Abstract
Background
A nonpedigreed male cat presented with epistaxis, severe bladder hemorrhage, and secondary urethral obstruction after cystocentesis.
Objectives
To characterize the phenotype of a cat with bleeding diathesis and use a precision medicine approach to identify the molecular genetic defect by whole genome sequencing.
Methods
Adenosine diphosphate (ADP) and arachidonic acid (AA)‐induced whole blood platelet aggregometry was performed in the affected cat and a healthy cat. Platelet activation, measured by P‐selectin expression, and surface integrin subunit β3 expression were evaluated by flow cytometry in the affected cat and healthy control. Total integrin subunit αIIb expression was assessed by western blot. Whole genome sequencing at 30× coverage was used to identify genetic variants that segregated in the affected cat compared to 194 cats from the 99 Lives Sequencing Consortium.
Results
Platelet aggregometry identified significant impairment in platelet aggregation in response to ADP and AA compared to the control cat. Targeted protein expression analyses by flow cytometry and immunoblot analysis determined that the surface expression and total expression of the integrin, αIIbβ3, was absent. Whole genome sequencing identified a homozygous c.1986delC frameshift variant in the integrin subunit αIIb (ITGA2B) gene that was not detected in the control population. The p.Pro662fs (ITGA2B P662X) variant terminates translation of the protein at the extracellular domain of the integrin prematurely, which is predicted to affect expression of the β3 unit.
Conclusions and Clinical Importance
This novel ITGA2B variant and the associated phenotype closely resemble Glanzmann's thrombasthenia, which has never been reported in cats.
Keywords: Glanzmann's thrombasthenia, integrin αIIbβ3, ITGA2B, whole genome sequencing
Abbreviations
- ADP
adenosine diphosphate
- AA
arachidonic acid
- BSA
bovine serum albumin
- GT
Glanzmann's thrombasthenia
- PCR
polymerase chain reaction
- vWF
Von Willebrand factor
- UTR
untranslated regions
1. INTRODUCTION
Congenital platelet disorders are well‐described causes of bleeding diathesis in human beings and dogs. Variants affecting adenosine diphosphate (ADP) storage in the dense granules (platelet delta‐storage pool disease) or expression of the platelet integrin, αIIbβ3, (Glanzmann's thrombasthenia [GT]) previously have been described in specific dog breeds. 1 To date, genetic variants resulting in platelet disorders have not been reported in cats. We combined the use of advanced platelet function assays and whole genome sequencing to characterize the phenotype and identify the genetic cause recurrent bleeding in a cat.
1.1. Cat information
A 6‐month old male, domestic short hair cat was presented to the Emergency and Critical Care Service of the Veterinary Medical Teaching Hospital at University of California, Davis for evaluation of severe hematuria 4 days after cystocentesis. Cystocentesis was performed previously to collect urine for urinalysis before a scheduled castration. On presentation, the cat was obtunded, with a pulse rate of 220 beats per minute, rectal temperature of 99°F (37.2°C), white mucous membranes, and undetectable capillary refill time. A large and turgid bladder could be palpated, and hemorrhagic urine was found around the prepuce. Point‐of‐care ultrasound examination showed a large intraluminal bladder mass 3.3 × 5.7 cm in diameter with a cavitary center suggestive of a hematoma. No free abdominal fluid was noted. Packed cell volume (PCV) and total protein concentration (TS) by refractometry were 9% and 4.2 g/dL, respectively. Estimated platelet count on blood smear evaluation was 1.50 × 108/mL with clumps. Point‐of‐care prothrombin time and activated partial thromboplastin time (Coag Dx Analyzer, Idexx Laboratories, Westbrook, Maine) were within normal limits. The cat first was stabilized by administration of 25 mL of type A and crossmatched‐compatible packed red blood cells, 60 mL of fresh frozen plasma and 10 μg desmopressin SC. After transfusion treatments, the cat's PCV and TS were 15% and 6.1 g/dL, respectively. Because of ongoing urethral obstruction, a urinary catheter was placed under sedation, followed by celiotomy and cystotomy under general anesthesia to remove the hematoma. No additional transfusions were required during or after surgery. The urinary bladder was biopsied intraoperatively and histopathology disclosed mild neutrophilic and lymphocytic cystitis with severe hemorrhage within the lamina propria and serosa of the urinary bladder. Given the inapparent cause of hemorrhage, plasma von Willebrand factor (vWF) antigen concentration was measured (108%; reference interval [RI], > 80%; Diagnostica, Stago SAS, France). The cat remained stable after surgery and was discharged without any medications.
Approximately 3 years after discharge from the initial hospital visit, the cat was presented for prolonged bilateral epistaxis (>24 hours), gingival bleeding, and intermittent subungual hemorrhage. A CBC disclosed mild thrombocytopenia (1.09 × 108/mL; RI, 1.80‐5.00 × 108/mL/μL). The epistaxis stopped without any intervention 48 hours after presentation and the cat was discharged on 250 mg Yunnan Baiyao orally once daily. At the time of writing, the cat continues to be on the same dose of Yunnan Baiyao without any apparent signs of bleeding.
2. MATERIALS AND METHODS
2.1. Phenotypic evaluation of the bleeding diathesis
Given the chronic and recurring nature of bleeding in the absence of prolonged clotting times and severe thrombocytopenia in this cat, further testing was performed to rule out congenital platelet disorders. A 4‐year‐old female spayed clinically healthy domestic shorthair cat served as control for platelet phenotype assays. Blood was collected from the medial saphenous vein and was immediately aliquoted into blood tubes containing 3.2% sodium citrate, >15 μg/mL IU hirudin (S‐Monovette, Sarstedt, Numbrecht, Germany), or EDTA. Protocols were approved by the University of California, Davis Institutional Animal Care and Use Committee (IACUC No: 20095).
2.2. Multiple electrode impedance platelet aggregometry
Hirudin‐anticoagulated blood was analyzed using multiple electrode impedance platelet aggregometry (Multiplate, Diapharma, West Chester, Ohio) 30 minutes after blood collection according to the manufacturer's instructions. Briefly, 300 μL of anticoagulated blood was incubated with 300 μL of 0.9% NaCl for 3 minutes at 37°C with continuous stirring, followed by the addition of 6.5 μM ADP (Roche, Basel, Switzerland) or 0.5 mM arachidonic acid (AA) (Roche, Basel, Switzerland). Each test cell incorporates a duplicate sensor for 2 simultaneous measurements, which served as an internal control. Platelet aggregation was reported as area under the curve over a 6‐minute interval. If the Pearson's correlation coefficient and difference between the 2 measurements were <0.98 or >20%, respectively, the measurements were repeated.
2.3. Flow cytometry
To further characterize platelet activation and integrin expression in the cat, we performed flow cytometry on platelet rich plasma (PRP) generated from the affected cat and the healthy control. Platelet rich plasma was generated as previously described. 2 The platelet count of PRP was first corrected to a final concentration of 1 × 107 cells/mL with Tyrode's hydroxyethyl piperazineethanesulfonic acid (HEPES) buffer (pH 7.2, 5 mM dextrose, no divalent cations) before stimulation with 20 μM ADP (Sigma‐Aldrich, St. Louis, Missouri) or 0.01 U/mL bovine α‐thrombin for 15 minutes at 37°C. Platelet rich plasma that was unstimulated (resting) served as biological negative control. All samples then were incubated with allophycocyanin‐conjugated polyclonal mouse anti‐human antibody to integrin β3 (CD61; 1:1000; clone VI‐PL2, Invitrogen, Carlsbed, California). Platelet P‐selectin (CD62P) was detected by monoclonal rat anti‐mouse antibody conjugated to fluorescein isothiocyanate (1:200;clone RB40.34, BD Pharmingen, San Jose, California) for 45 minutes at 37°C. Then, samples were fixed in 1% paraformaldehyde for 45 minutes at room temperature before analysis using a 5‐color flow cytometer (FC500, Beckmann Coulter, Pasadena, California). Compensation beads and monoclonal isotype immunoglobulin G1 kappa conjugated to matched experimental fluorochromes were used for compensation controls (BD CompBead, BD, San Jose, California). Platelets were identified by forward and side scatter properties by 0.9 and 3 μm calibration beads as previously described. 3 Gating boundaries were established by using fluorescence‐minus‐one controls to identify events positive for CD61 (integrin β3) and CD62P (P‐selectin) within the platelet gate (Figure 2A) as previously described. 4
FIGURE 2.
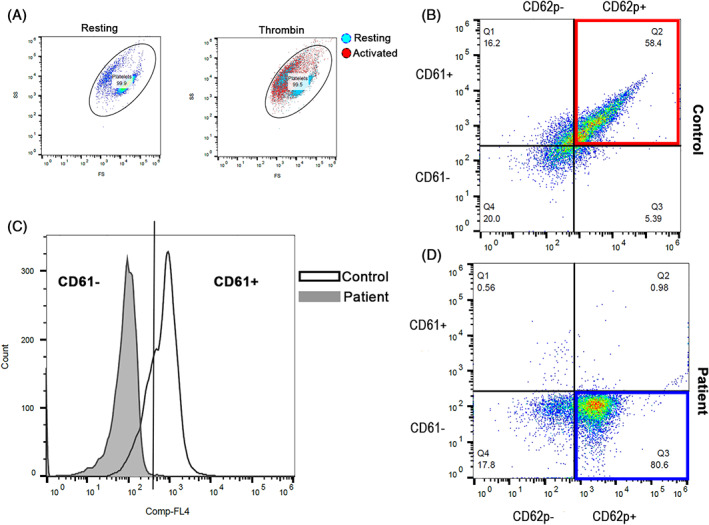
Representative scatter plot diagrams and histograms of flow cytometric in the cat with bleeding diathesis and a healthy control cat. A, Platelets were identified based on their forward‐(FS) and side‐scatter (SC) properties in unstimulated state (resting). Cat platelets underwent shape change in response to thrombin. Note the shift in FS and SC properties in thrombin‐activated platelets (red) compared to resting platelets (blue). B, Scatter dot plots demonstrating the co‐expression of P‐selectin (CD62P+) and integrin subunit β3 (CD61+) in adenosine diphosphate (ADP)‐activated platelets in the control cat (red box). C, A histogram illustrating the number of platelets expressing integrin subunit β3 (CD61+). Platelets from the cat (gray) failed to express integrin subunit β3 on the plasma membrane compared to platelets in a healthy cat (white). D, In contrast, despite the cat's ability to respond to ADP by expression P‐selectin (blue box) (CD62P+), no platelets co‐expressed P‐selectin and integrin subunit β3 (CD61−)
2.4. Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and western blot analysis
Based on whole genome sequencing results, we verified the intracellular expression of integrin subunit αIIb using western blot analysis. Platelets (1 × 108 cells/mL) from the patient and the control cat were lysed in 1× Laemmli buffer with dithiothreitol and boiled (100°C) for 5 minutes. Platelet lysate immediately was placed on ice (5 minutes) and protease inhibitor (ThermoFisher, Waltham, Massachusetts) was added before storage at −20°C until further analysis. Platelet lysate (30 μL) was separated on 7.5% polyacrylamide gel (Bio‐Rad, Hercules, California) by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred to polyvinylidene fluoride membranes (Bio‐Rad) before staining with 0.1% Ponceau S to confirm equal and adequate protein transfer. Membranes then were blocked in 5% bovine serum albumin (BSA) overnight at 4°C, washed 3 times in 1× tris‐buffered saline, 0.1% polysorbate 20, before incubating in rat anti‐mouse antibody to integrin αIIb (CD41a; 0.05 μg/mL, clone: eBioMWReg30, eBioscience, Waltham, Massachusetts), known to cross‐reacts in cats. 5 After washing, the membrane was incubated with goat anti‐rat antibody conjugated to horseradish peroxidase (1:20 000, ab97057, Abcam, Cambridge, Massachusetts) for 10 minutes at room temperature, before addition of chemiluminescent substrate (Advansta, Menlo Park, California) and imaged (FluorChem E system, Proteinsimple, San Josa, California). Membranes then were stripped, washed, and reblocked with 5% BSA (overnight, 4C) before being incubated in mouse anti‐beta actin antibody (1:500, ab8224, Abcam), followed by goat anti‐mouse secondary antibody conjugated to horseradish peroxidase (1:50 000, ab97023, Abcam). Blots were analyzed by chemiluminescence as described above.
2.5. Genotypic characterization of platelet disorder
2.5.1. Whole genome sequencing and variant detection
A whole genome sequencing approach was used to determine the genetic cause of this cat's phenotype. The DNA was extracted from EDTA‐anticoagulated whole blood using a commercially available kit (Qiagen Puregene, Germantown, Maryland) and following the manufacturer's protocol. To ensure the quality of DNA was adequate for whole genome sequencing, the sample first was quantified by spectrophotometry (NanoDrop One, ThermoFisher) and then run on a 2% agarose gel to further confirm DNA quality. Two micrograms of DNA from this cat were submitted for whole genome sequencing at the McDonnell Genome Institute of Washington University, Saint Louis, Missouri. A 450 bp insert KAPA polymerase chain reaction (PCR)‐free library was constructed and sequenced using an Illumina NovaSeq S4 instrument (Illumina, San Diego, California) to generate 150 bp paired‐end reads at approximately 30× coverage. The generated sequence file was processed as previously described (the affected cat raw data was submitted to National Center for Biotechnology Information short read archive under BioProject: PRJNA641132). 6
The variant call file was uploaded and analyzed using single nucleotide polymorphism SNP & Variation Suite commercial software (Golden Helix, Bozeman, Montana). The variant call file contained variants for our domestic shorthair cat sample as well as 194 unphenotyped whole genome sequenced cats of 44 different breeds from the 99 Lives Sequencing Consortium (http://felinegenetics.missouri.edu/99lives). Sequence analysis and variant calling for the 99 Lives Consortium was performed at the University of Missouri, College of Veterinary Medicine. Initially, variants with a call rate of <80% were removed from this analysis. After, variants were annotated using Ensembl Variant Effect Predictor v100 web interface and variants located in intergenic regions were removed from further analysis. 7 , 8 Only heterozygous or homozygous variants in the affected cat and homozygous reference variants in all other cats (n = 194) were further analyzed.
Variants located in exons, introns, and in the 3′ or 5′ untranslated regions (UTR) with the following effects were furthered analyzed: 5′UTR premature start codon gain variants, disruptive inframe insertion variants, frameshift variants, inframe deletion variants, missense variants, noncoding exon variants, splice acceptor or donor variants, splice region variants, and stop gained variants. Variants classified as modifiers or those that had a high, moderate, or low predicted effect on protein function or structure based on Ensembl IMPACT rating remained in the analysis. A modifier classified variant refers to noncoding variants for which the predicted effect is difficult to discern. A variant classified as having a high effect refers to a variant that has a disruptive impact on a protein causing either truncation or loss of function. A moderate classified variant can alter a protein's function and a low classified variant is considered benign and unlikely to alter the protein's function. Additionally, variants that had a sorting intolerant from tolerant (SIFT) score available and ≤0.05 were further analyzed. The SIFT score is a predictive measure that analyzes whether an amino acid substitution affects protein function based on amino acid properties. After filtering was complete, we searched for genes that previously have been associated with platelet disorders. Predicted effects on protein structure for the variants of interest were modeled using SWISS‐MODEL. 8 , 9 , 10 , 11
Primers were designed for the identified integrin subunit alpha 2b (ITGA2B) variant. The forward (TGCTCAGCTTCAATGTGTCC) and reverse (TCAAGATTGTGGGTTCACA) primers were used for the PCR following the manufacturer's protocol for LATaq with GC buffer (Takara Bio USA Inc, Mountain View, California). The PCR products were cleaned using ExoSap‐It Express (ThermoFisher) and submitted for Sanger sequencing to the University of California, Davis DNA Sequencing Facility (Davis, California). For cycle sequencing, an ABI BigDye Terminator v3.1 kit was utilized followed by sequencing on an ABI 3730 Genetic Analyzer (ThermoFisher). The chromatograms were analyzed using Lasergene Evolution Suite (DNAStar, Madison, Wisconsin).
3. RESULTS
3.1. Multiple electrode impedance platelet aggregometry
The ADP and AA‐induced platelet aggregation in the affected cat were 0 and 3 units, respectively. In the healthy control, ADP‐ and AA‐induced aggregations were 101 and 117 U, respectively (Figure 1). The absence of platelet aggregation in response to ADP and AA was supportive of severe platelet dysfunction in the affected cat.
FIGURE 1.
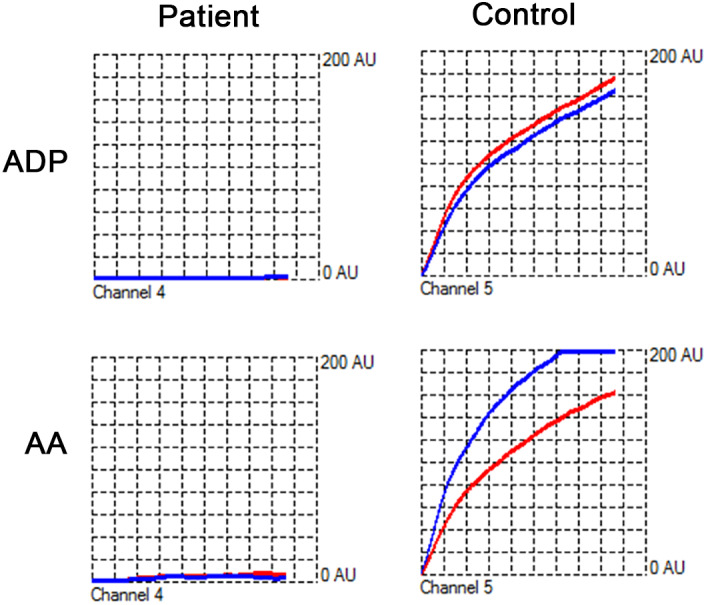
Representative tracings of adenosine diphosphate (ADP) and arachidonic acid (AA)‐induced whole blood platelet aggregometry in a cat with bleeding diathesis and a healthy control cat. No aggregation was detected upon activation by ADP or AA in the cat. Aggregation of platelets on the paired electrodes (red and blue curves) in response to ADP or AA resulted in gradual increase in electrical impedance, measured as aggregation units (AU) in the control cat
3.2. Flow cytometry
On flow cytometry, platelets from the affected cat displayed the usual characteristics of ex vivo platelet activation such as shape change and degranulation in response to thrombin (Figure 2A). However, the expression of surface integrin subunit β3 accounted for <2% on the affected cat's platelets compared to 84.3% in the control cat (Figure 2C). This finding indicates that the lack of aggregation in response to ADP and AA was a result of substantial downregulation of integrin subunit β3 on the membrane surface. Platelets from the affected cat, however, maintained the ability to undergo ADP‐mediated alpha granule secretion as evidenced by P‐selectin expression (Figure 2D, bottom panel).
3.3. Sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and western blot analysis
Total expression of integrin subunit αIIb, shown in western blot analysis (Figure 3) was consistent with surface β3 expression on flow cytometry. Although the control healthy cat expressed a detectable amount of integrin αIIb, platelets from the affected cat expressed trace to no integrin αIIb on immunoblot analysis.
FIGURE 3.
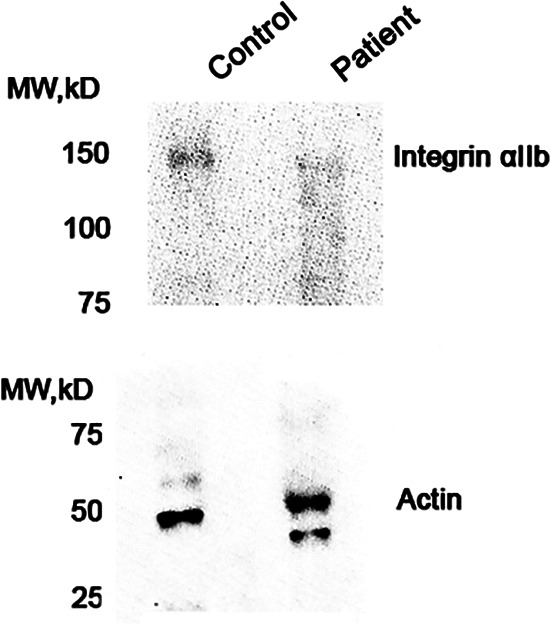
Representative immunoblot analysis of platelet integrin subunit αIIb in platelet lysates from a healthy control and the cat with bleeding diathesis. Molecular weight of integrin subunit αIIb is approximately 110 kDa. Immunoblot demonstrates that the cat's platelets did not express integrin subunit αIIb when compared to those in the healthy control. Beta actin (~42 kDa) was used as loading control. The analysis was repeated twice to ensure consistent results
3.4. Whole genome sequencing and variant discovery
A total of 89 817 458 variants were identified in the affected cat and in an additional 194 cats. Variants with a call rate <80% were removed and the remaining variants annotated using Ensembl VEP. Variants located in intergenic regions were removed from further analysis resulting in 32 506 712 variants. The affected cat sample genotype was used to further filter in which the affected cat can either be heterozygous or homozygous and all other cats (n = 194) were set to be the homozygous reference. This method was chosen because of the presumed rare nature of this disease in the general cat population. This approach resulted in 14 796 variants of which 517 variants in 383 genes met the filtering criteria described above (Table S1). Of these variants, 13 variants had a high Ensembl IMPACT rating that corresponds to variants predicted to result in a truncated protein or protein with loss of function (Table 1)]. Only the c.1986delC variant in the ITGA2B gene previously was associated with a platelet disorder, GT. The c.1986delC (p.Pro662fs) variant in exon 18 of the ITGA2B gene results in a cytosine deletion causing a reading frameshift and early termination. This frameshift variant truncates the protein structure at the genu resulting in a protein model that is predicted to lack the extracellular calf domains and the transmembrane domain (Figure 4B). The affected cat was homozygous for this variant based on whole genome sequencing and PCR genotyping (Figure 4C). The ITGA2B gene (Transcript ID: ENSFCAT00000003056.6) is located on chromosome E1:44 410 252‐44 4244 647 of the Felis catus 9.0 genome assembly.
TABLE 1.
Variants with a high predicted effect on protein structure based on Ensembl IMPACT rating identified in one domestic short hair cat
| Chr. | Position | Polyphen results | Functional class | Ref | Alt | Gene | Genotype | Protein Effect |
|---|---|---|---|---|---|---|---|---|
| A1 | 132227081‐132227082 | High | Splice donor | AC | A | DIMT1 | HO | N/A |
| A3 | 26189124‐26189124 | High | Frameshift | C | CTTTAAATTTTTTTTTTTTT | BPIFB6 | HET | p.(Lys133fs) |
| A3 | 26189131‐26189131 | High | Stop gained | G | GTTTTTATTTATTTTTGGGACAGAGAGAGACAGAGCA | BPIFB6 | HET | p.(Thr131_Met132ins24*) |
| A3 | 107759081‐107759081 | High | Stop gained | C | T | ITPRIPL1 | HET | p.(Trp194*) |
| B3 | 72098445‐72098446 | High | Frameshift | TG | T | LOC101099214 | HO | p.(Gly232fs) |
| B4 | 11371990‐11371990 | High | Splice donor | G | A | SEC61A2 | HO | N/A |
| B4 | 25705263‐25705263 | High | Stop gained | C | A | MKX | HO | p.(Glu73*) |
| C1 | 1568704‐1568721 | High | Frameshift | GTGGTTCTCCACGTGGAT | G | ACTRT2 | HO | p.(Trp338fs) |
| C1 | 79163959‐79163959 | High | Frameshift | C | CA | RPL5 | HET | p.(Ser12fs) |
| C1 | 152341977‐152341981 | High | Splice acceptor | CTGAA | C | RBMS1 | HO | N/A |
| C1 | 20958349‐20958349 | High | Stop gained | T | TAGACAGTACAGAAGCCAATGTGCAAAGCTCAGAAGTACACTGATCAGTTATGTGAGCTGCTTACTATCAGAAAAAAAATCTTGTAAGAAGATCCATATCC | STX12 | HET | p.(Ile216_Ile217ins39*) |
| D1 | 80922413‐80922413 | High | Stop gained | C | T | LOC101100590 | HET | p.(Arg286*) |
| E1 | 44416063‐44416064 | High | Frameshift | CG | C | ITGA2B | HO | p.Pro662fs |
FIGURE 4.
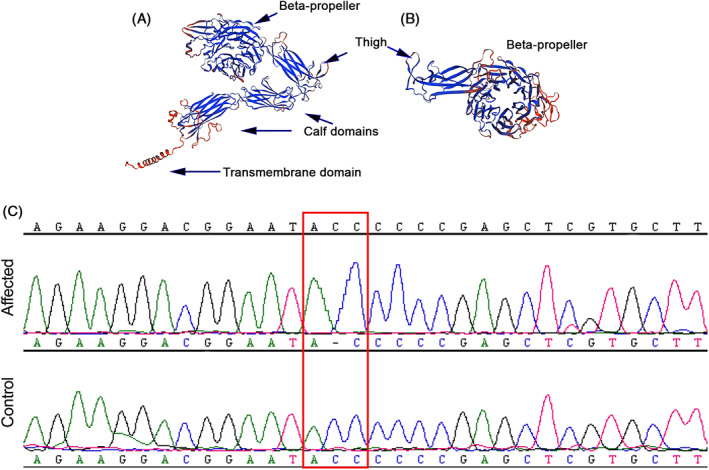
Illustration of the predicted wildtype protein structure (A) for integrin subunit αIIb, encoded by ITGA2B and truncated protein structure (B) for a cat that is homozygous for the ITGA2B P662X frameshift variant (C). Note how the truncated protein (B) has altered beta‐propeller and thigh domain and lacks the extracellular calf and transmembrane domains that are otherwise found in the wildtype protein structure. Additionally, note that in the chromatogram the affected cat has a deletion of a cytosine compared to the reference genome and unaffected cat
4. DISCUSSION
We describe here a novel variant, ITGA2B P662X, which affects expression of platelet integrin, αIIbβ3, caused impaired platelet aggregation and a bleeding diathesis in the affected cat. The platelet integrin, αIIbβ3, is a heterodimeric adhesion molecule that mediates cell‐to‐cell and cell‐to‐matrix interactions. Platelet aggregation, which is pivotal to hemostasis and thrombus formation, is mediated primarily by αIIbβ3. 12 In response to platelet agonists such as ADP, thromboxane A2 and thrombin, platelets initiate intracellular signaling cascades triggering the activation of the integrin from a low‐affinity to a high‐affinity state, a process known as inside‐out signaling. The activated αIIbβ3 then binds to extracellular matrix proteins such as fibrinogen, fibronectin or vWF to not only mediate platelet aggregation but also facilitate outside‐in signaling which further amplifies a range of cellular events essential for thrombus formation and consolidation. 13 The phenotype of the affected cat closely resembles type I GT in human beings, and GT in Great Pyrenees dogs. 14 Type I GT is characterized by a lack of expression of αIIb, β3, or both integrin subunits, impairment of platelet aggregation in the presence of platelet agonists and lack of fibrinogen in platelet α granules. Similar to dogs and humans with GT, the bleeding diathesis in our cat was mild but unpredictable. 14 Hemorrhage in affected cat only became life‐threatening after cystocentesis and subsequently was controlled after administration of fresh frozen plasma and desmopressin. Given our suspicion of a congenital platelet disorder in this cat during the initial hospitalization period, desmopressin was administered because of its ability to facilitate platelet adhesion by increasing plasma concentrations of vWF and factor VIII. In humans, desmopressin has been used as an adjunct treatment to improve hemostasis in people with congenital platelet disorders including GT. 15 Interestingly, no additional blood product was required during or after cystotomy in the affected cat. This observation highlights the importance of maintaining good hemostasis practice throughout invasive procedures in patients with GT. Because of the lack of readily available platelet products for cats and the financial burden associated with multiple transfusions, we preemptively treated our cat with Yunnan Baiyao, an herbal remedy with procoagulant effects. In non‐feline species, Yunnan Baiyao has been shown to reinforce clot strength and enhance ex vivo platelet granule secretion and expression of surface glycoproteins on platelets. 13 , 16 , 17 The hemostatic effects of Yunnan Baiyao on feline platelets and coagulation, however, remain poorly understood. 18 , 19
In our cat, a frameshift variant in exon 18 in the ITGA2B gene presumably resulted in truncation of the extracellular domain at the genu, a bending point between the thigh and calf‐1 domains within the heavy chain of the αIIb subunit (Figure 4). The absence of subunit β3 on the platelet surface, as initially detected by flow cytometry, is consistent with the variant identified in the ITGA2B gene. Although no consequential variants in ITGB3 were detected in our cat, studies using human megakaryocytes show that the assembly of both subunits occurs shortly after translation in the rough endoplasmic reticulum. Early interactions between both subunits are crucial for surface expression of the αIIbβ3 complex. 20 Only heterodimers assembled in the endoplasmic reticulum are transported to the Golgi apparatus, where additional modification occurs before export to the cell membrane. In this case, a defect in subunit αIIb likely resulted in failure of dimeric complex formation, causing complete absence of the αIIbβ3 complex on the platelet surface. 21 , 22
We illustrated the advantages of utilizing precision medicine to identify the genetic etiology of a disease in the affected cat. This approach was possible using next‐generation sequencing technologies with improved availability to genetic and genomic resources for both purebred and mixed breed cats. Specifically, the 99 Lives Sequencing Consortium has been an essential resource for high coverage whole genome sequenced cats of various breeds (http://felinegenetics.missouri.edu/99lives). This approach and database have been validated and utilized previously, but ours is the first report to identify a novel variant associated with a platelet disorder in an individual cat. 6 , 23 , 24 As precision medicine continues to be used in clinical settings, the genetic makeup of cats will become an integral part of their standard healthcare. Specifically, this case is an example of precision feline medicine, which guided us in implementing appropriate preventative and life‐saving treatment for this rare congenital platelet disorder.
CONFLICT OF INTEREST DECLARATION
Joshua Stern serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
University of California, Davis IACUC approval, #20095.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Variants that are either heterozygous or homozygous in our affected shorthair domestic cat when compared to 194 control samples that are wildtype.
ACKNOWLEDGMENTS
The authors acknowledge the 99 Lives Sequencing Consortium for providing a large control sample for this manuscript. Each member of the consortium has reviewed and approved this manuscript for publication. The 99 Lives Consortium consists of Reuben M. Buckley,1 Danielle Aberdein,2 Paulo C. Alves,3,4 Gregory S. Barsh,5,6 Rebecca R. Bellone,7 Tomas F. Bergström,8 Adam R. Boyko,9 Jeffrey A. Brockman,10 Margret L. Casal,11 Marta G. Castelhano,12 Ottmar Distl,13 Nicholas H. Dodman,14 N. Matthew Ellinwood,15 Jonathan E. Fogle,16 Oliver P. Forman,17 Dorian J. Garrick,2,15 Edward I. Ginns,18 Jens Häggström,19 Robert J. Harvey,20 Daisuke Hasegawa,21 Bianca Haase,22 Christopher R. Helps,23 Isabel Hernandez,24 Marjo K. Hytönen,25 Maria Kaukonen,25 Christopher B. Kaelin,5,6 Tomoki Kosho,26 Emilie Leclerc,27 Teri L. Lear,28 Tosso Leeb,29 Hannes Lohi,25 Maria Longeri,30 Mark A. Magnuson,31 Richard Malik,32 Shrinivasrao P. Mane,33 John S. Munday,2 William J. Murphy,34 Niels C. Pedersen,35 Simon M. Peterson‐Jones,36 Max F. Rothschild,15 Clare Rusbridge,37 Beth Shapiro,38 William F. Swanson,39 Karen A. Terio,40 Rory J. Todhunter,12 Wesley C. Warren,41 Elizabeth A. Wilcox,12 Julia H. Wildschutte,42 Yoshihiko Yu,21 Leslie A. Lyons.1 1Department of Veterinary Medicine and Surgery, College of Veterinary Medicine, University of Missouri, Columbia, MO, 65211. 2School of Veterinary Science, Massey University, Palmerston North 4442 New Zealand. 3CIBIO/InBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos/InBIO Associate Lab & Faculdade de Ciências, Universidade do Porto, Campus e Vairão, 4485‐661 Vila do Conde, Portugal. 4Wildlife Biology Program, University of Montana, Missoula, MT 59812. 5HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806. 6Department of Genetics, Stanford University, Stanford, CA 94305. 7Veterinary Genetics Laboratory, University of California, Davis, Davis, CA 95616. 8Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences, 750 07 Uppsala, Sweden. 9Department of Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853. 10Hill's Pet Nutrition Inc., Topeka, KS 66601. 11Reproduction, and Pediatrics, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA 19104. 12Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853. 13Institute for Animal Breeding and Genetics, University of Veterinary Medicine, Hannover, 30559, Hannover, Germany. 14Department of Clinical Sciences, Cummings School of Veterinary Medicine, Tufts University, Grafton, MA 01536. 15Department of Animal Science, College of Agriculture and Life Sciences, Iowa State University, Ames, IA 50011. 16College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607. 17WALTHAM Centre for Pet Nutrition, Freeby Lane, Waltham on the Wolds, Leicestershire, LE14 4RT UK. 18Department of Psychiatry, University of Massachusetts Medical School, Worcester, MA 01655. 19Department of Clinical Sciences, Faculty of Veterinary Medicine and Animal Science, Swedish University of Agricultural Sciences, Uppsala, SE‐750 07 Sweden. 20School of Health and Sport Sciences, University of the Sunshine Coast, Sippy Downs, QLD 4558, Australia. 21Department of Clinical Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo 180‐8602, Japan. 22Sydney School of Veterinary Science, Faculty of Science, University of Sydney, Sydney, NSW 2006, Australia. 23Langford Vets, University of Bristol, Langford, Bristol, BS40 5DU UK. 24Pediatrics and Medical Genetics Service, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853. 25Department of Veterinary Biosciences; Department of Medical Genetics, University of Helsinki and Folkhälsan Research Center, Helsinki 00014 Finland. 26Department of Medical Genetics, Center for Medical Genetics, Shinshu University Hospital, Matsumoto, Nagano 390‐8621, Japan. 27SPF ‐ Diana Pet food ‐ Symrise Group ‐ 56250 Elven, France. 28Department of Veterinary Science, University of Kentucky ‐ Lexington, Lexington, KY 40506 (In memoriam). 29Vetsuisse Faculty, Institute of Genetics, University of Bern, 3001 Bern, Switzerland. 30Dipartimento di Medicina Veterinaria, University of Milan, 20122 Milan, Italy. 31Departments of Molecular Physiology and Biophysics, Cell and Developmental Biology, and Medicine, Vanderbilt University, School of Medicine, Nashville, TN 37232. 32Centre for Veterinary Education, University of Sydney, Sydney, NSW 2006 Australia. 33Elanco Animal Health, Greenfield, IN 46140. 34Department of Veterinary Integrative Biosciences, College of Veterinary Medicine, Texas A&M University, College Station, TX 77845. 35Department of Medicine and Epidemiology, School of Veterinary Medicine, University of California at Davis, Davis, CA 95616. 36Small Animal Clinical Sciences, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48824. 37School of Veterinary Medicine, Faculty of Health & Medical Sciences, Univesity of Surrey, Guildford, Surrey, GU2 7AL, UK. 38Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, Santa Cruz, CA95064. 39Center for Conservation and Research of Endangered Wildlife (CREW), Cincinnati Zoo & Botanical Garden, Cincinnati, OH 45220. 40Zoological Pathology Program, University of Illinois, Brookfield, IL 60513. 41Division of Animal Sciences, College of Agriculture, Food and Natural Resources; School of Medicine, University of Missouri, Columbia, MO 65211. 42Bowling Green State University, Department of Biological Sciences, Bowling Green, OH 43403.
Li RHL, Ontiveros E, Nguyen N, Stern JA, Lee E, Hardy BT, the 99 Lives Cat Genome Consortium. Precision medicine identifies a pathogenic variant of the ITGA2B gene responsible for Glanzmann's thrombasthenia in a cat. J Vet Intern Med. 2020;34:2438–2446. 10.1111/jvim.15886
Funding information Center for Companion Animal Health, University of California, Davis, Grant/Award Number: 2019‐30‐F; Maxine Adler Graduate Fellowship for Eric Ontiveros
REFERENCE
- 1. Boudreaux MK, Lipscomb DL. Clinical, biochemical, and molecular aspects of Glanzmann's thrombasthenia in humans and dogs. Vet Pathol. 2001;38:249‐260. [DOI] [PubMed] [Google Scholar]
- 2. Li RHL, Stern JA, Ho V, Tablin F, Harris SP. Platelet activation and clopidogrel effects on ADP‐induced platelet activation in cats with or without the A31P mutation in MYBPC3. J Vet Intern Med. 2016;30:1619‐1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li RHL, Nguyen N, Tablin F. Canine platelets express functional toll‐like receptor‐4: lipopolysaccharide‐triggered platelet activation is dependent on adenosine diphosphate and thromboxane A2 in dogs. BMC Vet Res. 2019;15:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li RHL, Nguyen N, Rosati T, Jandrey K. Assessment of P2Y12 inhibition by clopidogrel in feline platelets using flow cytometry quantification of vasodilator‐stimulated phosphoprotein phosphorylation. Front Vet Sci. 2020;7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tablin F, Schumacher T, Pombo M, et al. Platelet activation in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:411‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ontiveros ES, Ueda Y, Harris SP, Stern JA, 99 Lives Consortium . Precision medicine validation: identifying the MYBPC3 A31P variant with whole‐genome sequencing in two Maine Coon cats with hypertrophic cardiomyopathy. J Feline Med Surg. 2019;21:1086‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zerbino DR, Achuthan P, Akanni W, et al. Ensembl 2018. Nucleic Acids Res. 2018;46:D754‐D761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLaren W, Gil L, Hunt SE, et al. The Ensembl variant effect predictor. Genome Biol. 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waterhouse A, Bertoni M, Bienert S, et al. SWISS‐MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296‐W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS‐MODEL and Swiss‐PdbViewer: a historical perspective. Electrophoresis. 2009;30(Suppl 1):S162‐S173. [DOI] [PubMed] [Google Scholar]
- 11. Benkert P, Biasini M, Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goggs R, Poole AW. Platelet signaling—a primer. J Vet Emerg Crit Care (San Antonio). 2012;22:5‐29. [DOI] [PubMed] [Google Scholar]
- 13. Durrant TN, van den Bosch MT, Hers I. Integrin alpha IIb beta3 outside‐in signaling. Blood. 2017;130:1607‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boudreaux MK, Kvam K, Dillon AR, et al. Type I Glanzmann's thrombasthenia in a Great Pyrenees dog. Vet Pathol. 1996;33:503‐511. [DOI] [PubMed] [Google Scholar]
- 15. DiMichele DM, Hathaway WE. Use of DDAVP in inherited and acquired platelet dysfunction. Am J Hematol. 1990;33:39‐45. [DOI] [PubMed] [Google Scholar]
- 16. Tansey C, Wiebe ML, Hybki GC, et al. A prospective evaluation of oral Yunnan Baiyao therapy on thromboleastographic parameters in apparently healthy dogs. J Vet Emerg Crit Care (San Antonio). 2018;28:221‐225. [DOI] [PubMed] [Google Scholar]
- 17. Chew EC. Yunnan Bai Yao‐induced platelet release in suspensions of washed platelets. Comp Med East West. 1977;5:271‐274. [DOI] [PubMed] [Google Scholar]
- 18. Patlogar JE, Tansey C, Wiebe M, et al. A prospective evaluation of oral Yunnan Baiyao therapy on thromboelastographic parameters in apparently healthy cats. J Vet Emerg Crit Care (San Antonio). 2019;29:611‐615. [DOI] [PubMed] [Google Scholar]
- 19. Chew EC. Effects of Yunnan Bai Yao on blood platelets: an ultrastructural study. Comp Med East West. 1977;5:169‐175. [DOI] [PubMed] [Google Scholar]
- 20. Duperray A, Berthier R, Chagnon E, et al. Biosynthesis and processing of platelet GPIIb‐IIIa in human megakaryocytes. J Cell Biol. 1987;104:1665‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Toole TE, Loftus JC, Plow EF, et al. Efficient surface expression of platelet GPIIb‐IIIa requires both subunits. Blood. 1989;74:14‐18. [PubMed] [Google Scholar]
- 22. Rosa JP, McEver RP. Processing and assembly of the integrin, glycoprotein IIb‐IIIa, in HEL cells. J Biol Chem. 1989;264:12596‐12603. [PubMed] [Google Scholar]
- 23. Aberdein D, Munday JS, Gandolfi B, et al. A FAS‐ligand variant associated with autoimmune lymphoproliferative syndrome in cats. Mamm Genome. 2017;28:47‐55. [DOI] [PubMed] [Google Scholar]
- 24. Mauler DA, Gandolfi B, Reinero CR, et al. Precision medicine in cats: novel Niemann‐Pick type C1 diagnosed by whole‐genome sequencing. J Vet Intern Med. 2017;31:539‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Variants that are either heterozygous or homozygous in our affected shorthair domestic cat when compared to 194 control samples that are wildtype.


