Abstract
Osteogenesis imperfecta (OI) is an inherited skeletal dysplasia characterized by bone fragility and skeletal deformities. While the majority of cases are associated with pathogenic variants in COL1A1 and COL1A2, the genes encoding type I collagen, up to 25% of cases are associated with other genes that function within the collagen biosynthesis pathway or are involved in osteoblast differentiation and bone mineralization. Clinically, OI is heterogeneous in features and variable in severity. In addition to the skeletal findings, it can affect multiple systems including dental and craniofacial abnormalities, muscle weakness, hearing loss, respiratory and cardiovascular complications. A multi-disciplinary approach to care is recommended to address not only the fractures, reduced mobility, growth and bone pain but also other extra-skeletal manifestations. While bisphosphonates remain the mainstay of treatment in OI, new strategies are being explored, such as sclerostin inhibitory antibodies and TGF beta inhibition, to address not only the low bone mineral density but also the inherent bone fragility. Studies in animal models have expanded the understanding of pathomechanisms of OI and, along with ongoing clinical trials, will allow to develop better therapeutic approaches for these patients.
1. Introduction
Osteogenesis imperfecta (OI or brittle bone disease) is a genetic disease characterized by bone fragility and increased risk of fractures. OI is most often caused by alterations in type I collagen (1). It is both a genetically and clinically heterogeneous disease with an estimated incidence of about 1 in 10,000 to 1 in 20,000 (1). Due to the systemic nature of the disease, individuals affected with OI can develop a number of additional symptoms and complications and thus require a multidisciplinary team of physicians for their care. In this short review, we briefly describe the genetic and clinical features of OI and then highlight some new advances in less studied aspects of the disease that significantly impact quality of life. We conclude with an overview of current treatment modalities and an update on ongoing clinical trials.
2. Inheritance and clinical aspects of OI
2.1. Types of OI, modes of inheritance, and effects on osteoblast function
Clinical forms of OI
The revised Nosology and Classification of Genetic Skeletal Disorders identifies 5 clinical forms of OI: non-deforming with persistently blue sclera (OI type I), perinatal lethal (OI type II), progressively deforming (OI type III), moderate (OI type IV), and with calcification of the interosseous membranes and/or hypertrophic callus (OI type V) (2). OI type I has the mildest phenotype; whereas individuals with OI type III are most severely affected (among patients surviving infancy), with multiple fractures, scoliosis, short stature, and restricted mobility.
Modes of inheritance and genetic features
OI can be inherited as a dominant, recessive, or X-linked disorder (Table 1) (1, 3). Most often, it is a dominant disease caused by pathogenic variants in either COL1A1 or COL1A2 (encoding components of type I collagen). Null alleles (i.e. deletions or splice variants that cause a shift in the reading frame, or truncating variants) in COL1A1 result in haploinsufficiency that is typically associated with mild OI (type I). Whereas missense (frequently glycine substitutions in a Gly-X-Y repeat) or splice mutations (that do not disrupt the reading frame) in COL1A1 or COL1A2 tend to give rise to either lethal, severe, or moderate OI (types II, III, or IV, respectively). A dominant form of the disease, although rarer, is caused by a recurrent pathogenic variant in the 5’untranslated region of IFITM5 (encoding the interferon-induced transmembrane protein 5, also known as BRIL) and classified as OI type V (4).
Table 1.
Current genetic classification of OI according to OMIM (Online Mendelian Inheritance in Man).
| OI TYPE | Gene | Chromosomal Location | OMIM # | Inheritance | Proposed Pathway or Mechanism |
|---|---|---|---|---|---|
| I | COL1A1, COL1A2 | 17q21.33 7q21.3 |
166200 | AD | Reduced amount of type I collagen |
| II | COL1A1, COL1A2 | 17q21.33 7q21.3 |
166210 | AD | Folding, secretion and mineralization of type I collagen |
| III | COL1A1, COL1A2 | 17q21.33 7q21.3 |
259420 | AD | Folding, secretion and mineralization of type I collagen |
| IV | COL1A1, COL1A2 | 17q21.33 7q21.3 |
166220 | AD | Folding, secretion and mineralization of type I collagen |
| V | IFITM5 | 11p15.5 | 610967 | AD | ECM mineralization |
| VI | SERPINF1 | 17p13.3 | 613982 | AR | ECM mineralization |
| VII | CRTAP | 3p22.3 | 610682 | AR | PTM and folding of fibrillar collagen |
| VIII | P3H1 | 1p34.2 | 610915 | AR | PTM and folding of fibrillar collagen |
| IX | PPIB | 15q22.31 | 259440 | AR | PTM and folding of fibrillar collagen |
| X | SERPINH1 | 11q13.5 | 613848 | AR | Folding and intracellular trafficking of collagen |
| XI | FKBP10 | 17q21.2 | 610968 | AR | Folding and intracellular trafficking of collagen |
| XII | SP7 | 12q13.13 | 613849 | AR | Abnormal osteoblast differentiation |
| XIII | BMP1 | 8p21.3 | 614856 | AR | Processing of collagen |
| XIV | TMEM38B | 9q31.2 | 615066 | AR | Ca2+ homeostasis in the ER |
| XV | WNT1 | 12q13.12 | 615220 | AR | WNT anabolic signaling |
| XVI | CREB3L1 | 11p11.2 | 616229 | AR | Protein quality control and ER stress response |
| XVII | SPARC | 5q33.1 | 616507 | AR | ECM mineralization |
| XVIII | TENT5A | 6q14.1 | 617952 | AR | Unknown |
| XIX | MBTPS2 | Xp22.12 | 301014 | XLR | Protein quality control and ER stress response |
| XX | MESD | 15q25.1 | 618644 | AR | WNT anabolic signaling |
AD = autosomal dominant; AR = autosomal recessive; XLR = X-linked recessive; ECM = extracellular matrix; PTM = post-translational modification.
In the last 15 years, pathogenic variants in several additional genes have been associated with recessive and X-linked forms of the disease (Table 1). Studying these genes has produced a wealth of information and novel insights into the process of bone formation and mineralization. Many of these genes encode proteins that play an important role in the folding and intracellular or extracellular post-translational modifications of type I collagen (e.g., CRTAP, P3H1, PPIB, and BMP1), or its intracellular trafficking (e.g., FKBP10 and SERPINH1), as well as in the quality control of protein synthesis and the endoplasmic reticulum (ER) stress response (e.g., CREB3L1 and MBTPS2) (1). Some genes encode proteins that are directly secreted by osteoblasts and can bind to collagen in the matrix and affect its mineralization during bone formation (e.g., SERPINF1 and SPARC) (1). Some play an important role in the bone anabolic function of the WNT canonical signaling pathway (e.g., LRP5, WNT1, and MESD) and, in addition to OI, can be associated with generalized osteoporosis (WNT1) or similar conditions (LRP5 in osteoporosis pseudoglioma syndrome) (1, 5). Other genes instead encode proteins with an intracellular or nuclear localization that affect osteoblast function in a way that is not yet fully understood (e.g., SP7 and TENT5A) (1, 6).
It is estimated that alterations in genes other than those encoding type I collagen are responsible for about 15%–25% of OI cases, with pathogenic alleles showing different geographic distribution (7, 8). The growing list of genes associated with rarer forms of OI gave rise to a classification system that determines OI subtypes based on the causative gene, and it is now up to OI type XX (Table 1) according to the Online Mendelian Inheritance in Man database (https://www.omim.org/). While such a genetic classification could become important, as interventions might be effective only in some OI types, the phenotypic classification has proven more useful in the clinical setting (1, 9).
Effect of OI on osteoblasts
Irrespective of the genetic cause of OI, bone-forming osteoblasts appear to be primarily affected. For instance, in cases of type I collagen mutations or mutations affecting proteins involved in its biosynthetic pathway, osteoblasts often show enlarged ER cisternae and ER stress, reduced secretion of type I collagen into the matrix, increased matrix mineralization, and defective matrix-to-cell signaling (1). Importantly, the disease in these cases is always systemic, because fibroblasts in other organs and body systems also synthesize abnormal type I collagen (e.g., in the eyes, lungs, and heart valves) (10). Mutations affecting genes unrelated to type I collagen synthesis appear to have deleterious effects on the differentiation and/or function of osteoblasts, which ultimately result in significantly decreased bone formation and thus brittle bones (1). Based on their typical normal lifecycle, select osteoblasts that do not die through apoptosis or do not become quiescent bone-lining cells will terminally differentiate into long-lived osteocytes and become embedded into mineralized bone. In OI, there is a higher density of osteocytes (11). While the mechanism of increased osteocyte density is not fully understood, it is consistent with findings in mouse models of increased TGFβ signaling which also exhibit high bone turnover and low bone mass (12). Interestingly, in at least two mouse models of OI, oim/oim and CrtapKO (Table 2), the osteocyte transcriptome is greatly dysregulated, including changes in both the WNT and TGFβ signaling (13). Studies in mouse models suggest that osteocytes play an important role in WNT signaling during bone development, and conditional deletion of Wnt1 in late osteoblasts/osteocytes in the Dmp1-Cre Wnt1fl/fl mice resulted in low bone mass phenotype and spontaneous fractures (14). These data suggest that osteocytes may contribute to the disease process.
Table 2.
OI mouse models discussed in this review with their genetic characteristics and some of the novel findings.
| OI Mouse Model | Affected Gene | Type of Mutation | Clinical OI Type (I-V) Modeled | Novel Findings discussed in this review |
|---|---|---|---|---|
| CrtapKO | Crtap | Loss of function - recessive | moderate to severe (III/IV) |
|
| Col1a2G610C/+ ‘Amish’ Col1a2tm1Mcbr | Col1a2 | Glycine substitution - dominant | Mild to moderate (I/IV) |
|
| oim/oim | Col1a2 | C-propeptide frameshift mutation - semi-dominant* | Severe (III) |
|
| Aga2 | Col1a1 | C-propeptide frameshift mutation - dominant | Severe (II/III) |
|
| Col1a1Jrt/+ | Col1a1 | Splice donor mutation causing exon 9 skipping - dominant | Severe (III) |
|
oim/+ mice also have low bone mass but their phenotype is mild.
2.2. Clinical manifestations of OI
Bone fragility and osteopenia in OI lead to recurrent fractures, fractures in atypical locations, and low-trauma fractures (including in utero fractures in severe forms of OI) (1, 9). At least in the more common and milder type of OI type I, fracture incidence is highest in the pediatric population and decreases with age (9). Deformities of the spine, long bones, and rib cage reduce mobility and cause respiratory complications (1, 9). Short stature is a common feature, especially in severe forms of OI, and to a lesser extent in the milder OI type I (9, 15). Anomalies of the cranio-cervical junction have been reported in up to 37% of patients and can cause serious complications (16, 17). As a consequence of the structural and functional skeletal abnormalities, bone pain is common. Evidence for this chronic pain has also been reported in the Col1a1JRT/+dominant mouse model of moderate OI (Table 2) (18).
OI is a systemic connective tissue disorder, and extra-skeletal manifestations can occur in tissues that express type I collagen, or they may develop secondarily to the skeletal alterations. These include blue-gray sclera, dental abnormalities, joint hypermobility, hearing loss, muscle weakness, cardiovascular complications, and pulmonary or respiratory problems (1, 9).
Craniofacial and dental issues
The abnormal development of craniofacial structures has been documented in all types of OI (17). A recent study using 3-dimensional imaging via cone beam computed tomography in patients with moderate to severe OI revealed hypoplastic lower face with maxillary retrusion and mandibular prognathism (17). Other findings included deviation of the nasal septum and alteration of the cranial base angle. These features may affect the airways, contributing to complications such as sleep apnea, and should be considered in the planning of orthodontic treatment (17). Dental abnormalities such as dentinogenesis imperfecta (brittle or discolored teeth), missing teeth, ectopic teeth, and dental malocclusion are common in OI and are more prevalent in the severe types of OI (19, 20). These abnormalities are associated with functional limitations and impact quality of life (20).
Craniofacial and dental defects are also described in mouse models of OI. Eimar et al. (21) studied Col1a1Jrt/+ mice and showed smaller head, class III malocclusion, shorter anterior cranial base with mandibular deviation and smaller masticatory region compared to control mice. In addition, abnormalities of dentin matrix and its mineralization and changes in the periodontal compartment were present. A more recent study of Crtap knockout (CrtapKO) mice, a model of OI type VII, demonstrated a brachycephalic skull shape with midface hypoplasia and a class III dental malocclusion. Interestingly, an in depth analysis of their teeth revealed changes in dentin volume, changes in cellular and acellular cementum, decreased alveolar bone volume and mineral density, bone–tooth ankylosis, and increased periodontal ligament space with ectopic calcifications of the periodontal ligament (22). The findings on periodontal tissue add significantly to the classic features of dentinogenesis imperfecta. While these craniofacial and dental phenotypes will need to be studied in other mouse models of OI, they point to the importance of early oral health examinations and care for patients with OI.
Hearing loss
Hearing loss is a recognized extra-skeletal manifestation of OI, and the mechanism is thought to involve abnormal mineralization and otosclerosis-like process in the inner ear (23). This has been primarily described in individuals with dominant COL1A1/COL1A2-related OI (23), and has also been reported in some recessive forms of OI (24, 25) and in OI mouse models (26, 27). Hearing loss may be conductive, sensorineural, or mixed, as well as unilateral or bilateral. Conductive hearing loss is most frequently found in the pediatric and adolescent populations, while mixed or sensorineural hearing loss are found predominantly in adults (23, 24, 28). When present, hearing impairment is in the milder range and of adult onset in most patients, with no clear relation to OI type or severity (23); however, one study identified a higher risk of early-onset hearing loss in moderate to severe forms of OI (24). The reported prevalence varies across studies; a recent study with a North American cohort of 312 individuals reported hearing impairment in 28% of OI cases (24). A similar prevalence was reported for a cohort in Brazil (28).
Muscle weakness
Muscle weakness, reduced muscle mass, and impaired muscle function have been described in mouse models of OI and in patients with both dominant and recessive forms of OI (29–31). This is not surprising, as type I collagen is a component of the connective tissue surrounding muscle fibers, as well as a component of tendons and ligaments. Muscle abnormalities may also result from the limited mobility of patients (29). Lastly, alteration in signal transduction, mechanotransduction, and paracrine interactions between the adjacent bone and muscle tissues have been proposed to contribute to the phenotype (30).
In the Col1a2G610C/+ mouse model of mild to moderate OI (Table 2), muscle function and locomotor activity were comparable to that of wild type littermates (32). In contrast, the oim/oim mouse model of more severe OI exhibited significantly reduced muscle mass and decreased muscle strength (33). This suggests a correlation between disease severity and muscle function in OI or perhaps a potential effect of α1(I) homotrimers that are produced in oim/oim mice on muscle formation and function (29, 30).
The latest study on muscle function in oim/oim mice showed mitochondrial dysfunction in the gastrocnemius muscle, with decreased mitochondrial citrate synthase activity and respiration rates (34). While the connection between type I collagen alterations and mitochondrial function in muscle is not yet clear, this is an interesting and novel observation, and further studies are needed to validate this finding. In humans, the muscle phenotype has been primarily studied in OI types I and IV, where decreased muscle size (OI type I) and deficits in muscle force and function (OI types I and IV) were described (29, 30). Evaluation is technically challenging in patients with moderate to severe OI, but the abnormal muscle function correlated with the severity of the skeletal phenotype (29).
Pulmonary function
Decreased respiratory function is another extra-skeletal manifestation of OI and is a leading cause of mortality, although it is poorly understood (35). The long-standing, conventional view is that respiratory function is restricted because of skeletal abnormalities that affect the chest wall, including rib and vertebral fractures, kyphoscoliosis, and short stature causing diaphragm restriction (36). These skeletal abnormalities may ultimately reduce alveolar ventilation because of lung compression, ineffective cough, poor secretion clearance, airway diseases such as asthma and sleep apnea, and cause low blood oxygen (36).
However, a small retrospective clinical study of individuals with OI (including types I, III, IV, VIII, and IX) showed that while pulmonary comorbidities were present in 40% of cases, there was no correlation between elevated FEV1/FVC ratio (utilized as a measure of restrictive pulmonary dysfunction) and the degree of scoliosis (37). This is further supported by the fact that patients with milder forms of OI without severe malformations of the chest cavity are still at higher risk of death due to respiratory complications (35, 38, 39). In multiple mouse models of OI primary pathological changes have been identified in the lungs (40–42). For instance, in the Aga2 mouse model of OI (Table 2) severe cardio-pulmonary complications could lead to perinatal lethality and an in vitro analysis showed several gene expression changes in both cardiac and pulmonary primary fibroblasts (40). In the lung cells these changes were consistent with increased inflammation, hypoxia and possibly hypertension (40). Baglole et al. (41) have shown pulmonary airspace enlargement and alterations in the diaphragm of Col1a1Jrt/+ mice. Recent work by Dimori et al. (10) in the CrtapKO mouse model of recessive OI showed that type I collagen in the lung had similar defects as in the bone and skin. Moreover, plethysmography and respiratory mechanics measurements in CrtapKO mice demonstrated intrinsic changes suggestive of tissue-level alterations in the resistive and elastic properties of the lungs, accompanied by significant alterations in pressure–volume (PV) curves. These results suggest altered mechanical properties consistent with a less-compliant respiratory system, increased dissipative properties, and increased tissue elasticity, while the PV data indicate altered pressure–volume relationships (10). These data support the presence of intrinsic defects in the respiratory system of both dominant and recessive mouse models of OI and hopefully will ultimately translate into improved health care for OI patients.
Short stature
Severe short stature is also a feature of OI and especially affects patients with OI type III or IV (15, 43, 44). This aspect of the disease is not well-studied, but it negatively impacts the quality of life of these patients.
Longitudinal bone growth is mediated by the proliferation and differentiation of chondrocytes within the epiphyseal growth plates. Because chondrocytes are characterized by the expression of fibrillar collagen type II and, upon becoming hypertrophic, of collagen type X, it is not clear how the alterations in type I collagen that cause OI negatively impact the biology and activity of these cells. However, Scheiber et al. (45) showed recently that murine hypertrophic chondrocytes also express type I collagen, and in the Col1a2G610C/+ mouse model of OI, these cells too (in addition to osteoblasts) had an abnormally large ER, which likely caused ER stress and a chondrocyte maturation defect. They also observed elongated growth plates, which translated into significantly shorter long bones (45). Therefore, at least in this mouse model of OI, dysfunctional hypertrophic chondrocytes contribute to short stature. Further studies are needed to confirm this observation in other mouse models of OI and to dissect the underlying mechanism(s) (e.g., defects in cartilage matrix mineralization, blood vessel invasion, or trans-differentiation of chondrocytes into osteoblasts).
Growth hormone therapy improved linear growth in patients with OI type IV, but did not show similar effect in the more severe OI type III (46), and there is not enough data to support the benefits of this treatment in OI. In children with OI that were given a systemic infusion of allogeneic bone marrow-derived mesenchymal cells, treatment improved linear growth velocity significantly for at least 6 months after infusion (47). Interestingly, miRNA-containing extracellular vesicles derived from mesenchymal stromal cells (MSC) were sufficient to stimulate chondrocyte proliferation and bone growth in the Col1a2G610C/+ mouse model of OI (48). It will be important to confirm the potential therapeutic effects of such MSC-derived extracellular vesicles, as this may represent an entirely novel approach to treating growth deficiency in OI.
3. Treatment of OI
The management of OI is primarily supportive and symptomatic and is tailored to the patient based on their age and OI type and severity. Some individuals with mild OI type I may only need to be monitored for complications; whereas, OI type III/IV patients will typically require multidisciplinary management with medications, physical therapy, occupational therapy, orthopaedic interventions, and follow up by other subspecialists. The treatment goals for OI are to improve bone strength, decrease fracture risk, decrease pain, increase mobility and functional independence, and prevent long-term complications. The management of OI has been reviewed extensively elsewhere (9, 49–52), so this review provides a brief update on current treatment modalities, ongoing clinical trials, and future therapeutic approaches (for a summary, see Table 3).
Table 3.
Summary table of current therapeutic approaches for OI.
| Therapeutic agent | Brand name | Mode of administration | Suggested mechanism | Notes |
|---|---|---|---|---|
| Bisphosphonates | Pamidronate, Alendronate, Risdronate, Zoledronic acid | Typically infusion, may be administered orally | Antiresorptive, inhibition of osteoclast activity | Mainstay of therapy in OI |
| Denosumab | Prolia (Amgen Inc., Thousand Oaks, CA, USA) | Subcutaneous injection | Antiresorptive, anti-RANKL antibody, inhibition of osteoclast activity | In clinical trial |
| Teriparatide | Forteo (Eli Lilly & Co., Indianapolis, IN, USA) | Subcutaneous injection | Anabolic, recombinant human parathyroid hormone | Therapy limited to 24 months, not approved in children |
| Sclerostin antibody | Romosozumab (Amgen Inc., Thousand Oaks, CA, USA), Blosozumab (Eli Lilly & Co., Indianapolis, IN, USA), BSP804 (Novartis, Basel, Switzerland) | Subcutaneous injection or infusion | Anabolic, Anti-sclerostin (an inhibitor of bone formation) | In clinical trial |
| TGFβ inhibitory antibody | Fresolimumab (Sanofi Genzyme, Cambridge, MA, USA) | Infusion | Targets excessive TGFβ signaling in bone | In clinical trial |
3.1. Therapeutic agents used in OI
Bisphosphonates
Bisphosphonates, which act by inhibiting osteoclast activity and bone resorption, are the mainstay of pharmacologic treatment in pediatric patients with OI. Bisphosphonates have been shown to consistently improve bone mineral density in patients with OI (9, 49, 53) and, to some extent, reduce fracture incidence (9, 51). During growth, they have a beneficial effect on reshaping vertebrae that have compression fracture deformities (9, 54). Other than the improvement in bone mass and architecture in growing children, the beneficial effects of bisphosphonates have not been conclusive, although a recent study indicated a positive effect on fracture incidence, scoliosis probability, and mobility in preadolescent patients with OI type I (53). Common side effects include acute phase infusion reaction (most typically during the first infusion), and transient hypocalcemia. Importantly, the effect of bisphosphonates has been less robust in OI compared to osteoporosis, especially in the more severe forms of OI, likely because it does not target the inherent defect in bone quality (55). Therefore, other treatment options, as discussed below, are being explored to address disease complications in pediatric and adult patients with moderate to severe types of OI.
Denosumab
Denosumab, an anti-RANKL (receptor activator of nuclear factor kappa-B ligand) antibody that inhibits osteoclast differentiation and function, is approved for treating osteoporosis in adults and is currently being studied for OI. Similar to bisphosphonates, it acts on osteoclasts to suppress bone resorption. Treatment with Denosumab has been shown to improve bone mineral density in patients with OI in a few small-scale studies. Specifically, it was studied in OI type VI which is a recessive form of OI that is poorly responsive to bisphosphonates (56). Its use was associated with significant risk of hypercalcemia and hypercalciuria (9). A clinical trial is currently ongoing to evaluate the safety and efficacy of Denosumab for OI (NCT03638128 and NCT02352753).
Teriparatide
Teriparatide, a PTH analogue (recombinant human parathyroid hormone 1–34) that induces anabolism in bone, significantly increased bone mineral density in adults with OI type I, but it was not as effective in moderate and severe forms of OI (57). Its clinical use is limited to adults, and is restricted to 24 months duration, because of the concern for osteosarcoma (observed in preclinical studies in rats) (9, 51, 52). Towards discontinuation of treatment, consolidation with an anti-resorptive agent should be considered to avoid the risk of accelerated bone loss (58).
Sclerostin inhibitory antibody
Sclerostin inhibitory antibody is an anabolic agent designed to target sclerostin, an inhibitor of bone formation via canonical WNT signaling pathway. Preclinical studies in mouse models of OI showed a positive effect on bone mass and strength (9, 51, 52). In a phase 2 clinical trial, adult OI patients exhibited increased bone formation, decreased bone resorption, and increased bone mineral density after a short-term dose-escalation trial with BPS804 anti-sclerostin antibody (59). The trial has been expanded to further assess the efficacy in a larger cohort of adult patients with OI types I, III, and IV (NCT03118570).
Transforming growth factor beta inhibition
Transforming growth factor beta (TGFβ) inhibition targets the excessive activation of TGFβ signaling that is implicated in regulating bone mass and fragility in OI (9, 49, 51, 52). Preclinical studies showed increased bone mass and bone strength in mouse models of OI treated with TGFβ inhibitory antibody (11). The safety and efficacy of Fresolimumab, a TGFβ inhibitory antibody, is currently being investigated in adult OI patients (NCT03064074).
Progenitor cell therapy
Progenitor cell therapy via transplantation of healthy progenitor stem cells has been proposed to specifically address the inherent bone fragility in OI. The rationale is that engrafted healthy donor cells will differentiate into osteoblasts that produce normal collagen (51). This has been tested in preclinical mouse models (60, 61) and in pediatric patients with severe OI, both prenatally (in utero) and postnatally (62–65). Different approaches taken include transplantation of hematopoietic stem cells, ex-vivo expanded mesenchymal stem cells and amniotic fluid stem cells. To date, cell therapy is promising but remains experimental due to inconsistent results, safety concerns, and ethical considerations.
3.2. Orthopaedic management of OI
Orthopaedic surgeons are often involved in the treatment of individuals with OI, particularly pediatric patients. These patients are medically complex and require a multidisciplinary team approach to optimize their outcomes; clear communication between the orthopaedist, endocrinologist, internist, anesthesiologist, and physical therapist is vital (66).
Fractures
Fracture care is one of the most common indications for orthopaedic consult in patients with OI. While these patients are more prone to fracture than the general population, fracture healing is also affected by the inherent poor bone quality and can be complicated by non-union and refracture (66, 67). Studies in dominant and recessive mouse models of OI show delayed fracture healing, and reduced strength of the healed bone (68, 69). While fractures, particularly in young children, may be treated nonoperatively with immobilization, this can result in muscle weakness, joint stiffness and disuse osteopenia. With this in mind, the surgeon should strive to minimize this time period.
Surgical treatment for fractures is indicated when closed reduction (i.e., straightening the bone) might not be successful (66). General orthopaedic principles include fixation of the entire bone, typically through an intramedullary implant (i.e. rigid or flexible nails). Stabilization with plates and screws should generally be avoided because rigid implants do not stabilize the entire length of bone. As a result, patients are prone to fracture above or below the implant because of stress riser formation (70, 71). When surgery is indicated, potential challenges with anesthesia should be considered, especially in patients with moderate to severe OI (72).
Static implants were initially used to treat fractures in OI. These were metallic rods placed in the intramedullary canal to splint the entire bone and allow a fracture or osteotomy to heal. However, this was problematic in pediatric patients because with growth the bone became longer than the implant, resulting in a bony section unsupported by the implant that predisposed the patient to fracture or deformity. As a result, telescoping implants were developed, and the Fassier–Duval (FD) telescoping rod is the latest iteration of this concept. Spahn et al. compared the survivorship of FD rods and static implants and found that static implants were 13.2 times more likely to fail than FD rods; this resulted in a total surgery rate that was 7.8 times higher for the static implant group than the FD group (71). The FD rod is currently the most commonly used telescoping implant in this demographic (71, 73) (see Figure 1).
Figure 1.
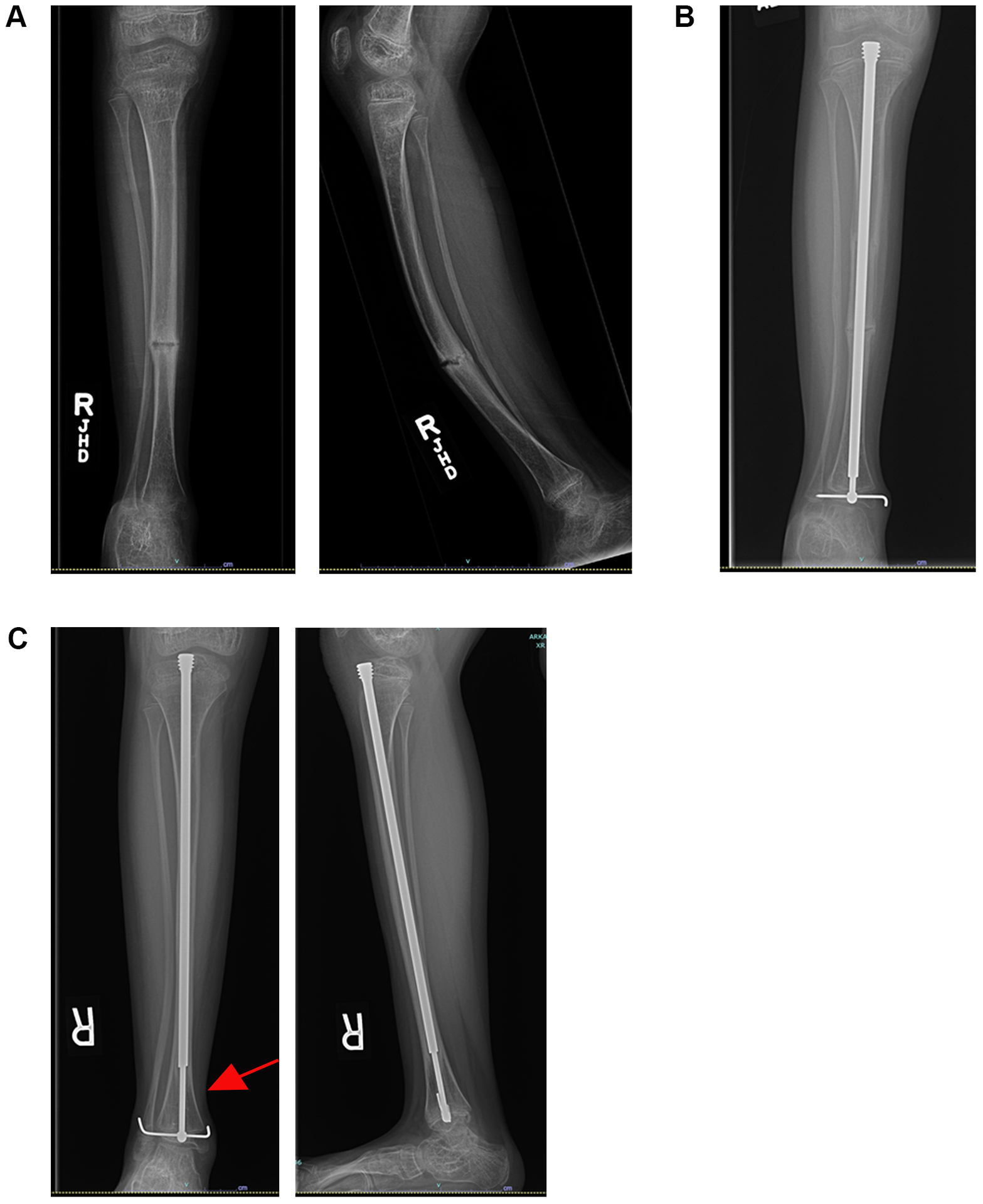
An 8-year-old female with osteogenesis imperfecta had severe anterior bowing of the tibia and recurrent fractures (A). This case failed nonoperative management with a brace. The patient underwent a 2-level open tibial osteotomy with placement of a Fassier–Duval telescoping rod (B). The patient is now over 1 year out from surgery. Note the increased growth and telescoping of the rod (C – red arrow). The osteotomies have healed well, and the patient is currently asymptomatic.
Skeletal deformities
Skeletal deformities, particularly bowing of the lower extremities, may also require a surgical consult. These deformities often become severe and predispose the patient to fractures; recurrent fractures can then worsen the deformity (74). Nonoperative treatment options include bracing. If this is not successful, surgery may be indicated. The goal of surgery is to restore mechanical alignment, decrease fracture incidence, and promote ambulation. This is typically accomplished with intramedullary implants and multiple osteotomies (70, 71).
Several techniques have been described to minimize the risk of bone nonunion (failure to heal) after surgery. These include minimizing soft-tissue stripping and avoiding the use of power saws to create an osteotomy, as the friction and subsequent heat can cause osteonecrosis and increase the risk of nonunion. The use of drills and osteotomes is preferred. Azzam et al. evaluated the experience of a single center using FD rods with osteotomes and a percutaneous technique in a cohort of 179 extremities. Good function at a 5-year follow up was noted based on 2 separate mobility scores. The authors were also able to address multiple bones during a single surgery with a low morbidity and short hospitalization (75).
Spinal abnormalities
Spinal abnormalities are common in OI, and include scoliosis, craniocervical junction abnormalities (such as basilar invagination and platybasia), spondylolysis (a defect or fracture in the segment of bone that joins the facet joints in the back of the vertebrae) and spondylolisthesis (a spine defect causing vertebral forward translation) at the lumbosacral junction. The first step in treatment is identification. As such, these patients merit regular spinal and neurological exams. When concerns arise, X-ray is indicated. While many patients may experience symptoms, some may not; certain craniocervical abnormalities may be asymptomatic. Therefore, some clinicians recommend a screening radiograph of the cervical spine by age 6 (76).
The boney abnormalities typical of OI pose unique challenges to the spine surgeon. Decreased bone mineral density can jeopardize implant grip, so bisphosphonates are often used preoperatively to improve bone quality for more robust implant attachment. Spine surgeons might also use preoperative or intraoperative traction to gradually straighten the spine with minimal force. Other tools include the maximization of fixation points in the spine to better distribute corrective forces, and the use of cement to augment pedicle screw fixation. Bone fusion is typically the goal of spine surgeries, and there is a theoretical risk of impaired bone fusion due to bisphosphonate treatment. There is no consensus on the use of bisphosphonates perioperatively, but some clinicians discontinue use for 4 months after surgery (76, 77).
Use of bisphosphonates
Bisphosphonates improve bone mineral density by inhibiting osteoclasts and decreasing bone turnover (70). As osteoclast function plays an important role in bone healing, the surgeon and treatment team must be mindful of the effects of these medications. Although concerns have been raised regarding delayed bone healing in patients treated with these medications, two separate studies showed that bisphosphonates did not adversely affect the healing of fractures (67, 78).
The effect of bisphosphonates on bone healing after osteotomy appears to be more controversial. Munns et al. found pamidronate therapy to be associated with delayed healing in patients with OI (78). The authors then adjusted their protocol by adding a 4-month bisphosphonate-free period after osteotomy and used an osteotome instead of a power saw for osteotomy to minimize heat necrosis. These changes decreased the delayed bone union rate (i.e. persistence of at least part of osteotomy line at 1 year) from 72% to 42% (79). As the operative technique changed in addition to a bisphosphate holiday, it is unclear if one or both of these factors led to a decrease in their delayed union rate. Azzam et al. noted a nonunion rate of 14.5% for osteotomy using a percutaneous technique and osteotomes. These patients were on cyclic bisphosphonate therapy, and infusions were not postponed after surgical procedures (75). In this cohort, bisphosphonate infusions were on a schedule and were given irrespective of orthopaedic surgery. The authors did not track the time period between bisphosphate infusion and surgery. Clearly, the use of bisphosphonates in the setting of elective osteotomies is a gray area, and further research is needed.
4. Conclusions
Great progress has been made in defining the molecular genetics underlying OI, and this has led to the identification of new therapeutic targets and clinical trials. Although a cure for OI is still to come, the health care of individuals with OI has improved. While the focus remains on the skeleton, as OI patients are living longer there is a need to focus on other aspects of the disease that can be associated with significant morbidity and negatively affect quality of life. These include hearing loss, reduced muscle function, and decreased respiratory fitness. It is expected that studying these comorbidities will lead to a better understanding of the role of type I collagen in both the development and homeostasis of these organ systems. Ultimately, this will allow a more complete appreciation of OI pathogenic processes and will enable the development of much needed targeted therapies for OI.
Funding
Ronit Marom is supported by the National Institutes of Health T32-GM007526-41, by the Rolanette and Berdon Lawrence Bone Disease Program of Texas award, and by the Baylor College of Medicine Chao Physician-Scientist award. Roy Morello is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number R03HD097559, the UAMS College of Medicine Research Scholar Pilot Grant Award in Child Health, and the National Institute of General Medical Sciences under award number P20 GM125503. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors declare no conflicts of interest.
References
- 1.Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017. Aug 18;3:17052. [DOI] [PubMed] [Google Scholar]
- 2.Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, Nishimura G, Robertson S, Sangiorgi L, Savarirayan R et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A. 2019. Dec;179(12):2393–419. [DOI] [PubMed] [Google Scholar]
- 3.Lindert U, Cabral WA, Ausavarat S, Tongkobpetch S, Ludin K, Barnes AM, Yeetong P, Weis M, Krabichler B, Srichomthong C et al. MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta. Nat Commun. 2016. Jul 06;7:11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semler O, Garbes L, Keupp K, Swan D, Zimmermann K, Becker J, Iden S, Wirth B, Eysel P, Koerber F et al. A mutation in the 5’-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet. 2012. August 10;91(2):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moosa S, Yamamoto GL, Garbes L, Keupp K, Beleza-Meireles A, Moreno CA, Valadares ER, de Sousa SB, Maia S, Saraiva J et al. Autosomal-Recessive Mutations in MESD Cause Osteogenesis Imperfecta. Am J Hum Genet. 2019. October 3;105(4):836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyard M, Bacrot S, Huber C, Di Rocco M, Goldenberg A, Aglan MS, Brunelle P, Temtamy S, Michot C, Otaify GA et al. FAM46A mutations are responsible for autosomal recessive osteogenesis imperfecta. J Med Genet. 2018. April;55(4):278–84. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes AM, Rocha-Braz MGM, Franca MM, Lerario AM, Simoes VRF, Zanardo EA, et al. The molecular landscape of osteogenesis imperfecta in a Brazilian tertiary service cohort. Osteoporos Int. 2020. Mar 2. [DOI] [PubMed] [Google Scholar]
- 8.Bardai G, Moffatt P, Glorieux FH, Rauch F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta: diagnostic yield and mutation spectrum. Osteoporos Int. 2016. December;27(12):3607–13. [DOI] [PubMed] [Google Scholar]
- 9.Tauer JT, Robinson ME, Rauch F. Osteogenesis Imperfecta: New Perspectives From Clinical and Translational Research. JBMR Plus. 2019. Aug;3(8):e10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimori M, Heard-Lipsmeyer ME, Byrum SD, Mackintosh SG, Kurten RC, Carroll JL, Morello R. Respiratory defects in the CrtapKO mouse model of Osteogenesis Imperfecta. Am J Physiol Lung Cell Mol Physiol. 2020. Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B et al. Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014. June;20(6):670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlebacher A, Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol. 1996. January;132(1–2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman SM, Dimori M, Heard-Lipsmeyer ME, Morello R. The Osteocyte Transcriptome Is Extensively Dysregulated in Mouse Models of Osteogenesis Imperfecta. JBMR Plus. 2019. Jul;3(7):e10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joeng KS, Lee YC, Lim J, Chen Y, Jiang MM, Munivez E, Ambrose C, Lee BH. Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest. 2017. June 30;127(7):2678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain M, Tam A, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D, Krischer J, Mullins M et al. Growth characteristics in individuals with osteogenesis imperfecta in North America: results from a multicenter study. Genet Med. 2019. February;21(2):275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arponen H, Makitie O, Haukka J, Ranta H, Ekholm M, Mayranpaa MK, Kaitila I, Waltimo-Sired J. Prevalence and natural course of craniocervical junction anomalies during growth in patients with osteogenesis imperfecta. J Bone Miner Res. 2012. May;27(5):1142–9. [DOI] [PubMed] [Google Scholar]
- 17.Reznikov N, Dagdeviren D, Tamimi F, Glorieux F, Rauch F, Retrouvey JM. Cone-Beam Computed Tomography of Osteogenesis Imperfecta Types III and IV: Three-Dimensional Evaluation of Craniofacial Features and Upper Airways. JBMR Plus. 2019. Jun;3(6):e10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelaziz DM, Abdullah S, Magnussen C, Ribeiro-da-Silva A, Komarova SV, Rauch F, Stone LS. Behavioral signs of pain and functional impairment in a mouse model of osteogenesis imperfecta. Bone. 2015. December;81:400–6. [DOI] [PubMed] [Google Scholar]
- 19.Marcal FF, Ribeiro EM, Costa FWG, Fonteles CSR, Teles GS, de Barros Silva PG, Chaves CM, Rodrigues Ribeiro T. Dental alterations on panoramic radiographs of patients with osteogenesis imperfecta in relation to clinical diagnosis, severity, and bisphosphonate regimen aspects: a STROBE-compliant case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019. Dec;128(6):621–30. [DOI] [PubMed] [Google Scholar]
- 20.Najirad M, Ma MS, Rauch F, Sutton VR, Lee B, Retrouvey JM, Members of the BBD, Esfandiari S. Oral health-related quality of life in children and adolescents with osteogenesis imperfecta: cross-sectional study. Orphanet J Rare Dis. 2018. Oct 25;13(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eimar H, Tamimi F, Retrouvey JM, Rauch F, Aubin JE, McKee MD. Craniofacial and Dental Defects in the Col1a1Jrt/+ Mouse Model of Osteogenesis Imperfecta. J Dent Res. 2016. July;95(7):761–8. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Lenhart SA, Chu EY, Chavez MB, Wimer HF, Dimori M, Somerman MJ, Morello R, Foster BL, Hatch NE. Dental and Craniofacial Defects in the Crtap(−/−) Mouse Model of Osteogenesis Imperfecta Type VII. Dev Dyn. 2020. Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carre F, Achard S, Rouillon I, Parodi M, Loundon N. Hearing impairment and osteogenesis imperfecta: Literature review. Eur Ann Otorhinolaryngol Head Neck Dis. 2019. Oct;136(5):379–83. [DOI] [PubMed] [Google Scholar]
- 24.Machol K, Hadley TD, Schmidt J, Cuthbertson D, Traboulsi H, Silva RC, Citron C, Khan S, Citron K, Carter E et al. Hearing loss in individuals with osteogenesis imperfecta in North America: Results from a multicenter study. Am J Med Genet A. 2019. December 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiscaletti M, Biggin A, Bennetts B, Wong K, Briody J, Pacey V, Birman C, Munns CF. Novel variant in Sp7/Osx associated with recessive osteogenesis imperfecta with bone fragility and hearing impairment. Bone. 2018. May;110:66–75. [DOI] [PubMed] [Google Scholar]
- 26.Pokidysheva E, Tufa S, Bresee C, Brigande JV, Bachinger HP. Prolyl 3-hydroxylase-1 null mice exhibit hearing impairment and abnormal morphology of the middle ear bone joints. Matrix Biol. 2013. January;32(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stankovic KM, Kristiansen AG, Bizaki A, Lister M, Adams JC, McKenna MJ. Studies of otic capsule morphology and gene expression in the Mov13 mouse--an animal model of type I osteogenesis imperfecta. Audiol Neurootol. 2007;12(5):334–43. [DOI] [PubMed] [Google Scholar]
- 28.da Costa Otavio AC, Teixeira AR, Felix TM, Rosito LPS, da Costa SS. Osteogenesis imperfecta and hearing loss: an analysis of patients attended at a benchmark treatment center in southern Brazil. Eur Arch Otorhinolaryngol. 2020. Jan 31. [DOI] [PubMed] [Google Scholar]
- 29.Veilleux LN, Trejo P, Rauch F. Muscle abnormalities in osteogenesis imperfecta. J Musculoskelet Neuronal Interact. 2017. Jun 1;17(2):1–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips CL, Jeong Y. Osteogenesis Imperfecta: Muscle-Bone Interactions when Bi-directionally Compromised. Curr Osteoporos Rep. 2018. August;16(4):478–89. [DOI] [PubMed] [Google Scholar]
- 31.Xu XJ, Lv F, Song YW, Li LJ, Asan, Wei XX, Zhao XL, Jiang Y, Wang O, Xing XP et al. Novel mutations in BMP1 induce a rare type of osteogenesis imperfecta. Clin Chim Acta. 2019. February;489:21–8. [DOI] [PubMed] [Google Scholar]
- 32.Jeong Y, Carleton SM, Gentry BA, Yao X, Ferreira JA, Salamango DJ, Weis MA, Oestreich AK, Williams AM, McCray MG et al. Hindlimb Skeletal Muscle Function and Skeletal Quality and Strength in +/G610C Mice With and Without Weight-Bearing Exercise. J Bone Miner Res. 2015. October;30(10):1874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gentry BA, Ferreira JA, McCambridge AJ, Brown M, Phillips CL. Skeletal muscle weakness in osteogenesis imperfecta mice. Matrix Biol. 2010. September;29(7):638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gremminger VL, Jeong Y, Cunningham RP, Meers GM, Rector RS, Phillips CL. Compromised Exercise Capacity and Mitochondrial Dysfunction in the Osteogenesis Imperfecta Murine (oim) Mouse Model. J Bone Miner Res. 2019. September;34(9):1646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkestad L, Hald JD, Canudas-Romo V, Gram J, Hermann AP, Langdahl B, Abrahamsen B, Brixen K. Mortality and Causes of Death in Patients With Osteogenesis Imperfecta: A Register-Based Nationwide Cohort Study. J Bone Miner Res. 2016. June 27. [DOI] [PubMed] [Google Scholar]
- 36.Sandhaus R Pulmonary Function in Osteogenesis Imperfecta In: Shapiro J, editor. Osteogenesis Imperfecta: a translational approach to brittle bone disease: Elsevier; 2013. p. 335–41. [Google Scholar]
- 37.Bronheim R, Khan S, Carter E, Sandhaus RA, Raggio C. Scoliosis and Cardiopulmonary Outcomes in Osteogenesis Imperfecta Patients. Spine. 2019. Aug 1;44(15):1057–63. [DOI] [PubMed] [Google Scholar]
- 38.Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, Schneider R. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine. 1999. Aug 15;24(16):1673–8. [DOI] [PubMed] [Google Scholar]
- 39.McAllion SJ, Paterson CR. Causes of death in osteogenesis imperfecta. J Clin Pathol. 1996. August;49(8):627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiele F, Cohrs CM, Flor A, Lisse TS, Przemeck GK, Horsch M, Schrewe A, Gailus-Durner V, Ivandic B, Katus HA et al. Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum Molec Genet. 2012. Aug 15;21(16):3535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baglole CJ, Liang F, Traboulsi H, de Souza AR, Giordano C, Tauer JT, Rauch F, Petrof BJ. Pulmonary and diaphragmatic pathology in collagen alpha1 type I (COL1A1) mutant mice with osteogenesis imperfecta. Pediatr Res. 2018. March 14. [DOI] [PubMed] [Google Scholar]
- 42.Baldridge D, Lennington J, Weis M, Homan EP, Jiang MM, Munivez E, Keene DR, Hogue WR, Pyott S, Byers PH et al. Generalized connective tissue disease in Crtap−/− mouse. PLoS One. 2010;5(5):e10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lund AM, Muller J, Skovby F. Anthropometry of patients with osteogenesis imperfecta. Arch Dis Child. 1999. June;80(6):524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barber LA, Abbott C, Nakhate V, Do AND, Blissett AR, Marini JC. Longitudinal growth curves for children with classical osteogenesis imperfecta (types III and IV) caused by structural pathogenic variants in type I collagen. Genet Med. 2019. May;21(5):1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheiber AL, Guess AJ, Kaito T, Abzug JM, Enomoto-Iwamoto M, Leikin S, Iwamoto M, Otsuru S. Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem Biophys Res Commun. 2019. January 29;509(1):235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marini JC, Hopkins E, Glorieux FH, Chrousos GP, Reynolds JC, Gundberg CM, Reing CM. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003. February;18(2):237–43. [DOI] [PubMed] [Google Scholar]
- 47.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002. June 25;99(13):8932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otsuru S, Desbourdes L, Guess AJ, Hofmann TJ, Relation T, Kaito T, Dominici M, Iwamoto M, Horwitz EM. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy. 2018. January;20(1):62–73. [DOI] [PubMed] [Google Scholar]
- 49.Tournis S, Dede AD. Osteogenesis imperfecta - A clinical update. Metabolism. 2018. March;80:27–37. [DOI] [PubMed] [Google Scholar]
- 50.Palomo T, Vilaca T, Lazaretti-Castro M. Osteogenesis imperfecta: diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes. 2017. December;24(6):381–8. [DOI] [PubMed] [Google Scholar]
- 51.Morello R Osteogenesis imperfecta and therapeutics. Matrix Biol. 2018. Oct;71–72:294–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi V, Lee B, Marom R. Osteogenesis imperfecta: advancements in genetics and treatment. Curr Opin Pediatr. 2019. December;31(6):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bains JS, Carter EM, Citron KP, Boskey AL, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D et al. A Multicenter Observational Cohort Study to Evaluate the Effects of Bisphosphonate Exposure on Bone Mineral Density and Other Health Outcomes in Osteogenesis Imperfecta. JBMR Plus. 2019. May;3(5):e10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li LJ, Zheng WB, Zhao DC, Yu W, Wang O, Jiang Y, Xia WB, Li M. Effects of zoledronic acid on vertebral shape of children and adolescents with osteogenesis imperfecta. Bone. 2019. October;127:164–71. [DOI] [PubMed] [Google Scholar]
- 55.Marom R, Lee YC, Grafe I, Lee B. Pharmacological and biological therapeutic strategies for osteogenesis imperfecta. Am J Med Genet C Semin Med Genet. 2016. December;172(4):367–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoyer-Kuhn H, Netzer C, Koerber F, Schoenau E, Semler O. Two years’ experience with denosumab for children with osteogenesis imperfecta type VI. Orphanet J Rare Dis. 2014. Sep 26;9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orwoll ES, Shapiro J, Veith S, Wang Y, Lapidus J, Vanek C, Reeder JL, Keaveny TM, Lee DC, Mullins MA et al. Evaluation of teriparatide treatment in adults with osteogenesis imperfecta. J Clin Invest. 2014. February;124(2):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Laing TF, McGowan JA, Rosen CJ, PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. New Engl J Med. 2005. August 11;353(6):555–65. [DOI] [PubMed] [Google Scholar]
- 59.Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, Junker U, Ruckle J, Seefried L, Winkle PJ. BPS804 Anti-Sclerostin Antibody in Adults With Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. J Bone Miner Res. 2017. July;32(7):1496–504. [DOI] [PubMed] [Google Scholar]
- 60.Sinder BP, Novak S, Wee NKY, Basile M, Maye P, Matthews BG, Kalajzic I. Engraftment of skeletal progenitor cells by bone directed transplantation improves osteogenesis imperfecta murine bone phenotype. Stem Cells. 2019. December 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee LR, Peacock L, Ginn SL, Cantrill LC, Cheng TL, Little DG, Munns CF, Schindeler A. Bone Marrow Transplantation for Treatment of the Col1a2(+/G610C) Osteogenesis Imperfecta Mouse Model. Calcif Tissue Int. 2019. April;104(4):426–36. [DOI] [PubMed] [Google Scholar]
- 62.Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, Hofmann TJ, Veronesi, Dominici M, Iwamoto M et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 2012. August 30;120(9):1933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gotherstrom C, Westgren M, Shaw SW, Astrom E, Biswas A, Byers PH, Mattar CNZ, Graham GE, Taslimi J, Ewald U et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med. 2014. February;3(2):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005. June 15;79(11):1607–14. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandra DL, Shaw SS, Shangaris P, Loukogeorgakis S, Guillot PV, Coppi PD, David AL. In utero therapy for congenital disorders using amniotic fluid stem cells. Front Pharmacol. 2014;5:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kocher MS, Shapiro F. Osteogenesis imperfecta. The Journal of the American Academy of Orthopaedic Surgeons. 1998. Jul-Aug;6(4):225–36. [DOI] [PubMed] [Google Scholar]
- 67.Pizones J, Plotkin H, Parra-Garcia JI, Alvarez P, Gutierrez P, Bueno A, Fernandez-Arroyo A. Bone healing in children with osteogenesis imperfecta treated with bisphosphonates. J Pediatr Orthop. 2005. May-Jun;25(3):332–5. [DOI] [PubMed] [Google Scholar]
- 68.Besio R, Maruelli S, Battaglia S, Leoni L, Villani S, Layrolle P, Rossi A, Trichet V, Forlino A. Early Fracture Healing is Delayed in the Col1a2(+/G610C) Osteogenesis Imperfecta Murine Model. Calcif Tissue Int. 2018. December;103(6):653–62. [DOI] [PubMed] [Google Scholar]
- 69.Zieba J, Munivez E, Castellon A, Jiang MM, Dawson B, Ambrose CG, Lee B. Fracture Healing in Collagen-Related Preclinical Models of Osteogenesis Imperfecta. J Bone Miner Res. 2020. Feb 13. [DOI] [PubMed] [Google Scholar]
- 70.Burnei G, Vlad C, Georgescu I, Gavriliu TS, Dan D. Osteogenesis imperfecta: diagnosis and treatment. The Journal of the American Academy of Orthopaedic Surgeons. 2008. June;16(6):356–66. [DOI] [PubMed] [Google Scholar]
- 71.Spahn KM, Mickel T, Carry PM, Brazell CJ, Whalen K, Georgopoulos G, Miller NH. Fassier-Duval Rods are Associated With Superior Probability of Survival Compared With Static Implants in a Cohort of Children With Osteogenesis Imperfecta Deformities. J Pediatr Orthop. 2019. May/Jun;39(5):e392–e6. [DOI] [PubMed] [Google Scholar]
- 72.Rothschild L, Goeller JK, Voronov P, Barabanova A, Smith P. Anesthesia in children with osteogenesis imperfecta: Retrospective chart review of 83 patients and 205 anesthetics over 7 years. Paediatr Anaesth. 2018. Nov;28(11):1050–8. [DOI] [PubMed] [Google Scholar]
- 73.Persiani P, Martini L, Ranaldi FM, Zambrano A, Celli M, Celli L, D’Eufemia P, Villani C. Elastic intramedullary nailing of the femur fracture in patients affected by osteogenesis imperfecta type 3: Indications, limits and pitfalls. Injury. 2019. Jul;50 Suppl 2:S52–S6. [DOI] [PubMed] [Google Scholar]
- 74.Krakow D Osteogenesis Imperfecta In: Copel JADAM, Feltovich H, Gratacos E, Krakow D, Odibo AO, Platt LD, Tutschek B, editor. Obstetric Imaging: Fetal Diagnosis and Care. Second ed: Elsevier; 2018. p. 270–3. [Google Scholar]
- 75.Azzam KA, Rush ET, Burke BR, Nabower AM, Esposito PW. Mid-term Results of Femoral and Tibial Osteotomies and Fassier-Duval Nailing in Children With Osteogenesis Imperfecta. J Pediatr Orthop. 2018. July;38(6):331–6. [DOI] [PubMed] [Google Scholar]
- 76.Wallace MJ, Kruse RW, Shah SA. The Spine in Patients With Osteogenesis Imperfecta. The Journal of the American Academy of Orthopaedic Surgeons. 2017. February;25(2):100–9. [DOI] [PubMed] [Google Scholar]
- 77.O’Donnell C, Bloch N, Michael N, Erickson M, Garg S. Management of Scoliosis in Children with Osteogenesis Imperfecta. JBJS Rev. 2017. Jul;5(7):e8. [DOI] [PubMed] [Google Scholar]
- 78.Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004. November;19(11):1779–86. [DOI] [PubMed] [Google Scholar]
- 79.Anam EA, Rauch F, Glorieux FH, Fassier F, Hamdy R. Osteotomy Healing in Children With Osteogenesis Imperfecta Receiving Bisphosphonate Treatment. J Bone Miner Res. 2015. August;30(8):1362–8. [DOI] [PubMed] [Google Scholar]


