42 |
Notes: N = 458.
Abbreviation: CI, confidence interval.
4. DISCUSSION
Ours is the first study to explore RSD and epilepsy in cats by analyzing data from a multicenter primary care research database in the UK. Our study of 285 547 cats attending UK primary care practices in 2013 reports a 1‐year period prevalence of 0.16% for RSD and 0.04% for epilepsy as classified by primary care veterinary practitioners. These results confirm that although RSD are less frequently diagnosed in cats than in dogs, 7 , 8 , 37 RSD are a relatively common clinical presentation for cats evaluated by veterinary practitioners, which is consistent with previous reports. 38 , 39 Numerically, despite the relatively low prevalence of these disorders, an estimated 10.9 million cats are owned in the UK with 24% of UK adults owning a cat, 40 which equates to a substantial number of potentially affected cats in the UK. Our findings are substantially lower than the 2.1% and 3.5% prevalences of seizures previously reported in referral feline populations. 3 , 4 This difference likely reflects inherent differences in caseloads between primary care and referral practices, 6 and mirrors a discrepancy described in Germany where the primary care period prevalence of dogs presenting with seizures was 0.43% compared to 1.78% in a referral population. 41 Considering the differences between cats and dogs, although the prevalence for RSD in cats is considerably lower than the prevalence in dogs with at least 1 seizure reported previously, 8 the inclusion criteria for RSD in our study required cats to have had at least 2 seizure events separated by at least 24 hours, an epilepsy diagnosis, or treatment using ASD. Given that toxicity and metabolic disturbances (reactive seizures) are responsible for many single seizure events, 3 , 38 , 39 this situation may account for a proportion of the differences reported between the species. Additionally, recognition of seizures in cats often is complicated by the heterogenous semiology of the condition in this species, 4 , 13 , 42 , 43 such that the true proportion of cats with RSD may be higher than that reported by owners. Furthermore, compared to dogs, many UK cats spend a considerable proportion of their time outdoors, where owners are less likely to witness seizure events. 39
Despite substantial progress in recent decades on the classification of neurological diseases in companion animals 5 along with efforts to formally define internationally‐accepted diagnostic lexicons and definitions, 19 reluctance remains for primary care practitioners to evaluate and identify their neurological clinical caseloads. 44 This reluctance for standardized diagnosis recording means that many primary care cases are recorded using phenotypic signature (ie, seizure disorder) rather than a formal diagnosis term (eg, epilepsy). This phenomenon was highlighted in a recent study of seizure disorders in dogs presenting to primary care clinics where only 10.7% of idiopathic epilepsy cases that met IVETF criteria were recorded as epilepsy or idiopathic epilepsy in the clinical records. 45 One impact for scientific research arising from this tendency toward informal diagnosis recording is that studies that rely on formally‐recorded diagnosis terms are likely to substantially underreport the true frequency of these disorders. Consequently, and in an effort to include all true cases in our study, we chose not to rely on formal diagnosis terms such as epilepsy or idiopathic epilepsy as recorded in the clinical notes but instead to focus more on a phenotypic signature that would identify cats with RSD.
A formal diagnosis of epilepsy was recorded by the first opinion veterinary practitioner for 114 (24.89%) of the 458 RSD cases, although it is likely that many more of these cases met the internationally‐agreed definition for epilepsy despite not being recorded as such by the attending veterinarians. 19 The precise clinical criteria applied for the epilepsy diagnoses recorded in the clinical records was not explored in our study, but these could be the topic of future research exploring how closely primary care clinicians apply IVETF diagnosis guidelines. 44 It is likely that a final pathophysiological rationale for the disorder was not reached in many of the cases that were not recorded as epilepsy, and therefore the attending veterinarians recorded the condition based on its phenotypic signature rather than spuriously recording a biomedical diagnosis term that they could not be confident was correct. Our study applied the diagnosis term “epilepsy” to include subclassifications including idiopathic and structural epilepsy as well as epilepsy of unknown cause. 19
Diagnosis of “epilepsy” in veterinary medicine classically requires ≥2 seizures that are 24 hours apart and cases without a known underlying cause generally are recorded as “idiopathic epilepsy.” 46 As with all idiopathic conditions, idiopathic epilepsy is a diagnosis of exclusion, and therefore relies on the clinical acumen and resources available to the relevant veterinary teams. 47 In dogs aged 6 months to 6 years with normal interictal neurological examination, lack of clinically relevant abnormalities on routine blood serum biochemical and urinalysis tests with typical seizure presentation is consistent with Tier I level of diagnostic confidence. 44 A similar consensus view on diagnostic confidence for cats currently is not available. In cats, idiopathic epilepsy historically has been considered relatively rare, but in recent years more investigators have used the term routinely for cats and reported that up to 57% of cats with epilepsy might be classified as having idiopathic epilepsy. 13 It is conceivable that general veterinary practitioners may feel reluctant to formally diagnose epilepsy or idiopathic epilepsy in cats because of a combination of factors, including their limited confidence in performing a complete neurological examination in cats, the longstanding traditional belief that cats do not commonly have idiopathic epilepsy, and a belief that access to advanced imaging is essential to exclude other causes. Epilepsy was discussed as a possible diagnosis in a further 69 cats in our study, which could increase the proportion of RSD cases with a diagnosis of epilepsy to 183/458 cats (39.95%), although this figure could be substantially lower because cats >7 years of age are more likely to have structural epilepsy. 43 Cats with RSD cats that have a normal interictal neurological examination not infrequently are identified as having structural epilepsy based on advanced imaging, with 12.2% having clinically relevant magnetic resonance imaging (MRI) abnormalities reported in 1 study. 48 This proportion is similar to the 11.8% of clinically relevant MRI findings reported in dogs with normal interictal neurological examinations. 49 Thus, it is likely that some cats diagnosed with epilepsy in our study may have had an unrecognized structural cause that advanced imaging would have elucidated.
The median age at first recorded seizure event for RSD overall in our study was 8.9 years, which was comparatively higher than the median age of 6.4 years at first recorded seizure event for the subset of cats with RSD that we recorded as having epilepsy. Results of the risk factor modeling highlight the age group that is most likely to be diagnosed with epilepsy from among the RSD caseloads: cats aged 3 to 6 years had 3.32 times the odds of diagnosis compared with cats aged <3 years old. This tendency to diagnose epilepsy in younger cats among RSD caseloads is consistent with most previous studies, which reported cats diagnosed with epilepsy as younger than cats with structural causes. The reported ages of 3.4 to 4.6 years for epilepsy are lower than the 8.1 to 9.2 years reported for cats with structural causes. 3 , 4 , 20 , 43 , 48 , 50 Compared with cats <3 years of age, the odds of a diagnosis of epilepsy was higher in all older age groups (except for cats aged 12‐15 years). Therefore, although 3 to 6 years may be the peak time at which most cases of idiopathic epilepsy in cats are diagnosed, this condition should not be excluded for diagnosis in older animals simply because of their age. However, the odds of diagnosis of epilepsy in both the overall population and among the RSD caseloads suggest that cats <3 years of age have lower odds of diagnosis with epilepsy. These findings for the age at which epilepsy is most likely to affect cats can serve as a benchmark for practitioners when prioritizing differential causes of RSD in cats. Furthermore, this information may assist in the future development of guidelines in cats for the classification and diagnosis of idiopathic epilepsy.
Although the odds of diagnosis specifically with epilepsy were highest in cats aged 3 to 6 years, the odds of diagnosis with RSD in general increased progressively with age. Structural causes of RSD are reported in 40% to 70% of affected cats. 13 The RSD caseload in our study likely includes several underlying conditions, including neoplastic and vascular disorders that predominantly affect older animals. 9 , 43 Additionally, although recognized cases of reactive seizures were excluded from our study, some may have remained given that not all cases were comprehensively evaluated by complete serum biochemistry, urinalysis and blood pressure measurement. Because reactive seizures are also predominantly caused by conditions that preferentially affect older animals, 39 potential retention of some misclassified reactive seizure cases in our study may have increased the apparent probability of RSD in older cats. Seizures therefore should be considered as a common cause of paroxysmal events in cats, particularly with increasing age. This information may offer primary practitioners evidence with which to advise medical and possibly advanced imaging studies in cases in which seizures are suspected.
Insured cats had 1.49 times the odds of diagnosis with RSD compared with uninsured cats in our study. Animals that are insured are more likely to be presented to veterinary practices for investigation of medical problems because financial barriers to presentation are decreased. 51 Although it is also possible that a small proportion of cats may have become insured because their owners suspected a health problem, it is more likely that the higher diagnosis rates in insured cats reflect greater awareness of disease in insured cats rather than truly higher rates of inherent disease. The association between insurance and diagnosis was even higher for epilepsy, with insured cats showing 2.38 times the odds of a diagnosis of epilepsy compared with uninsured cats. Similar associations with increased diagnosis in insured animals have been reported previously for several disorders including diabetes mellitus in cats (×2.0), 52 lipoma in dogs (×1.78), 53 hyperadrenocorticism in dogs (×4.0), 54 and corneal ulceration in dogs (×1.6). 55 This insurance bias must be considered carefully when generalizing the results of studies that are based entirely on data from insured animals. 37 We did not report the proportion of animals referred for specialist management or that underwent advanced imaging, but it would have been useful to know these because a normal brain MRI and cerebrospinal fluid (CSF) analysis would increase confidence in a diagnosis of idiopathic epilepsy and insured animals are more likely to undergo this level of diagnostic evaluation.
Our findings do not support an association between breed and RSD in cats. The prevalence of RSD was not statistically different between crossbred cats (0.17%) and purebred cats (0.13%, P = .16). Breeds in our study with the highest RSD prevalence were Foreign (breed prevalence, 1.89%) and Burmilla (0.84%), but these results should be interpreted with caution because only 1 affected individual was represented in each of these breeds. The relatively low proportion of individual purebred cat breeds in the UK means that our study was grossly underpowered to evaluate breed as a risk factor. Given that the demographic data reported in our study show that crossbred cats comprise the majority of the UK pet cat population, this factor will always make it challenging to achieve good statistical power for breed comparisons in cats, even with access to increasingly large research datasets over time. 25 , 26 , 27 However, ownership of purebred cats currently is increasing, which may facilitate breed‐based research in cats in the future. 56
Specifically in relation to epilepsy, the results of univariable analysis in our study indicated that Burmese, Birman, and British Short Hair cats to have 3.27, 2.14, and 1.85 times the odds, respectively, of epilepsy compared to crossbreed cats, but the breed variable was not retained on multivariable analysis. The low counts of affected animals of these breeds in our study precludes firm conclusions from being drawn about breed risk, but these breeds perhaps should be considered specifically in future explorations of genetic factors for epilepsy of cats. A previous referral study in the UK reported that pedigreed cats had 5.68 times the odds of epilepsy compared with nonpedigreed cats. 43 However, unlike the situation in dogs, specific breeds have not yet been linked to epilepsy in cats, and genetic epilepsy only has been reported in study colonies of cats. 11 , 17 , 18 A study of audiogenic reflex seizures in cats reported that a large number of affected cats were Birmans. 57 Audiogenic reflex seizures are proposed to have a genetic basis despite their geriatric onset and possibly represent a genetic type of epilepsy in cats. 57 Further investigation of genetic causes of epilepsy in cats would benefit from similar development of rigorous diagnostic criteria of epilepsy of unknown cause in cats, as has been achieved in dogs. 5 , 19
No evidence was found of an association between sex and either RSD or epilepsy in cats in our study. This finding is consistent with previous studies in cats. 3 , 4 In dogs, males may have increased odds of developing seizure disorders, 7 , 8 , 58 but this observation is complicated by effects of neutering as well as different sex predisposition to seizures among specific dog breeds. 58 , 59
Despite the large numbers of cats affected by RSD in our study, no antiseizure medications currently are licensed for use in cats in the UK. Several studies have shown that cats can respond well to medical treatment and furthermore that stopping treatment leads to recurrence of seizure activity in up to 75% of affected cats. 13 , 60 Our findings should prompt manufacturers to consider steps to undertake the licensing of antiseizure medications in cats and encourage practitioners to consider medical treatment of affected animals.
Our study had some limitations. Despite there being an estimated 1 million more owned cats than dogs in the UK, 40 far fewer cats are registered at veterinary practices than dogs. 26 , 61 Although our study aimed to provide reliable estimates for the prevalence and demographics of RSD‐affected cats, many owned cats are not under routine veterinary care and thus our results should be interpreted with caution and not generalized. Barriers to regular veterinary presentation of cats may include lower numbers of insured cats than dogs (ie, 7% vs 19% of animals attending VetCompass participating practices) and perceived difficulties in medicating cats. Diagnosis of epilepsy in our study relied on the opinion of the primary care practitioner rather than the formal classification guidelines as laid out by IVETF, which were published after data collection for our study. 5 , 19
Only fair to moderate interobserver agreement has been found between neurologists and nonspecialists when diagnosing seizure activity in dogs and cats 15 and, as discussed above, poor concordance has been shown between primary care and retrospective classification of epilepsy cases in dogs. 45 Improvement in future studies using similar datasets would include extraction of information pertaining to ancillary testing so as to categorize animals into an IVEFT Tier 1 level of confidence of diagnosis of epilepsy. In addition to the clinical acumen of the individual veterinary practitioner and the available diagnostic tests, an accurate description of events by owners is also essential to generate the initial index of suspicion of seizure activity, 62 but such ability is likely to vary substantially among owners.
5. CONCLUSION
Our large retrospective study using a multicenter primary practice dataset found a 0.16% prevalence of RSD in cats, highlighting that these disorders are not uncommon in this species. Recurrent seizure disorders increase in prevalence with increasing age. No evidence was found for sex and breed associations with RSD in cats, which may reflect multiple etiologies in this species. Epilepsy was recorded in 0.04% of cats by primary care practitioners, with cats aged 3 to 6 years being the most likely age group of cats with RSD to be diagnosed with epilepsy. Future studies using expanded datasets are needed to fully explore questions related to semiology and clinical outcomes. The veterinary neurology community should take efforts to better define the term “epilepsy” in cats and develop diagnostic criteria.
CONFLICT OF INTEREST DECLARATION
Holger Volk in last 5 years served as contract researcher for: Nestle 2012‐2014 and 2017‐2020—dietary modification of epilepsy in dogs; Boehringer Ingelheim 2014‐2015—investigating the effects of imepitoin behavioral, physiologic and owner reported indicators of anxiety in dogs treated for idiopathic epilepsy; CASE BBSRC PhD studentship 2012‐2016—metabolic profiling of epilepsy in dogs; American Kennel Club American Health Foundation, 2016‐2020—Investigating the Effect of a Ketogenic Medium Chain Triglycerides Supplement on the treatment of Canine Idiopathic Epilepsy and its behavioral comorbidities; BBSRC 2017‐2020—Investigating the relationship between epilepsy, drug‐resistance and affective disorders in the domestic dog BB/P001874/1; provided continuous education for veterinary surgeons sponsored by Nestle, Boehringer Ingelheim and Virbac in the last 5 years.
Dan O'Neill was the supervisor on a study of seizures in dogs in 2018 supported by Bayer.
None of these COI relate to feline seizure disorders or epilepsy. The authors did not allow any bias from any of these COI to affect the design, analysis or writing of the current paper.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
O'Neill DG, Phillipps SA, Egan JR, Brodbelt D, Church DB, Volk HA. Epidemiology of recurrent seizure disorders and epilepsy in cats under primary veterinary care in the United Kingdom. J Vet Intern Med. 2020;34:2582–2594. 10.1111/jvim.15881
REFERENCES
- 1. Egenvall A, Nødtvedt A, Haggstrom J, et al. Mortality of life‐insured Swedish cats during 1999‐2006: age, breed, sex, and diagnosis. J Vet Intern Med. 2009;23:1175‐1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Neill DG, Church DB, McGreevy PD, et al. Prevalence of disorders recorded in cats attending primary‐care veterinary practices in England. Vet J. 2014;202:286‐291. [DOI] [PubMed] [Google Scholar]
- 3. Schriefl S, Steinberg TA, Matiasek K, et al. Etiologic classification of seizures, signalment, clinical signs, and outcome in cats with seizure disorders: 91 cases (2000–2004). J Am Vet Med Assoc. 2008;233:1591‐1597. [DOI] [PubMed] [Google Scholar]
- 4. Pákozdy Á, Leschnik M, Sarchahi AA, et al. Clinical comparison of primary versus secondary epilepsy in 125 cats. J Feline Med Surg. 2010;12:910‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volk HA. Editorial—International veterinary epilepsy task force consensus reports on epilepsy definition, classification and terminology, affected dog breeds, diagnosis, treatment, outcome measures of therapeutic trials, neuroimaging and neuropathology in companion animals. BMC Vet Res. 2015;11:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartlett PC, Van Buren JW, Neterer M, et al. Disease surveillance and referral bias in the veterinary medical database. Prev Vet Med. 2010;94:264‐271. [DOI] [PubMed] [Google Scholar]
- 7. Kearsley‐Fleet L, O'Neill DG, Volk HA, et al. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec. 2013;172:338. [DOI] [PubMed] [Google Scholar]
- 8. Erlen A, Potschka H, Volk HA, et al. Seizure occurrence in dogs under primary veterinary care in the UK: prevalence and risk factors. J Vet Intern Med. 2018;32:1665‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pakozdy A, Halasz P, Klang A. Epilepsy in cats: theory and practice. J Vet Intern Med. 2014;28:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer RMA. Survival in feline epilepsy: the long and short of it. Vet Rec. 2017;181:477. [DOI] [PubMed] [Google Scholar]
- 11. Hasegawa D, Pakozdy A, Volk HA. Differentiating structural from idiopathic epilepsy in cats. Vet Rec. 2017;180:608. [DOI] [PubMed] [Google Scholar]
- 12. Kline KL. Feline epilepsy. Clin Tech Small Anim Pract. 1998;13:152‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hazenfratz M, Taylor SM. Recurrent seizures in cats: diagnostic approach—when is it idiopathic epilepsy? J Feline Med Surg. 2018;20:811‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavely JA. Pediatric seizure disorders in dogs and cats. Vet Clin Small Anim Pract. 2014;44:275‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Packer RMA, Berendt M, Bhatti S, et al. Inter‐observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non‐specialists. BMC Vet Res. 2015;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mariani CL. Terminology and classification of seizures and epilepsy in veterinary patients. Top Companion Anim Med. 2013;28:34‐41. [DOI] [PubMed] [Google Scholar]
- 17. Kuwabara T, Hasegawa D, Ogawa F, et al. A familial spontaneous epileptic feline strain: a novel model of idiopathic/genetic epilepsy. Epilepsy Res. 2010;92:85‐88. [DOI] [PubMed] [Google Scholar]
- 18. Hamamoto Y, Hasegawa D, Mizoguchi S, et al. Changes in the interictal and early postictal diffusion and perfusion magnetic resonance parameters in familial spontaneous epileptic cats. Epilepsy Res. 2017;133:76‐82. [DOI] [PubMed] [Google Scholar]
- 19. Berendt M, Farquhar RG, Mandigers PJJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wahle AM, Brühschwein A, Matiasek K, et al. Clinical characterization of epilepsy of unknown cause in cats. J Vet Intern Med. 2014;28:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robinson PN, Köhler S, Bauer S, et al. The human phenotype ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83:610‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyalina S, Percha B, LePendu P, et al. Identifying phenotypic signatures of neuropsychiatric disorders from electronic medical records. J Am Med Inform Assoc. 2013;20:e297‐e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGreevy P, Thomson P, Dhand N, et al. VetCompass Australia: a national big data collection system for veterinary science. Animals. 2017;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. PETscan. Utrecht University; 2020.
- 25. VetCompass Australia. Sydney: The University of Sydney; 2020.
- 26. VetCompass . VetCompass™ Programme. London: RVC Electronic Media Unit; 2020.
- 27. SAVSNET. University of Liverpool; 2020.
- 28. O'Neill D, Church D, McGreevy P, et al. Approaches to canine health surveillance. Canine Genet Epidemiol. 2014;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The VeNom Coding Group . VeNom Veterinary Nomenclature. VeNom Coding Group; 2020.
- 30. Epi Info 7 CDC . Centers for Disease Control and Prevention (US): Introducing Epi Info 7. Atlanta, Georgia: CDC; 2019.
- 31. Murray JK, Browne WJ, Roberts MA, et al. Number and ownership profiles of cats and dogs in the UK. Vet Rec. 2010;166:163‐168. [DOI] [PubMed] [Google Scholar]
- 32. Scott M, Flaherty D, Currall J. Statistics: how many? J Small Anim Pract. 2012;53:372‐376. [DOI] [PubMed] [Google Scholar]
- 33. Robinson R. Genetics for Cat Breeders. 3rd ed. Oxford: Pergamon; 1991. [Google Scholar]
- 34. Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Canada: VER; 2009. [Google Scholar]
- 35. Kirkwood BR, Sterne JAC. Essential Medical Statistics. 2nd ed. Oxford: Blackwell Science; 2003. [Google Scholar]
- 36. Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 37. Heske L, Nødtvedt A, Jäderlund KH, et al. A cohort study of epilepsy among 665,000 insured dogs: incidence, mortality and survival after diagnosis. Vet J. 2014;202:471‐476. [DOI] [PubMed] [Google Scholar]
- 38. Qahwash M, Heller HLB. Seizure etiologic classification and long‐term outcome for cats with juvenile‐onset seizures. J Am Vet Med Assoc. 2018;253:763‐767. [DOI] [PubMed] [Google Scholar]
- 39. Kwiatkowska M, Tipold A, Huenerfauth E, et al. Clinical risk factors for early seizure recurrence in dogs hospitalized for seizure evaluation. J Vet Intern Med. 2018;32:757‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. PDSA . PAW PDSA Animal Wellbeing Report 2019; 2019.
- 41. Tauber C‐A. Epidemiologische Untersuchungen zum Vorkommen von Epilepsie bei Hund und Katze in der Kleintierpraxis. München: Ludwig‐Maximilians‐Universität München; 2017. [Google Scholar]
- 42. Packer RMA, Volk HA. Epilepsy beyond seizures: a review of the impact of epilepsy and its comorbidities on health‐related quality of life in dogs. Vet Rec. 2015;177:306‐315. [DOI] [PubMed] [Google Scholar]
- 43. Stanciu G‐D, Packer RMA, Pakozdy A, et al. Clinical reasoning in feline epilepsy: which combination of clinical information is useful? Vet J. 2017;225:9‐12. [DOI] [PubMed] [Google Scholar]
- 44. De Risio L, Bhatti S, Muñana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Erlen A, Potschka H, Volk HA, et al. Seizures in dogs under primary veterinary care in the UK: etiology, diagnostics and clinical management. J Vet Intern Med. 2020; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhatti SFM, De Risio L, Muñana K, et al. International veterinary epilepsy task force consensus proposal: medical treatment of canine epilepsy in Europe. BMC Vet Res. 2015;11:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fred HL. The diagnosis of exclusion: an ongoing uncertainty. Tex Heart Inst J. 2013;40:379‐381. [PMC free article] [PubMed] [Google Scholar]
- 48. Raimondi F, Shihab N, Gutierrez‐Quintana R, et al. Magnetic resonance imaging findings in epileptic cats with a normal interictal neurological examination: 188 cases. Vet Rec. 2017;180:610. [DOI] [PubMed] [Google Scholar]
- 49. Smith PM, Talbot CE, Jeffery ND. Findings on low‐field cranial MR images in epileptic dogs that lack interictal neurological deficits. Vet J. 2008;176:320‐325. [DOI] [PubMed] [Google Scholar]
- 50. Szelecsenyi AC, Giger U, Golini L, et al. Survival in 76 cats with epilepsy of unknown cause: a retrospective study. Vet Rec. 2017;181:479‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egenvall A, Nødtvedt A, Penell J, et al. Insurance data for research in companion animals: benefits and limitations. Acta Vet Scand. 2009;51:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Neill DG, Gostelow R, Orme C, et al. Epidemiology of diabetes mellitus among 193,435 cats attending primary‐care veterinary practices in England. J Vet Intern Med. 2016;30:964‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Neill DG, Corah CH, Church DB, et al. Lipoma in dogs under primary veterinary care in the UK: prevalence and breed associations. Canine Genet Epidemiol. 2018;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Neill DG, Scudder C, Faire JM, et al. Epidemiology of hyperadrenocorticism among 210,824 dogs attending primary‐care veterinary practices in the UK from 2009 to 2014. J Small Anim Pract. 2016;57:365‐373. [DOI] [PubMed] [Google Scholar]
- 55. O'Neill DG, Lee MM, Brodbelt DC, et al. Corneal ulcerative disease in dogs under primary veterinary care in England: epidemiology and clinical management. Canine Genet Epidemiol. 2017;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Malik R. Genetic diseases of cats. J Feline Med Surg. 2001;3:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lowrie M, Bessant C, Harvey RJ, et al. Audiogenic reflex seizures in cats. J Feline Med Surg. 2015;18:328‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Van Meervenne SAE, Volk HA, Matiasek K, et al. The influence of sex hormones on seizures in dogs and humans. Vet J. 2014;201:15‐20. [DOI] [PubMed] [Google Scholar]
- 59. Hülsmeyer V‐I, Fischer A, Mandigers PJJ, et al. International veterinary epilepsy task force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. 2015;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Platt S. Feline seizure control. J Am Anim Hosp Assoc. 2001;37:515‐517. [DOI] [PubMed] [Google Scholar]
- 61. Sánchez‐Vizcaíno F, Noble P‐JM, Jones PH, et al. Demographics of dogs, cats, and rabbits attending veterinary practices in Great Britain as recorded in their electronic health records. BMC Vet Res. 2017;13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Risio L, Newton R, Freeman J, et al. Idiopathic epilepsy in the Italian Spinone in the United Kingdom: prevalence, clinical characteristics, and predictors of survival and seizure remission. J Vet Intern Med. 2015;29:917‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]


