Obesity is one of the strongest predictors of hypertension, but how obesity increases blood pressure is currently unknown. Obesity affects the metabolism and function of kidney, liver, adipose, heart, brain and blood vessels and a clearly defined mechanism would aid in the development of better anti-hypertensives for obese subjects.
In this issue of Circulation, Ottolini and colleagues1 report on the ability of obesity in both mice and humans to compromise the function of Transient Receptor Potential cation channel subfamily V member 4 (or TRPV4 and V the vanilloid subfamily of channels). TRPV4 is a calcium permeable, non-selective cation channel ubiquitously expressed in many cell types2. TRPV4 has a range of cell-specific functions, but in endothelial cells (EC) it is important for transducing signals from agonists and shear stress to promote vasodilation3. TRPV4 is part of a signaling complex that includes the scaffolding molecule, AKAP150 which coordinates the binding of kinases (PKC and PKA) and TRPV4 to regulate channel activity4.
ECs regulate blood vessel tone by buffering vasoconstrictor forces and by promoting vasodilation. This is achieved via two major calcium-dependent pathways, the release of nitric oxide (NO) and endothelium-derived hyperpolarization (EDH), which are both regulated by TRPV4 activity. Balance between the respective contributions of NO and EDH pathways varies along the vascular tree with vasodilator tone in the thoracic aorta mediated exclusively by NO and in smaller resistance vessels it is mediated by a combination of NO and EDH. Myoendothelial junctions or projections (MEJs/MEPs) are specialized microdomains in resistance arteries where ECs penetrate the internal elastic lamina to make contact with underlying VSMCs. MEJs are thought to transmit EDH to the underlying VSMC to reduce vascular tone and thus reduce blood pressure.
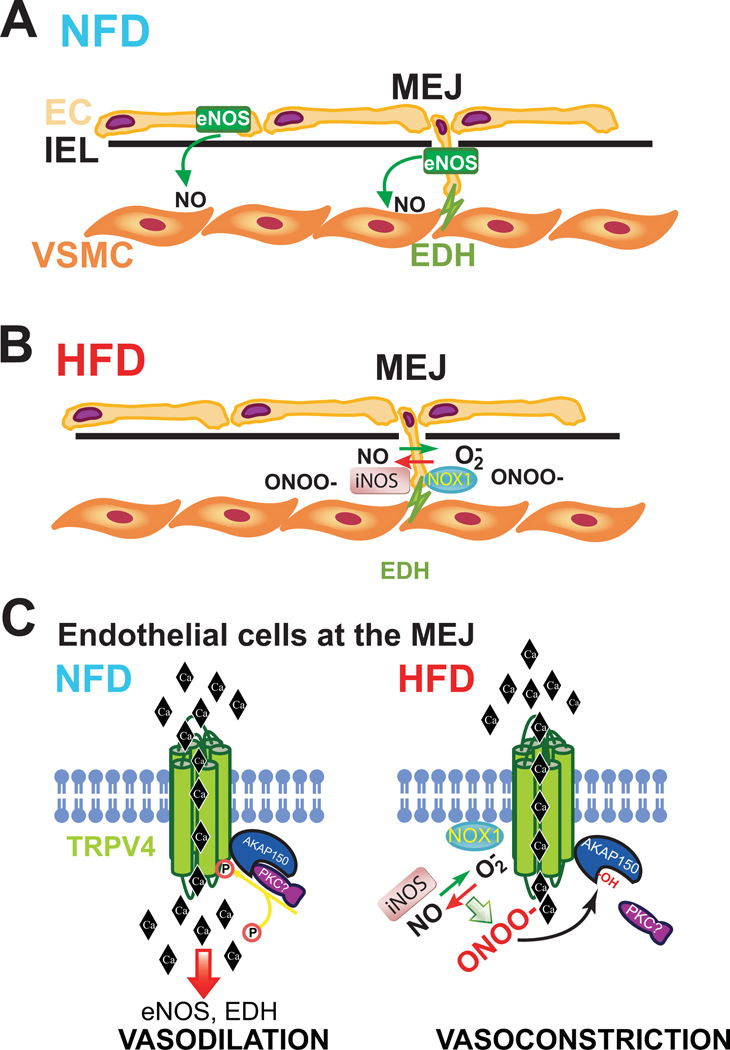
Ottolini and colleagues1 find that TRPV4 and AKAP150 are not uniformly expressed in all EC, but are instead concentrated in MEJs. Using novel EC-specific TRPV4 and AKAP150 knockout mice, they show impaired relaxation of 3rd order mesenteric resistance arteries and increased blood pressure at baseline. Using a high fat diet to induce obesity in WT mice, the authors find reduced TRPV4 channel activity, reduced carbachol- and TRPV4 agonist- induced vasodilation, and increased blood pressure despite normal expression of TRPV4 and AKAP150. Comparable reductions in endothelium-dependent vasodilation, EC TRPV4 channel activity and calcium flux were also seen in resistance blood vessels from obese humans. Delving deeper into the mechanisms, the authors find that inhibition of nitric oxide synthases (NOS) reduces vasodilation to a TRPV4 agonist in mice fed a normal diet. In contrast, in obese mice on a high fat diet, inhibition of NOS paradoxically enhances vasodilation. Blood vessels from obese mice have increased nitrosative stress and antioxidants targeting peroxynitrite (ONOO-), but not other oxidants (H2O2, HOCl), improve channel function and vasodilation. This data suggests that increased ONOO- is responsible for the deficits in TRPV4 function and the authors identify the sources of ROS and NO, as Nox1 and iNOS respectively, which are also found localized to MEJs. Inhibition of Nox1 and iNOS improved TRPV4 channel function and endothelium-dependent vasodilation in HFD mice and surprisingly, inhibition of iNOS and NOS decreased blood pressure. Exogenous ONOO- directly impaired TRPV4 function and evidence of cysteine oxidation to sulfenic acid was observed specifically in MEJs of HFD mice. The authors show that changes in TRPV4 activity were not due to loss of channel number, and reductionist experiments in HEK cells demonstrate that it is the impaired ability of AKAP150 to activate TRPV4 and more specifically a role for Cys36 oxidation within the PKC binding site of AKAP150. Using proximity ligation strategies to localize interacting proteins, obesity did not alter TRPV4 and AKAP150 binding, but instead disrupted binding of PKC to AKAP150. A summary of these mechanisms is shown in Figure 1A–C.
Figure 1. Schematic of mechanisms underlying HFD induced endothelial function.
Pathways of vasodilation originating in endothelial cell (EC) and transmitted to vascular smooth muscle cells (VSMC) via diffusion of nitric oxide (NO) from endothelial nitric oxide synthase (eNOS) in luminal EC of conduit arteries and via NO and endothelium-derived hyperpolarization (EDH) at myoendothelial junctions (MEJ) in resistance blood vessels. A. Pathways operational in the blood vessels of mice on a normal fat diet. B. Mice on a high fat diet have compromised NO and EDH via increased expression of iNOS and Nox1 and formation of ONOO-. C. In EC at the MEJ, a HFD induces iNOS and Nox1 expression, increase ONOO- and oxidation (-OH) of a critical cysteine residue within AKAP150 which prevents PKC binding and thus phosphorylation (P) of TRPV4. Dephosphorylated TRPV4 reduces calcium entry in EC, promoting vasoconstriction and hypertension.
This is an elegant, technically impressive and comprehensive study. However, its major findings interweave with an established body of literature that invites important questions. The first of these is the model of obesity in mice. While a 60% fat diet is effective at rapidly inducing weight gain, this diet does not mimic that of humans (~35% fat) nor the composition of dietary fat5 and the amount of sugar. In the literature there are reports of varying degrees of hypertension in obese HFD mice, with some showing a range of increases6 and others no change, suggesting that additional variables such as the time on diet, changes in the microbiome, genetic drift of mice, sex etc. may be required for BP changes. Indeed, a very recent study by Greenstein and colleagues using an identical HFD model (Male C57BL6/J, 60% fat Research Diets Inc. albeit longer duration , 21 versus 14 weeks) and an identical approach, found no change in endothelial TRPV4 activity at MEJs and no differences in carbachol or TRPV4 agonist-induced vasodilation of 3rd order mesenteric vessels. Instead they observed enhanced vascular tone secondary to impaired activation of BK potassium channels in VSMCs7. These differences in very closely related studies may reflect the diverse nature of obesity in both mice and men. Alternatively, mechanisms in addition to obesity may be responsible for the differences in vascular dysfunction. Confirmation of some of the key mechanisms of endothelial dysfunction in obese human resistance vessels was an important strength of the study by Ottolini and colleagues1.
A similar mechanism, disruption of AKAP150: TRPV4 interaction, has been shown to impair endothelial function in a model of AngiotensinII-induced hypertension8. Although Nox1 was not investigated in that study, it has been shown by others to mediate AngiotensinII-mediated increases in blood pressure and Nox1-derived O2- promoted endothelial dysfunction by scavenging NO9. In contrast, Ottolini and colleagues1 propose that ONOO-, formed from the interaction of iNOS-derived NO and Nox1-derived O2-, mediates dysfunction of ECs at MEJs via inactivation of TRPV4 channels. It remains unclear why formation of ONOO- would lead to endothelial dysfunction in both settings via different mechanisms, but this may reflect important differences in the macro- versus micro-circulation i.e. MEJs. The biochemistry of ONOO- formation at MEJs is also in need of greater scrutiny. The dogma of NOS biology is that ECs express eNOS, but not iNOS, and that iNOS is almost exclusively expressed by immune cells. Moreover, the tightly coupled calcium-dependent activity of eNOS produces discrete amounts of NO that can efficiently signal between EC and SMC. In contrast, iNOS is a constitutively active, more loosely coupled, large volume producer of NO and ONOO- and in macrophages this is important for host-defense. Whether Nox1 can stimulate additional ONOO- from iNOS remains to be determined. The vascular actions of ONOO-, at least acutely, are also debatable. Numerous studies have shown that the ONOO- donor, SIN-1 relaxes blood vessels and lowers blood pressure and iNOS has a well-established role in the robust blood pressure drop in sepsis. The ability of iNOS (1400W) and NOS (L-NNA) inhibitors to decrease blood pressure in this study is remarkable and not seen in WT mice or other models of obesity10. Given the ability of TRPV4 to stimulate eNOS-derived NO, it is paradoxical that NOS inhibitors administered to TRPV4 knockout mice elicit greater increases in blood pressure11. This data suggests that TRPV4 plays more complex roles in the regulation of nitric oxide synthases and blood pressure. Uric acid, which scavenges ONOO-, and TEMPOL, a SOD mimetic, both reversed TRPV4 dysfunction in HFD mice. Whether uric acid, at concentrations in normal blood (~ 200μM), can scavenge ONOO- and influence obesity-dependent hypertension is not known. Although TEMPOL is effective at reducing blood pressure in hypertensive mice, antioxidants have not been an effective approach to lowering blood pressure in humans. Finally, it should be noted that systemic delivery of NOS inhibitors impacts more than the vasculature. Renal NOS activity is elevated by increased perfusion in obese animals as they try to off-load excess volume. Blockade of NOS in such animals generally increases pressure, even if NOS activity in the vasculature is depressed12.
A key component of the proposed mechanism is the importance of PKC to the activation of TRPV4 channels via AKAP150. Numerous studies have shown that PKC is deleterious to endothelial function in the setting of obesity and diabetes. PKC can activate Nox enzymes including Nox1 as well as phosphorylate eNOS at Thr495 to reduce NO release and increase O2-. Identification of the isoform mediating TRPV4 phosphorylation and activation in endothelial cells would be an important way to distinguish between these mechanisms.
In summary, the study by Ottolini and colleagues1 improves our understanding of the mechanistic links between obesity, endothelial and blood vessel dysfunction and the development of hypertension. Compartmentalization of endothelial TRPV4 channels and AKAP150 along with Nox1 and iNOS in MEJ is a novel mechanism that impairs endothelial function in obesity and elevates blood pressure. However, there are a number of important unresolved issues. Animal models of obesity are imperfect and like in humans, obesity phenotypes in mice can be diverse and involve distinct mechanisms of vascular and endothelial dysfunction. The role of TRPV4 channels is clearly more complex as TRPV4 and AKAP150 have important and, at times contrasting, cell specific roles in regulating vascular function. Endothelial knockout of both TRPV4 and AKAP150 result in hypertension whereas global knockouts result in no change in or reduced blood pressure, respectively13, 14 and pharmacological inhibitors of TRPV4 can suppress EC inflammation and improve vasodilation in inflammatory settings15. The validity of TRPV4 as a potential anti-hypertensive target merits continued investigation, but how best to promote the antihypertensive actions of TRPV4? Strategies to preserve the phosphorylation status of TRPV4 in EC may be useful in guiding a therapeutic strategy to improve endothelial function and hypertension in obesity.
Footnotes
Conflict of Interest Disclosures
None
References cited
- 1.Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE and Sonkusare SK. Local Peroxynitrite Impairs Endothelial TRPV4 Channels and Elevates Blood Pressure in Obesity. Circulation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JP, Cibelli M, Urban L, Nilius B, McGeown JG and Nagy I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev. 2016;96:911–973. [DOI] [PubMed] [Google Scholar]
- 3.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC and Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan HC, Zhang X and McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes (Lond). 2019;43:1491–1492. [DOI] [PubMed] [Google Scholar]
- 6.Williams TD, Chambers JB, Roberts LM, Henderson RP and Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–778. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein AS, Kadir S, Csato V, Sugden SA, Baylie RA, Eisner DA and Nelson MT. Disruption of Pressure-Induced Ca(2+) Spark Vasoregulation of Resistance Arteries, Rather Than Endothelial Dysfunction, Underlies Obesity-Related Hypertension. Hypertension. 2020;75:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF and Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal. 2014;7:ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyazawa T, Zeng F, Wang S, Fan X, Cheng H, Yang H, Bian A, Fogo AB and Harris RC. Low nitric oxide bioavailability upregulates renal heparin binding EGF-like growth factor expression. Kidney Int. 2013;84:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W and Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polichnowski AJ, Licea-Vargas H, Picken M, Long J, Bisla R, Williamson GA, Bidani AK and Griffin KA. Glomerulosclerosis in the diet-induced obesity model correlates with sensitivity to nitric oxide inhibition but not glomerular hyperfiltration or hypertrophy. Am J Physiol Renal Physiol. 2015;309:F791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL and Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–1074. [DOI] [PubMed] [Google Scholar]
- 14.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS and Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. [DOI] [PubMed] [Google Scholar]
- 15.Dalsgaard T, Sonkusare SK, Teuscher C, Poynter ME and Nelson MT. Pharmacological inhibitors of TRPV4 channels reduce cytokine production, restore endothelial function and increase survival in septic mice. Sci Rep. 2016;6:33841. [DOI] [PMC free article] [PubMed] [Google Scholar]