Abstract
Nanotechnology is enjoying an impressive growth and the global nanotechnology industry is expected to exceed US$ 125 billion by 2024. Based on these successes, there are notions that we have known enough and efforts on engineered nanomaterial (ENM) environmental health and safety (nano-EHS) research should be in a back burner. However, there are recent events showing that it is not the case. FDA found ferumoxytol (carbohydrate-coated superparamagnetic iron oxide (SPIO) nanoparticle) for anemia treatment could induce lethal anaphylactic reactions. EU will categorize TiO2 as category 2 carcinogen due to its inhalation hazard and France banned use of TiO2 (E171) in food from Jan 1st 2020 on its carcinogenic potential. Although nano-industry is seemingly in a healthy state, growth could be hindered for the lack of certainty and more nano-EHS research is needed for the sustainable growth of nano-industry. Herein, we elaborate the current knowledge gaps and the way forward.
Keywords: Engineered nanomaterials, Environmental health and safety, Health impact, Sustainable development, Nano-industry
Introduction
Nanotechnology is enjoying an impressive growth since the announcement of National Nanotechnology Initiative (NNI) two decades ago. With mounting investment in nanotechnology R&D, the global nanotechnology industry is expected to exceed US$ 125 Billion mark by 2024.[1] Nanotechnology is currently widely used in commercial products, which has a broad and fundamental impact on nearly all sectors of global economy, including electronics, energy, biomedical, cosmetics, defense, automotive and agriculture among others.[1] The top three applications of nanotechnology, electronics, energy and biomedical, account for over 70% share of the global nanotechnology market and the largest application for nanotechnology is electronics. If categorized by components, nanoparticles accounts for largest share of the global nanomaterial market at 85%, with nanotools (e.g., nanolithography tools and scanning probe microscopes) accounting for second highest share followed by nanodevices including nanosensors and nanoelectronics.
As a major growth area under the global economy setting, it is necessary to ensure the sustainable development and application of nanotechnology in the different industries worldwide.[2] Despite the impressive growth curve, maintaining the momentum will require overcoming many hurdles and address concerns from the perspectives of different stakeholders, e.g., reducing production costs of nanomaterials, incorporating nanocomponents into the existing infrastructure, increasing societal awareness of nanomaterials and nanotechnology, and importantly, understanding and mitigating the potential risks of engineered nanomaterials (ENMs) to the environment and human health.[3]
In terms of environmental health and safety aspect of nanotechnology (nano-EHS), we have come a long way in comparison to the accelerating point over 20 years ago. For example, we have paid more attention to physicochemical characterization of ENMs for nano-EHS, we have known more about the behavior of ENMs in biological relevant environment, such as formation of protein and lipid corona, agglomeration, dissolution, transformation, etc., we have expanded our understanding of ENM impacts in many organisms and animal models, even human health impacts in occupational settings, and we have identified major ENM properties that are likely drivers for potential adverse outcomes. Based on the successes of the technology and the seemingly lack of ENM-induced major adverse outcomes in the environment and human health, there are notions that are gaining speed that we have known enough and efforts on nano-EHS should be just in a back burner. However, there are some recent events showing that this is not the case and proactive research are still much in need to safeguard the sustainable development of nanotechnology. One example is on titanium dioxide (TiO2), the European Commission (EC) is reported to be advancing to classify TiO2 as a category 2 carcinogen due to its inhalation hazard.[4] The classification will apply to liquids as well as powders “containing 1% or more of TiO2 in the form of or incorporated in particles with aerodynamic diameter ≤ 10 μm.” Furthermore, France has banned the use of TiO2 in food beginning on January 1, 2020.[5] This decision was based experimental findings on E171 (TiO2 food additive used to whiten various products, including chewing gum, cake icing, and candy, etc.), which has been shown to be able to damage the intestinal cells and disrupt the gut microbiome that can cause cancer. It is worth noting that although there is evidence to show the carcinogenic potential of TiO2, there are also many reports that showed negative or inconclusive results. The question is how to make regulatory decisions by weighing the existing and sometimes contradictory evidence. In parallel to the TiO2 case in EU, The US Food and Drug Administration (FDA) has put stricter warnings and contraindications for the anemia drug ferumoxytol (carbohydrate-coated superparamagnetic iron oxide (SPIO) nanoparticle-based drug given intravenously for iron replacement to hospitalized patients with chronic kidney disease).[6] The FDA found 79 anaphylactic reactions between June 2009 and June 30, 2014. Eighteen of the 79 patients died despite immediate medical intervention. It is worth noting that half of the anaphylactic reactions occurred with the first dose and the mechanism responsible for these hypersensitivity responses is still unclear. These events show that there are still much unknowns and uncertainty surrounding the nano-EHS research, and the lack of certainty (exposure route, realistic exposure dose, duration, biological effects especially on carcinogenicity) or mechanistic understanding of the toxicological effects could impact the long-term sustainability of the nanotechnology considering the exposure of ENMs to the environment and humans will only increase due to the rapidly expanding use of nano-products. Although the nanotechnology industry is seemingly healthy right now, the growth could be hindered for lack of certainty on nano-EHS and more research is need to be done for the sustainable growth of nano-industries. Herein, we will describe the current knowledge gaps and challenges in nano-EHS research based on what we have achieved to date.
Achievements in nano-EHS research
Nano-EHS or nanosafety received much attention from the start of the NNI.[7] At that time, the prevailing question was whether nanomaterials are more toxic than their bulk form, if so, what material properties were responsible for the toxicity.[8] It took some time for the field to perform detailed physicochemical characterization of the ENMs for nano-EHS and develop tests beyond using the cell viability assays to use more mechanism-based systemic safety assessment. The beginning of major efforts on nano-EHS research at national and international levels coincides with the major paradigm change in the broader field of toxicology. A case in point is the toxicity testing of chemicals, which was heavily bent to “gold standard” animal tests that are inefficient, costly, time consuming, and the limited testing results are mostly descriptive without ample mechanistic understanding. So it becomes evident that this animal dominant approach is unsustainable not only for the 100,000 plus chemicals but also for the ENMs with numerous physicochemical property variations such as chemical composition, size, shape, aspect ratio, charge, dissolution, hydrophobicity/hydrophilicity, surface functionalization, etc. as well as their diverse uses in nanocomposites and nanoproducts. In 2007, the US National Academy of Sciences landmark published a report titled: “Toxicity Testing in the 21st Century: A Vision and a Strategy”,[9] which advocates increased efficiency of toxicity testing by transitioning from qualitative, descriptive animal testing to quantitative, mechanistic, and pathway-based toxicity testing in human cells or cell lines using high-throughput approaches. Limited and focused animal tests will be used to validate the in vitro results and iteration of the processes will lead to mechanistic understanding of toxicant-induced diseases. It is a big challenge for the research community to transition from animal testing to high throughput, mechanism-based alternative methods. Taking consideration of this rational approach for chemical toxicity, nano-EHS will also benefit enormously if high throughput screening, alternative testing, and predictive toxicological approaches could be established. Indeed, the nano-EHS moved quickly to establish the 21st century toxicity testing strategy specifically tailored for ENMs. This includes, nanomaterial libraries that provide property variations that could be used to link the specific physicochemical characteristics to toxicity, high throughput screening systems (e.g., cell culture, C elegans, zebrafish, etc.), in vitro mechanism of toxicity based assays (oxidative stress, NLRP3 inflammasome activation, ER stress, autophagy, apoptosis, pyroptosis, etc.), computational models to build structure activity relationships (SAR), and limited but focused in vivo validations to establish predictive toxicology paradigms and adverse outcome pathways (AOPs) to facilitate regulatory decision making.[10–17] In addition, the field begins to use un-biased multi-omics approach (e.g., metabolomics, lipidomics, epigenomics, etc.) that discovers new mode of action, especially at lower dose range that has higher environmental relevance.[18–20] Over the years, much progress has been achieved by the nanosafety community, which has greatly enhanced our understanding on the major physicochemical properties that could lead to toxicity, molecular and cellular mechanisms of toxicity induced by ENMs, different exposure routes and dosimetry, animal models as well as implications to human health (Figure 1), however, there are many knowledge gaps and challenges still remain.[11, 21–24]
Figure 1. Examples of mechanistic toxicological responses for major types of ENMs.
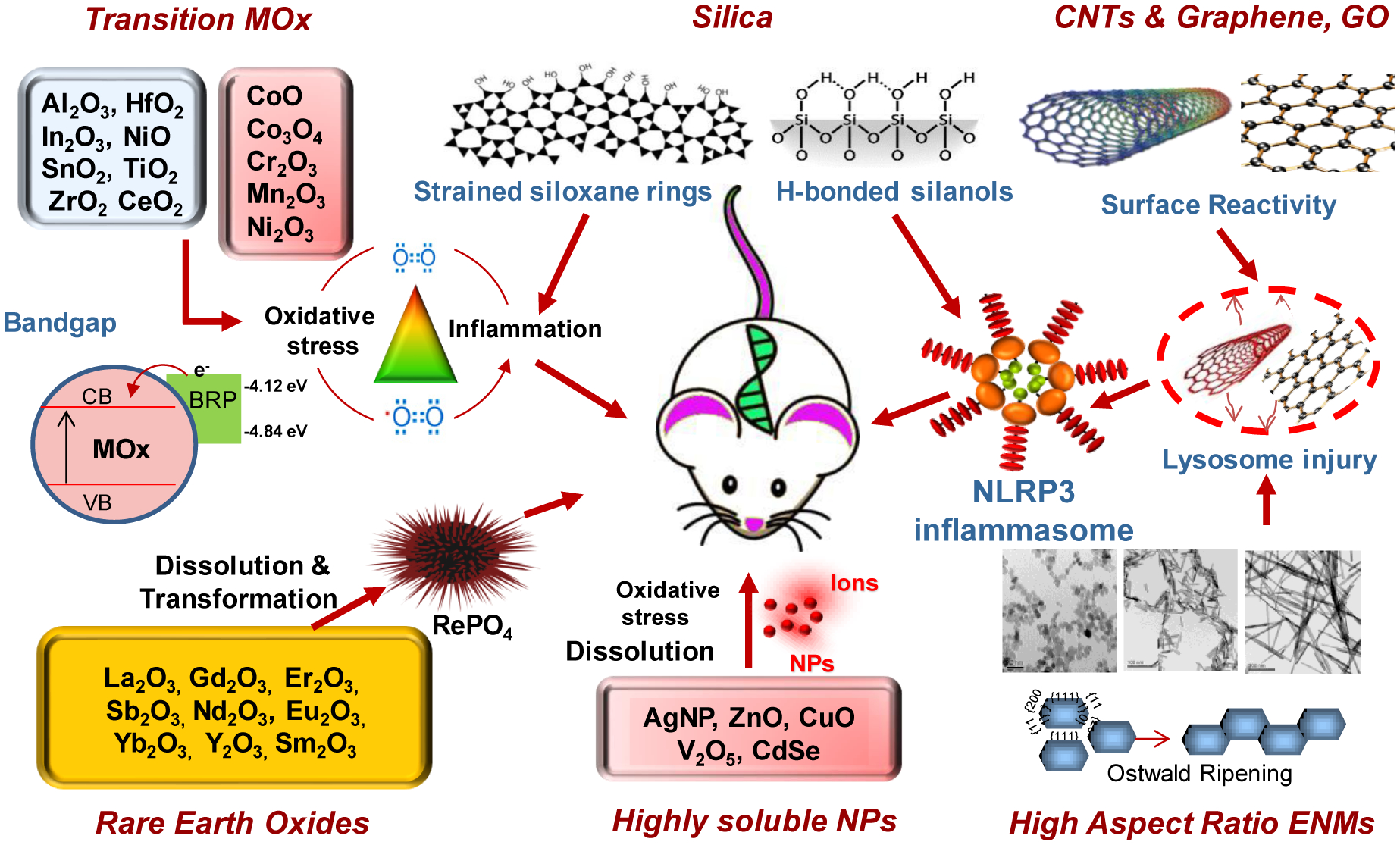
These include transition metal oxide nanomaterials, silica-based nanomaterials, carbon nanotubes, graphene and graphene oxide, high aspect ratio materials, and highly soluble metal and metal oxides, rare-earth oxides. Using ENM libraries, properties of the ENMs (bandgap, dissolution, surface reactivity, aspect ratio, transformation, etc.) could be linked to mechanism-based in vitro (oxidative stress, lysosomal damage, NLRP3 inflammasome activation, cell death, etc.) and in vivo (e.g., lung inflammation and fibrosis) toxicity in a predictive fashion.
Current knowledge gaps and challenges in nano-EHS research
With the rapidly increasing invention, production and use of ENMs in nanoproducts, their incidental, accidental or intentional release into the environment and exposure to humans are inevitable. Diverse types of ENMs (e.g., Ag, CuO, CeO2, carbon nanotubes (CNTs), TiO2, SiO2, etc.) have been detected in various environmental media including the air, water, soil. Meanwhile, the current wastewater treatment and waste management facilities are not equipped to remove ENMs and prevent their re-entry into the environment.[25–26] Additionally, nanotechnology has been applied to substantially increase the production of crops through the use of nanosized fertilizers and herbicides.[27–28] The use of ENMs in agriculture would give rise to the release of large quantity of ENMs into the environment and possibly, the food chain. In addition, ENMs are increasingly used in biomedicine for imaging and therapeutic purposes, for example, fluorescent core-shell silica nanoparticle, magnetic iron oxide nanoparticles, and gold nanoshells have been approved by FDA for clinical trials. Thus, ENM exposure to the environment and humans is occurring in an unprecedented and diverse fashion. This issue becomes more complicated by the synthesis and introduction of new generation of ENMs.[2] Even though substantial efforts have been devoted and significant progresses have been made to nano-EHS research, there is still a long way to fully grasp the potential impacts associated with even the existing ENMs, not to mention the ones in the development pipeline.[29–30] A largely overlooked issue is the nano-EHS impacts under realistic exposure scenarios considering their environmental transformation, realistic doses, and chronic effects, especially on the potential of carcinogenesis that has generated many uncertainties.[31] Under these circumstances, nano-EHS research is still very much in need and effective communications among researchers, industries, government, and other stakeholders are critical for the risk management and sustainable development of nanotechnology.
1. Determining realistic exposure scenarios and dosage.
A critically important step in nano-EHS is determining realistic concentrations of ENM in real-life environment and potential exposure pathways. This is currently a major challenge due to the complex real-life working or living environment with confounding co-contaminants existing as mixtures. To identify, characterize, and quantify the ENMs under complicated environmental conditions is not trivial. i) Nanotechnology industries are developing fast, and increasing new ENMs and ENM-enabled products are being developed at a rapid pace. We are facing not only the pre-existed ENMs in pristine form such as carbon nanotubes (single walled and multiwall), 2D graphene and graphene oxide, metals (e.g., Ag, Cu, Pt) and metal oxides (CuO, ZnO, TiO2, CeO2, SiO2, etc.), but also emerging ENMs (e.g., 2D ENMs (carbides and nitrides (MXenes), black phosphorus, transition metal dichalcogenides (TMDCs)), as well as ENMs in nanocomposites and other nano-products that can be released upon degradation.[32–35] Thus, it is difficult to predict the forms and dosage considering the various types of ENMs that will likely to be released and exposed to the environment and humans. For example, recent findings clearly show that ENMs released from nanocomposites may exhibit significantly different properties in the environment compared to their as-synthesized, pristine counterparts.[36–37] However, because typical nanocomposites contain low concentrations of ENMs (1–5% w/w) and they are incorporated in the matrix material, so there are only limited release of free ENMs from nanocomposites and most ENMs are contained inside nanocomposite fragments under environmental relevant degradation conditions (sanding, UV irradiation, sun light exposure, etc.). As a result, the nanocomposite toxicity is dominated by the matrix material rather than the ENMs used as fillers [38] ii) The results from ENM hazard evaluations are often skewed by the use of unrealistic exposure doses, reducing the real-life relevance of the studies. To date, studies on fate, transport and effects of ENMs were often conducted with ENM concentrations or doses orders of magnitude higher than what has been reported under the typical environmental conditions, thus it is not clear the relevance of these studies in terms of the implications to the environment and human health.[39–43] However, it is worth noting that despite the high doses used in vitro and in vivo studies, valuable information has been obtained on the mechanism of toxicity and potential adverse effects for ENMs, and measures have been taken to cover a wider dose range for in vitro and in vivo studies and use more sensitive methods such as toxicogenomics to study ENM effects at lower doses.[18, 20] To better predict the realistic concentrations of ENMs, models and approaches have been developed based on the estimation of global and regional production, application and life-cycle of ENMs.[44–45] Further improvement and fine-tuning of these models by taking into account the real-life measurements would facilitate the development of ENM hazard assessment under environmentally relevant conditions. iii) It is also important to choose appropriate exposure routes for animal studies. For example, most of the studies that uses animal models focused on lung toxicity because it is one of the most likely exposure pathways for dry ENMs that can be easily aerosolized during synthesis, handling, and packaging. Furthermore, ENM suspensions could become aerosolized during mixing, processing, and sonication in the laboratory or manufacture settings. In addition, some ENMs are released in the air, e.g., CeO2 are used in diesel fuels and released in the exhaust of vehicles. Notably, pulmonary research is not limited to lung toxicity alone, it also serves as a surrogate that can reflect the general hazard of ENMs that may be relevant for other tissues and organs. However, there is a need to go beyond the lung and study effects to other systems based on their potential use and exposure routes. For instance, graphene oxide is mostly researched to serve as a drug carrier for its biomedical applications, which means intravenous administration is the most relevant exposure route rather than inhalation and the toxicity will mostly likely to happen at the accumulation sites (liver, spleen, kidney, lung, etc.) depending on the biodistributions.[20, 39] iv) Another major hurdle for understanding realistic exposure of ENMs is a lack of analytical tools that can be used to characterize and quantify ENMs from complex mixtures, including identification, content, forms of existence in complex media (e.g., food, dust, slurry, soil, etc.). For this purpose, although useful methods are being developed including single-particle ICP-MS, single-particle ICP-TOFMS and automated electron microscopy,[46–51] portable, easy to use, accurate and reliable methods are very much in need to characterize and quantify diverse ENMs in the complex environmental media.
2. Elucidation of transformation processes.
To precisely assess nano-EHS, it is critical to understand the specific speciation and properties of ENMs to which biological systems are exposed. To date, most studies were conducted primarily using pristine nanomaterials, which is reasonable and necessary to build the baseline toxicity profiles for ENMs. However, ENMs are seldom existing in their pristine form for long except when they are first synthesized under controlled conditions. It becomes clear that many physical, chemical and biological transformation processes can happen to pristine ENMs when they leave the controlled environment, which will change their properties as well as the subsequent biological effects. The properties will also change when ENMs are integrated into nanoproducts, so there is a need for companies to report on how ENMs are being used in the consumer products, which will help understand the potential property changes, degradation and release, as well as the exposure scenarios. ENMs rarely stay put under various environmental conditions or inside cells, tissues, and organs, even for those conventionally considered to be stable (e.g., SiO2, MoS2, CdSe, graphene oxide, rare earth oxides, Ag, etc.). The first thing that happens when ENMs are introduced to the biological environments are the formation of protein/lipid corona, which will change the properties of ENMs immediately and impact the bioavailability and biodistribution as well as safety profiles.[52–56] and transformations (morphology, speciation, oxidation/reduction states, dissolution, recrystallization/reformation, etc.) can remarkably alter their physicochemical properties, and consequently, their fate, transport and biological effects.[39, 57–61] As the transformation of ENMs has increasingly been reported [57–58, 62–65], more attention should be paid on dissecting how the properties of ENMs change would impact their toxic effects. In addition to transformation to the ENMs, ENMs will have the opportunity to interact with other environmental agents and pollutants when released into the environment, including heavy metals, organic chemicals, natural organic matter, etc. Given the large surface area of ENMs, they can readily adsorb various contaminants simultaneously on their surface.[66–68] Similar to air pollution particles, this association or binding process can alter the physicochemical properties, bioavailability, biodistribution, and toxicological effects of ENMs as well as impact the toxicity of the contaminants on the surface of ENMs (additive, subtractive or synergistic).[69–70] Thus, ENM–contaminant interactions, as well as how environmental conditions may complicate such interactions, are important factors that may impact the adverse outcomes to environmental organisms or human beings. To date, only sporadic studies have considered this topic, and more research is needed on this front.[68, 71]
3. Health impacts under realistic exposure conditions.
Considering the abovementioned limitations in the knowledge of realistic exposure and transformation of ENMs, evaluation of the currently available data on nano-EHS should be taken with a grain of salt. i) To date, most studies in the literature (in particular, those on human health) were carried out using as synthesized, pristine ENMs, which are unlikely to be found in the environment expect under certain occupational settings; these studies may not reflect realistic exposure scenarios. Thus, more nano-EHS studies should focus on the types of materials that will more likely be found in the real-life environment (e.g., mixture of ENMs with other contaminants, debris of nanocomposites, etc.). The study should also consider the chemical and biological transformation of ENMs as mentioned previously. ii) Most studies were conducted using unrealistically high doses/concentrations of ENMs, which would induce obvious toxic effects but cannot provide answers to address more realistic exposure scenarios, which is long-term effects under low doses. To this end, health effects under exposure at environmentally relevant doses/concentrations should be the focus of future studies, and attention should be paid to long-term/chronic effects (e.g., carcinogenic potentials), including possible effects that influence reproductive systems and cross-generational effects of genetic and epigenetic origin. iii) While inhalation or lung exposure dominates the nano-EHS research, exposure through intravenous exposure routes, gastrointestinal tract, ocular, and dermal exposure routes in various animal models should be considered under the occupational and therapeutic exposure settings.[65, 72] There are studies on the biodistribution of Ag nanoparticles in pregnant rodents, which showed silver is able to affect the uterine artery function and cross the placenta barrier to reach the fetus. These findings suggest that ENMs may affect fetus development, however, more research is needed to fully grasp the impacts.[73–74] iv) As mentioned above, exposure of ENMs is often accompanied by the co-existing environmental pollutants, which may result in elevated risks of both ENMs and contaminants. Studies have found that the bioavailability of certain contaminants can be greatly increased by ENMs due to their “Trojan Horse” effects.[27, 75] However, toxicological effects resulting from the interaction between ENMs and contaminants have been largely overlooked due to the incomplete understanding on the effects of ENMs themselves. As we know more about the behavior of ENMs, future research could wade into the impacts of complicated mixtures. v) Most previous studies concerned the direct toxicity to primarily targeted tissues or organs after ENM exposure. Future work should also take into consideration the secondary effects in tissues and organs distant from the primary target sites.[40, 76–77] vi) In addition, more association and epidemiological studies are needed, especially in an occupational exposure setting to build the link of ENM exposure to health impacts for workers. This requires more accurate dose and exposure measurements for ENMs in the working environment, coverage of more physiological or disease markers, determination of the causal relationships, as wells as increase in sample sizes.[78–79] Moreover, more selective biomarkers including epigenetic surrogates [80–81] should be identified to better reflect the biological responses to ENMs exposure.
4. Developing tools and frameworks for nano-EHS.
Since the nanotechnology became the forefront of research and development, laws and regulations have been installed to ensure the safe use of the emerging technology. The Europe Union installed REACH (Regulation concerning the Registration, evaluation, Authorization and Restriction of Chemicals) that requires companies to provide information on nano-EHS. The new Toxic Substance Control Act (TSCA) approved in the US in 2016 also requires the toxicity testing for all chemicals including ENMs. The Organization for Economic Co-operation and Development (OECD) has been active to establish guidance and manuals for the member states on hazard and exposure assessment of ENMs over the years [82]. Although much progress has been made on the nano-EHS field, the quality of research is uneven and discrepancies or even conflicting results are common in the literature, which leads to confusion to the research community, the public, regulators and nano-industries. To confront these issues, there is still a need to develop standard or reference ENMs for read-across and comparing results, and it is still important to provide appropriate material characterization, biological characterization, and experimental protocol details regarding the biological behavior, safety and therapeutic use of ENMs to improve reproducibility in nano-EHS research.[21] i) For physicochemical characterization of ENM properties, there are widely available tools for determining chemical compositions, primary particle size, morphology, hydrodynamic size and charge, etc., however, quantitative determination of certain ENMs and properties, e.g. the carbon-based ENM content in tissues and surface free energy of ENMs, is challenging. Recently, novel analytical methods have been developed such as using mass spectrometry imaging (laser desorption/ionization mass spectrometry imaging (LDI-MSI)) to determine the sub-organ distribution of carbon nanomaterials and a maximum particle dispersion (MPD) method for quantitatively determining the surface free energy of a wide range of micro- and nanoparticles.[83–84] ii)The current in vitro assays for EHS were derived mostly from those traditionally developed for chemicals; however, some ENMs could interfere with the assays. For instance, many nanoparticles distort the MTT/MTS cell viability assay and lactate dehydrogenase (LDH) release assays that are detected based on absorbance and introduce artifacts.[85–86] Therefore, guidelines should be recommended to choose the appropriate assays for various ENMs, and other complementary assays using a different detection mode (fluorescence or bioluminescence) may be needed for certain types of ENMs. Moreover, additional alternative test strategies and multi-omics technology should be utilized more for nano-EHS assessment, which includes high throughput screening, predictive toxicology, genomics/epigenetics/metabolomics, and in silico approaches, to move nano-EHS from descriptive science to mechanism-based toxicology.[11, 13, 18, 20, 87] Especially, it is important to link the physicochemical properties of ENMs to the toxicological outcomes.[10–12, 16, 87–88] iii) After years of development, there are still enormous obstacles and limitations for the current computational models to precisely characterize nanotoxicology. Thus, computational models (e.g. nano-oriented Quantitative Structure–Activity Relationship models, Nano-QSAR) should be improved to identify the key characteristics that dictate the nano-EHS.[16, 89–91] Furthermore, more meta-analyses, computational simulation (molecular dynamics simulations) and predictive studies should be carried out to tease out the underlying SAR in controlling the behaviors and biological effects of ENMs.[92–96] This would guide the safer design of ENMs, which targets the properties that induce toxicity to reduce the potential hazard while maintaining the beneficial properties of ENMs.[41–43, 57–58, 61, 97–98] In addition, the nanosafety research could provide useful information on development of nanomedicine, For example, while high aspect ratio materials including aluminum oxyhydroxide (AlOOH) nanorods and cellulose nanocrystals and nanofibers could induce pro-inflammatory effects by triggering NLRP3 inflammasome activation in macrophages and dendritic cells (DC), these ENMs have been shown to be able to serve as adjuvant to boost immune responses by inducing DC maturation, which has the potential to be used in vaccines. Graphene oxide (GO) possesses carbon radicals on its surface that could attack plasma membrane and induce lipid peroxidation, leading to cell death. The same feature has been used to develop GO coatings on medical devices including glass or catheter surface to inhibit the growth of antibiotic resistant bacteria. Similarly, the property of GO could be used to enhance cancer killing by anticancer drugs in a synergistic fashion through the mechanism of membrane disruption.[42, 60, 99–100] Considering recent advances, an updated framework of nano-EHS is described in Figure 2.
Figure 2. Focus of future nano-EHS research.
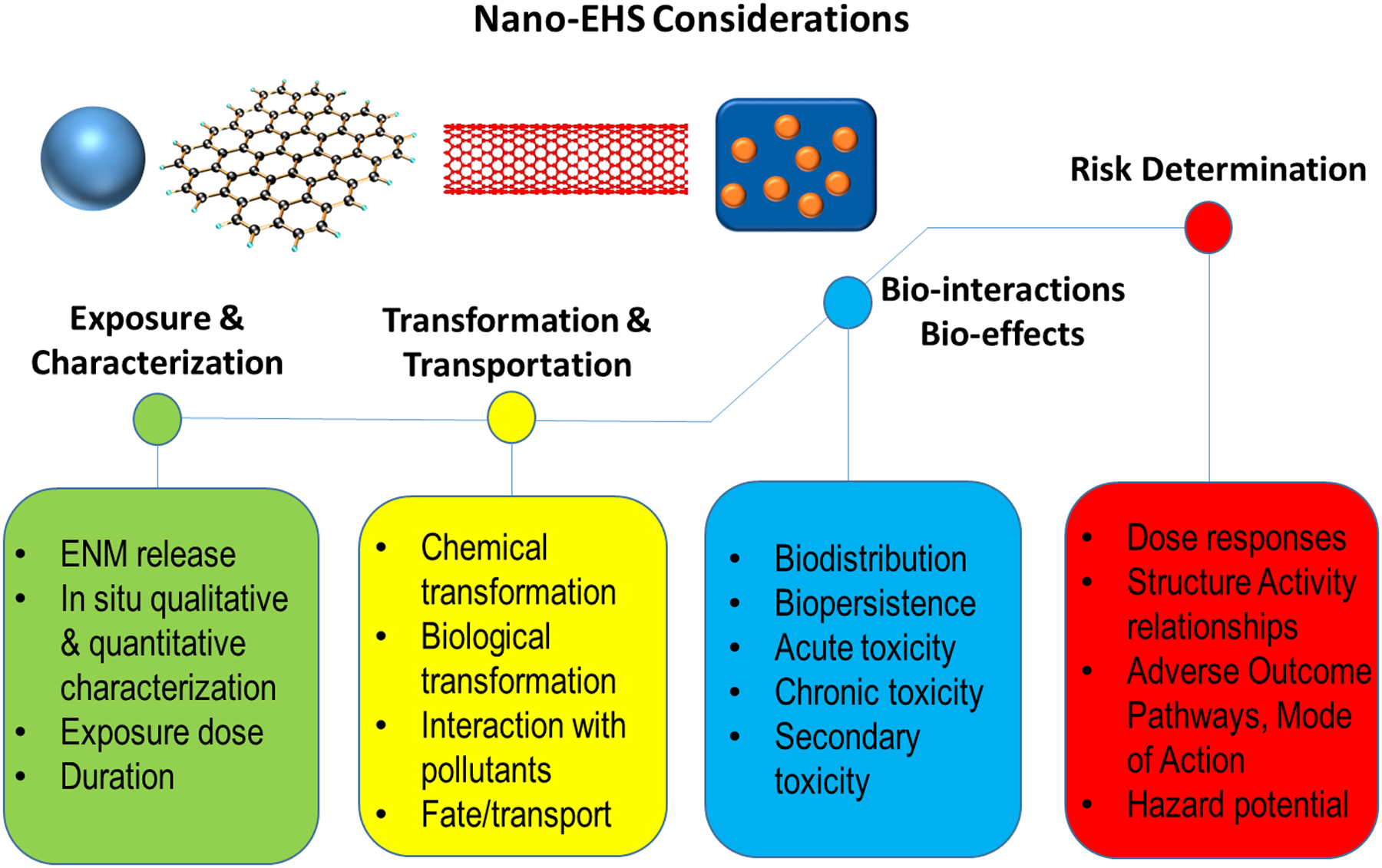
Increasing number of ENMs are produced including pristine nanomaterials and nanocomposites. It is important to determine the realistic exposure including doses and exposure routes, their transformation behavior, biodistribution and biological or pathological responses. The mechanisms of toxicity and adverse outcome pathways will facilitate the risk assessment of ENMs.
Conclusions and perspectives.
Substantial progress has been made on nano-EHS, which plays an important role in facilitating the rapid growth of nano-industries to date. However, uncertainties or lack of understanding on nanosafety issues, e.g., carcinogenic potential of TiO2 used in food and iron oxide induced anaphylaxis, can serve as wakeup calls for all the stakeholders that it is still important to continue to improve our understanding on ENM effects to the environment and human health. To deal with the challenge of increasing invention and production of ENMs, 21st century toxicological approaches including use of standard ENMs and ENM libraries, predictive toxicology, alternative testing strategies, high throughput screening, data analysis and modeling, and continued discovery on the mechanism of toxicity and AOPs using multi-omics approaches are critical for nano-EHS. The knowledge on the SAR can be used to design and produce safer ENMs by modifying or changing the physicochemical properties. Through an iterative approach, these efforts would yield safer nano-products, reducing the uncertainties and alleviate the public concern over ENM and nano-product safety, which will drive the sustainable growth of nano-industry in the future.
Acknowledgements
This work was supported under grants from the National Natural Science Foundation of China (grant numbers: 21637004 and 21920102007), the Beijing Natural Science Foundation (grant number: 8191002) and the international collaboration key grant from the Chinese Academy of Sciences (grant number: 121311KYSB20190010). TX was supported by supported by NIH U01 ES027237 and R01 HL139379.
Footnotes
Competing interests
There is no potential conflict of interests to disclose.
References:
- 1.Wiseguyreports.com, Nanotechnology Market 2019 Global Industry - Key Players, Size, Trends, Opportunities, Growth Analysis and Forecast to 2024. 2019
- 2.Murphy F; Mullins M; Hester K; Gelwick A; Scott-Fordsmand JJ; Maynard T, Nat Nanotechnol 2017, 12, 717. [DOI] [PubMed] [Google Scholar]
- 3.Valsamijones E; Lynch I, Science 2015, 350, 388. [DOI] [PubMed] [Google Scholar]
- 4.European Chemicals Agency, Titanium dioxide proposed to be classified as suspected of causing cancer when inhaled. 2017
- 5.US Department of Agriculture, France bans Titanium Dioxide in food products by January 2020. 2019
- 6.Food and Drug Administration, Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). 2015
- 7.Oberdorster G; Oberdorster E; Oberdorster J, Environ Health Perspect 2005, 113, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberdorster G, Int Arch Occup Environ Health 2001, 74, 1. [DOI] [PubMed] [Google Scholar]
- 9.National Academy of Sciences, Toxicity Testing in the 21st Century: A Vision and a Strategy. 2007
- 10.Nel A; Xia T; Madler L; Li N, Science 2006, 311, 622. [DOI] [PubMed] [Google Scholar]
- 11.Nel A; Xia T; Meng H; Wang X; Lin S; Ji Z; Zhang H, Acc Chem Res 2013, 46, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nel AE; Madler L; Velegol D; Xia T; Hoek EM; Somasundaran P; Klaessig F; Castranova V; Thompson M, Nat Mater 2009, 8, 543. [DOI] [PubMed] [Google Scholar]
- 13.Nel AE; Nasser E; Godwin H; Avery D; Bahadori T; Bergeson L; Beryt E; Bonner JC; Boverhof D; Carter J; Castranova V; DeShazo JR; Hussain SM; Kane AB; Klaessig F; Kuempel E; Lafranconi M; Landsiedel R; Malloy T; Miller MB; Morris J; Moss K; Oberdorster G; Pinkerton K; Pleus RC; Shatkin JA; Thomas R; Tolaymat T; Wang A; Wong J, ACS Nano 2013, 7, 6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B; Wang X; Ji Z; Li R; Xia T, Small 2013, 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun B; Wang X; Ji Z; Wang M; Liao YP; Chang CH; Li R; Zhang H; Nel AE; Xia T, Small 2015, 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X; Sun B; Liu S; Xia T, NanoImpact 2017, 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia T; Malasarn D; Lin S; Ji Z; Zhang H; Miller RJ; Keller AA; Nisbet RM; Harthorn BH; Godwin HA; Lenihan HS; Liu R; Gardea-Torresdey J; Cohen Y; Madler L; Holden PA; Zink JI; Nel AE, Small 2013, 9, [DOI] [PubMed] [Google Scholar]
- 18.Cui L; Wang X; Sun B; Xia T; Hu S, ACS Nano 2019, 13, 13065. [DOI] [PubMed] [Google Scholar]
- 19.Guo C; Robertson S; Weber RJM; Buckley A; Warren J; Hodgson A; Rappoport JZ; Ignatyev K; Meldrum K; Romer I; Macchiarulo S; Chipman JK; Marczylo T; Leonard MO; Gant TW; Viant MR; Smith R, Nanotoxicology 2019, 13, 733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y; Feng W; Liu R; Xia T; Liu S, ACS Nano 2020, 14, 877. [DOI] [PubMed] [Google Scholar]
- 21.Leong HS; Butler KS; Brinker CJ; Azzawi M; Conlan S; Dufes C; Owen A; Rannard S; Scott C; Chen C; Dobrovolskaia MA; Kozlov SV; Prina-Mello A; Schmid R; Wick P; Caputo F; Boisseau P; Crist RM; McNeil SE; Fadeel B; Tran L; Hansen SF; Hartmann NB; Clausen LPW; Skjolding LM; Baun A; Agerstrand M; Gu Z; Lamprou DA; Hoskins C; Huang L; Song W; Cao H; Liu X; Jandt KD; Jiang W; Kim BYS; Wheeler KE; Chetwynd AJ; Lynch I; Moghimi SM; Nel A; Xia T; Weiss PS; Sarmento B; das Neves J; Santos HA; Santos L; Mitragotri S; Little S; Peer D; Amiji MM; Alonso MJ; Petri-Fink A; Balog S; Lee A; Drasler B; Rothen-Rutishauser B; Wilhelm S; Acar H; Harrison RG; Mao C; Mukherjee P; Ramesh R; McNally LR; Busatto S; Wolfram J; Bergese P; Ferrari M; Fang RH; Zhang L; Zheng J; Peng C; Du B; Yu M; Charron DM; Zheng G; Pastore C, Nat Nanotechnol 2019, 14, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh AV; Laux P; Luch A; Sudrik C; Wiehr S; Wild AM; Santomauro G; Bill J; Sitti M, Toxicol Mech Methods 2019, 29, 378. [DOI] [PubMed] [Google Scholar]
- 23.Fadeel B, Front Immunol 2019, 10, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu G; Xia T; Zhou W; Zhang X; Zhang H; Hu L; Shi J; Yu XF; Jiang G, Chem Rev 2020, 120, 2288. [DOI] [PubMed] [Google Scholar]
- 25.Kiser MA; Westerhoff P; Benn T; Wang Y; Pérez-Rivera J; Hristovski K, Environ. Sci. Technol 2009, 43, 6757. [DOI] [PubMed] [Google Scholar]
- 26.Shi X; Li Z; Chen W; Qiang L; Xia J; Chen M; Zhu L; Alvarez PJJ, NanoImpact 2016, 3–4, 96 [Google Scholar]
- 27.Qiang L; Chen M; Zhu L; Wu W; Wang Q, Environ. Sci. Technol 2016, 50, 11627. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues SM; Demokritou P; Dokoozlian N; Hendren CO; Karn B; Mauter MS; Sadik OA; Safarpour M; Unrine JM; Viers J, Environ Sci-Nano 2017, 4, 767 [Google Scholar]
- 29.Maynard A; Rejeski D, Nature 2009, 460, 174. [DOI] [PubMed] [Google Scholar]
- 30.Walker W; Bosso C; Eckelman M; Isaacs J; Pourzahedi L, J Nanopart Res 2015, 17, 344 [Google Scholar]
- 31.National Nanotechnology Initiative, Quantifying Exposure to Engineered Nanomaterials (QEEN) from Manufactured Products, Addressing Environmental, Health, and Safety Implications. 2015
- 32.De Volder MF; Tawfick SH; Baughman RH; Hart AJ, Science 2013, 339, 535. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y; Fang Q; Chen B, Environ. Sci. Technol 2015, 49, 67. [DOI] [PubMed] [Google Scholar]
- 34.Prateek; Thakur VK; Gupta RK, Chem Rev 2016, 116, 4260. [DOI] [PubMed] [Google Scholar]
- 35.Gottschalk F; Sonderer T; Scholz RW; Nowack B, Environ. Sci. Technol 2009, 43, 9216. [DOI] [PubMed] [Google Scholar]
- 36.Schlagenhauf L; Buerkithurnherr T; Kuo YY; Wichser A; Nüesch F; Wick P; Wang J, Environ. Sci. Technol 2015, 49, 10616. [DOI] [PubMed] [Google Scholar]
- 37.Pillai KV; Gray PJ; Tien CC; Bleher R; Sung LP; Duncan TV, Environ Sci-Nano 2016, 3, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amorim MJB; Lin S; Schlich K; Navas JM; Brunelli A; Neubauer N; Vilsmeier K; Costa AL; Gondikas A; Xia T; Galbis L; Badetti E; Marcomini A; Hristozov D; Kammer FV; Hund-Rinke K; Scott-Fordsmand JJ; Nel A; Wohlleben W, Environ. Sci. Technol 2018, 52, 1514. [DOI] [PubMed] [Google Scholar]
- 39.Li R; Guiney LM; Chang CH; Mansukhani ND; Ji Z; Wang X; Liao YP; Jiang W; Sun B; Hersam MC; Nel AE; Xia T, ACS Nano 2018, 12, 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J; Li R; Qu G; Liu H; Yan B; Xia T; Liu Y; Liu S, Nanoscale 2016, 8, 18070. [DOI] [PubMed] [Google Scholar]
- 41.Sun B; Pokhrel S; Dunphy DR; Zhang H; Ji Z; Wang X; Wang M; Liao YP; Chang CH; Dong J; Li R; Madler L; Brinker CJ; Nel AE; Xia T, ACS Nano 2015, 9, 9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X; Mansukhani ND; Guiney LM; Ji Z; Chang CH; Wang M; Liao YP; Song TB; Sun B; Li R; Xia T; Hersam MC; Nel AE, Small 2015, 11, 5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X; Xia T; Duch MC; Ji Z; Zhang H; Li R; Sun B; Lin S; Meng H; Liao YP; Wang M; Song TB; Yang Y; Hersam MC; Nel AE, Nano Lett 2012, 12, 3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottschalk F; Sun T; Nowack B, Environ Pollut 2013, 181, 287. [DOI] [PubMed] [Google Scholar]
- 45.Mueller NC; Nowack B, Environ. Sci. Technol 2008, 42, 4447. [DOI] [PubMed] [Google Scholar]
- 46.Bitragunta SP; Palani SG; Gopala A; Sarkar SK; Kandukuri VR, Bulletin of environmental contamination and toxicology 2017, 98, 595. [DOI] [PubMed] [Google Scholar]
- 47.Costa-Fernández JM; Menéndez-Miranda M; Bouzas-Ramos D; Encinar JR; Sanz-Medel A, Trac-Trend Anal Chem 2016, 84, 139 [Google Scholar]
- 48.Doudrick K; Herckes P; Westerhoff P, Environ. Sci. Technol 2015, 46, 12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattarozzi M; Suman M; Cascio C; Calestani D; Weigel S; Undas A; Peters R, Analytical and bioanalytical chemistry 2017, 409, 63. [DOI] [PubMed] [Google Scholar]
- 50.Neubauer N; Scifo L; Navratilova J; Gondikas A; Mackevica A; Borschneck D; Chaurand P; Vidal V; Rose J; von der Kammer F; Wohlleben W, Environ. Sci. Technol 2017, 51, 11669. [DOI] [PubMed] [Google Scholar]
- 51.Witzler M; Küllmer F; Hirtz A; Guenther K, J Agr Food Chem 2016, 64, 4165. [DOI] [PubMed] [Google Scholar]
- 52.Cedervall T; Lynch I; Lindman S; Berggard T; Thulin E; Nilsson H; Dawson KA; Linse S, Proc Natl Acad Sci U S A 2007, 104, 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly PM; Aberg C; Polo E; O’Connell A; Cookman J; Fallon J; Krpetic Z; Dawson KA, Nat Nanotechnol 2015, 10, 472. [DOI] [PubMed] [Google Scholar]
- 54.Lundqvist M; Stigler J; Elia G; Lynch I; Cedervall T; Dawson KA, Proc Natl Acad Sci U S A 2008, 105, 14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persaud I; Shannahan JH; Raghavendra AJ; Alsaleh NB; Podila R; Brown JM, Ecotoxicol Environ Saf 2019, 170, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raghavendra AJ; Fritz K; Fu S; Brown JM; Podila R; Shannahan JH, Sci Rep 2017, 7, 8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li R; Ji Z; Chang CH; Dunphy DR; Cai X; Meng H; Zhang H; Sun B; Wang X; Dong J; Lin S; Wang M; Liao YP; Brinker CJ; Nel A; Xia T, ACS Nano 2014, 8, 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R; Ji Z; Dong J; Chang CH; Wang X; Sun B; Wang M; Liao YP; Zink JI; Nel AE; Xia T, ACS Nano 2015, 9, 3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li R; Ji Z; Qin H; Kang X; Sun B; Wang M; Chang CH; Wang X; Zhang H; Zou H; Nel AE; Xia T, ACS Nano 2014, 8, 10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li R; Mansukhani ND; Guiney LM; Ji Z; Zhao Y; Chang CH; French CT; Miller JF; Hersam MC; Nel AE; Xia T, ACS Nano 2016, 10, 10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li R; Wang X; Ji Z; Sun B; Zhang H; Chang CH; Lin S; Meng H; Liao YP; Wang M; Li Z; Hwang AA; Song TB; Xu R; Yang Y; Zink JI; Nel AE; Xia T, ACS Nano 2013, 7, 2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lowry GV; Gregory KB; Apte SC; Lead JR, Environ. Sci. Technol 2012, 46, 6891. [DOI] [PubMed] [Google Scholar]
- 63.Zhao J; Liu F; Wang Z; Cao X; Xing B, Environ. Sci. Technol 2015, 49, 2849. [DOI] [PubMed] [Google Scholar]
- 64.Wang L; Zhang T; Li P; Huang W; Tang J; Wang P; Liu J; Yuan Q; Bai R; Li B; Zhang K; Zhao Y; Chen C, ACS Nano 2015, 9, 6532. [DOI] [PubMed] [Google Scholar]
- 65.Qi Y; Liu Y; Xia T; Xu A; Liu SJ; Chen W, Npg Asia Mater 2018, 10, 385 [Google Scholar]
- 66.Yang K; Xing B, Chem Rev 2010, 110, 5989. [DOI] [PubMed] [Google Scholar]
- 67.Zou Y; Wang X; Khan A, Environ. Sci. Technol 2016, 50, 7290. [DOI] [PubMed] [Google Scholar]
- 68.Thomas AG; Syres KL, Chem Soc Rev 2012, 43, 4207. [DOI] [PubMed] [Google Scholar]
- 69.De LTR; Hawthorne J; Deng Y; Xing B; Cai W; Newman LA; Wang Q; Ma X; Hamdi H; White JC, Environ. Sci. Technol 2013, 47, 12539. [DOI] [PubMed] [Google Scholar]
- 70.Fan W; Peng R; Li X; Ren J; Liu T; Wang X, Water Res 2016, 105, 129. [DOI] [PubMed] [Google Scholar]
- 71.He X; Aker WG; Fu PP; Hwang HM, Environ Sci-Nano 2015, 2, 564 [Google Scholar]
- 72.Chamorro S; Gutierrez L; Vaquero MP; Verdoy D; Salas G; Luengo Y; Brenes A; Jose Teran F, Nanotechnology 2015, 26, 205101. [DOI] [PubMed] [Google Scholar]
- 73.Fennell TR; Mortensen NP; Black SR; Snyder RW; Levine KE; Poitras E; Harrington JM; Wingard CJ; Holland NA; Pathmasiri W; Sumner SC, J Appl Toxicol 2017, 37, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vidanapathirana AK; Thompson LC; Herco M; Odom J; Sumner SJ; Fennell TR; Brown JM; Wingard CJ, Reprod Toxicol 2018, 75, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song M; Wang F; Zeng L; Yin J; Wang H; Jiang G, Environ. Sci. Technol 2014, 48, 13978. [DOI] [PubMed] [Google Scholar]
- 76.Ma J; Li R; Liu Y; Qu G; Liu J; Guo W; Song H; Li X; Liu Y; Xia T; Yan B; Liu S, Small 2017, 1603830. [DOI] [PubMed] [Google Scholar]
- 77.Holland NA; Thompson LC; Vidanapathirana AK; Urankar RN; Lust RM; Fennell TR; Wingard CJ, Part Fibre Toxicol 2016, 13, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liou SH; Tsou TC; Wang SL; Li LA; Chiang HC; Li WF; Lin PP; Lai CH; Lee HL; Lin MH; Hsu JH; Chen CR; Shih TS; Liao HY; Chung YT, J Nanopart Res 2012, 14, 878 [Google Scholar]
- 79.Schubauer-Berigan MK; Dahm MM; Erdely A; Beard JD; Eileen Birch M; Evans DE; Fernback JE; Mercer RR; Bertke SJ; Eye T; de Perio MA, Part Fibre Toxicol 2018, 15, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y; Xu M; Zhang J; Ma J; Gao M; Zhang Z; Xu Y; Liu S, Adv Mater 2017, 29, 1604580. [DOI] [PubMed] [Google Scholar]
- 81.Gao M; Zhao B; Chen M; Liu Y; Xu M; Wang Z; Liu S; Zhang C, Biomaterials 2017, 130, 14. [DOI] [PubMed] [Google Scholar]
- 82.OECD, Series on the Safety of Manufactured Nanomaterials. 2020
- 83.Cao Z; Tsai SN; Zuo YY, Anal Chem 2019, 91, 12819. [DOI] [PubMed] [Google Scholar]
- 84.Chen S; Xiong C; Liu H; Wan Q; Hou J; He Q; Badu-Tawiah A; Nie Z, Nat Nanotechnol 2015, 10, 176. [DOI] [PubMed] [Google Scholar]
- 85.Holder AL; Goth-Goldstein R; Lucas D; Koshland CP, Chem Res Toxicol 2012, 25, 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia T; Hamilton RF; Bonner JC; Crandall ED; Elder A; Fazlollahi F; Girtsman TA; Kim K; Mitra S; Ntim SA; Orr G; Tagmount M; Taylor AJ; Telesca D; Tolic A; Vulpe CD; Walker AJ; Wang X; Witzmann FA; Wu N; Xie Y; Zink JI; Nel A; Holian A, Environ Health Perspect 2013, 121, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mirshafiee V; Jiang W; Sun B; Wang X; Xia T, Mol Ther 2017, 25, 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Q; Wang X; Xia T, Analytical and bioanalytical chemistry 2018, 410, 6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu R; France B; George S; Rallo R; Zhang H; Xia T; Nel AE; Bradley K; Cohen Y, Analyst 2014, 139, 943. [DOI] [PubMed] [Google Scholar]
- 90.Liu R; Liu HH; Ji Z; Chang CH; Xia T; Nel AE; Cohen Y, ACS Nano 2015, 9, 9303. [DOI] [PubMed] [Google Scholar]
- 91.Liu R; Zhang HY; Ji ZX; Rallo R; Xia T; Chang CH; Nel A; Cohen Y, Nanoscale 2013, 5, 5644. [DOI] [PubMed] [Google Scholar]
- 92.Gajewicz A, Nanoscale 2017, 9, 8435. [DOI] [PubMed] [Google Scholar]
- 93.Oh E LR, Nel A, Gemill KB, Bilal M, Cohen Y, Medintz IL, Nat Nanotechnol 2016, 11, 479. [DOI] [PubMed] [Google Scholar]
- 94.Tu Y; Lv M; Xiu P; Huynh T; Zhang M; Castelli M; Liu Z; Huang Q; Fan C; Fang H; Zhou R, Nat Nanotechnol 2013, 8, 594. [DOI] [PubMed] [Google Scholar]
- 95.Xu Y; Li S; Luo Z; Ren H; Zhang X; Huang F; Zuo YY; Yue T, Langmuir 2018, 34, 9054. [DOI] [PubMed] [Google Scholar]
- 96.Xu Y; Luo Z; Li S; Li W; Zhang X; Zuo YY; Huang F; Yue T, Nanoscale 2017, 9, 10193. [DOI] [PubMed] [Google Scholar]
- 97.George S; Pokhrel S; Xia T; Gilbert B; Ji Z; Schowalter M; Rosenauer A; Damoiseaux R; Bradley KA; Madler L; Nel AE, ACS Nano 2010, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X; Chang CH; Jiang J; Liu Q; Liao YP; Lu J; Li L; Liu X; Kim J; Ahmed A; Nel AE; Xia T, Small 2019, 15, e1901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun B; Ji Z; Liao YP; Chang CH; Wang X; Ku J; Xue C; Mirshafiee V; Xia T, ACS Appl Mater Inter 2017, 9, 21697. [DOI] [PubMed] [Google Scholar]
- 100.Sun B; Ji Z; Liao YP; Wang M; Wang X; Dong J; Chang CH; Li R; Zhang H; Nel AE; Xia T, ACS Nano 2013, 7, 10834. [DOI] [PMC free article] [PubMed] [Google Scholar]


