Abstract
Background
Hyperlipasemia is frequent in critically ill people without evidence of acute pancreatitis (AP), and has been associated with increased morbidity and mortality.
Objective
To evaluate the prevalence of hyperlipasemia at admission and development of hyperlipasemia during hospitalization in critically ill dogs, explore factors associated with hyperlipasemia, and evaluate association with outcome.
Animals
Critically ill, client owned dogs (n = 1360), presented on emergency and admitted to the intensive care unit, that had 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase activity measured within 24 hours of admission.
Methods
Retrospective cross‐sectional study of clinical and laboratory records.
Results
The DGGR lipase activity was increased >3× the upper reference limit at admission in 216/1360 (16%) dogs, of which 70/216 (32%) had a clinical diagnosis of AP. Other primary conditions associated with hyperlipasemia were renal, endocrine, and immune‐mediated diseases, and upper airway obstruction. Predictors of hyperlipasemia at admission were prior glucocorticoid administration, vomiting and abdominal pain, increased age, plasma bilirubin and creatinine concentrations, and decreased hematocrit. Of dogs with repeat measurements, 78/345 (23%) had significantly increased lipase during hospitalization, of which 13/78 (17%) had a clinical diagnosis of AP. Other primary conditions associated with in‐hospital hyperlipasemia were renal and immune‐mediated disorders. Predictors of developing hyperlipasemia during hospitalization were hemodialysis events, increased plasma bilirubin and creatinine concentrations, and decreased hematocrit. Hyperlipasemia both at admission and during hospitalization was associated with longer hospitalization and higher mortality.
Conclusions and Clinical Importance
Significant DGGR‐hyperlipasemia is frequent in critically ill dogs and associated with a variety of nonpancreatic conditions and negative outcome.
Keywords: canine, cPL, DGGR, ICU, lipase
Abbreviations
- ALP
alkaline phosphatase activity
- ALT
alanine transaminase activity
- AP
acute pancreatitis
- CI
confidence interval
- cPL
canine pancreatic‐specific lipase
- DGGR
1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester
- DKA
diabetic ketoacidosis
- gGT
γ‐glutamyl transferase activity
- GLDH
glutamate dehydrogenase activity
- HL group
hyperlipasemic group
- ICU
intensive care unit
- IQR
interquartile range
- NIL group
nonsignificant increase in lipase group
- NL group
nonhyperlipasemic group
- SIL group
significant increase in lipase group
- URL
upper reference limit
1. INTRODUCTION
Increased serum pancreatic enzyme activities, namely hyperlipasemia and hyperamylasemia, occur in 14%‐80% of people with no prior pancreatic disease in intensive care units (ICU), depending on study population and enzyme activity cut‐offs. 1 , 2 , 3 Some of these patients may develop acute pancreatitis (AP) while undergoing treatment for an unrelated disorder, mainly because of splanchnic ischemia, sepsis, or drug administration. However, only up to 35% of patients with pancreatic hyperenzymemia have clinical and imaging findings consistent with AP. 2 , 4 , 5 In others, clinically irrelevant hyperenzymemia is suspected and is believed to be associated with a variety of disorders, including head injury or intracranial events, hepatobiliary disease, malignancy, diabetic ketoacidosis (DKA), bowel obstruction, perforation, or both, and renal disease. 1 , 3 , 4 , 6 , 7 , 8 Some studies have reported longer hospital stays 4 or higher mortality 6 in patients with pancreatic hyperenzymemia, but others contradict these findings. 9 Given that a diagnosis of AP in both people and dogs relies largely on a combination of clinical signs, measurement of pancreatic enzymes, and diagnostic imaging, 10 distinguishing true AP from clinically irrelevant hyperenzymemia is particularly challenging in critically ill individuals in the face of comorbidities with clinical signs that may mimic AP.
The most commonly used assays in the diagnostic evaluation of AP in people are serum amylase and lipase activities, 10 but canine pancreatic‐specific lipase concentration (cPL) is currently regarded as the most accurate blood test in dogs, whereby concentrations ≥400 μg/L are reported to have sensitivities and specificities of 21%‐78% and 80%‐100%, respectively, with sensitivity increasing with severity of disease. 11 , 12 , 13 , 14 , 15 A point‐of‐care test (SNAP cPL) for patient‐side measurement of cPL has been developed, using a cutoff of ≥200 μg/L, which reportedly has higher sensitivity (82%‐93%) but lower specificity (59%‐74%). 13 , 16 These studies also indicated poor performance of the 1,2‐diglyceride lipase assay for the diagnosis of AP. However, evaluation of the more recently validated 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay 17 found high agreement with cPL. 18
Besides AP, conditions associated with increased lipase activity or cPL in dogs include septic peritonitis, gastrointestinal foreign body, gastric dilatation‐volvulus, gastroenteritis, pancreatic or hepatic neoplasia, cardiac disease, DKA, obesity, Ehrlichia canis or Babesia rossi infection, hyperadrenocorticism, and glucocorticoid administration. 16 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Some of these disorders may cause secondary AP because of diffuse abdominal inflammation, pancreatic hypoperfusion or ischemia and reperfusion. 16 However, the clinical relevance of hyperlipasemia in many cases remains unclear, as does the influence of renal disease on lipase activity.
Although an association between hyperlipasemia and certain specific conditions has been investigated in dogs, the prevalence of hyperlipasemia in critically ill dogs in the ICU is unknown, and factors associated with hyperlipasemia in these dogs remain to be elucidated. Given the incorporation of lipase assays in routine biochemical profiles at some institutions, including ours, such knowledge is important to help assess the relevance of hyperlipasemia in critically ill dogs.
Therefore, our aims were to: (a) evaluate the prevalence of DGGR‐hyperlipasemia in critically ill dogs at admission and during hospitalization, (b) determine factors associated with hyperlipasemia, and (c) evaluate the association between hyperlipasemia and duration of hospitalization and mortality. We hypothesized that DGGR‐hyperlipasemia is frequent in critically ill dogs with disorders unrelated to AP and is associated with increased duration of hospitalization and mortality.
2. METHODS
The electronic medical records and laboratory database were retrospectively searched for dogs presented to the Small Animal Clinic, Vetsuisse Faculty, University of Berne, Switzerland between April 2015 and April 2017. All dogs presented during this period were included in the study if they were considered critically ill and if a plasma biochemical profile that included DGGR‐lipase was performed within 24 hours of admission. Dogs were considered critically ill based on emergency presentation and immediate admission to the ICU, regardless of clinical signs or affected organ system. For dogs that were admitted on >1 occasion during the study period, only the most recent admission was included.
Data collected included signalment, clinical signs at admission considered consistent with AP (anorexia, vomiting, abdominal pain, diarrhea), 30 clinical diagnosis, treatment with glucocorticoids within 7 days before admission, duration of hospitalization, and outcome defined as discharge or death or euthanasia. All clinical diagnoses of AP were made by a board‐certified internal medicine or emergency and critical care specialist, or an intern or resident under their close supervision. Cases were considered to be primary AP if a clinical diagnosis of AP was recorded as the primary clinical diagnosis, and secondary AP if it was suspected to have developed during hospitalization in dogs with another primary clinical diagnosis. Results of ultrasonographic examination, performed by a board‐certified radiologist or a diagnostic imaging resident under direct supervision of a board‐certified radiologist, were included if performed within 48 hours of admission.
Clinicopathologic data collected included initial and all further measurements of DGGR‐lipase activity using a previously validated assay, 17 as well as simultaneous automated measurements of hematocrit (ADVIA 2120i, Siemens Healthcare, Zürich, Switzerland) and selected biochemistry analytes (Cobas c501, Roche Diagnostics, Basel, Switzerland), including alanine transaminase activity (ALT), albumin, alkaline phosphatase activity (ALP), bilirubin, creatinine, C‐reactive protein, γ‐glutamyl transferase activity (gGT), and glutamate dehydrogenase activity (GLDH). Samples were submitted for simultaneous Spec cPL measurements (IDEXX Diavet, Bäch, Switzerland) at the clinicians' discretion; published cut‐offs for normal results (0‐200 μg/L), equivocal (201‐399 μg/L), and consistent with pancreatitis (≥400 μg/L) were used. 15 In addition, abdominal surgery, anesthesia and hemodialysis performed between initial and subsequent DGGR‐lipase measurements were documented.
Dogs were categorized into the hyperlipasemic (HL) group if DGGR‐lipase activity measured within 24 hours of admission exceeded 3× the URL (>324 U/L) and into the nonhyperlipasemic (NL) group if they had DGGR‐lipase activity within 3× of the upper reference limit (URL, <325 U/L). This cut‐off was chosen based on the definition of clinically relevant hyperlipasemia used in previous studies in people and dogs. 5 , 7 , 8 , 10 , 30 , 31 For those dogs with subsequent DGGR‐lipase measurements during hospitalization, the first result measured within 24 hours of admission served as baseline, and only the highest repeated measured activity was additionally included in the data analysis. A clinically relevant in‐hospital increase in DGGR‐lipase was arbitrarily defined as a value >3× the URL and also >2× the value at admission, and dogs were thus further grouped as nonsignificant increase in lipase (NIL) group or significant increase in lipase (SIL) group (Figure 1).
FIGURE 1.
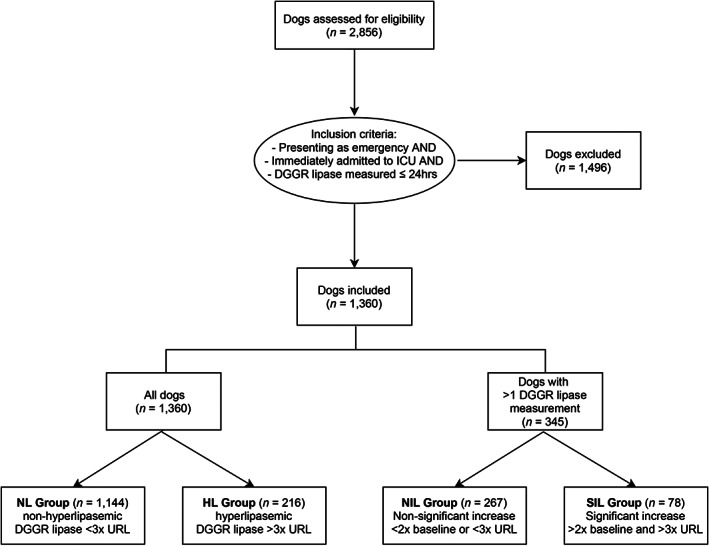
Flow diagram documenting case enrollment and classification. Dogs initially assessed for eligibility included all dogs presented to our institution between April 2015 and April 2017 which had a DGGR lipase measurement performed in our laboratory as part of their current visit. URL, upper reference limit
2.1. Statistical analysis
Statistical analysis was performed using MedCalc Statistical Software version 17.8.1. Data were assessed for normality using D'Agostino‐Pearson tests and by evaluation of normal plots. Because most data were non‐normally distributed, nonparametric analyses were performed. Comparisons between groups were analyzed using chi‐squared tests and Mann‐Whitney tests for categorical and ordinal data, respectively. The association between demographic, clinical and clinicopathologic factors and lipase groups was evaluated using univariate logistic regression. Predictors significantly associated with lipase groups were further analyzed using multivariate logistic regression in a stepwise selection fashion. Spearman's rank correlation was used to assess the association between DGGR‐lipase activity and plasma creatinine concentrations. Cohen's kappa was used to assess agreement between a clinical and sonographic diagnosis of AP. Statistical significance was set at P < .05 throughout.
3. RESULTS
3.1. Study population demographics
During the study period, 4074 DGGR‐lipase assays were performed on 2856 dogs. Of these, 1360 dogs met the inclusion criteria. Dogs represented 172 breeds, the most common being mixed breed (n = 191), Labrador Retriever (n = 79), French Bulldog (n = 55), Yorkshire Terrier (n = 54), Chihuahua (n = 48), Golden Retriever (n = 47), Bernese Mountain dog (n = 44), Jack Russell terrier (n = 43), and German Shepherd (n = 35). Data regarding age, sex, and body weight at admission were missing from the medical records in 11, 3, and 77 dogs, respectively. Data from the remaining dogs are presented in Table 1.
TABLE 1.
Clinical data from 1360 critically ill dogs with DGGR‐lipase activities at admission within 3× (NL group) and above 3× (HL group) the upper reference limit
| Variable | All dogs | NL group | HL group | P value |
|---|---|---|---|---|
| Number of dogs | 1360 | 1144 (84%) | 216 (16%) | |
| DGGR‐lipase, U/L | ||||
| range | 7‐19 700 | 7‐295 | 369‐19 700 | |
| median (IQR) | 71 (38‐174) | 59 (35‐113) | 1140 (566‐2081) | |
| Age, median (IQR), years | 7.5 (3.6‐10.3) | 7.2 (3.3‐10.1) | 8.7 (5.5‐10.9) | <.001 |
| Body weight, median (IQR), kg | 18.0 (7.8‐30.0) | 18.5 (7.9‐30.0) | 14.9 (7.4‐30.0) | .342 |
| Sex | ||||
| Female | 647 (433 spayed) | 556 (367 spayed) | 91 (66 spayed) | .08 |
| Male | 710 (341 castrated) | 585 (269 castrated) | 125 (72 castrated) | |
| Clinical signs at presentation | ||||
| Anorexia | 393 (29%) | 318 (28%) | 75 (35%) | .04 |
| Vomiting | 575 (42%) | 452 (40%) | 123 (57%) | <.001 |
| Diarrhea | 322 (24%) | 254 (22%) | 68 (32%) | .003 |
| Abdominal pain | 233 (17%) | 182 (16%) | 51 (24%) | .006 |
| Diagnosis of acute pancreatitis | ||||
| Clinical | 95 (7%) | 25 (2%) | 70 (32%) | <.001 |
| Sonographic | 102/720 (14%) | 55/572 (10%) | 47/148 (32%) | <.001 |
| Duration of hospitalization, median (IQR), days | 4 (3‐7) | 4 (3‐6) | 5 (3‐8) | .006 |
| Mortality | 325 (24%) | 255 (22%) | 70 (32%) | .001 |
Abbreviation: IQR, interquartile range.
3.2. Differences between lipase groups
The DGGR‐lipase activity measured at admission ranged from 7 to 19 700 U/L. A total of 1144 (84%) dogs were assigned to the NL group, of which 843 (62%) and 301 (22%) had DGGR‐lipase activities below the URL and between 1× and 3× the URL, respectively. A further 216 (16%) dogs, with DGGR‐lipase activities >3× the URL, were assigned to the HL group (Table 1, Figure 1).
The median age of dogs in the NL group was slightly lower than in the HL group, but no difference between the groups was found for sex or body weight (Table 1). Dogs in the HL group were significantly more likely to have clinical signs considered consistent with AP than were dogs in the NL group (Table 1), and more commonly had several of these signs (data not shown). Data regarding previous drug administration were available for 1357 dogs. A total of 93 dogs had received glucocorticoids, of which 69/1142 (6%) and 24/215 (11%) were in the NL and HL groups, respectively. Glucocorticoids administered before presentation included both prednisolone and dexamethasone, and were prescribed to dogs in all disease categories, but predominantly in dogs with immune‐mediated and lung disease, as well as gastroenteritis.
Plasma albumin concentration and hematocrit were significantly lower in dogs of the HL group, whereas ALT, ALP, bilirubin, creatinine, gGT, and GLDH were significantly higher (P < .001; Supplemental Table 1).
3.3. Predictors of lipase groups
Demographic data (age, sex, body weight), clinical signs at admission, prior glucocorticoid administration, and clinicopathologic data found to be significant predictors of HL group in univariate analyses were age, prior administration of glucocorticoids, anorexia, vomiting, diarrhea, abdominal pain, creatinine, bilirubin, ALT, ALP, gGT, GLDH, and hematocrit (data not shown). In multivariate analysis, only age, vomiting, abdominal pain, prior administration of glucocorticoids, creatinine, bilirubin, and hematocrit were found to be independent predictors of lipase group (Table 2). The correlation (Spearman's rank) between DGGR‐lipase activity and plasma creatinine concentrations in all dogs was poor at 0.22 (95% confidence intervals [CI], 0.17‐0.27).
TABLE 2.
Multivariate logistic regression of demographic, clinical, and clinicopathologic data independently associated with HL group
| Independent variable (predictor) | Coefficient | SE | P value | OR | 95% CI |
|---|---|---|---|---|---|
| Age, years | 0.090 | 0.021 | <.001 | 1.09 | 1.05‐1.14 |
| History of vomiting | 0.602 | 0.170 | <.001 | 1.83 | 1.31‐2.55 |
| Abdominal pain on presentation | 0.396 | 0.198 | .05 | 1.49 | 1.01‐2.19 |
| Prior administration of glucocorticoids | 0.808 | 0.271 | .003 | 2.24 | 1.32‐3.82 |
| Creatinine (mg/dL) | 0.166 | 0.027 | <.001 | 1.18 | 1.12‐1.24 |
| Bilirubin (mg/dL) | 0.085 | 0.027 | .002 | 1.09 | 1.03‐1.15 |
| Hematocrit (%) | −0.021 | 0.007 | .004 | 0.98 | 0.97‐0.99 |
| constant | −2.329 | 0.358 | <.001 |
Notes: Predictor variables analyzed in univariate analysis but found nonsignificant and not included in the model were albumin, anorexia at presentation, alkaline phosphatase, alanine aminotransferase, diarrhea at presentation, gamma glutamate transferase, and glutamate dehydrogenase.
Abbreviations: CI, confidence interval; OR, odds ratio (adjusted).
3.4. Clinical and sonographic diagnoses
A total of 95 (7%) dogs were considered to have clinical AP either as a primary or secondary disorder. Of these, 70 were classified in the HL group (Table 1). Thus, 146/216 (68%) dogs in the HL group were not diagnosed with AP and 25/1144 (2%) dogs in the NL group were diagnosed with AP. Nonetheless, a clinical diagnosis of AP was significantly associated with lipase group (Table 1), and the median DGGR‐lipase activity in dogs with and without a diagnosis of AP was 1136 U/L (interquartile range [IQR], 258‐2280 U/L) and 65 U/L (IQR, 37‐143 U/L), respectively.
For the 720 dogs that underwent abdominal ultrasound examination within 48 hours of admission, 102 (14%) were given a sonographic diagnosis of AP. This outcome was significantly associated with lipase group (Table 1), and the median lipase activity in dogs with and without a sonographic diagnosis of AP was 245 U/L (IQR, 74‐1542 U/L) and 83 U/L (IQR, 41‐186 U/L), respectively. The kappa agreement between a clinical and a sonographic diagnosis of AP was 0.47 (95% CI, 0.37‐0.56).
A clinical diagnosis of primary AP was given to 61 (4.5%) dogs. These included 46/216 (21%) and 15/1144 (1.3%) dogs in the HL and NL groups, respectively. The most common other primary diseases in the HL group were renal, endocrine (predominantly diabetes mellitus and hypoadrenocorticism) and immune‐mediated disorders, as well as upper airway obstruction (Figure 2). A list of diseases included as “others” is provided in Supplemental Table 2.
FIGURE 2.
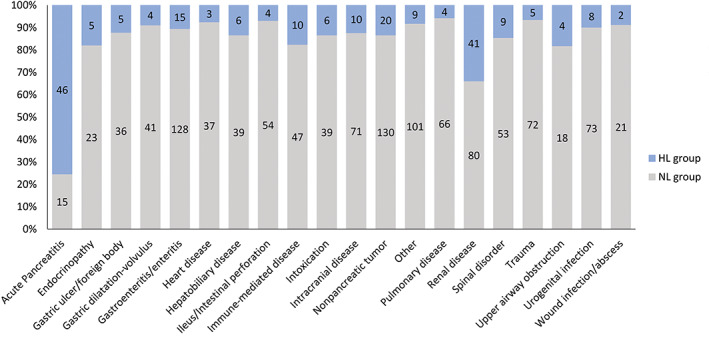
Primary disorders associated with admission DGGR‐lipase activities below (NL; gray) and above (HL; blue) 3× the upper reference limit in critically‐ill dogs. Numbers of dogs in each group are shown within bars
3.5. Duration of hospitalization and outcome
The duration of hospitalization ranged from 1 to 35 days, with shorter median hospitalization in the NL group compared to the HL group (Table 1). Overall, mortality was 24%, and differed slightly but significantly between groups (Table 1, Figure 3A) with mortality likelihood ratios of 0.91 (95% CI, 0.86‐0.97) and 1.53 (95% CI, 1.18‐1.97) in the NL and HL groups, respectively.
FIGURE 3.
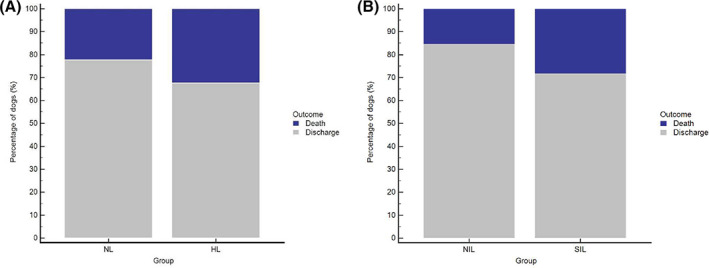
A, Outcome of 1360 critically ill dogs with DGGR‐lipase activities at admission within 3× (NL group) and above 3× (HL group) the upper reference limit (dark blue, death; gray, discharge). Mortality differed significantly between groups (P = .001). B, Outcome of 345 critically ill dogs with nonsignificant (NIL) and significant (SIL) increases in DGGR‐lipase activities during hospitalization (dark blue, death; gray, discharge). Mortality differed significantly between groups (P = .01)
3.6. Dogs with repeated measurements of DGGR‐lipase activity
Repeat DGGR‐lipase measurements were performed 518 times in 345 dogs during hospitalization with activities ranging from 8 to 20 700 U/L (Table 3). In 126/345 (37%) dogs, the maximum repeat value was greater than 2× the value at admission. In 78/345 (23%) dogs, values also were >3× the URL, and these dogs were assigned to the significant increase group (SIL). The remaining 267 dogs were assigned to the nonsignificant increase group (NIL). Hematocrit was significantly lower in dogs of the SIL group, whereas plasma bilirubin and creatinine concentrations were significantly higher (P < .001; Supplemental Table 3).
TABLE 3.
Clinical data and outcome associated with nonsignificant (NIL) and significant (SIL) increases in DGGR‐lipase activities during hospitalization in 345 critically ill dogs
| Variable | All dogs | NIL group | SIL group | P value |
|---|---|---|---|---|
| Number of dogs | 345 | 267 | 78 | |
| Admission DGGR‐lipase, U/L | ||||
| Range | 7‐15 616 | 7‐15 616 | 18‐4746 | |
| Median (IQR) | 95 (43‐263) | 76 (39‐262) | 139 (71‐281) | |
| Repeat DGGR‐lipase, U/L | ||||
| Range | 8‐20 700 | 8‐13 700 | 433‐20 700 | |
| Median (IQR) | 143 (56‐895) | 94 (45‐207) | 1656 (962‐3732) | |
| Clinical diagnosis of acute pancreatitis | 53 (15%) | 40 (15%) | 13 (17%) | <.001 |
| Events between lipase measurements | ||||
| Anesthesia | 37 (11%) | 31 (12%) | 6 (8%) | .326 |
| Abdominal surgery | 56 (16%) | 41 (15%) | 15 (19%) | .415 |
| Hemodialysis | 21 (6%) | 1 (0%) | 20 (26%) | <.001 |
| Duration of hospitalization, median (IQR), days | 7 (5‐11) | 7 (5‐9) | 10 (7‐14) | <.001 |
| Mortality | 63 (18%) | 41 (15%) | 22 (28%) | .01 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; CRP, C‐reactive protein; gGT, gamma glutamyl transferase; GLDH, glutamate dehydrogenase; IQR, interquartile range.
3.7. Predictors of in‐hospital increase in lipase
Demographic data, clinical signs at admission, clinicopathologic data, and anesthesia, surgery or hemodialysis events found to be predictors of SIL group in univariate analyses were creatinine, bilirubin, and hematocrit measured simultaneously with the repeat DGGR‐lipase measurement, and hemodialysis between initial and repeat measurements (data not shown). These 4 predictors were maintained as independent predictors of SIL group in multivariate analysis (Table 4).
TABLE 4.
Multivariate logistic regression of demographic, clinical, and clinicopathologic data independently associated with SIL group
| Independent variable (predictor) | Coefficient | SE | P value | OR | 95% CI |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.307 | 0.085 | <.001 | 1.36 | 1.15‐1.61 |
| Bilirubin (mg/dL) | 0.125 | 0.052 | .02 | 1.13 | 1.02‐1.26 |
| Hematocrit (%) | −0.054 | 0.018 | .003 | 0.95 | 0.92‐0.98 |
| Hemodialysis | 2.484 | 1.122 | .03 | 11.99 | 1.33‐108.05 |
| Constant | −2.299 | 0.643 | .643 |
Abbreviations: CI, confidence interval; OR, odds ratio (adjusted).
3.8. Clinical diagnoses in repeat lipase groups
For dogs with repeated measurement of DGGR‐lipase, 53/345 (15%) dogs were considered to have clinical AP, of which only 13/78 (17%) dogs were in the SIL group (Table 3). A primary diagnosis of AP was given to 31/345 dogs with repeat DGGR‐lipase measurements. These were 3/78 (4%) and 28/267 (10%) dogs in the SIL and NIL groups, respectively. The most common other primary diseases in the SIL group were renal and immune‐mediated disorders (Figure 4). A list of diseases included as “others” is provided in Supplemental Table 4. No association was found between groups and anesthesia or abdominal surgery between initial and repeat DGGR‐lipase measurements, but 20/21 dogs undergoing hemodialysis were in the SIL group (Table 3).
FIGURE 4.
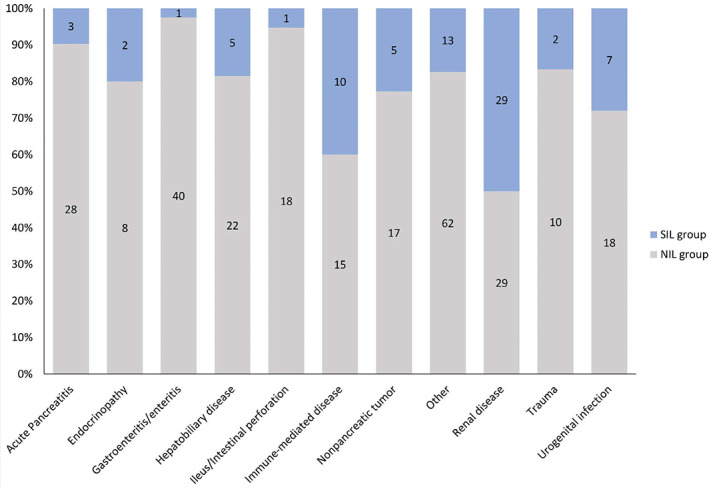
Primary disorders associated with nonsignificant (NIL; gray) and significant (SIL; blue) increases in DGGR‐lipase activity during hospitalization in 345 critically ill dogs. Numbers of dogs in each group are shown within bars
3.9. Duration of hospitalization and outcome in repeat lipase groups
Significantly shorter hospitalization was recorded in the NIL group compared to the SIL group (Table 3). Overall mortality of dogs with repeat lipase measurements was 18% and differed significantly between groups (Table 3, Figure 3B), with mortality likelihood ratios of 0.81 (95% CI, 0.67‐0.98) for the NIL group and 1.76 (95% CI, 1.17‐2.65) for the SIL group.
3.10. Simultaneous measurements of cPL
In 71/1360 dogs, cPL was measured from the same sample used to measure DGGR‐lipase. The cPL was 0‐200 μg/L in 38 samples, 201‐399 μg/L in 8 samples and ≥400 μg/L in 25 samples. Of dogs with cPL ≥400 μg/L, 20/25 (80%) were in the HL group, whereas all dogs with cPL <400 μg/L were in the NL group (Figure 5).
FIGURE 5.
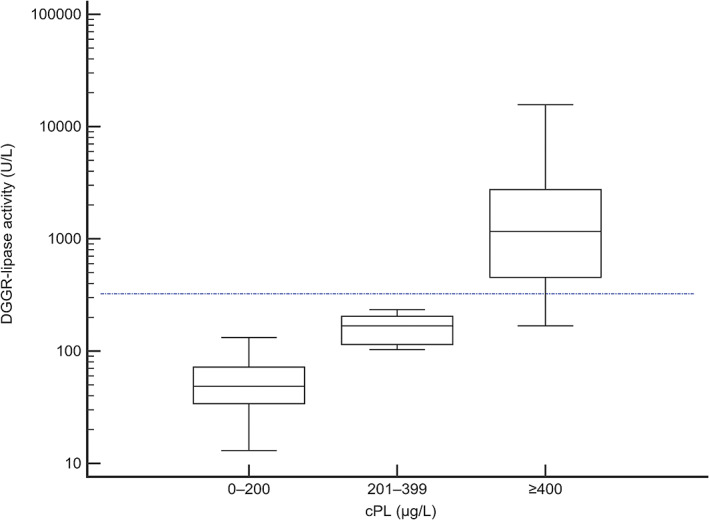
Results of simultaneous measurements of DGGR‐lipase and cPL measured in 71 critically‐ill dogs. The blue dashed line indicates 3× the upper reference limit for DGGR‐lipase activity used to denote significant increased activity
4. DISCUSSION
To our knowledge, this study is the first to evaluate the prevalence of and factors associated with DGGR‐hyperlipasemia in a large cohort of critically ill dogs. We found an increase in lipase activity >3 URL in 16% of the examined population at admission, of which only approximately one‐third was suspected to have AP based on clinical and ultrasonographic findings. This finding suggests that significantly increased DGGR‐lipase activity is common in critically ill dogs at admission without overt clinical AP. This finding is comparable to studies in people in which 14%‐80% of patients admitted to ICU had hyperlipasemia, with only up to 35% meeting criteria to diagnose AP. 1 , 2 , 3 , 4 , 5
The most common nonpancreatic disorder associated with an increased lipase activity in our study was renal disease, despite poor correlation between DGGR‐lipase activity and plasma creatinine concentration. The effect of decreased GFR, because of naturally occurring or experimentally induced acute kidney injury and chronic kidney disease on lipase activity has been variably reported in the literature, with older reports suggesting that lipase activity is increased in renal disease, 32 but newer studies not documenting significant effects of experimentally induced acute kidney injury 33 and chronic renal failure 34 on lipase activity and cPL. The kidneys are considered to be a major site for lipase metabolism, likely through a combination of glomerular filtration, tubular reabsorption, and enzyme inactivation, 35 which would explain why hyperlipasemia cannot be accounted for by decreased GFR alone. In our study, despite choosing a conservative cut‐off for DGGR‐lipase activity, renal disease remained a common diagnosis in dogs with hyperlipasemia, and was furthermore associated with a significant increase in lipase activity during hospitalization. Noteworthy was that patients undergoing hemodialysis frequently experienced a marked increase in DGGR‐lipase activity during hospitalization. Increased cPL recently has been reported in a large proportion of dogs undergoing hemodialysis for acute kidney injury, but cPL was measured at any time within 7 days before or after admission and no further testing (such as imaging) was described to confirm the diagnosis of pancreatitis. 36 People undergoing dialysis, particularly peritoneal dialysis, have been reported to have higher risk for developing pancreatitis. 37 Furthermore, heparin used as an anticoagulant during hemodialysis also has been shown to stimulate lipoprotein lipase and thus cause significant increases in lipase activity in absence of pancreatitis. 38 In our study population, citrate more commonly was used as an anticoagulant for dogs undergoing dialysis. Therefore, we hypothesize that the significant in‐hospital increase in DGGR‐lipase activity observed in 20/21 dogs in our study is more likely a reflection of subclinical pancreatic damage than of lipoprotein lipase induction. Possible reasons for this conclusion extrapolated from human medicine include inflammation secondary to the underlying disorder causing renal injury, multiorgan failure, and ischemia or hypoperfusion because of changes in plasma volume. 4 , 9 , 39
Other primary nonpancreatic disorders frequently associated with significantly increased DGGR‐lipase activities in our study were endocrinopathies, immune‐mediated disorders, and upper airway obstruction. Diabetes mellitus and AP are common comorbidities; a previous study found diabetes mellitus in 36% of dogs diagnosed with AP, 40 and another identified cPL concentrations >400 μg/L in 73% of dogs with DKA. 24 In people, type 2 diabetes mellitus is associated with increased risk of AP, 41 but nonspecific hyperlipasemia without evidence of AP on cross‐sectional imaging also was common in patients with DKA. 42 These studies suggest that although AP is associated with diabetes mellitus and DKA in dogs and people, nonspecific increases in pancreatic enzymes apparently also occur, and both may partly explain the association between increased DGGR‐lipase activity and endocrinopathies observed in our study. Diabetes mellitus and other endocrinopathies, such as hyperadrenocorticism and hypothyroidism, are associated with AP, possibly because of a combination of hyperlipidemia, decreased insulin sensitivity, and increased proinflammatory cytokines. 24 , 43
Increased cPL with questionable clinical relevance previously has been described in dogs with various immune‐mediated disorders treated with prednisolone. 29 Although drug administration could be suspected to be a major contributing factor, other studies have shown that steroid administration did not cause an increase in cPL concentrations above reference intervals in healthy dogs. 44 , 45 We did observe a significantly higher number of patients with prior corticosteroid administration in the HL group, but this finding may reflect the underlying primary disease, including immune‐mediated disorders and hypoadrenocorticism, rather than direct drug effects.
Hyperlipasemia has to our knowledge not yet been described in association with upper airway obstruction in dogs, but abnormalities of the upper gastrointestinal tract have been identified in the majority of brachycephalic dogs with upper respiratory syndrome, including patients without gastrointestinal clinical signs. 46 Despite that study not specifically examining the pancreas, it raises the question of subclinical AP possibly being an explanation for the relatively high prevalence of upper airway obstruction in the HL group of our study. The latter however could also simply reflect the high prevalence of French Bulldogs in our patient population, rather than a true association with pancreatitis.
In addition to the substantial number of dogs presenting with DGGR‐hyperlipasemia, 23% of our dogs with repeated measurements had a significant in‐hospital increase in DGGR‐lipase activity. This phenomenon also has been reported in people admitted to ICU, and is hypothesized to be linked to underlying inflammation, hypoperfusion, and multiorgan failure. 4 , 9 , 39 One study found that hyperlipasemia was associated with anemia, increased serum creatinine concentration, and hyperbilirubinemia, and thus was interpreted as a pancreatic manifestation of multisystem organ failure. 4 This possibility also could explain the association of higher plasma bilirubin and creatinine concentrations and lower hematocrit in our dogs with DGGR‐hyperlipasemia at admission or during hospitalization. It must be noted however that dogs with clinically unstable conditions such as hypotension, multiple organ dysfunction, and sepsis are more likely to have repeated plasma biochemistry performed, which possibly has artificially inflated the prevalence of SIL in our retrospective study.
Hyperlipasemia and in‐hospital increases in DGGR‐lipase activity were associated with both increased duration of hospitalization and mortality in our study. People with hyperlipasemia are reported to have increased duration of hospitalization, but the effect on mortality is variable. 4 , 6 , 9 However, comparison of mortality rates in our population is confounded by euthanasia in some dogs because of perceived poor prognosis or financial constraints, for which we could not account appropriately because of the retrospective nature of the study. As such, severity of illness scoring or intention‐to‐treat analysis in dogs instead of mortality may be more objective tools for the assessment of outcome in future studies. Moreover, duration of hospitalization and mortality may differ depending on the type of clinical setting, and thus findings from our study cannot be assumed to reflect expected findings in disparate settings.
Our work had several other limitations, the main limitation being the retrospective study design, which did not allow for the ultrasound evaluation or the clinical assessment for pancreatitis to be standardized. In absence of histopathology, the diagnosis of pancreatitis in dogs relies on the combination of nonspecific clinical signs, pancreatic enzyme assays and ultrasonographic findings. The latter has not proven to be a reliable diagnostic tool for pancreatitis, with weak to fair agreement between ultrasonographic diagnosis and presence of hyperlipasemia in previous studies, 18 , 47 with our findings also supporting this conclusion. Therefore, the true prevalence of AP in our population unfortunately could not be reliably established. Lipase activity often is criticized as being too nonspecific to reliably predict AP, compared to cPL, which currently is considered the biomarker of choice for AP with reported specificities of 66%‐100%, depending on cut‐off and study design. 11 , 12 , 13 , 14 However, previous studies 18 found excellent agreement between cPL and DGGR‐lipase, which also seemed to be true in our patient population. The high proportion of hyperlipasemic animals without a diagnosis of AP in our study therefore would suggest that cPL is less specific than previously thought when applied to a population of critically ill dogs with a clinical picture comparable to that of AP. Because lipase and cPL results strongly influenced the clinician's decision to rule in or rule out pancreatitis, this likely represents a bias in our study, which may have overestimated the prevalence of AP, further decreasing the specificity of these tests. Another major limitation of our study was the lack of a scoring system such as Acute Patient Physiologic and Laboratory Evaluation (APPLE) scores at admission to evaluate the severity of illness. This score was not determined for the animals enrolled in our study because of inconsistent availability of data required to formulate such a score. However, dogs hospitalized in our ICU are the most severely ill patients in our hospital.
On the other hand, the large and broad study population represents a major strength of our study. Because DGGR‐lipase activity measurement is incorporated in our plasma biochemical profile for dogs, the assay is performed on almost every dog admitted to the ICU, regardless of the clinician's index of suspicion for AP. In contrast, at our institution, because we do not routinely use the cage‐side SNAP cPL test, cPL is a send‐off test only performed on specific request, presumably based on a clinician's desire to rule in or out a diagnosis of AP.
In conclusion, critically ill dogs have a relatively high occurrence of DGGR‐hyperlipasemia both at admission and developing during hospitalization despite a relatively low prevalence of either clinical or sonographic AP. Factors associated with DGGR‐hyperlipasemia include primary renal disease and hemodialysis, endocrinopathies, immune‐mediated disorders, upper airway obstruction, as well as previous treatment with corticosteroids. Hyperlipasemia >3× the URL is associated with longer hospitalization and mortality in critically ill dogs, and may be more a marker of multiple organ dysfunction or ischemia than of clinically relevant AP. As such, clinicians should be wary of making a diagnosis of AP based solely on DGGR‐lipase or cPL in critically ill dogs.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Supplemental tables.
ACKNOWLEDGMENT
Parts of this study were presented to the University of Bern as part of the requirements of the doctoral thesis of the first author (Julia Prümmer) in May 2018.
Prümmer JK, Howard J, Grandt LM, Obrador de Aguilar R, Meneses F, Peters LM. Hyperlipasemia in critically ill dogs with and without acute pancreatitis: Prevalence, underlying diseases, predictors, and outcome. J Vet Intern Med. 2020;34:2319–2329. 10.1111/jvim.15902
REFERENCES
- 1. Muniraj T, Dang S, Pitchumoni CS. Pancreatitis or not?—elevated lipase and amylase in ICU patients. J Crit Care. 2015;30:1370‐1375. [DOI] [PubMed] [Google Scholar]
- 2. Hardt PD, Mayer K, Ewald N. Exocrine pancreatic involvement in critically ill patients. Curr Opin Clin Nutr Metab Care. 2009;12:168‐174. [DOI] [PubMed] [Google Scholar]
- 3. Agrawal A, Alagusundarmoorthy SS, Jasdanwala S. Pancreatic involvement in critically ill patients. J Pancreas (Online). 2015;16:346‐355. [Google Scholar]
- 4. Manjuck J, Zein J, Carpati C, Astiz M. Clinical significance of increased lipase levels on admission to the ICU. Chest. 2005;127:246‐250. [DOI] [PubMed] [Google Scholar]
- 5. Denz C, Siegel L, Lehmann KJ, Dagorn JC, Fiedler F. Is hyperlipasemia in critically ill patients of clinical importance? An observational CT study. Intensive Care Med. 2007;33:1633‐1636. [DOI] [PubMed] [Google Scholar]
- 6. Lee CC, Chung WY, Shih YH. Elevated amylase and lipase levels in the neurosurgery intensive care unit. J Chin Med Assoc. 2010;73:8‐14. [DOI] [PubMed] [Google Scholar]
- 7. Cohen J, MacArthur KL, Atsawarungruangkit A, et al. Defining the diagnostic value of hyperlipasemia for acute pancreatitis in the critically ill. Pancreatology. 2017;17:176‐181. [DOI] [PubMed] [Google Scholar]
- 8. Nair S, Yadav D, Pitchumoni CS. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol. 2000;95:2795‐2800. [DOI] [PubMed] [Google Scholar]
- 9. Chaari A, Hakim KA, Rashed N, et al. Factors associated with increased pancreatic enzymes in septic patients: a prospective study. J Intensive Care. 2017;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400‐1415. 1416. [DOI] [PubMed] [Google Scholar]
- 11. Trivedi S, Marks SL, Kass PH, et al. Sensitivity and specificity of canine pancreas‐specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J Vet Intern Med. 2011;25:1241‐1247. [DOI] [PubMed] [Google Scholar]
- 12. Mansfield CS, Anderson GA, O'Hara AJ. Association between canine pancreatic‐specific lipase and histologic exocrine pancreatic inflammation in dogs: assessing specificity. J Vet Diagn Invest. 2012;24:312‐318. [DOI] [PubMed] [Google Scholar]
- 13. McCord K, Morley PS, Armstrong J, et al. A multi‐institutional study evaluating the diagnostic utility of the spec cPL and SNAP(R) cPL in clinical acute pancreatitis in 84 dogs. J Vet Intern Med. 2012;26:888‐896. [DOI] [PubMed] [Google Scholar]
- 14. Neilson‐Carley SC, Robertson JE, Newman SJ, et al. Specificity of a canine pancreas‐specific lipase assay for diagnosing pancreatitis in dogs without clinical or histologic evidence of the disease. Am J Vet Res. 2011;72:302‐307. [DOI] [PubMed] [Google Scholar]
- 15. Steiner JM, Newman S, Xenoulis P, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther. 2008;9:263‐273. [PubMed] [Google Scholar]
- 16. Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and Spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Emerg Crit Care (San Antonio). 2014;24:135‐143. [DOI] [PubMed] [Google Scholar]
- 17. Graca R, Messick J, McCullough S, Barger A, Hoffmann W. Validation and diagnostic efficacy of a lipase assay using the substrate 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′ methyl resorufin)‐ester for the diagnosis of acute pancreatitis in dogs. Vet Clin Pathol. 2005;34:39‐43. [DOI] [PubMed] [Google Scholar]
- 18. Kook PH, Kohler N, Hartnack S, Riond B, Reusch CE. Agreement of serum Spec cPL with the 1,2‐o‐dilauryl‐rac‐glycero glutaric acid‐(6′‐methylresorufin) ester (DGGR) lipase assay and with pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med. 2014;28:863‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Israeli I, Steiner J, Segev G, et al. Serum pepsinogen‐A, canine pancreatic lipase immunoreactivity, and C‐reactive protein as prognostic markers in dogs with gastric dilatation‐volvulus. J Vet Intern Med. 2012;26:920‐928. [DOI] [PubMed] [Google Scholar]
- 20. Rallis TS, Koutinas AF, Kritsepi M, et al. Serum lipase activity in young dogs with acute enteritis or gastroenteritis. Vet Clin Pathol. 1996;25:65‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalli IV, Adamama‐Moraitou KK, Patsika MN, et al. Prevalence of increased canine pancreas‐specific lipase concentrations in young dogs with parvovirus enteritis. Vet Clin Pathol. 2017;46:111‐119. [DOI] [PubMed] [Google Scholar]
- 22. Quigley KA, Jackson ML, Haines DM. Hyperlipasemia in 6 dogs with pancreatic or hepatic neoplasia: evidence for tumor lipase production. Vet Clin Pathol. 2001;30:114‐120. [DOI] [PubMed] [Google Scholar]
- 23. Han D, Choi R, Hyun C. Canine pancreatic‐specific lipase concentrations in dogs with heart failure and chronic mitral valvular insufficiency. J Vet Intern Med. 2015;29:180‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolton TA, Cook A, Steiner JM, Fosgate GT. Pancreatic lipase immunoreactivity in serum of dogs with diabetic ketoacidosis. J Vet Intern Med. 2016;30:958‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verkest KR, Fleeman LM, Morton JM, et al. Association of postprandial serum triglyceride concentration and serum canine pancreatic lipase immunoreactivity in overweight and obese dogs. J Vet Intern Med. 2012;26:46‐53. [DOI] [PubMed] [Google Scholar]
- 26. Mylonakis ME, Xenoulis PG, Theodorou K, et al. Serum canine pancreatic lipase immunoreactivity in experimentally induced and naturally occurring canine monocytic ehrlichiosis (Ehrlichia canis). Vet Microbiol. 2014;169:198‐202. [DOI] [PubMed] [Google Scholar]
- 27. Koster LS, Steiner JM, Suchodolski JS, et al. Serum canine pancreatic‐specific lipase concentrations in dogs with naturally occurring Babesia rossi infection. J S Afr Vet Assoc. 2015;86:E1‐E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mawby DI, Whittemore JC, Fecteau KA. Canine pancreatic‐specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med. 2014;28:1244‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohta H, Morita T, Yokoyama N, et al. Serial measurement of pancreatic lipase immunoreactivity concentration in dogs with immune‐mediated disease treated with prednisolone. J Small Anim Pract. 2017;58:342‐347. [DOI] [PubMed] [Google Scholar]
- 30. Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56:13‐26. [DOI] [PubMed] [Google Scholar]
- 31. Steiner JM. Diagnosis of pancreatitis. Vet Clin North Am Small Anim Pract. 2003;33:1181‐1195. [DOI] [PubMed] [Google Scholar]
- 32. Wagner AE, Macy DW. Nephelometric determination of serum amylase and lipase in naturally occurring azotemia in the dog. Am J Vet Res. 1982;43:697‐699. [PubMed] [Google Scholar]
- 33. Hulsebosch SE, Palm CA, Segev G, Cowgill LD, Kass PH, Marks SL. Evaluation of canine pancreas‐specific lipase activity, lipase activity, and trypsin‐like immunoreactivity in an experimental model of acute kidney injury in dogs. J Vet Intern Med. 2016;30:192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steiner JM, Finco DR, Williams DA. Serum lipase activity and canine pancreatic lipase immunoreactivity (cPLI) concentration in dogs with experimentally induced chronic renal failure. Vet Res. 2010;3:58‐63. [Google Scholar]
- 35. Jacobs RM. Renal disposition of amylase, lipase, and lysozyme in the dog. Vet Pathol. 1988;25:443‐449. [DOI] [PubMed] [Google Scholar]
- 36. Takada K, Palm CA, Epstein SE, Cowgill LD. Assessment of canine pancreas‐specific lipase and outcomes in dogs with hemodialysis‐dependent acute kidney injury. J Vet Intern Med. 2018;32:722‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lankisch PG, Weber‐Dany B, Maisonneuve P, et al. Frequency and severity of acute pancreatitis in chronic dialysis patients. Nephrol Dial Transplant. 2008;23:1401‐1405. [DOI] [PubMed] [Google Scholar]
- 38. Montalto G, Soresi M, Carroccio A, et al. Influence of haemodialysis on lipase activity. Eur J Clin Chem Clin Biochem. 1997;35:237‐238. [PubMed] [Google Scholar]
- 39. Chaari A, Abdel Hakim K, Bousselmi K, et al. Pancreatic injury in patients with septic shock: a literature review. World J Gastrointest Oncol. 2016;8:526‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Papa K, Mathe A, Abonyi‐Toth Z, et al. Occurrence, clinical features and outcome of canine pancreatitis (80 cases). Acta Vet Hung. 2011;59:37‐52. [DOI] [PubMed] [Google Scholar]
- 41. Yang L, He Z, Tang X, Liu J. Type 2 diabetes mellitus and the risk of acute pancreatitis: a meta‐analysis. Eur J Gastroenterol Hepatol. 2013;25:225‐231. [DOI] [PubMed] [Google Scholar]
- 42. Yadav D, Nair S, Norkus EP, Pitchumoni CS. Nonspecific hyperamylasemia and hyperlipasemia in diabetic ketoacidosis: incidence and correlation with biochemical abnormalities. Am J Gastroenterol. 2000;95:3123‐3128. [DOI] [PubMed] [Google Scholar]
- 43. Mansfield C. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top Companion Anim Med. 2012;27:123‐132. [DOI] [PubMed] [Google Scholar]
- 44. Steiner JM, Teague SR, Lees GE, Willard MD, Williams DA, Ruaux CG. Stability of canine pancreatic lipase immunoreactivity concentration in serum samples and effects of long‐term administration of prednisone to dogs on serum canine pancreatic lipase immunoreactivity concentrations. Am J Vet Res. 2009;70:1001‐1005. [DOI] [PubMed] [Google Scholar]
- 45. Ohta H, Kojima K, Yokoyama N, et al. Effects of immunosuppressive prednisolone therapy on pancreatic tissue and concentration of canine pancreatic lipase immunoreactivity in healthy dogs. Can J Vet Res. 2018;82:278‐286. [PMC free article] [PubMed] [Google Scholar]
- 46. Poncet CM, Dupre GP, Freiche VG, Estrada MM, Poubanne YA, Bouvy BM. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract. 2005;46:273‐279. [DOI] [PubMed] [Google Scholar]
- 47. Cridge H, Sullivant AM, Wills RW, Lee AM. Association between abdominal ultrasound findings, the specific canine pancreatic lipase assay, clinical severity indices, and clinical diagnosis in dogs with pancreatitis. J Vet Intern Med. 2020;34:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental tables.


