Abstract
Over the last century, a great deal of effort and resources have been poured into the development of vaccines to protect against malaria, particularly targeting the most widely spread and deadly species of the human-infecting parasites: Plasmodium falciparum. Many of the known proteins the parasite uses to invade human cells have been tested as vaccine candidates. However, precisely because of the importance and immune visibility of these proteins, they tend to be very diverse, and in many cases redundant, which limits their efficacy in vaccine development. With the advent of genomics and constantly improving sequencing technologies, an increasingly clear picture is emerging of the vast genomic diversity of parasites from different geographic areas. This diversity is distributed throughout the genome and includes most of the vaccine candidates tested so far, playing an important role in the low efficacy achieved. Genomics is a powerful tool to search for genes that comply with the most desirable attributes of vaccine targets, allowing us to evaluate function, immunogenicity and also diversity in the worldwide parasite populations. Even predicting how this diversity might evolve and spread in the future becomes possible, and can inform novel vaccine efforts.
Keywords: Plasmodium falciparum, malaria, vaccine, variation, diversity, genomics, sequencing
1. Introduction
Although preventable and curable, malaria is one of the most severe worldwide public health problems, causing crippling disease leading to approximately half a million deaths every year. The intense efforts for malaria intervention deployed so far have achieved a substantial decrease in the incidence of the disease, which has encouraged ambitious plans for malaria elimination by the World Health Organisation. However, recent years have witnessed a stagnation of progress in the reduction of cases [1], compounded by a number of factors such as conflict, poverty and political instability.
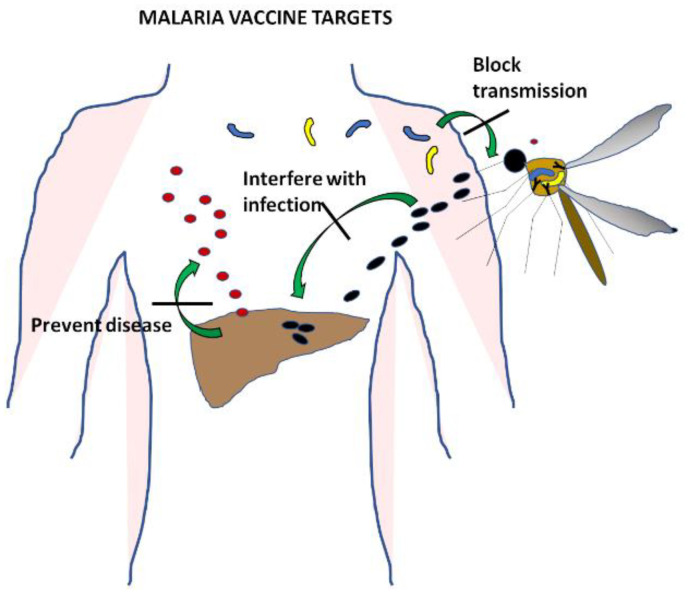
Malaria is a complex infectious disease caused by five different species of Plasmodium parasites. Plasmodium vivax and Plasmodium falciparum are the most common, though their distribution around the world does not completely overlap. Plasmodium vivax and Plasmodium ovale form hypnozoites, latent hepatic forms that are difficult to clear and constitute a reservoir of parasites that maintains the infection in the population. Plasmodium knowlesi has a shorter life cycle and as a consequence a very quick onset of clinical symptoms. The most severe disease is caused by Plasmodium falciparum, and much effort has been devoted over decades of research to the development of a vaccine specific to this species [2]. All stages of the parasite in the human host have been targeted (Figure 1) with the aim of designing vaccines that prevent infection by directing the immune response to the sporozoites, that avoid clinical symptoms and the spread of disease by using the antigens expressed during the blood cycle, or that stop transmission by targeting gametocytes.
Figure 1.
Malaria parasite life cycle. Stages of malaria infection that are the focus of vaccine development. Interfering with the infection of hepatocytes would prevent the disease altogether. Blocking the blood stage would stop clinical symptoms and also prevent transmission of the disease. Targeting gametocytes of early stages in the mosquito would avoid infection spread from person to person without affecting the development of clinical disease.
In this genomic era, the completion of the whole sequence of the Plasmodium falciparum genome [3] has revealed the full set of genes, paving the way to start deciphering their function in the different stages of the life cycle of the parasite. This knowledge has enhanced the understanding of the disease, and started providing a genomic landscape of the parasite in different regions of the world [4]. Being able to sequence parasite genomes from vast numbers of clinical samples allows for associations with characteristics such as drug resistance as well as an overall view of its distribution and spread [5]. Parasite evolution, population distribution and dynamics in different regions of the world [6,7,8] can also be estimated with genomic information. Importantly, these studies are revealing the complexity of parasite diversity, providing a detailed insight into the genetic variation of parasite proteins that are at the centre of vaccine development.
This review summarises the main vaccine developments for Plasmodium falciparum, particularly focusing on those that have reached clinical trials, providing efficacy data in humans [2] (Table 1). The results of these efforts are considered in light of the vast sequencing data of P. falciparum acquired over the last decade to discuss the current understanding of genomic variation and its impact on vaccine efficacy and development.
Table 1.
Recent clinical trials of Plasmodium falciparum vaccines with efficacy data available. Orange: pre-erythrocytic proteins; red: whole sporozoite vaccines; green: blood stage proteins; blue: multi-stage vaccines.
| Antigen | Vaccine | Strain | Homologous | Heterologous | Trial | Reference |
|---|---|---|---|---|---|---|
| Multi Epitope TRAP | ME-TRAP | T9/96 | 21% | Phase I/Iia: NCT00890760 | [9] | |
| 13% | Phase I/Iia CHMI NCT01623557 | [10] | ||||
| 13% | CHMI | [11] | ||||
| 67% | Phase Iib: NCT1666925 | [12] | ||||
| 10.30% | ISRCTN05221133 | [13] | ||||
| No protection | Phase I/IIb: NCT01635647 | [14] | ||||
| CSP | RTS,S/AS01 | 3D7 | 36.30% | [15] | ||
| 34.80% | Phase III: NCT00866619 | [16] | ||||
| 30.10% | [17] | |||||
| 27% | [18] | |||||
| 32.1–53.7% | Phase III: NCT02207816 | [19] | ||||
| 86.70% | CHMI Phase Iia: NCT01857869 | [20] | ||||
| 55–76% | Phase IIa: NCT03143218 | [21] | ||||
| Me-TRAP+RTS,S | 82.40% | CHMI NCT01883609 | [22] | |||
| Whole Sporozoite | PfSPZ | NF54 | 100% | CHMI NCT01441167 | [23] | |
| 100% | CHMI NCT00442377 | [24] | ||||
| 100% | CHMI NCT02613520 | [25] | ||||
| 29% | Phase I: NCT01988636 | [26] | ||||
| 20% | CHMI NCT02132299 | [27] | ||||
| 92.30% | 80% | CHMI NCT02215707 | [28] | |||
| 100% | CHMI NCT02115516 | [29] | ||||
| 100% | 11–20% | CHMI NCT02098590 | [30] | |||
| AMA1 | FMP2.1 | 3D7 | 64.30% | 20% | Phase II: NCT00460525 | [31] |
| 24% | 9.90% | Phase II: NCT00460525 | [32] | |||
| 0% | CHMI NCT02044198 | [33] | ||||
| 0% | CHMI NCT00385047 | [34] | ||||
| AMA1+MSP1 | ChAd63-MVA | 3D7 | 11% | CHMI NCT01142765 | [35] | |
| MSP3- GLURP | GMZ2 | FVO / F32 | 14% | PACTR2010060002033537 | [36] | |
| 0% | CHMI PACTR201503001038304 | [37] | ||||
| SERA5 | BK-SE36 | Honduras-1 | 75% | Phase Ib: ISRCTN71619711 | [38] | |
| CSP+AMA1 | NMRC-M3V-Ad-PfCA | 3D7 | 0% | Phase I/IIa: NCT00392015 | [39] | |
| 27% | CHMI NCT00870987 | [40] |
2. The Infection: The Sporozoite
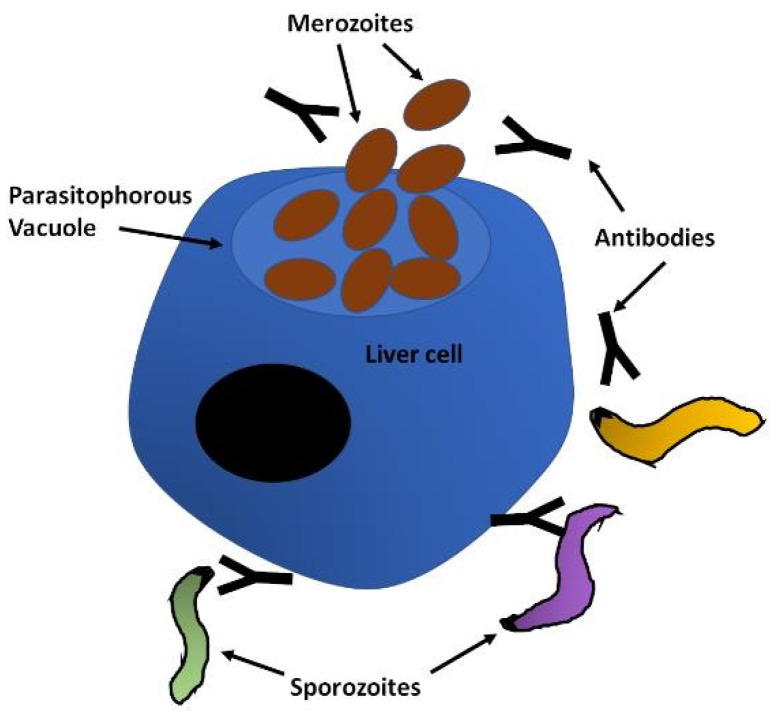
Human malaria infections start when the sporozoite form of the parasite is deposited in the skin by the infected mosquito during its blood meal. The sporozoites move randomly in the skin until they contact the endothelium of lymphatic or blood circulation, which can take between 1 and 3 h. Importantly, during this time they are exposed to the immune system, particularly if they enter the lymph. Sporozoites have to traverse epithelial cells and cross the endothelial barrier to access the blood vessel, which involves the sporozoite microneme protein essential for cell traversal SPECT1 and 2 [41], cell traversal protein for ookinetes and sporozoites CelTOS [42] and a phospholipase (PL) [43,44]. Once in the circulation, the sporozoites are rapidly carried to the liver, where they are arrested by binding to the highly sulphated heparan sulphate proteoglycans (HSPGs) of the liver sinusoid through the circumsporozoite protein (CSP), which densely covers the surface of the sporozoites [45]. Access to the sinusoid is facilitated by the fenestrated endothelium, which allows the sporozoites to leave the blood stream migrating through or between endothelial cells. Upon contact with the hepatocytes, motility becomes essential for invasion and the thrombospondin-related anonymous protein (TRAP) is a key component of the actinomyosin motor of the sporozoite [46,47]. After migration around several hepatocytes, the sporozoite adheres to and penetrates the target cell in a process involving TRAP, CSP and apical membrane antigen 1 (AMA-1), establishing itself within a parasitophorous vacuole (PV) [48,49], where the transformation into the merozoites that will initiate the blood cycle takes place [50,51] (Figure 2).
Figure 2.
The liver stage. Sporozoites leave the skin and migrate to the liver, where they invade hepatocytes. Surface proteins of the sporozoite are involved in the migration, recognition and invasion of hepatocytes. These are represented by the dense black outline of the sporozoites and increased density in the apical region, and include mainly CSP, TRAP and AMA-1 at this stage. After the intracellular replication of the parasite the first merozoites that will initiate the blood infection are released into the blood stream. Pre-erythrocytic vaccines aim to induce an immune response, humoral and cellular, to interfere with invasion and trigger elimination of the parasite, respectively. CSP (circumsporozoite protein); TRAP (thrombospondin-related anonymous protein); AMA-1 (apical membrane antigen 1).
Targeting this stage for vaccine development has several advantages. While Plasmodium is an obligate intracellular parasite, during this stage it is free in the blood stream for several hours and therefore accessible to the immune system. Furthermore, as much as 20% of the inoculum enters the lymph, where exposure to the immune system and stimulation of T lymphocytes can be very effective [52,53]. Many of the unsuccessful sporozoites are removed from the skin by antigen presenting cells such as dendritic cells, providing additional immune stimulation opportunities. Together with the low number of sporozoites inoculated, this could make an immune response very effective at eliminating the parasite. Targeting this stage would prevent infection all together, avoiding clinical symptoms and blocking transmission by eliminating the human reservoir of the parasite [54]. Many of the known pre-erythrocytic proteins important for hepatocyte invasion and survival in the liver have been exploited for vaccine development [55].
The key protein for the motility and invasion of hepatocytes as well as mosquito salivary glands, TRAP, is expressed in the micronemes and on the surface of sporozoites. Numerous studies have described TRAP as a target of cellular and humoral immunity, and this response is correlated with a reduced risk of clinical malaria [56] and sterile protective immunity [57]. A multi-epitope vaccine was constructed with the aim of inducing a broader immune response more representative of the parasite.
The most extensively tested of these vaccines is ME-TRAP. This is a multiepitope string that includes CD8 and CD4 T-cell epitopes from CSP, EXP-1 (exported protein 1), LSA (liver stage antigen) 1 and 3 and STARP (sporozoite threonine and aparagine-rich protein) fused to full length TRAP from the P. falciparum strain T9/96 from Thailand. All these proteins have been demonstrated to induce the immune system, and some have been trialled individually without great success [58]. Trials with ME-TRAP, assessing efficacy with a CHMI using the heterologous strain 3D7, showed 10% to 33% partial protection and 10% to 20% sterile protection [9,10,11,59]. Natural infections after vaccination of adult volunteers reduced the risk of infection by 67% [12]. Another study found an efficacy of 10% in Gambia [13], while in Kenyan children no significant difference between the control and vaccinated groups was observed [60]. Despite a non-significant efficacy in Senegal, the vaccine showed promise in Gambian and Burkinabe children, but when expanded to a hyperendemic area of Burkina Faso, no significant protective efficacy could be shown in children with previous exposure to malaria [14]. One of the reasons attributed to the failure of TRAP-based vaccines was the antigenic diversity of the protein. The vaccine TRAP comes from the Thai strain T9/96 [61] that differs by 6% from the amino acid sequence of the 3D7 strain, including major differences in the number of repeats of the PPN sequence. The extent of nucleotide variation in TRAP correlates with the transmission intensity in endemic areas. Accordingly, the highest diversity was found in African countries, particularly Gambia, Senegal and Uganda, where the potential for multiclonal infection is considerable. Furthermore, high polymorphisms were found in some of the B- and T-cell epitopes, whereas the functional domains tend to be more conserved [62]. Among the components of the ME string, LSA-1 was found to have significant non-synonymous variation [63], though none of these changes affected T-cell epitopes [64]. The comparison of STARP among isolates worldwide revealed 20 haplotypes of the coding sequence but low levels of codon changes, and some regions of the gene were perfectly conserved [65]. An overview of genome-wide variation in over 600 samples worldwide provided in Plasmoview [66] shows a high degree of variation in TRAP as well as EXP1, LSA-1 and LSA-3, though the latter shows some conservation in the C-terminus. STARP, on the other hand, seems fairly conserved, with most variation contained within two defined regions. The most extensive variation was observed in CSP with hardly any conserved areas, as described in detail below.
The major surface antigen of the sporozoite, CSP, has been known to induce antibodies that can confer protective immunity against malaria, and a mechanism of targeting the parasite in the skin stage has been proposed [67]. CSP has three domains: a conserved amino-terminal region, a central B-cell epitope containing 37–44 NANP amino acid repeat sequences, and a polymorphic carboxy-terminal region that elicits a T-cell response. This protein has been the centre of many attempts to design an effective vaccine. A range of versions were used in vaccine design, some minimal, containing a three-unit repeat peptide from the central region of the protein [68], combinations of epitopes [69] and long synthetic peptides [70], but the most extensively tested and the only licensed anti-malaria vaccine to date is RTS,S/AS01. It consists of 19 NANP repeats and the C-terminal region of CSP from the 3D7/NF54 P. falciparum lab strain, fused with the hepatitis B surface protein and reconstituted with a novel adjuvant AS01. A series of trials showing safety, immunogenicity and efficacy against infection led to a large-scale phase III trial involving over 15,000 children throughout Africa [15,16,17,18,19]. Clinical malaria was reduced by 28%, and a booster applied 18 months after the third dose increased efficacy to 36.3%. CHMI trials comparing the dose regimens of vaccination showed an improved efficacy ranging from 55% to 86% against infection with a homologous 3D7 parasite [20,21]. In order to assess whether the modest efficacy of the vaccine in protecting against natural infections is due to allele specificity, samples from 7000 vaccinated and non-vaccinated children were sequenced, revealing a reduction in vaccine efficacy from 50%, for parasites with a perfect match to the vaccine protein, to 33% when amino acid differences were present [71]. A wider comparison of samples from other continents found 393 unique PfCSP haplotypes, and only 5.3% of the sequences identified in Africa and 0.25% in Asia corresponded to the 3D7 haplotype [72]. The global worldwide frequency of the 3D7 haplotype in the vaccine CSP region was calculated at 1.71%. In the analysis, the N-terminus showed much lower diversity across continents [73], which can be also observed in PlasmoView [66]. Indeed, comparing variability in the three regions of CSP, the C-terminus polymorphisms are most complex in the African continent, and the central repeat region shows great diversity of haplotypes, including the number of repeats, which is higher in Oceania and Asia, while the N-terminus is the most conserved, amounting mainly to a 57 bp insertion very frequent in Asia, Oceania and South America [73]. The lower diversity in the N-terminal region of the protein, which is functionally important and plays a role in immunity, makes the inclusion of this region in an improved version of the vaccine an attractive possibility [74]. A combination of ME-TRAP and RTS,S has also been tested in a CHMI with homologous parasites, but no improvement of the efficacy achieved with the best formulation of RTS,S alone was found [22].
CelTOS, one of the P. falciparum cell-traversal proteins, is present in the mosquito stage ookinetes as well as sporozoites. It was identified by naturally acquired antibodies in an endemic region as associated with protection against symptomatic infection [75]. A recombinant vaccine of CelTOS was tested in mouse models, in which it induced both humoral and cellular immune responses and sterile protection [76,77]. The P. berghei-expressing P. falciparum CelTOS tested in this model showed inhibition of both sporozoite infection and ookinete to oocyst development in the mosquito [78]. A recombinant vaccine using a 522 bp fragment from the 3D7 lab strain (PfCelTOS FMP012) was tested in two phase I clinical trials to monitor the elicited immune response and assess efficacy in controlled human malaria infection (CHMI), but the results have not been published. The genetic diversity of P. falciparum full length CelTOS evidenced 39 non-synonymous SNPs in 34 positions in isolates worldwide, in comparison with 3D7, amounting to 66 haplotypes [79]. Nevertheless, it was determined that most of the predicted B- and T-cell epitopes are in conserved regions of the protein, while most of the variation concentrates in the C-terminus. The assessment of the efficacy of this vaccine will prove very interesting, particularly in the context of heterologous infections.
3. Whole Sporozoite Vaccines
Immunisation with dead or inactivated whole organisms is the most widely used strategy to induce a preventive immune response against infectious agents. It was also applied to malaria as early as 1945 in studies with P. knowlesi in monkeys [80], P. vivax in humans [81] and P. berghei in mice [82]. These attempts showed that it is possible to use whole parasite immunisation for malaria, which could be a more effective way to elicit a wider and more diverse response than that achieved with individual proteins.
Three types of interventions have been tested: the injection of irradiation-attenuated parasites, genetically attenuated parasites and live parasites under drug pressure. The attenuated sporozoites arrest development in the liver, while the live parasites reach the blood but are wiped out by the drug before establishing a blood infection.
The most clinically developed P. falciparum sporozoite (PfSPZ) vaccine consists of metabolically active, irradiation-inactivated sporozoites from the lab strain NF54. Controlled infections proved that the vaccine can provide sterile immunity when volunteers are challenged with the homologous parasite [23,24,25]. The vaccine was subsequently tested for performance against naturally occurring infections in Mali [26] and Tanzania [27], where efficacy dropped to 29% and 20%, respectively. This was a clear indication that the lab strain NF54 vaccine is much less effective in protecting against heterologous parasites. A study was set up specifically to address this issue with a controlled infection with either homologous 3D7 parasites or the heterologous 7G8 from Brazil [28]. The results showed a 92% protection against 3D7 (12 of 13) after 3 weeks that dropped to 70% (7 of 10) after 24 weeks, while the protection against 7G8 was 80% (4 of 5) 3 weeks after immunization, which dropped to 10% (1 of 10) after 24 weeks. Though numbers in this study were small, there is an indication that the vaccine is less efficient against heterologous parasites and that immunity wanes off more rapidly with time.
In order to avoid potential damage of the SPZs by the irradiation process, which can have a negative impact on the vaccination efficacy, genetic inactivation was developed. These parasites can progress further through the liver stages and are therefore deemed more effective at triggering an immune response. Attenuation is achieved by deleting crucial genes involved in parasite development and, as a consequence, arrest in the liver stage [83]. The first clinical trial with this strategy was performed using parasites lacking p52 and p36, but there was a breakthrough leading to infection [84]. Genetic attenuation was improved by deleting p52, p36 and sap1, producing PfGAP3KO parasites that arrest early in the liver stage [85]. No efficacy results are available yet for these vaccines, but given that they originate from genetically engineered parasites, they are likely to rely on specific lab strains. It remains to be determined whether in this form, the stimulation of an immune response can be strong enough to be effective against a wider range of parasites.
Immunisation with live parasites under prophylaxis has been performed using mosquitoes infected with chloroquine-sensitive NF54 parasites to deliver parasites to volunteers, together with chloroquine treatment. Challenge with the homologous parasite showed 100% sterile immunity in the vaccinated participants, while the non-vaccinated individuals all developed the infection [24,29]. A comparison of the protection with this vaccination strategy against challenges with the genetically distinct clones NF135.C10 from Cambodia and NF166.C8 from Guinea was performed [30]. Consistent with the previous studies, vaccination provided 100% sterile immunity to the homologous parasite, but achieved 10 to 20% protection against the heterologous clones. Whole genome sequencing using various platforms was used to compare the vaccine strain NF54 with the clone 3D7, as well as strains used for heterologous challenges (7G8, NF166.C8 and NF135.C10), and also with a collection of clinical isolates from around the world [86]. As expected, not much variation was detected between NF54 and the 3D7 clone derived from it, but thousands of SNPs, indels and small structural variations, many of which fall in immunologically important regions, were identified in comparisons with the heterologous strains. These results make a clear case for the profound impact of parasite diversity on vaccine development.
4. The Disease: The Merozoite
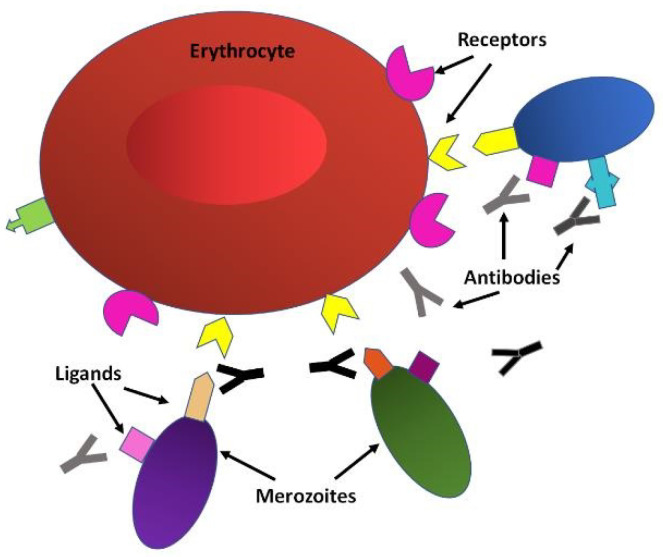
Once the merozoites transition from the liver to the bloodstream, they infect erythrocytes, initiating the blood cycle. This is a complex process involving many parasite proteins and host cell receptors interactions that are highly species-specific. P. falciparum uses several invasion pathways, some of which are redundant and interchangeable [87,88]. The first step is the attachment to the erythrocyte through merozoite surface proteins (MSPs). MSP1 is a major player in initial attachment, forming a complex with merozoite surface protein duffy binding ligands (MSPDBL1 and MSPDBL2) and mediating interactions with erythrocyte proteoglycans and membrane proteins [89]. Other proteins of this family, MSP3, MSP6 and MSP7, are also part of this complex, and MSP2 and MSP4 seem to be essential for invasion as well [90]. The merozoite rotates until the apical end contacts the erythrocyte membrane, secreting invasion ligands from the apical organelles, the rhoptries and micronemes. These are erythrocyte binding antigens (EBA-175, EBA-140, EBA181 and EBL1) and reticulocyte binding-like homologues (Rh2a, Rh2b, Rh4 and Rh5) that interact specifically with erythrocyte receptors [91]. The secretion of apical membrane antigen 1 (AMA1) from the micronemes forms a tight-junction together with the rhoptry neck protein 2 RON2, initiating internalisation of the merozoite powered by an actin–myosin motor that pulls the erythrocyte membrane around the parasite [91]. During this process, parasite surface proteins including AMA1 and MSP1 are cleaved by proteases and shed into the erythrocyte and the blood stream. Then the parasite grows to trophozoite and schizont, which will burst out of the erythrocyte, releasing merozoites which will continue the infection.
Targeting the blood cycle for vaccine development is attractive because this is the stage that causes most of the clinical symptoms of the disease (Figure 3). It is also when merozoites are periodically released into the bloodstream and therefore briefly free and accessible to the immune system and unsuccessful merozoites linger for even longer periods of time. Furthermore, egress and invasion result in the release of parasite proteins that can strengthen the immune response. The proteins involved in erythrocyte invasion are ideal targets for vaccine development because antibodies against them have been shown to interfere with this process [90,92]. Interference with the blood cycle would prevent disease and potentially also transmission.
Figure 3.
The blood cycle. Merozoites with different haplotypes (represented by different colours) of surface ligands (represented by different shapes) bind to erythrocyte receptors to start the invasion process. Erythrocytic stage vaccines aim to induce antibodies that recognise the parasite’s ligands to interfere with invasion and mediate the elimination of the parasite.
The invasion protein AMA1 is a leading target for vaccine development because of its high immunogenicity and the ability of antibodies against it to block invasion [93]. An AMA1 vaccine was trialled in Malian children [31] resulting in similar infection rates in the vaccinated and non-vaccinated groups (48.4% and 54.4%, respectively), for an efficacy of 17%. A higher level of protection was reported for cases of infection with a parasite homologous to the vaccine strain (64%), but both waned with time to 24% with homologous and 10% heterologous parasites in a 24 month follow-up study [32]. Disappointingly, the AMA1 vaccine showed no impact on the rate of experimental infections in controlled infection trials with the homologous strain 3D7 [33,34]. AMA1 is highly polymorphic; the genetic diversity includes amino acid changes in regions involved in invasion [66,94], and the extensive variation has been described even at the regional level [95,96]. This protein was combined with MSP1 in an attempt to increase efficacy, but protection levels against CHMI with the homologous parasite remained low (11%) [35], suggesting that antigenic polymorphism cannot be overcome by multiepitope designs.
To overcome AMA1 polymorphism, a diversity-covering formulation, AMA1-DiCo, was designed incorporating three variants to represent the major haplotypes based on 355 sequences available at the time, to provide broader protection [97]. A phase Ia/Ib trial was conducted with French and Burkinabe volunteers that reported the induction of antibodies reactive to parasites from different strains [98]. A combination with its natural partner in the invasion process, PfRon2, in an attempt to increase the potency of the IgG antibodies induced has also been tried [99]. However, though this combination was shown to improve the protection conferred by AMA1 alone to P. falciparum infection in Aotus monkeys [100], Ron2 did not improve cross-strain antibodies in humans, nor in vitro growth inhibition [99]. These data suggest it would be very difficult to cover the worldwide haplotypes in order to confer a global protection against malaria.
The merozoite protein MSP3 was identified as a vaccine candidate by the association of antibodies against it with parasite growth inhibition and protection in passive antibody transfer experiments [101]. While MSP3 is a highly variable protein, the C-terminus has been shown to be relatively conserved among parasite isolates [102,103]. A vaccine was developed with the C-terminus of MSP3 from P. falciparum Fc27 or 3D7, strains and several clinical trials have showed the induction of specific antibodies and cellular immunity [104,105,106,107]. Naturally developed antibodies against MSP3 are associated with a reduced risk of infection [108], but allele-specific antibodies showed a stronger correlation with protection than antibodies to the relatively conserved C-terminus. A diversity study in Thailand found two major haplotypes, designated 3D7 and K1, whose contribution to an effective immune response argues in favour of the inclusion of these sequences in a vaccine formulation. However, expanding the analysis to a wider collection of isolates from around the world painted a much more complex picture of the prevalent haplotypes in different areas [109], indicating that the two major haplotypes K1 and 3D7 are unlikely to cover the global variation in this protein.
In a renewed effort, the C-terminus of MSP3 was fused with a region of the glutamate-rich protein GLURP in the GMZ2 vaccine. GLURP is expressed through the pre-erythrocytic and erythrocytic stages, and has three major regions (GLURP94–489 (R0), GLURP489–705 (R1), and GLURP705–1178 (R2)) that elicit IgG responses. A strong correlation between IgG anti R0 and R2 with protection against malaria infection was reported [110]. However, a phase II trial in various countries in Africa showed an efficacy of 14% and 27% against severe malaria [36], while a CHMI trial with a homologous parasite failed to confer any protection against a PfSPZ challenge [37]. The low efficacy is likely to be related to the high variability of GLURP, whose only region of relative conservation is R2 [66].
Other more conserved surface proteins are being tested for their capacity to elicit a protective immune response—the trophozoite exported protein TEX1 and the serine repeat antigen 5 (SERA5) [66]. TEX1 is exported to Maurer’s Clefts in the erythrocyte and its function has not yet been determined. A highly conserved segment from TEX1, Pf27, that corresponds to a sequence predicted to assume a random coiled coil of 104 aminoacids (P27A), was found to be highly immunogenic and have growth inhibitory capacity in assays in vitro [111]. The genetic diversity of this peptide was limited to three non-synonymous SNPs, of which two were present in only one isolate examined worldwide [112]. There are great expectations for further trials with this peptide.
SERA5 is an abundant blood stage antigen present in the lumen of the parasitophorous vacuole and released into the blood stream following schizont egress. It was selected for clinical development on the basis of epidemiological studies showing high antibody titres that inversely correlated with malaria symptoms and severe disease [113]. In vitro studies of parasite growth inhibition by specific antibodies and animal studies demonstrated protection against P. falciparum challenge in non-human primates [114]. A recombinant form of the SERA5 N-terminal domain, SE36, based on the P. falciparum Honduras-1 strain showed significant protective efficacies only for more severe diseases, suggesting that BK-SE36 could have a disease-ameliorating rather than preventive effect [38]. The analysis of 445 near full-length P. falciparum SERA5 sequences from nine countries in Africa, Southeast Asia, Oceania and South America revealed extensive variations in the number of octamer repeat (OR) and serine repeat (SR) regions, as well as substantial levels of single nucleotide polymorphism (SNP) in non-repeat regions. Remarkably, a 14 amino acid sequence of SERA5 (amino acids 59-72) that is known to be the in vitro target of parasite growth inhibitory antibodies was found to be perfectly conserved in all 445 worldwide isolates of P. falciparum evaluated [115].
More recently, Rh5 emerged as the essential ligand for the P. falciparum invasion of erythrocytes [116], which makes it an ideal vaccine candidate that circumvents all the redundant alternative invasion pathways the parasite has devised through evolution. Rh5 has been found to be relatively conserved in isolates from several countries [117], and also more globally, with most of the diversity localised to the central region of the protein [66]. A full length Rh5 vaccine from the P. falciparum 3D7 strain induced antibodies in mice that were capable of inhibiting worldwide strains of the parasite in vitro [118]. A phase Ia clinical trial showed that an Rh5 vaccine induces antibodies with cross-strain in vitro growth inhibition activity [119]. Further trials to demonstrate the efficacy of the vaccine are ongoing, and given the essentiality of this protein, there is great interest in the outcome.
5. Whole Asexual Parasites Vaccine
With the aim of maximising antigen presentation to the immune system, a vaccine consisting of blood stage chemically inactivated asexual parasites was designed. The whole parasite approach is intended to induce immunity to a high number of parasite antigens, including unknown ones together with a mixture of conserved and variable proteins. In vivo studies in Aotus monkeys showed that the chemical inactivation of the FVO P. falciparum parasite is effective, and that vaccination induces parasite-specific T-cells, but not antibodies [120]. In the first clinical study of this kind, malaria-naïve participants received one dose of chemically inactivated 7G8 P. falciparum parasites [121]. The results showed a variable IgM response, lymphocytic proliferation induced by homologous as well as heterologous parasites, and an increase in various cytokines, including IFN-gamma. There are no data yet about the parasiticidal and protective capacity of the immune response induced by this vaccine. It will be crucial to test the cross-strain protection levels of a whole parasite vaccine to evaluate if the inclusion of all antigenic proteins can overcome the high genetic diversity of worldwide P. falciparum strains.
6. Transmission: The Gametocytes
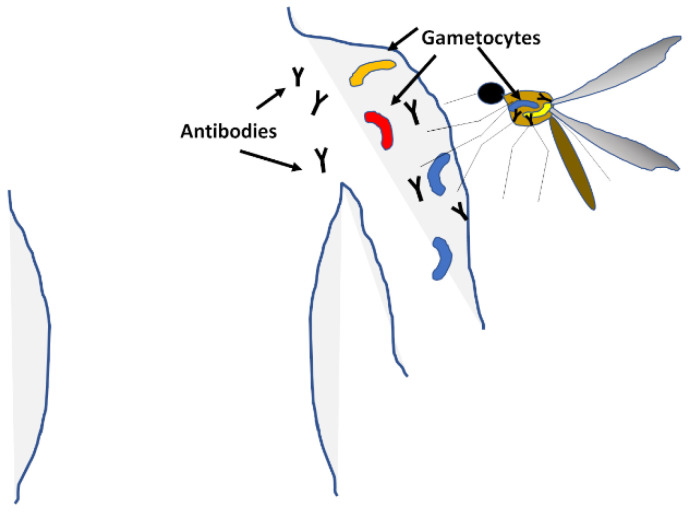
Gametocytes differentiate in the blood when infection is high or the health of the human host deteriorates, putting in jeopardy the survival of the parasite (Figure 4). Female and male stages are taken by the mosquito during the blood meal, which mate in the mosquito to form a zygote, and it is at this point that genetic recombination can happen [122]. The development in the mosquito culminates with the formation of sporozoites that migrate to the salivary glands and are deposited in the next human host during the mosquito’s next blood meal [123]. Vaccines targeting the gametocyte stage aim at reducing or stopping the transmission of malaria from one person to the next, rather than preventing clinical disease in any one person. Interruption of transmission has been proven many times throughout the history of the battle with malaria to be very effective at reducing the overall burden of the disease; these include the fumigations in 40 s–60 s to reduce mosquito populations, and more recently the introduction of insecticide-impregnated bednets to prevent mosquito bites. However, these initiatives proved to have complications, such as the toxicity of the insecticides, the resistance of the mosquitoes to them and changes in mosquito behaviour to diurnal habits. Targeting the gametocytes is one potential avenue to stop transmission and lower the burden of malaria in communities.
Figure 4.
Transmission. Male and female gametocytes form in the blood stream and migrate to the skin, where they are taken up by feeding mosquitoes. Transmission-blocking vaccines aim to mount an immune response against the gametocytes or the early development stages in the mosquito to eliminate the sexual stages and reduce the oocyte load in the vector.
A variety of proteins with different patterns of expression have been used to induce a transmission-blocking immune response. The antigens Pfs230C and Pfs48/45 are expressed in gametocytes within the human host, while Pfs25 and Pfs28 are expressed in the late gametocyte, zygote and oocyst stages of the parasite in the mosquito midgut. Some novel proteins have also been considered, including the male gametocyte HAP2, the female gametocyte Pfs47 and the mosquito APN1 [124].
Of these proteins, vaccines prepared with NF54 sequences for Pfs25 and Pfs230C conferred a complete blockage of NF54 P. falciparum parasites in A. stephensi mosquitoes [125], reducing oocyst counts in an antibody concentration-dependent manner. Tests for efficacy against heterologous parasites were also performed using isolates from children in Burkina Faso. The efficacy of Pfs25 in inducing transmission-blocking antibodies with activity against heterologous parasites was confirmed with P. falciparum isolates from Thailand [126]. The sequencing of Pfs25 among isolates revealed that this protein is highly conserved. Wider sequencing studies of 329 isolates from various countries in Africa and Asia confirmed the conservation of Pfs25, detecting only 1 polymorphism in the region associated with the binding of transmission-blocking antibodies [127]. This was in contrast with Pfs48/45, which showed eight non-synonymous mutations, four of which affected transmission-blocking epitopes. Though Pfs230C also demonstrated complete transmission blocking activity, it was found to be much more diverse than Pfs25 [4], so heterologous activity with a wider selection of geographical isolates would need to be established. The high conservation of Pfs25 and its effectiveness in inducing transmission-blocking antibodies show potential for this strategy to control malaria.
7. Multi-Stage Vaccine
A widely tested synthetic multiepitope and multistage vaccine against P. falciparum was developed in Colombia over 30 years ago. The synthetic P. falciparum vaccine 66 (SPf66) consisted of peptides derived from the Colombian FCB-2 strain, including three merozoite proteins: MSP1, SERA, and an unidentified molecule and the NANP repeat from the sporozoite CSP [128]. The vaccine was trialled in Colombia [129] and neighbouring countries Venezuela [130] and Ecuador [131], with a reported efficacy of the vaccine ranging between 35 and 60%. However, when tested further afield in the Rondonia state in Brazil [132], the efficacy dropped to 14% for the first episode of malaria. When testing was expanded to different continents, the drop in efficacy went from 31% in Tanzania [133] to 8% in Gambia [134], and no protection was reported in Thailand [135]. Though all these trials were set up and evaluated differently, most of them reported the induction of an immune response to the vaccine, which makes a compelling case for the role of parasite genetic diversity in the drop in vaccine efficacy in different geographic regions.
Another vaccine targeting various stages of the parasite was NYVAC-Pf7, composed of seven antigens derived from the P. falciparum 3D7 strain including three pre-erythrocytic (CSP, TRAP and LSA-1), three blood stage (MSP1, SERA and AMA1) and the Pfs25 sexual stage antigen [136]. This vaccine was tested in Phase I/IIa trials showing high levels of cellular immunity even if the antibody response was overall weak [137]. CHMI with the homologous 3D7 strain resulted in 1/35 sterile protection and a significant reduction in the time to parasitemia. More recently, a combination of AMA1 and CSP in the NMRC-M3V-Ad-PfCA vaccine showed no [39] or modest protection [40]. A more complex combination, containing constructs with AMA1-DiCo and Pfs25 together with either CSP, MSP1 or Rh5, was used in the immunisation of rabbits [138]. Efficacy assays with rabbit antibodies showed promise, but evidenced a need to improve the induction of reactive antibodies. Potentially, the use of AMA-DiCo would give these formulations a broader coverage, but as noted above, it is unlikely to be worldwide.
8. Lessons for the Future
The ideal vaccine should induce universal, long-term protection with the fewest possible doses. The current vaccines discussed here, as well as many previous attempts and trials, have been developed from P. falciparum lab strains. In most cases, the efficacy was promising for homologous strains but declined significantly with increasingly geographically distant heterologous parasites. Single-antigen vaccines stimulate an immune response of variable strength and that is generally strain-specific. Multi-stage vaccines improve the potency of the immune response, but not the cross-reactivity. Whole parasite strategies elicit strong and broader immunity, including conserved epitopes, but though sterile protection can be achieved with homologous strains, protection against heterologous parasites drops significantly. Designs covering some of the diversity of specific antigens show a broader antibody response, but generally also highlight the difficulty in achieving overall coverage for a universal vaccine.
We have advanced a long way from the initial anti-Plasmodium falciparum malaria vaccines, which viewed the species as one parasite. Antigen diversity has started being increasingly appreciated, particularly since the P. falciparum genomic sequence has been completed [139], but the full extent of genomic variation was only unveiled by the vast sequencing of worldwide parasite samples in the last decade. With these data, there is now a much more complete picture of the genomic characteristics of parasites in different regions, the prevalence of multiclonal infections, and the dynamics of parasite populations that will necessarily have an impact on the efficacy of vaccine efforts. The availability of these vast sets of genomic data and the advent of informatics capabilities must be taken full advantage of in order to guide the identification of vaccine candidates and design new approaches. The existing resources allow an in-depth analysis of genetic diversity and the categorising of the world haplotypes into groups with common features. The sequence analysis can also be complemented with data from immunological studies to select the most immunogenic and conserved peptides.
Perhaps a design including peptides from several antigens, each one covering the major haplotypes, would increase strain coverage, since the haplotypes for each peptide are unlikely to overlap the same strains. A synthetic vaccine of this kind gives more flexibility than a whole parasite approach, where the number of strains that can be included is limited. However, gene editing technologies, guided by genomic data, could perhaps be exploited to introduce the most prominent haplotypes of different antigens into one engineered ‘universal parasite’. Alternatively, it might become more feasible to consider regional vaccines covering specifically local haplotypes.
The latest data and technology will be useful to identify specific epitopes that are conserved enough to be used in a vaccine either on their own or in a combination of haplotypes to trigger an efficient immune response. This needs to be accompanied by the development of novel tests to measure and assess the efficacy of the new generation vaccines to bring them as close to humans as possible before deploying clinical trials. Some of the recent efforts are already using these advances to improve the chances of defeating this parasite.
Acknowledgments
This work was funded by the Wellcome Sanger Institute and much gratitude is owed to Julian Rayner for his support.
Abbreviations
| CHMI | Controlled human malaria infection |
| SPECT1 | Sporozoite microneme protein essential for cell traversal |
| CeLTOS | Cell traversal protein for ookinetes and sporozoites |
| PL | Phospholipase |
| HSPGs | Heparan sulphate proteoglycans |
| CSP | Circumsporozoite protein |
| TRAP | Thrombospondin-related anonymous protein |
| AMA-1 | Apical membrane antigen 1 |
| PV | Parasitophorous vacuole |
| EXP-1 | Exported protein 1 |
| LSA | Liver stage antigen |
| STARP | Sporozoite threonine and asparagine-rich protein |
| ME-TRAP | Multi epitope TRAP |
| PfSPZ | Plasmodium falciparum sporozoite |
| PfGAP3KO | P. falciparum genetically attenuated parasite P52, p36 and sap1 deleted |
| MSP | Merozoite surface protein |
| MSPDBL | Merozoite surface protein duffy binding ligand |
| EBA | Erythrocyte binding antigen |
| Rh | Reticulocyte binding-like homologue |
| RON2 | Rhoptry neck protein 2 |
| AMA1-DiCo | Apical membrane Aantigen 1 diversity covering |
| GLURP | Glutamate-rich protein |
| GMZ2 | vaccine formulation with MSP3 and GLURP |
| TEX1 | Trophozoite exported protein |
| SERA5 | Serine repeat antigen 5 |
| SPf66 | Synthetic P. falciparum vaccine 66 |
| NYVAC-Pf7 | P. falciparum hepta-vaccine |
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organisation World Malaria Report 2018. [(accessed on 1 October 2020)]; Available online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/
- 2.World Health Organisation . Tables of Malaria Vaccine Projects Globally. World Health Organisation; Geneva, Switzerland: 2017. [Google Scholar]
- 3.Gardner M.J., Hall N., Fung E., White O., Berriman M., Hyman R.W., Carlton J.M., Pain A., Nelson K.E., Bowman S., et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manske M., Miotto O., Campino S., Auburn S., Almagro-Garcia J., Maslen G., O′Brien J., Djimde A., Doumbo O., Zongo I., et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton W.L., Amato R., van der Pluijm R.W., Jacob C.G., Quang H.H., Thuy-Nhien N.T., Hien T.T., Hongvanthong B., Chindavongsa K., Mayxay M., et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: A genomic epidemiology study. Lancet Infect. Dis. 2019;19:943–951. doi: 10.1016/S1473-3099(19)30392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campino S., Auburn S., Kivinen K., Zongo I., Ouedraogo J.B., Mangano V., Djimde A., Doumbo O.K., Kiara S.M., Nzila A., et al. Population genetic analysis of Plasmodium falciparum parasites using a customized Illumina GoldenGate genotyping assay. PLoS ONE. 2011;6:e20251. doi: 10.1371/journal.pone.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudson A., Gonzalez-Casabianca F., Feged-Rivadeneira A., Pedreros M.F., Aponte S., Olaya A., Castillo C.F., Mancilla E., Piamba-Dorado A., Sanchez-Pedraza R., et al. Spatio-temporal dynamics of Plasmodium falciparum transmission within a spatial unit on the Colombian Pacific Coast. Sci. Rep. 2020;10:3756. doi: 10.1038/s41598-020-60676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amambua-Ngwa A., Amenga-Etego L., Kamau E., Amato R., Ghansah A., Golassa L., Randrianarivelojosia M., Ishengoma D., Apinjoh T., Maiga-Ascofare O., et al. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science. 2019;365:813–816. doi: 10.1126/science.aav5427. [DOI] [PubMed] [Google Scholar]
- 9.Ewer K.J., O′Hara G.A., Duncan C.J., Collins K.A., Sheehy S.H., Reyes-Sandoval A., Goodman A.L., Edwards N.J., Elias S.C., Halstead F.D., et al. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat. Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgson S.H., Ewer K.J., Bliss C.M., Edwards N.J., Rampling T., Anagnostou N.A., de Barra E., Havelock T., Bowyer G., Poulton I.D., et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J. Infect. Dis. 2015;211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunachie S.J., Walther M., Epstein J.E., Keating S., Berthoud T., Andrews L., Andersen R.F., Bejon P., Goonetilleke N., Poulton I., et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 2006;74:5933–5942. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogwang C., Kimani D., Edwards N.J., Roberts R., Mwacharo J., Bowyer G., Bliss C., Hodgson S.H., Njuguna P., Viebig N.K., et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci. Transl. Med. 2015;7:286re285. doi: 10.1126/scitranslmed.aaa2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorthy V.S., Imoukhuede E.B., Milligan P., Bojang K., Keating S., Kaye P., Pinder M., Gilbert S.C., Walraven G., Greenwood B.M., et al. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiono A.B., Nebie I., Anagnostou N., Coulibaly A.S., Bowyer G., Lam E., Bougouma E.C., Ouedraogo A., Yaro J.B.B., Barry A., et al. First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLoS ONE. 2018;13:e0208328. doi: 10.1371/journal.pone.0208328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rts S.C.T.P. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rts S.C.T.P., Agnandji S.T., Lell B., Soulanoudjingar S.S., Fernandes J.F., Abossolo B.P., Conzelmann C., Methogo B.G., Doucka Y., Flamen A., et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 17.Rts S.C.T.P., Agnandji S.T., Lell B., Fernandes J.F., Abossolo B.P., Methogo B.G., Kabwende A.L., Adegnika A.A., Mordmuller B., Issifou S., et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012;367:2284–2295. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rts S.C.T.P. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: A phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 2014;11:e1001685. doi: 10.1371/journal.pmed.1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinto H., Otieno W., Gesase S., Sorgho H., Otieno L., Liheluka E., Valea I., Sing′oei V., Malabeja A., Valia D., et al. Long-term incidence of severe malaria following RTS,S/AS01 vaccination in children and infants in Africa: An open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect. Dis. 2019;19:821–832. doi: 10.1016/S1473-3099(19)30300-7. [DOI] [PubMed] [Google Scholar]
- 20.Regules J.A., Cicatelli S.B., Bennett J.W., Paolino K.M., Twomey P.S., Moon J.E., Kathcart A.K., Hauns K.D., Komisar J.L., Qabar A.N., et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J. Infect. Dis. 2016;214:762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 21.Moon J.E., Ockenhouse C., Regules J.A., Vekemans J., Lee C., Chuang I., Traskine M., Jongert E., Ivinson K., Morelle D., et al. A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS,S/AS01E and RTS,S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rampling T., Ewer K.J., Bowyer G., Bliss C.M., Edwards N.J., Wright D., Payne R.O., Venkatraman N., de Barra E., Snudden C.M., et al. Safety and High Level Efficacy of the Combination Malaria Vaccine Regimen of RTS,S/AS01B With Chimpanzee Adenovirus 63 and Modified Vaccinia Ankara Vectored Vaccines Expressing ME-TRAP. J. Infect. Dis. 2016;214:772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seder R.A., Chang L.J., Enama M.E., Zephir K.L., Sarwar U.N., Gordon I.J., Holman L.A., James E.R., Billingsley P.F., Gunasekera A., et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 24.Roestenberg M., McCall M., Hopman J., Wiersma J., Luty A.J., van Gemert G.J., van de Vegte-Bolmer M., van Schaijk B., Teelen K., Arens T., et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 25.Jongo S.A., Church L.W.P., Mtoro A.T., Chakravarty S., Ruben A.J., Swanson Ii P.A., Kassim K.R., Mpina M., Tumbo A.M., Milando F.A., et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ Vaccine in Tanzanian adults. Clin. Infect. Dis. 2019 doi: 10.1093/cid/ciz1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sissoko M.S., Healy S.A., Katile A., Omaswa F., Zaidi I., Gabriel E.E., Kamate B., Samake Y., Guindo M.A., Dolo A., et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect. Dis. 2017;17:498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jongo S.A., Shekalaghe S.A., Church L.W.P., Ruben A.J., Schindler T., Zenklusen I., Rutishauser T., Rothen J., Tumbo A., Mkindi C., et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am. J. Trop. Med. Hyg. 2018;99:338–349. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein J.E., Paolino K.M., Richie T.L., Sedegah M., Singer A., Ruben A.J., Chakravarty S., Stafford A., Ruck R.C., Eappen A.G., et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. 2017;2:e89154. doi: 10.1172/jci.insight.89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mordmuller B., Surat G., Lagler H., Chakravarty S., Ishizuka A.S., Lalremruata A., Gmeiner M., Campo J.J., Esen M., Ruben A.J., et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542:445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walk J., Reuling I.J., Behet M.C., Meerstein-Kessel L., Graumans W., van Gemert G.J., Siebelink-Stoter R., van de Vegte-Bolmer M., Janssen T., Teelen K., et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: A double-blind randomized controlled clinical trial. BMC Med. 2017;15:168. doi: 10.1186/s12916-017-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thera M.A., Doumbo O.K., Coulibaly D., Laurens M.B., Ouattara A., Kone A.K., Guindo A.B., Traore K., Traore I., Kouriba B., et al. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 2011;365:1004–1013. doi: 10.1056/NEJMoa1008115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurens M.B., Thera M.A., Coulibaly D., Ouattara A., Kone A.K., Guindo A.B., Traore K., Traore I., Kouriba B., Diallo D.A., et al. Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in malian children: 24-month follow-up of a randomized, double-blinded phase 2 trial. PLoS ONE. 2013;8:e79323. doi: 10.1371/journal.pone.0079323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne R.O., Milne K.H., Elias S.C., Edwards N.J., Douglas A.D., Brown R.E., Silk S.E., Biswas S., Miura K., Roberts R., et al. Demonstration of the Blood-Stage Plasmodium falciparum Controlled Human Malaria Infection Model to Assess Efficacy of the P. falciparum Apical Membrane Antigen 1 Vaccine, FMP2.1/AS01. J. Infect. Dis. 2016;213:1743–1751. doi: 10.1093/infdis/jiw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spring M.D., Cummings J.F., Ockenhouse C.F., Dutta S., Reidler R., Angov E., Bergmann-Leitner E., Stewart V.A., Bittner S., Juompan L., et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4:e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehy S.H., Duncan C.J., Elias S.C., Choudhary P., Biswas S., Halstead F.D., Collins K.A., Edwards N.J., Douglas A.D., Anagnostou N.A., et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: Assessment of efficacy against mosquito bite challenge in humans. Mol. Ther. 2012;20:2355–2368. doi: 10.1038/mt.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirima S.B., Mordmuller B., Milligan P., Ngoa U.A., Kironde F., Atuguba F., Tiono A.B., Issifou S., Kaddumukasa M., Bangre O., et al. A phase 2b randomized, controlled trial of the efficacy of the GMZ2 malaria vaccine in African children. Vaccine. 2016;34:4536–4542. doi: 10.1016/j.vaccine.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 37.Dejon-Agobe J.C., Ateba-Ngoa U., Lalremruata A., Homoet A., Engelhorn J., Nouatin O.P., Edoa J.R., Fernandes J.F., Esen M., Mouwenda Y.D., et al. Controlled Human Malaria Infection of Healthy Adults With Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2. Clin. Infect. Dis. 2019;69:1377–1384. doi: 10.1093/cid/ciy1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacpac N.M., Ntege E., Yeka A., Balikagala B., Suzuki N., Shirai H., Yagi M., Ito K., Fukushima W., Hirota Y., et al. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS ONE. 2013;8:e64073. doi: 10.1371/journal.pone.0064073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamminga C., Sedegah M., Maiolatesi S., Fedders C., Reyes S., Reyes A., Vasquez C., Alcorta Y., Chuang I., Spring M., et al. Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection. Hum. Vaccin Immunother. 2013;9:2165–2177. doi: 10.4161/hv.24941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chuang I., Sedegah M., Cicatelli S., Spring M., Polhemus M., Tamminga C., Patterson N., Guerrero M., Bennett J.W., McGrath S., et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS ONE. 2013;8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang A.S.P., O’Neill M.T., Jennison C., Lopaticki S., Allison C.C., Armistead J.S., Erickson S.M., Rogers K.L., Ellisdon A.M., Whisstock J.C., et al. Cell Traversal Activity Is Important for Plasmodium falciparum Liver Infection in Humanized Mice. Cell Rep. 2017;18:3105–3116. doi: 10.1016/j.celrep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 42.Jimah J.R., Salinas N.D., Sala-Rabanal M., Jones N.G., Sibley L.D., Nichols C.G., Schlesinger P.H., Tolia N.H. Malaria parasite CelTOS targets the inner leaflet of cell membranes for pore-dependent disruption. Elife. 2016;5 doi: 10.7554/eLife.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinnis P., Coppi A. A long and winding road: The Plasmodium sporozoite’s journey in the mammalian host. Parasitol. Int. 2007;56:171–178. doi: 10.1016/j.parint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar H., Tolia N.H. Getting in: The structural biology of malaria invasion. PLoS Pathog. 2019;15:e1007943. doi: 10.1371/journal.ppat.1007943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppi A., Natarajan R., Pradel G., Bennett B.L., James E.R., Roggero M.A., Corradin G., Persson C., Tewari R., Sinnis P. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J. Exp. Med. 2011;208:341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dundas K., Shears M.J., Sun Y., Hopp C.S., Crosnier C., Metcalf T., Girling G., Sinnis P., Billker O., Wright G.J. Alpha-v-containing integrins are host receptors for the Plasmodium falciparum sporozoite surface protein, TRAP. Proc. Natl. Acad. Sci. USA. 2018;115:4477–4482. doi: 10.1073/pnas.1719660115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pradel G., Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33:1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 48.Ishino T., Yano K., Chinzei Y., Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meis J.F., Verhave J.P., Jap P.H., Sinden R.E., Meuwissen J.H. Ultrastructural observations on the infection of rat liver by Plasmodium berghei sporozoites in vivo. J. Protozool. 1983;30:361–366. doi: 10.1111/j.1550-7408.1983.tb02931.x. [DOI] [PubMed] [Google Scholar]
- 50.Kori L.D., Valecha N., Anvikar A.R. Insights into the early liver stage biology of Plasmodium. J. Vector Borne Dis. 2018;55:9–13. doi: 10.4103/0972-9062.234631. [DOI] [PubMed] [Google Scholar]
- 51.Duffy P.E., Sahu T., Akue A., Milman N., Anderson C. Pre-erythrocytic malaria vaccines: Identifying the targets. Expert Rev. Vaccines. 2012;11:1261–1280. doi: 10.1586/erv.12.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obeid M., Franetich J.F., Lorthiois A., Gego A., Gruner A.C., Tefit M., Boucheix C., Snounou G., Mazier D. Skin-draining lymph node priming is sufficient to induce sterile immunity against pre-erythrocytic malaria. EMBO Mol. Med. 2013;5:250–263. doi: 10.1002/emmm.201201677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radtke A.J., Kastenmuller W., Espinosa D.A., Gerner M.Y., Tse S.W., Sinnis P., Germain R.N., Zavala F.P., Cockburn I.A. Lymph-node resident CD8alpha+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathog. 2015;11:e1004637. doi: 10.1371/journal.ppat.1004637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinnis P., Zavala F. The skin: Where malaria infection and the host immune response begin. Semin. Immunopathol. 2012;34:787–792. doi: 10.1007/s00281-012-0345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bettencourt P. Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens. Front. Immunol. 2020;11:190. doi: 10.3389/fimmu.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Todryk S.M., Bejon P., Mwangi T., Plebanski M., Urban B., Marsh K., Hill A.V., Flanagan K.L. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3:e2027. doi: 10.1371/journal.pone.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trieu A., Kayala M.A., Burk C., Molina D.M., Freilich D.A., Richie T.L., Baldi P., Felgner P.L., Doolan D.L. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol. Cell Proteom. 2011;10:M111.007948. doi: 10.1074/mcp.M111.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ken Tucker A.R.N., Kotraiah V., Phares T.W., Tsuji M., Nardin E.H., Gutierrez G.M. Current Topics in Imalaria. IntechOpen; London, UK: 2016. Pre-Erythrocytic Vaccine Candidates in Malaria. [DOI] [Google Scholar]
- 59.Webster D.P., Dunachie S., Vuola J.M., Berthoud T., Keating S., Laidlaw S.M., McConkey S.J., Poulton I., Andrews L., Andersen R.F., et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA. 2005;102:4836–4841. doi: 10.1073/pnas.0406381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bejon P., Mwacharo J., Kai O.K., Todryk S., Keating S., Lang T., Gilbert S.C., Peshu N., Marsh K., Hill A.V. Immunogenicity of the candidate malaria vaccines FP9 and modified vaccinia virus Ankara encoding the pre-erythrocytic antigen ME-TRAP in 1-6 year old children in a malaria endemic area. Vaccine. 2006;24:4709–4715. doi: 10.1016/j.vaccine.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 61.Tredgold R.H., Elgamal M. A study of the incorporation of cytochrome oxidase into planar synthetic membranes. Biochim. Biophys. Acta. 1979;555:381–387. doi: 10.1016/0005-2736(79)90392-4. [DOI] [PubMed] [Google Scholar]
- 62.Mehrizi A.A., Jafari Zadeh A., Zakeri S., Djadid N.D. Population genetic structure analysis of thrombospondin-related adhesive protein (TRAP) as a vaccine candidate antigen in worldwide Plasmodium falciparum isolates. Infect. Genet. Evol. 2020;80:104197. doi: 10.1016/j.meegid.2020.104197. [DOI] [PubMed] [Google Scholar]
- 63.Yang C., Shi Y.P., Udhayakumar V., Alpers M.P., Povoa M.M., Hawley W.A., Collins W.E., Lal A.A. Sequence variations in the non-repetitive regions of the liver stage-specific antigen-1 (LSA-1) of Plasmodium falciparum from field isolates. Mol. Biochem. Parasitol. 1995;71:291–294. doi: 10.1016/0166-6851(95)00069-D. [DOI] [PubMed] [Google Scholar]
- 64.Ravichandran M., Doolan D.L., Cox-Singh J., Hoffman S.L., Singh B. Research note: HLA degenerate T-cell epitopes from Plasmodium falciparum liver stage-specific antigen 1 (LSA-1) are highly conserved in isolates from geographically distinct areas. Parasite Immunol. 2000;22:469–473. doi: 10.1046/j.1365-3024.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 65.Jongwutiwes S., Putaporntip C., Karnchaisri K., Seethamchai S., Hongsrimuang T., Kanbara H. Positive selection on the Plasmodium falciparum sporozoite threonine-asparagine-rich protein: Analysis of isolates mainly from low endemic areas. Gene. 2008;410:139–146. doi: 10.1016/j.gene.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Preston M.D., Assefa S.A., Ocholla H., Sutherland C.J., Borrmann S., Nzila A., Michon P., Hien T.T., Bousema T., Drakeley C.J., et al. PlasmoView: A web-based resource to visualise global Plasmodium falciparum genomic variation. J. Infect. Dis. 2014;209:1808–1815. doi: 10.1093/infdis/jit812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aliprandini E., Tavares J., Panatieri R.H., Thiberge S., Yamamoto M.M., Silvie O., Ishino T., Yuda M., Dartevelle S., Traincard F., et al. Cytotoxic anti-circumsporozoite antibodies target malaria sporozoites in the host skin. Nat. Microbiol. 2018;3:1224–1233. doi: 10.1038/s41564-018-0254-z. [DOI] [PubMed] [Google Scholar]
- 68.Herrington D.A., Clyde D.F., Davis J.R., Baqar S., Murphy J.R., Cortese J.F., Bank R.S., Nardin E., DiJohn D., Nussenzweig R.S., et al. Human studies with synthetic peptide sporozoite vaccine (NANP)3-TT and immunization with irradiated sporozoites. Bull. World Health Organ. 1990;68:33–37. [PMC free article] [PubMed] [Google Scholar]
- 69.Walther M., Dunachie S., Keating S., Vuola J.M., Berthoud T., Schmidt A., Maier C., Andrews L., Andersen R.F., Gilbert S., et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23:857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 70.Audran R., Lurati-Ruiz F., Genton B., Blythman H.E., Ofori-Anyinam O., Reymond C., Corradin G., Spertini F. The synthetic Plasmodium falciparum circumsporozoite peptide PfCS102 as a malaria vaccine candidate: A randomized controlled phase I trial. PLoS ONE. 2009;4:e7304. doi: 10.1371/journal.pone.0007304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neafsey D.E., Juraska M., Bedford T., Benkeser D., Valim C., Griggs A., Lievens M., Abdulla S., Adjei S., Agbenyega T., et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. N. Engl. J. Med. 2015;373:2025–2037. doi: 10.1056/NEJMoa1505819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pringle J.C., Carpi G., Almagro-Garcia J., Zhu S.J., Kobayashi T., Mulenga M., Bobanga T., Chaponda M., Moss W.J., Norris D.E. RTS,S/AS01 malaria vaccine mismatch observed among Plasmodium falciparum isolates from southern and central Africa and globally. Sci. Rep. 2018;8:6622. doi: 10.1038/s41598-018-24585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le H.G., Kang J.M., Moe M., Jun H., Thai T.L., Lee J., Myint M.K., Lin K., Sohn W.M., Shin H.J., et al. Genetic polymorphism and natural selection of circumsporozoite surface protein in Plasmodium falciparum field isolates from Myanmar. Malar. J. 2018;17:361. doi: 10.1186/s12936-018-2513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pance A. How elusive can a malaria vaccine be? Nat. Rev. Microbiol. 2019;17:129. doi: 10.1038/s41579-018-0148-3. [DOI] [PubMed] [Google Scholar]
- 75.Kanoi B.N., Takashima E., Morita M., White M.T., Palacpac N.M., Ntege E.H., Balikagala B., Yeka A., Egwang T.G., Horii T., et al. Antibody profiles to wheat germ cell-free system synthesized Plasmodium falciparum proteins correlate with protection from symptomatic malaria in Uganda. Vaccine. 2017;35:873–881. doi: 10.1016/j.vaccine.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Bergmann-Leitner E.S., Mease R.M., De La Vega P., Savranskaya T., Polhemus M., Ockenhouse C., Angov E. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS ONE. 2010;5:e12294. doi: 10.1371/journal.pone.0012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergmann-Leitner E.S., Legler P.M., Savranskaya T., Ockenhouse C.F., Angov E. Cellular and humoral immune effector mechanisms required for sterile protection against sporozoite challenge induced with the novel malaria vaccine candidate CelTOS. Vaccine. 2011;29:5940–5949. doi: 10.1016/j.vaccine.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 78.Espinosa D.A., Vega-Rodriguez J., Flores-Garcia Y., Noe A.R., Munoz C., Coleman R., Bruck T., Haney K., Stevens A., Retallack D., et al. The Plasmodium falciparum Cell-Traversal Protein for Ookinetes and Sporozoites as a Candidate for Preerythrocytic and Transmission-Blocking Vaccines. Infect. Immun. 2017;85 doi: 10.1128/IAI.00498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pirahmadi S., Zakeri S., Mehrizi A.A., Djadid N.D. Analysis of genetic diversity and population structure of gene encoding cell-traversal protein for ookinetes and sporozoites (CelTOS) vaccine candidate antigen in global Plasmodium falciparum populations. Infect. Genet. Evol. 2018;59:113–125. doi: 10.1016/j.meegid.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 80.Freund J., Thomson K.J., Sommer H.E., Walter A.W., Schenkein E.L. Immunization of Rhesus Monkeys against Malarial Infection (P. Knowlesi) with Killed Parasites and Adjuvants. Science. 1945;102:202–204. doi: 10.1126/science.102.2643.202. [DOI] [PubMed] [Google Scholar]
- 81.Heidelberger M., Mayer M.M., Demarest C.R. Studies in human malaria; the preparation of vaccines and suspensions containing plasmodia. J. Immunol. 1946;52:325–330. [PubMed] [Google Scholar]
- 82.Nussenzweig R.S., Vanderberg J., Most H., Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 83.Vaughan A.M., Wang R., Kappe S.H. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum. Vaccin. 2010;6:107–113. doi: 10.4161/hv.6.1.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spring M., Murphy J., Nielsen R., Dowler M., Bennett J.W., Zarling S., Williams J., de la Vega P., Ware L., Komisar J., et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine. 2013;31:4975–4983. doi: 10.1016/j.vaccine.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 85.Kublin J.G., Mikolajczak S.A., Sack B.K., Fishbaugher M.E., Seilie A., Shelton L., VonGoedert T., Firat M., Magee S., Fritzen E., et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aad9099. [DOI] [PubMed] [Google Scholar]
- 86.Moser K.A., Drabek E.F., Dwivedi A., Stucke E.M., Crabtree J., Dara A., Shah Z., Adams M., Li T., Rodrigues P.T., et al. Strains used in whole organism Plasmodium falciparum vaccine trials differ in genome structure, sequence, and immunogenic potential. Genome Med. 2020;12:6. doi: 10.1186/s13073-019-0708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowman A.F., Berry D., Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J. Cell Biol. 2012;198:961–971. doi: 10.1083/jcb.201206112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duraisingh M.T., DeSimone T., Jennings C., Refour P., Wu C. Erythrocyte invasion by Plasmodium falciparum: Multiple ligand-receptor interactions and phenotypic switching. Subcell. Biochem. 2008;47:46–57. doi: 10.1007/978-0-387-78267-6_3. [DOI] [PubMed] [Google Scholar]
- 89.Lin C.S., Uboldi A.D., Marapana D., Czabotar P.E., Epp C., Bujard H., Taylor N.L., Perugini M.A., Hodder A.N., Cowman A.F. The merozoite surface protein 1 complex is a platform for binding to human erythrocytes by Plasmodium falciparum. J. Biol. Chem. 2014;289:25655–25669. doi: 10.1074/jbc.M114.586495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beeson J.G., Drew D.R., Boyle M.J., Feng G., Fowkes F.J., Richards J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. Fems Microbiol. Rev. 2016;40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weiss G.E., Gilson P.R., Taechalertpaisarn T., Tham W.H., de Jong N.W., Harvey K.L., Fowkes F.J., Barlow P.N., Rayner J.C., Wright G.J., et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 2015;11:e1004670. doi: 10.1371/journal.ppat.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salamanca D.R., Gomez M., Camargo A., Cuy-Chaparro L., Molina-Franky J., Reyes C., Patarroyo M.A., Patarroyo M.E. Plasmodium falciparum Blood Stage Antimalarial Vaccines: An Analysis of Ongoing Clinical Trials and New Perspectives Related to Synthetic Vaccines. Front. Microbiol. 2019;10:2712. doi: 10.3389/fmicb.2019.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polley S.D., Mwangi T., Kocken C.H., Thomas A.W., Dutta S., Lanar D.E., Remarque E., Ross A., Williams T.N., Mwambingu G., et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 94.Takala S.L., Coulibaly D., Thera M.A., Batchelor A.H., Cummings M.P., Escalante A.A., Ouattara A., Traore K., Niangaly A., Djimde A.A., et al. Extreme polymorphism in a vaccine antigen and risk of clinical malaria: Implications for vaccine development. Sci. Transl. Med. 2009;1:2ra5. doi: 10.1126/scitranslmed.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller R.H., Hathaway N.J., Kharabora O., Mwandagalirwa K., Tshefu A., Meshnick S.R., Taylor S.M., Juliano J.J., Stewart V.A., Bailey J.A. A deep sequencing approach to estimate Plasmodium falciparum complexity of infection (COI) and explore apical membrane antigen 1 diversity. Malar. J. 2017;16:490. doi: 10.1186/s12936-017-2137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lumkul L., Sawaswong V., Simpalipan P., Kaewthamasorn M., Harnyuttanakorn P., Pattaradilokrat S. Unraveling Haplotype Diversity of the Apical Membrane Antigen-1 Gene in Plasmodium falciparum Populations in Thailand. Korean J. Parasitol. 2018;56:153–165. doi: 10.3347/kjp.2018.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Remarque E.J., Faber B.W., Kocken C.H., Thomas A.W. A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect. Immun. 2008;76:2660–2670. doi: 10.1128/IAI.00170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sirima S.B., Durier C., Kara L., Houard S., Gansane A., Loulergue P., Bahuaud M., Benhamouda N., Nebie I., Faber B., et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel(R) in European and African adults: A phase 1a/1b, randomized, double-blind multi-centre trial. Vaccine. 2017;35:6218–6227. doi: 10.1016/j.vaccine.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 99.Spiegel H., Boes A., Fendel R., Reimann A., Schillberg S., Fischer R. Immunization with the Malaria Diversity-Covering Blood-Stage Vaccine Candidate Plasmodium falciparum Apical Membrane Antigen 1 DiCo in Complex with Its Natural Ligand PfRon2 Does Not Improve the In Vitro Efficacy. Front. Immunol. 2017;8:743. doi: 10.3389/fimmu.2017.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srinivasan P., Baldeviano G.C., Miura K., Diouf A., Ventocilla J.A., Leiva K.P., Lugo-Roman L., Lucas C., Orr-Gonzalez S., Zhu D., et al. A malaria vaccine protects Aotus monkeys against virulent Plasmodium falciparum infection. npj Vaccines. 2017;2 doi: 10.1038/s41541-017-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh S., Soe S., Mejia J.P., Roussilhon C., Theisen M., Corradin G., Druilhe P. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 2004;190:1010–1018. doi: 10.1086/423208. [DOI] [PubMed] [Google Scholar]
- 102.McColl D.J., Anders R.F. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3) Mol. Biochem. Parasitol. 1997;90:21–31. doi: 10.1016/S0166-6851(97)00130-8. [DOI] [PubMed] [Google Scholar]
- 103.Nebie I., Diarra A., Ouedraogo A., Tiono A.B., Konate A.T., Gansane A., Soulama I., Cousens S., Leroy O., Sirima S.B. Humoral and cell-mediated immunity to MSP3 peptides in adults immunized with MSP3 in malaria endemic area, Burkina Faso. Parasite Immunol. 2009;31:474–480. doi: 10.1111/j.1365-3024.2009.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Audran R., Cachat M., Lurati F., Soe S., Leroy O., Corradin G., Druilhe P., Spertini F. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect. Immun. 2005;73:8017–8026. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sirima S.B., Nebie I., Ouedraogo A., Tiono A.B., Konate A.T., Gansane A., Derme A.I., Diarra A., Ouedraogo A., Soulama I., et al. Safety and immunogenicity of the Plasmodium falciparum merozoite surface protein-3 long synthetic peptide (MSP3-LSP) malaria vaccine in healthy, semi-immune adult males in Burkina Faso, West Africa. Vaccine. 2007;25:2723–2732. doi: 10.1016/j.vaccine.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 106.Lusingu J.P., Gesase S., Msham S., Francis F., Lemnge M., Seth M., Sembuche S., Rutta A., Minja D., Segeja M.D., et al. Satisfactory safety and immunogenicity of MSP3 malaria vaccine candidate in Tanzanian children aged 12-24 months. Malar. J. 2009;8:163. doi: 10.1186/1475-2875-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sirima S.B., Tiono A.B., Ouedraogo A., Diarra A., Ouedraogo A.L., Yaro J.B., Ouedraogo E., Gansane A., Bougouma E.C., Konate A.T., et al. Safety and immunogenicity of the malaria vaccine candidate MSP3 long synthetic peptide in 12-24 months-old Burkinabe children. PLoS ONE. 2009;4:e7549. doi: 10.1371/journal.pone.0007549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Polley S.D., Tetteh K.K., Lloyd J.M., Akpogheneta O.J., Greenwood B.M., Bojang K.A., Conway D.J. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J. Infect. Dis. 2007;195:279–287. doi: 10.1086/509806. [DOI] [PubMed] [Google Scholar]
- 109.Pattaradilokrat S., Sawaswong V., Simpalipan P., Kaewthamasorn M., Siripoon N., Harnyuttanakorn P. Genetic diversity of the merozoite surface protein-3 gene in Plasmodium falciparum populations in Thailand. Malar. J. 2016;15:517. doi: 10.1186/s12936-016-1566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oeuvray C., Theisen M., Rogier C., Trape J.F., Jepsen S., Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 2000;68:2617–2620. doi: 10.1128/IAI.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steiner-Monard V., Kamaka K., Karoui O., Roethlisberger S., Audran R., Daubenberger C., Fayet-Mello A., Erdmann-Voisin A., Felger I., Geiger K., et al. The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers. Clin. Infect. Dis. 2019;68:466–474. doi: 10.1093/cid/ciy514. [DOI] [PubMed] [Google Scholar]
- 112.Olugbile S., Kulangara C., Bang G., Bertholet S., Suzarte E., Villard V., Frank G., Audran R., Razaname A., Nebie I., et al. Vaccine potentials of an intrinsically unstructured fragment derived from the blood stage-associated Plasmodium falciparum protein PFF0165c. Infect. Immun. 2009;77:5701–5709. doi: 10.1128/IAI.00652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Okech B., Mujuzi G., Ogwal A., Shirai H., Horii T., Egwang T.G. High titers of IgG antibodies against Plasmodium falciparum serine repeat antigen 5 (SERA5) are associated with protection against severe malaria in Ugandan children. Am. J. Trop. Med. Hyg. 2006;74:191–197. doi: 10.4269/ajtmh.2006.74.191. [DOI] [PubMed] [Google Scholar]
- 114.Horii T., Shirai H., Jie L., Ishii K.J., Palacpac N.Q., Tougan T., Hato M., Ohta N., Bobogare A., Arakaki N., et al. Evidences of protection against blood-stage infection of Plasmodium falciparum by the novel protein vaccine SE36. Parasitol. Int. 2010;59:380–386. doi: 10.1016/j.parint.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 115.Tanabe K., Arisue N., Palacpac N.M., Yagi M., Tougan T., Honma H., Ferreira M.U., Farnert A., Bjorkman A., Kaneko A., et al. Geographic differentiation of polymorphism in the Plasmodium falciparum malaria vaccine candidate gene SERA5. Vaccine. 2012;30:1583–1593. doi: 10.1016/j.vaccine.2011.12.124. [DOI] [PubMed] [Google Scholar]
- 116.Crosnier C., Bustamante L.Y., Bartholdson S.J., Bei A.K., Theron M., Uchikawa M., Mboup S., Ndir O., Kwiatkowski D.P., Duraisingh M.T., et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bustamante L.Y., Bartholdson S.J., Crosnier C., Campos M.G., Wanaguru M., Nguon C., Kwiatkowski D.P., Wright G.J., Rayner J.C. A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants. Vaccine. 2013;31:373–379. doi: 10.1016/j.vaccine.2012.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Douglas A.D., Williams A.R., Illingworth J.J., Kamuyu G., Biswas S., Goodman A.L., Wyllie D.H., Crosnier C., Miura K., Wright G.J., et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat. Commun. 2011;2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Payne R.O., Silk S.E., Elias S.C., Miura K., Diouf A., Galaway F., de Graaf H., Brendish N.J., Poulton I.D., Griffiths O.J., et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De S.L., Stanisic D.I., van Breda K., Bellete B., Harris I., McCallum F., Edstein M.D., Good M.F. Persistence and immunogenicity of chemically attenuated blood stage Plasmodium falciparum in Aotus monkeys. Int J. Parasitol. 2016;46:581–591. doi: 10.1016/j.ijpara.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Stanisic D.I., Fink J., Mayer J., Coghill S., Gore L., Liu X.Q., El-Deeb I., Rodriguez I.B., Powell J., Willemsen N.M., et al. Vaccination with chemically attenuated Plasmodium falciparum asexual blood-stage parasites induces parasite-specific cellular immune responses in malaria-naive volunteers: A pilot study. BMC Med. 2018;16:184. doi: 10.1186/s12916-018-1173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Campino S., Marin-Menendez A., Kemp A., Cross N., Drought L., Otto T.D., Benavente E.D., Ravenhall M., Schwach F., Girling G., et al. A forward genetic screen reveals a primary role for Plasmodium falciparum Reticulocyte Binding Protein Homologue 2a and 2b in determining alternative erythrocyte invasion pathways. PLoS Pathog. 2018;14:e1007436. doi: 10.1371/journal.ppat.1007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 124.Acquah F.K., Adjah J., Williamson K.C., Amoah L.E. Transmission-Blocking Vaccines: Old Friends and New Prospects. Infect. Immun. 2019;87 doi: 10.1128/IAI.00775-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kapulu M.C., Da D.F., Miura K., Li Y., Blagborough A.M., Churcher T.S., Nikolaeva D., Williams A.R., Goodman A.L., Sangare I., et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci. Rep. 2015;5:11193. doi: 10.1038/srep11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Da D.F., Dixit S., Sattabonkot J., Mu J., Abate L., Ramineni B., Ouedraogo J.B., MacDonald N.J., Fay M.P., Su X.Z., et al. Anti-Pfs25 human plasma reduces transmission of Plasmodium falciparum isolates that have diverse genetic backgrounds. Infect. Immun. 2013;81:1984–1989. doi: 10.1128/IAI.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Juliano J.J., Parobek C.M., Brazeau N.F., Ngasala B., Randrianarivelojosia M., Lon C., Mwandagalirwa K., Tshefu A., Dhar R., Das B.K., et al. Pooled Amplicon Deep Sequencing of Candidate Plasmodium falciparum Transmission-Blocking Vaccine Antigens. Am. J. Trop. Med. Hyg. 2016;94:143–146. doi: 10.4269/ajtmh.15-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Patarroyo M.E., Amador R., Clavijo P., Moreno A., Guzman F., Romero P., Tascon R., Franco A., Murillo L.A., Ponton G., et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332:158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 129.Valero M.V., Amador L.R., Galindo C., Figueroa J., Bello M.S., Murillo L.A., Mora A.L., Patarroyo G., Rocha C.L., Rojas M., et al. Vaccination with SPf66, a chemically synthesised vaccine, against Plasmodium falciparum malaria in Colombia. Lancet. 1993;341:705–710. doi: 10.1016/0140-6736(93)90483-W. [DOI] [PubMed] [Google Scholar]
- 130.Noya O., Gabaldon Berti Y., Alarcon de Noya B., Borges R., Zerpa N., Urbaez J.D., Madonna A., Garrido E., Jimenez M.A., Borges R.E., et al. A population-based clinical trial with the SPf66 synthetic Plasmodium falciparum malaria vaccine in Venezuela. J. Infect. Dis. 1994;170:396–402. doi: 10.1093/infdis/170.2.396. [DOI] [PubMed] [Google Scholar]
- 131.Sempertegui F., Estrella B., Moscoso J., Piedrahita L., Hernandez D., Gaybor J., Naranjo P., Mancero O., Arias S., Bernal R., et al. Safety, immunogenicity and protective effect of the SPf66 malaria synthetic vaccine against Plasmodium falciparum infection in a randomized double-blind placebo-controlled field trial in an endemic area of Ecuador. Vaccine. 1994;12:337–342. doi: 10.1016/0264-410X(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 132.Urdaneta M., Prata A., Struchiner C.J., Tosta C.E., Tauil P., Boulos M. Evaluation of SPf66 malaria vaccine efficacy in Brazil. Am. J. Trop. Med. Hyg. 1998;58:378–385. doi: 10.4269/ajtmh.1998.58.378. [DOI] [PubMed] [Google Scholar]
- 133.Alonso P.L., Smith T., Schellenberg J.R., Masanja H., Mwankusye S., Urassa H., Bastos de Azevedo I., Chongela J., Kobero S., Menendez C., et al. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet. 1994;344:1175–1181. doi: 10.1016/S0140-6736(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 134.D′Alessandro U., Leach A., Drakeley C.J., Bennett S., Olaleye B.O., Fegan G.W., Jawara M., Langerock P., George M.O., Targett G.A., et al. Efficacy trial of malaria vaccine SPf66 in Gambian infants. Lancet. 1995;346:462–467. doi: 10.1016/S0140-6736(95)91321-1. [DOI] [PubMed] [Google Scholar]
- 135.Nosten F., Luxemburger C., Kyle D.E., Ballou W.R., Wittes J., Wah E., Chongsuphajaisiddhi T., Gordon D.M., White N.J., Sadoff J.C., et al. Randomised double-blind placebo-controlled trial of SPf66 malaria vaccine in children in northwestern Thailand. Shoklo SPf66 Malaria Vaccine Trial Group. Lancet. 1996;348:701–707. doi: 10.1016/S0140-6736(96)04465-0. [DOI] [PubMed] [Google Scholar]
- 136.Tine J.A., Lanar D.E., Smith D.M., Wellde B.T., Schultheiss P., Ware L.A., Kauffman E.B., Wirtz R.A., De Taisne C., Hui G.S., et al. NYVAC-Pf7: A poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect. Immun. 1996;64:3833–3844. doi: 10.1128/IAI.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ockenhouse C.F., Sun P.F., Lanar D.E., Wellde B.T., Hall B.T., Kester K., Stoute J.A., Magill A., Krzych U., Farley L., et al. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 1998;177:1664–1673. doi: 10.1086/515331. [DOI] [PubMed] [Google Scholar]
- 138.Boes A., Spiegel H., Kastilan R., Bethke S., Voepel N., Chudobova I., Bolscher J.M., Dechering K.J., Fendel R., Buyel J.F., et al. Analysis of the dose-dependent stage-specific in vitro efficacy of a multi-stage malaria vaccine candidate cocktail. Malar. J. 2016;15:279. doi: 10.1186/s12936-016-1328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ouattara A., Barry A.E., Dutta S., Remarque E.J., Beeson J.G., Plowe C.V. Designing malaria vaccines to circumvent antigen variability. Vaccine. 2015;33:7506–7512. doi: 10.1016/j.vaccine.2015.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]