Abstract
Background:
Older adults with acute myocardial infarction (AMI) have higher prevalence of functional impairments, including deficits in cognition, strength, and sensory domains, than their younger counterparts.
Objective:
To develop and evaluate the prognostic utility of a risk model for 6-month post-AMI mortality in older adults that incorporates information about functional impairments.
Design:
Prospective cohort study. (ClinicalTrials.gov: NCT01755052)
Setting:
94 hospitals throughout the United States.
Participants:
3006 persons aged 75 years or older who were hospitalized with AMI and discharged alive.
Measurements:
Functional impairments were assessed during hospitalization via direct measurement (cognition, mobility, muscle strength) or self-report (vision, hearing). Clinical variables associated with mortality in prior risk models were ascertained by chart review. Seventy-two candidate variables were selected for inclusion, and backward selection and Bayesian model averaging were used to derive (n = 2004 participants) and validate (n = 1002 participants) a model for 6-month mortality.
Results:
Participants’ mean age was 81.5 years, 44.4% were women, and 10.5% were nonwhite. There were 266 deaths (8.8%) within 6 months. The final risk model included 15 variables, 4 of which were not included in prior risk models: hearing impairment, mobility impairment, weight loss, and lower patient-reported health status. The model was well calibrated (Hosmer–Lemeshow P > 0.05) and showed good discrimination (area under the curve for the validation cohort = 0.84). Adding functional impairments significantly improved model performance, as evidenced by category-free net reclassification improvement indices of 0.21 (P = 0.008) for hearing impairment and 0.26 (P < 0.001) for mobility impairment.
Limitation:
The model was not externally validated.
Conclusion:
A newly developed model for 6-month post-AMI mortality in older adults was well calibrated and had good discriminatory ability. This model may be useful in decision making at hospital discharge.
Primary Funding Source:
National Heart, Lung, and Blood Institute of the National Institutes of Health.
One third of patients hospitalized for acute myocardial infarction (AMI) in the United States are older adults (aged ≥75 years) (1), and the number of incident AMI cases among older adults is projected to double over the next several decades (2). Advanced age is a strong determinant of post-AMI mortality in the 6 months after hospital discharge (3–5). Compared with their younger counterparts, older adults have more comorbid diseases, lower physiologic reserve, and more functional impairments (including in physical capabilities and cognition), which may contribute to their higher risk for post-AMI mortality. However, there is considerable heterogeneity among older adults, and some have exceptional outcomes (6, 7).
Although clinical practice guidelines endorse routine use of AMI mortality risk models to assist with decision making (8), current models were derived from younger cohorts and may have limited discrimination (9) and calibration (10) in older adults. Physiologic changes with aging may have important prognostic value, and several small studies have shown that functional impairments related to these changes may be independently associated with post-AMI mortality (11, 12). Although a recent joint scientific statement from several cardiology and geriatrics professional societies emphasized “a need for risk-stratification tools relevant to older adults” (13), no AMI mortality risk models that consider these impairments have been developed specifically for the older adult population.
Our primary objective was to develop and validate a 6-month mortality risk model for use in older adults at the time of discharge from hospitalization for AMI. Our model selection process considered functional impairments as well as traditional demographic and clinical variables to assess whether functional impairments would have sufficiently high prognostic utility to be retained in the final model. We evaluated the incremental prognostic utility of adding information about functional impairments to traditional cardiovascular risk factors and compared the model’s performance with that of the existing Global Registry of Acute Coronary Events (GRACE) 6-month mortality risk score (4).
Methods
This article follows the guidelines in the TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) consensus document (14).
Data Source
We used data from the SILVER-AMI (Comprehensive Evaluation of Risk in Older Adults with AMI) study. This prospective observational cohort study involved 3041 patients aged 75 years or older who were hospitalized with AMI at 94 U.S. hospitals. Participants were enrolled from 11 January 2013 to 28 October 2016. Institutional review board approval was obtained at all centers, and informed consent was obtained from participants or their designated proxies.
Participants
The design of SILVER-AMI has been described previously (15). Patients aged 75 years or older were enrolled if they met the criteria in the Third Universal Definition of MI (16). Patients were excluded if they were transferred to the study hospital from another hospital after more than 24 hours. We did not exclude participants with cognitive impairment, but we did screen for capacity (17) and used proxy respondents when necessary. Because our objective was to model risk for postdischarge death, we excluded those who died in the hospital (n = 35), retaining a final sample of 3006 participants for analysis.
Study Procedures
At the time of hospitalization, participants underwent a baseline assessment, including demographic characteristics, symptoms, health status (12-Item Short Form Health Survey), functional impairments (including cognition, vision, hearing, muscle strength, and mobility), and other conditions common with aging (such as unintentional weight loss). Fifty-two percent of assessments occurred within the first 2 days of hospitalization. Medical record review included details of initial presentation, comorbidities, medications, cardiac procedures, and laboratory results.
Outcome
The primary outcome was death within 6 months of hospital discharge, which was ascertained via medical records (in-hospital death), death certificates (out-of-hospital death), or obituaries (out-of-hospital death). When these were not available (<5% of cases), mortality information was obtained through secondary report, such as from a family member. All deaths were double-adjudicated by physician investigators who reviewed source documents to determine the cause, which was classified as cardiovascular (attributable to coronary disease, heart failure, AMI, valvular heart disease, arrhythmia, venous thrombosis, peripheral artery disease, stroke, or complications of a cardiac procedure), noncardiovascular, or unknown. Mortality status was definitively obtained for 99.9% of participants.
Predictors at Baseline
Functional impairments and other conditions common with aging were selected on the basis of the geriatrics literature and ease of measurement in the hospital setting and are defined in Table 1 of Supplement 1 (available at Annals.org). The following impairments and conditions were performance-based: general cognitive function (Telephone Interview for Cognitive Status) (18), verbal fluency (Controlled Oral Word Association Test) (19), upper-extremity strength (handheld dynamometer [B&L Engineering]) (20, 21), and functional mobility (Timed Up and Go [TUG]) (22). The TUG was measured according to a standard protocol that involved the participant standing from a chair, walking 10 feet at his or her usual pace, turning around, walking back to the chair, and sitting. The following impairments and conditions were assessed via self-report: visual impairment (Visual Function Questionnaire) (23), hearing impairment (global question about impairments imposed by hearing) (24), unintentional weight loss (>10 lb in the prior year), activities of daily living disability (bathing, dressing, rising from chair, or ambulating) (25), depressive symptoms (Patient Health Questionnaire 8) (26), and fall history in the prior year. Cut points were selected on the basis of previously validated thresholds; if the definitive threshold was ambiguous, consensus was reached among study team members on the basis of the best available evidence.
Statistical Analysis
The 3006 participants were randomly assigned using simple random sampling, as implemented in the SAS SURVEYSELECT procedure, such that two thirds (n = 2004) were chosen for derivation and one third (n = 1002) were assigned to validation. This allowed sufficient power to develop and validate the risk prediction model, and the random nature of the process protected against selection bias.
We initially selected 72 candidate variables for inclusion (Table 2 of Supplement 1) based on existing risk models for adverse clinical events after AMI hospitalization (4, 27–29), the study’s objective of evaluating functional impairments and other conditions common with aging that could influence mortality risk, and clinical judgment of the study investigators with regard to other variables that might influence postdischarge mortality.
We generated descriptive statistics for all participants (that is, for the derivation and validation cohorts combined), using means for continuous variables and percentages for categorical variables (Table 1). We also assessed baseline characteristics of the derivation and validation cohorts separately (Table 3 of Supplement 1). When feasible, we used continuous variables to retain statistical power, although some were converted into categorical variables for clinical interpretability. Thresholds were chosen on the basis of clinical relevance and distributions. From our initial list, we omitted variables with missingness greater than 20% and those with extremely low (<5%) or high (>95%) prevalence (the full rationale is described in the Statistical Methods section of Supplement 1). All variables except ejection fraction (9.2%) and functional mobility (15.9%) had missingness less than 5%. After confirming the absence of collinearity between variables and assuming that missing values were missing at random, we multiply imputed the data 20 times. All variables were imputed using fully conditional specification (30), drawing from the 72 candidates eligible for model selection. Details of the imputation process are provided in the Statistical Methods section of Supplement 1.
Table 1.
Participant Characteristics at Baseline
| Characteristic | Deceased at 180 Days (n = 266) | Alive at 180 Days (n = 2740) | ||
|---|---|---|---|---|
| Mean (SD) or Number (Percentage) | Missing Data, n (%) | Mean (SD) or Number (Percentage) | Missing Data, n (%) | |
| Demographic characteristics | ||||
| Mean age (SD), y | 83.7 (5.8) | 0 (0.0) | 81.3 (4.9) | 0 (0.0) |
| Male, n (%) | 140 (52.6) | 0 (0.0) | 1531 (55.9) | 0 (0.0) |
| Nonwhite race, n (%) | 43 (16.4) | 3 (1.1) | 274 (10.2) | 45 (1.6) |
| Married/living with partner, n (%) | 107 (40.4) | 1 (0.4) | 1407 (51.4) | 4 (0.1) |
| Education ≤12 y, n (%) | 170 (64.6) | 3 (1.1) | 1537 (56.6) | 23 (0.8) |
| Medical history, n (%) | ||||
| Hypertension | 247 (92.9) | 0 (0.0) | 2319 (84.6) | 0 (0.0) |
| Dyslipidemia | 165 (62.0) | 0 (0.0) | 1733 (63.3) | 0 (0.0) |
| Current or ever-smoker | 160 (61.3) | 5 (1.9) | 1506 (55.4) | 19 (0.7) |
| Arrhythmia | 86 (32.3) | 0 (0.0) | 663 (24.2) | 0 (0.0) |
| Heart failure | 96 (36.1) | 0 (0.0) | 467 (17.0) | 0 (0.0) |
| Peripheral artery disease | 58 (21.8) | 0 (0.0) | 305 (11.1) | 0 (0.0) |
| Valvular disease | 50 (18.8) | 0 (0.0) | 299 (10.9) | 0 (0.0) |
| Stroke | 64 (24.1) | 0 (0.0) | 404 (14.7) | 0 (0.0) |
| Diabetes mellitus | 112 (42.1) | 0 (0.0) | 1004 (36.7) | 0 (0.0) |
| Chronic obstructive pulmonary disease | 59 (22.2) | 0 (0.0) | 367 (13.4) | 0 (0.0) |
| Sleep apnea | 36 (13.5) | 0 (0.0) | 213 (7.8) | 0 (0.0) |
| Prior myocardial infarction | 84 (31.6) | 0 (0.0) | 735 (26.8) | 0 (0.0) |
| Prior revascularization procedure | 113 (42.5) | 0 (0.0) | 1107 (40.4) | 0 (0.0) |
| Presentation characteristics | ||||
| STEMI, n (%) | 53 (19.9) | 0 (0.0) | 738 (26.9) | 0 (0.0) |
| Chest pain as presenting symptom, n (%) | 102 (40) | 13 (4.9) | 1109 (41.8) | 86 (3.1) |
| ≥6 h from symptoms to presentation, n (%) | 131 (49.6) | 2 (0.8) | 1140 (41.8) | 14 (0.5) |
| Mean body mass index (SD), kg/m2 | 26.5 (5.8) | 0 (0.0) | 27.6 (5.3) | 0 (0.0) |
| Killip class II to IV, n (%) | 70 (26.3) | 0 (0.0) | 322 (11.8) | 0 (0.0) |
| Mean initial systolic BP (SD), mm Hg | 136.3 (28.8) | 0 (0.0) | 146.8 (30.8) | 0 (0.0) |
| Mean initial diastolic BP (SD), mm Hg | 74.1 (17.0) | 0 (0.0) | 78.5 (17.7) | 0 (0.0) |
| Mean initial heart rate (SD), beats/min | 89.3 (21.9) | 0 (0.0) | 83.0 (22.7) | 0 (0.0) |
| Atrial fibrillation, n (%) | 68 (25.6) | 0 (0.0) | 478 (17.5) | 0 (0.0) |
| Mean initial hemoglobin level (SD), g/L | 118 (21) | 0 (0.0) | 129 (20) | 8 (0.3) |
| Mean initial leukocyte count (SD), × 109 cells/L | 10.7 (6.7) | 0 (0.0) | 9.5 (4.7) | 11 (0.4) |
| Mean eGFR (SD), mL/min/1.73 m2 | 44.1 (21.6) | 0 (0.0) | 55.7 (19.5) | 2 (0.1) |
| Mean TIMI score (SD) | ||||
| NSTEMI | 4.3 (1.2) | 0 (0.0) | 4.6 (1.2) | 0 (0.0) |
| STEMI | 6.8 (1.8) | 0 (0.0) | 6.0 (1.6) | 14 (1.9) |
| Mean GRACE ACS score (SD) | 162.4 (22.9) | 0 (0.0) | 143.8 (21.8) | 2 (0.1) |
| In-hospital diagnostics, therapies, and complications, n (%) | ||||
| Left ventricular ejection fraction | 30 (11.3) | 247 (9.0) | ||
| Normal (>50%) | 90 (38.1) | 1437 (57.6) | ||
| Mildly reduced (40%–50%) | 56 (23.7) | 541 (21.7) | ||
| Moderately reduced (30%–40%) | 54 (22.9) | 339 (13.6) | ||
| Severely reduced (<30%) | 36 (15.3) | 176 (7.1) | ||
| Medications within first 24 h | ||||
| Aspirin | 249 (93.6) | 0 (0.0) | 2627 (95.9) | 1 (0.0) |
| Antiplatelet agent (P2Y12 inhibitor) | 147 (55.3) | 0 (0.0) | 1726 (63.0) | 1 (0.0) |
| β-Blocker | 206 (77.4) | 0 (0.0) | 2159 (78.8) | 1 (0.0) |
| Statin | 182 (68.4) | 0 (0.0) | 2090 (76.3) | 1 (0.0) |
| Intravenous antithrombotic agent | 0 (0.0) | 1 (0.0) | ||
| None | 62 (23.3) | 453 (16.5) | ||
| Heparin or bivalirudin | 194 (72.9) | 2004 (73.2) | ||
| Heparin or bivalirudin plus glycoprotein IIb/IIIa | 10 (3.8) | 282 (10.3) | ||
| Revascularization status | 0 (0.0) | 0 (0.0) | ||
| No cardiac catheterization | 97 (36.5) | 361 (13.2) | ||
| Cardiac catheterization only | 56 (21.1) | 438 (16.0) | ||
| Cardiac catheterization with PCI | 101 (38.0) | 1599 (58.4) | ||
| Coronary artery bypass graft surgery | 12 (4.5) | 342 (12.5) | ||
| In-hospital complications | ||||
| Bleeding | 71 (26.7) | 0 (0.0) | 702 (25.6) | 0 (0.0) |
| Acute kidney injury | 99 (37.2) | 0 (0.0) | 593 (21.7) | 2 (0.1) |
| Heart failure | 63 (23.7) | 0 (0.0) | 351 (12.8) | 0 (0.0) |
| Functional impairments and conditions common in aging | ||||
| Cognitive impairment, n (%) | 7 (2.6) | 42 (1.5) | ||
| None (TICS score ≥27) | 173 (66.8) | 2278 (84.4) | ||
| Mild (TICS score 23–26) | 44 (17.0) | 270 (10.0) | ||
| Moderate or severe (TICS score ≤22) | 42 (16.2) | 150 (5.6) | ||
| Mean total “S” words (SD) (COWAT) | 8.2 (4.8) | 15 (5.6) | 9.8 (4.7) | 44 (1.6) |
| Clinically significant vision impairment, n (%) | 40 (15.1) | 1 (0.4) | 216 (7.9) | 4 (0.1) |
| Clinically significant hearing impairment, n (%) | 52 (19.8) | 3 (1.1) | 352 (13.0) | 35 (1.3) |
| Unintentional weight loss >10 lb in past year, n (%) | 102 (38.8) | 3 (1.1) | 569 (20.9) | 14 (0.5) |
| Any ADL disability, n (%) | 75 (28.3) | 1 (0.4) | 337 (12.3) | 0 (0.0) |
| >1 fall in past year, n (%) | 70 (26.9) | 6 (2.3) | 523 (19.1) | 6 (0.2) |
| Weak grip strength, n (%) | 192 (78.4) | 21 (7.9) | 1615 (61.3) | 107 (3.9) |
| Functional mobility (based on Timed Up and Go), n (%) | 39 (14.7) | 440 (16.1) | ||
| Completed in ≤15 s | 37 (16.3) | 828 (36.0) | ||
| Completed in >15 and ≤25 s | 30 (13.2) | 589 (25.6) | ||
| Completed in >25 s | 46 (20.3) | 437 (19.0) | ||
| Unable to complete | 114 (50.2) | 446 (19.4) | ||
| SF-12: general health question (4 categories) | 4 (1.5) | 2 (0.1) | ||
| Excellent or very good | 38 (14.5) | 804 (29.4) | ||
| Good | 72 (27.5) | 1034 (37.8) | ||
| Fair | 91 (34.7) | 669 (24.4) | ||
| Poor | 61 (23.3) | 231 (8.4) | ||
| Depressive symptoms (PHQ-8 score ≥10) | 56 (22.7) | 19 (7.1) | 366 (13.8) | 80 (2.9) |
ACS = acute coronary syndrome; ADL = activity of daily living; BP = blood pressure; COWAT = Controlled Oral Word Association Test; eGFR = estimated glomerular filtration rate; GRACE = Global Registry of Acute Coronary Events; NSTEMI = non–ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention; PHQ-8 = Patient Health Questionnaire 8; SF-12 = 12-Item Short Form Health Survey; STEMI = ST-segment elevation myocardial infarction; TICS = Telephone Interview for Cognitive Status; TIMI = Thrombolysis In Myocardial Infarction.
Our process for model selection and estimation used imputed derivation data and was previously described in a methods paper using an outcome of death within 30 days of discharge in the SILVER-AMI cohort (31). We first reduced the number of candidate variables by applying multivariable logistic regression with backward selection to an aggregate data set of the 20 imputations, retaining the 30 variables with the strongest adjusted associations with the outcome. We subsequently applied Bayesian model averaging with multivariable logistic regression to these final candidates in each of the multiply imputed data sets. We previously demonstrated the utility of Bayesian model averaging to inform retention of variables differentially selected across imputed data sets in the SILVER-AMI cohort (31). We prespecified that the final predictors would be those exhibiting a positive posterior probability in at least half of the imputations. These predictors were subsequently examined for linearity and used in a multivariable model fit separately to each imputation using generalized estimating equations to adjust for clustering of patients within hospitals, with the coefficients estimated from the separate imputations combined using Rubin rules to yield final coefficients (32). Because Bayesian model averaging was used to select variables rather than P values, some model terms did not have P values below 0.05 (33).
Discrimination and calibration of the final model were evaluated in the derivation and validation cohorts using the c-statistic and the Hosmer–Lemeshow goodness-of-fit statistic, respectively. Observed and predicted probabilities of the outcome were calculated for deciles of risk obtained by applying the final model to the validation data. We evaluated the incremental value of separately adding functional impairments to the model using category-free (continuous) net reclassification improvement (NRI) indices (34).
The GRACE score, which was developed for 6-month post-AMI mortality, includes the following factors: age, history of AMI, heart failure, increased heart rate at presentation, low systolic blood pressure at presentation, elevated initial serum creatinine level, elevated initial levels of serum cardiac biomarkers, ST-segment depression on presenting electrocardiogram, and not undergoing percutaneous coronary intervention in the hospital (4). Predictive performance of the SILVER-AMI and GRACE risk scores was compared in several ways. The difference in area under the curve (AUC) for the SILVER-AMI and GRACE risk scores (evaluated in the SILVER-AMI validation cohort) was tested using the methods of DeLong and colleagues (35). We also compared specificity, positive predictive value, and negative predictive value of the GRACE and SILVER-AMI models as applied to the SILVER-AMI validation data for selected values of sensitivity. Kaplan–Meier curves were generated based on quartiles of predicted probability of death from the GRACE and SILVER-AMI models as applied to the SILVER-AMI derivation and validation data sets and compared using log-rank statistics. Finally, integrated predictiveness curves from application of both models to the SILVER-AMI validation data were compared (36).
Analyses were performed using SAS, version 9.4 (SAS Institute), except for the Bayesian model averaging, for which the BMA package in R (R Foundation for Statistical Computing) was used (37). We developed a Web-based calculator derived from model effect estimates for bedside prognostication.
Role of the Funding Source
The National Heart, Lung, and Blood Institute of the National Institutes of Health supported this study but had no role in its design, conduct, or reporting.
Results
Baseline Characteristics
Baseline characteristics of the study participants are shown in Table 1. Mean age was 81.5 years (SD, 5.0; range, 75 to 101 years) (Figure 1 of Supplement 1), 44.4% were women, 10.5% were nonwhite, and 2.8% had proxy consent. The most common chronic medical conditions were hypertension (85.3%), dyslipidemia (63.2%), and diabetes (37.2%). Most participants (73.7%) presented with non–ST-segment elevation AMI, and most underwent invasive coronary angiography (84.6% in the overall sample, 97.0% in the ST-segment elevation AMI subgroup, and 80.2% in the non–ST-segment elevation AMI subgroup). The most common functional impairments were weak grip (62.8%) and impaired mobility based on a TUG greater than 15 seconds (65.7%). Baseline characteristics are shown separately for the derivation and validation cohorts in Table 3 of Supplement 1.
Mortality at 6 Months
Overall, 266 participants (8.8%) who were alive at hospital discharge died within 6 months (184 [9.2%] in the derivation cohort and 82 [8.2%] in the validation cohort). Cause of death was adjudicated as cardiovascular in 41.7% of these participants, noncardiovascular in 24.4%, and unknown in 33.8%. Participants who died were older (83.7 vs. 81.3 years), more likely to be nonwhite (16.4% vs. 10.2%), and more likely to present with non–ST-segment elevation AMI (80.1% vs. 73.1%) and had a higher burden of comorbid disease, including history of heart failure (36.1% vs. 17.0%), peripheral artery disease (21.8% vs. 11.1%), and stroke (24.1% vs. 14.7%) (Table 1). They were also more likely to have in-hospital acute kidney injury (37.2% vs. 21.7%) and heart failure (23.7% vs. 12.8%). Several functional impairments and other conditions common with aging were more common among decedents, including cognitive impairment (33.2% vs. 15.6%), unintentional weight loss (38.8% vs. 20.9%), and impaired mobility based on a TUG greater than 15 seconds (83.7% vs. 64.0%).
Multivariable Results
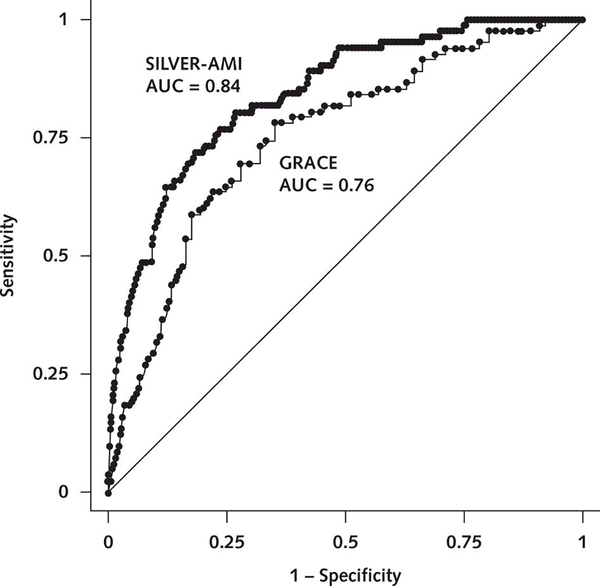
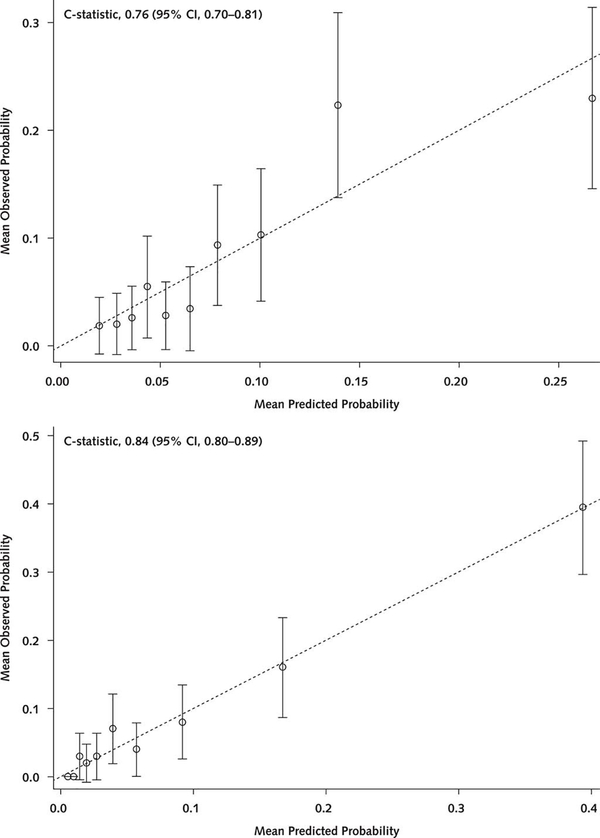
In the multivariable model, 15 factors were associated with 6-month mortality (Table 4 of Supplement 1). The strongest factors (based on standardized β-coefficients) included receipt of in-hospital coronary artery bypass graft (CABG), age, length of stay, self-reported health status, and unintentional weight loss. Among these, CABG was protective (odds ratio [OR], 0.15 [95% CI, 0.07 to 0.31]), whereas the others were associated with increased risk (OR for age [per year], 1.06 [CI, 1.02 to 1.11]; OR for length of stay [per day], 1.05 [CI, 1.02 to 1.08]; OR for self-reported health status [per level of worsening], 1.34 [CI, 1.11 to 1.61]; OR for unintentional weight loss, 1.69 [CI, 1.19 to 2.39]). Model discrimination was good (AUC = 0.82 in the derivation cohort and 0.84 in the validation cohort). Compared with the GRACE mortality risk score (4) (evaluated in the SILVER cohort), discrimination was improved (AUC = 0.84 vs. 0.76; P < 0.001) (Figure 1). The model was also well calibrated (Figure 2). Standard operating characteristics from application of the SILVER-AMI and GRACE models to the SILVER-AMI validation cohort are shown in Tables 2 and 3. Specificity and positive predictive values were higher, although still modest, for the SILVER-AMI model across selected values of sensitivity (details are provided in the Statistical Methods section of Supplement 1).
Figure 1.
Receiver-operating characteristic curves for the SILVER-AMI and GRACE mortality risk models. In our validation cohort, we compared the AUCs for the SILVER-AMI 6-month mortality risk model and the GRACE risk model (which was previously developed for post-AMI 6-month mortality). The GRACE risk model includes the following factors: age, development (or history) of heart failure, peripheral vascular disease, systolic blood pressure, Killip class, initial serum creatinine concentration, elevated initial cardiac markers, cardiac arrest on admission, and ST-segment deviation. The diagonal green line indicates 50% discrimination and is provided to show how much each model improves on purely random assignment of risk. As shown, the SILVER-AMI mortality risk model showed superior discrimination compared with the GRACE model (AUC = 0.84 vs. 0.76; P < 0.001). AMI = acute myocardial infarction; AUC = area under the curve; GRACE = Global Registry of Acute Coronary Events; SILVER-AMI = Comprehensive Evaluation of Risk in Older Adults with AMI.
Figure 2.
Calibration plot for the SILVER-AMI mortality risk model. Shown are deciles of observed 6-month risk versus predicted 6-month mortality, with the GRACE model applied to the SILVER-AMI validation cohort (top) and the SILVER-AMI model applied to the SILVER-AMI validation cohort (bottom). Error bars represent 95% CIs. AMI = acute myocardial infarction; GRACE = Global Registry of Acute Coronary Events; SILVER-AMI = Comprehensive Evaluation of Risk in Older Adults with AMI.
Table 2.
Standard Operating Characteristics from Application of the GRACE Model to the SILVER-AMI Validation Cohort for Death Within 180 Days (n = 1002)
| Characteristic | Sensitivity (95% CI)* |
||||
|---|---|---|---|---|---|
| 0.70 (0.67–0.72) | 0.74 (0.71–0.77) | 0.81 (0.78–0.83) | 0.85 (0.83–0.88) | 0.92 (0.90–0.93) | |
| Specificity (95% CI) | 0.72 (0.70–0.75) | 0.67 (0.64–0.70) | 0.57 (0.54–0.60) | 0.43 (0.40–0.46) | 0.34 (0.31–0.37) |
| Positive predictive value (95% CI) | 0.18 (0.16–0.21) | 0.17 (0.14–0.20) | 0.14 (0.12–0.17) | 0.12 (0.10–0.14) | 0.11 (0.10–0.13) |
| Negative predictive value (95% CI) | 0.96 (0.95–0.98) | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 0.98 (0.97–0.99) |
AMI = acute myocardial infarction; GRACE = Global Registry of Acute Coronary Events; SILVER-AMI = Comprehensive Evaluation of Risk in Older Adults with AMI.
Due to risk model differences, sensitivity values in Tables 2 and 3 are statistically equivalent rather than numerically identical.
Table 3.
Standard Operating Characteristics from Application of the SILVER-AMI Model to the SILVER-AMI Validation Cohort for Death Within 180 Days (n = 1002)
| Characteristic | Sensitivity (95% CI)* |
||||
|---|---|---|---|---|---|
| 0.71 (0.68–0.74) | 0.76 (0.73–0.78) | 0.80 (0.78–0.83) | 0.85 (0.83–0.88) | 0.90 (0.88–0.92) | |
| Specificity (95% CI) | 0.82 (0.80–0.85) | 0.77 (0.75–0.80) | 0.73 (0.71–0.76) | 0.60 (0.57–0.63) | 0.55 (0.52–0.58) |
| Positive predictive value (95% CI) | 0.26 (0.24–0.29) | 0.23 (0.20–0.26) | 0.21 (0.19–0.24) | 0.16 (0.14–0.18) | 0.15 (0.13–0.17) |
| Negative predictive value (95% CI) | 0.97 (0.96–0.98) | 0.97 (0.96–0.98) | 0.98 (0.97–0.99) | 0.98 (0.97–0.99) | 0.98 (0.98–0.99) |
AMI = acute myocardial infarction; SILVER-AMI = Comprehensive Evaluation of Risk in Older Adults with AMI.
Due to risk model differences, sensitivity values in Tables 2 and 3 are statistically equivalent rather than numerically identical.
Kaplan–Meier curves for time to death by quartiles of predicted probabilities from the GRACE and SILVER-AMI scores in both the derivation and validation cohorts are provided in Figure 2 of Supplement 1. In both cohorts, there was larger separation between the third and fourth quartiles in SILVER-AMI relative to GRACE, indicating that SILVER-AMI assigns higher probabilities to persons most likely to die. Integrated predictiveness curves for SILVER-AMI and GRACE (Figure 3 of Supplement 1) show that for predicted probabilities below the observed outcome incidence (8.2%), SILVER-AMI consistently yields lower risk, and for values above the observed outcome incidence, SILVER-AMI consistently yields higher risk, reflecting its ability to better identify patients at either risk level.
The addition of functional impairments significantly improved the predictive ability of the model, as measured by category-free NRI indices (38). The TUG yielded an event NRI of 16% (CI, 11% to 22%) and a nonevent NRI of 10% (CI, 9% to 12%) for a combined NRI of 0.26 (P < 0.001), and hearing impairment yielded an event NRI of −4% (CI, −7% to −1%) and a nonevent NRI of 25% (CI, 23% to 27%) for a combined NRI of 0.21 (P = 0.008).
We used the results from our multivariable model to develop a Web-based calculator for 6-month mortality after discharge, which is available at www.silverscore.org (Figure 4 of Supplement 1). To illustrate, consider 2 hypothetical patients, both aged 85 years, who undergo percutaneous coronary intervention and have an admission heart rate of 90 beats/min, estimated glomerular filtration rate of 60 mL/min/1.73 m2, hemoglobin level of 120 g/L, mildly decreased ejection fraction, length of stay of 5 days, no peripheral artery disease, and no sleep apnea (Figure 5 of Supplement 1). If the first patient reports that hearing interferes with activities “a lot,” has fair health status, has a TUG greater than 25 seconds, and reports unintentional weight loss of more than 10 pounds in the past year, the predicted risk for death within 6 months is 22% using the SILVER-AMI model. Conversely, if the second patient reports that hearing interferes with activities “a little,” has good health status, has a TUG of 15 seconds or less, and denies unintentional weight loss of more than 10 pounds in the past year, the predicted risk for death within 6 months is 5% using the SILVER-AMI model.
Discussion
In our sample of patients aged 75 years or older, 15 risk factors were selected for inclusion in the prediction model of death within 6 months of hospitalization for AMI. These included comorbid diseases, laboratory values, in-hospital procedures, and functional impairments. Our model had good discrimination and was well calibrated. Functional impairments enhanced the predictive ability of the model, as demonstrated by their retention in the final model and by the NRI indices. We are unaware of any other postdischarge AMI mortality risk models specifically developed for older adults.
Risk prediction is critical to informed decision making for older adults with AMI and, in the context of discharge planning, can help identify high-risk patients who may benefit from closer outpatient monitoring and potentially more aggressive secondary preventive measures, including multidrug lipid-lowering therapy and cardiac rehabilitation. In addition, for patients at the highest risk, setting expectations for patients and family members and early involvement of palliative care may be appropriate.
The GRACE 6-month mortality model is the most commonly cited model for prediction of mortality after hospital discharge in the setting of AMI (4, 10, 29). It was developed from a registry of patients with acute coronary syndrome (AMI and unstable angina) who had a mean age of 65 years (4). Like the SILVER-AMI model, the GRACE model excluded patients who died in the hospital. Several variables in the GRACE model (age, heart rate, serum creatinine level, and revascularization status) were also retained in the final SILVER-AMI model, reflecting shared risk factors that are clinically plausible predictors of mortality. When we applied the GRACE risk score in the SILVER-AMI validation cohort, we found discrimination similar to that in the original GRACE validation cohort (AUC = 0.76), indicating that the score performs reasonably well in our cohort and suggesting that many factors identified by GRACE denote risk across the age spectrum. Although the SILVER-AMI risk score showed better predictive accuracy than the GRACE model in our validation cohort, some degradation of performance is expected when a risk score is applied to an external data set. Applying the SILVER-AMI score to a different population (that is, external validation) may yield less favorable estimates of performance. The GRACE and SILVER-AMI scores may also give different estimates of risk for some patients. As illustrated by the hypothetical examples, patients with functional impairments have substantially higher mortality risk according to the SILVER-AMI risk score, and these are not considered in the GRACE score. Although the importance of functional impairments is supported by their retention in the final SILVER-AMI model and their NRIs, the SILVER-AMI score has not been externally validated. It is therefore important that estimates be used judiciously and incorporated into the totality of clinical information, including response to and tolerance of therapies and goals of care.
Our study was designed to explicitly consider functional impairments and other conditions common with aging given that these factors are independently associated with adverse post-AMI outcomes (11, 12). Two impairments were retained in our final risk model: hearing impairment and mobility. The association between hearing impairment and mortality may be mediated by patients’ difficulty in comprehending complex medication and follow-up instructions at the time of hospital discharge, which may interfere with subsequent care (39, 40). We selected a mobility measure (TUG) that is a key indicator of frailty (41). Frail patients are at increased risk for death, likely due to an inability to maintain homeostasis in the settings of acute events (such as infection or injury) and chronic functional decline (42, 43). Weight loss, another factor retained in our model, is also a common frailty indicator and may be a marker of underlying advanced disease.
Of note, CABG was protective in our model. Although this may seem counterintuitive given that patients undergoing CABG typically have more severe atherosclerosis and a higher incidence of in-hospital complications, similar findings have been reported in other cohorts (44). The most likely explanation is selection bias given that patients referred for CABG were judged by surgeons as robust enough to withstand a major operation. We captured a broad range of comorbidities and functional impairments, but this selection process may not have been fully accounted for in our multivariable model.
Several limitations of our study deserve consideration. First, although we internally validated our prediction model, we did not evaluate its performance in an external data set. Second, rates of vision and hearing impairment in our sample were lower than in other published studies (45, 46), perhaps because these impairments posed a barrier to study participation. Furthermore, the mortality rate in SILVER-AMI was lower than in some cohort studies of older adults (47, 48), which may reflect lower mortality with improvements in AMI care over time (49, 50) or possibly a healthy enrollee effect. Third, our model requires information on functional impairments, which are not typically assessed as part of routine inpatient care. Collectively, the assessments required for our model should take less than 10 minutes to complete. Fourth, we were unable to distinguish between cardiovascular and noncardiovascular causes of death in about a third of participants. Inaccuracies in cause-of-death reporting are common (51), and assigning a single primary cause may be even more challenging in older populations. Fifth, the SILVER-AMI model is characterized by a modest positive predictive value, an inherent limitation of population-based models of low-incidence outcomes (52, 53). Finally, we recognize that there is a potential for misuse of risk models by health systems (for example, to justify withholding care). However, we are unaware of any such misuse with other risk models, and we believe it is important to provide patients and clinicians with information about their predicted outcomes based on the highest-quality data.
In conclusion, we developed a novel risk model for mortality within 6 months of hospitalization for AMI in patients aged 75 years or older that considered traditional risk factors, functional impairments, and other conditions common with aging. We found that several factors relevant to older adults and not considered in prior AMI risk models were independently associated with mortality. Our model was well calibrated; had good discrimination; and, with use of a Web-based calculator, can be used to inform prognostication at the time of hospital discharge.
Supplementary Material
Supplement 2. Study Protocol
Supplement 1. Supplemental Materials
Acknowledgments
Grant Support: This research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (R01HL115295). This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG021342). The project described in the article used REDCap (Research Electronic Data Capture), which is supported by the National Center for Advancing Translational Sciences of the NIH through grant UL1 TR00000. Dr. Dodson is the recipient of Patient-Oriented Research Career Development Award K23-AG052463 from the National Institute on Aging of the NIH. Dr. Hajduk was supported by a training grant from the National Institute on Aging (T32-AG19134). Dr. Nanna is supported by NIH training grant T32-HL069749-15. Dr. Gill is the recipient of an Academic Leadership Award (K07-AG043587) from the National Institute on Aging.
Footnotes
Disclosures: Dr. Krumholz reports personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Arnold & Porter, Ben C. Martin Law Firm, Facebook, and the National Center for Cardiovascular Diseases, Beijing; ownership (with spouse) of Hugo; contracts from the Centers for Medicare & Medicaid Services; and grants from Medtronic, the U.S. Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information outside the submitted work. Dr. Chaudhry reports personal fees from the CVS Caremark Clinical Program for the state of Connecticut outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M19-0974.
Reproducible Research Statement: Study protocol: See Supplement 2 (available at Annals.org). Statistical code and data set: Available from Dr. Chaudhry (e-mail, Sarwat.chaudhry@yale.edu).
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
John A. Dodson, New York University School of Medicine, New York, New York.
Alexandra Hajduk, Yale School of Medicine, New Haven, Connecticut.
Mary Geda, Yale School of Medicine, New Haven, Connecticut.
Harlan M. Krumholz, Yale New Haven Hospital, Yale School of Medicine, and Yale School of Public Health, New Haven, Connecticut.
Terrence E. Murphy, Yale School of Medicine, New Haven, Connecticut.
Sui Tsang, Yale School of Medicine, New Haven, Connecticut.
Mary E. Tinetti, Yale School of Medicine, New Haven, Connecticut.
Michael G. Nanna, Duke University School of Medicine, Durham, North Carolina.
Richard McNamara, Spectrum Health, Grand Rapids, Michigan.
Thomas M. Gill, Yale School of Medicine, New Haven, Connecticut.
Sarwat I. Chaudhry, Yale School of Medicine, New Haven, Connecticut.
References
- 1.Wright RS, Anderson JL, Adams CD, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011;57:e215–367. doi: 10.1016/j.jacc.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 2.Odden MC, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.e5. 10.1016/j.amjmed.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharacholou SM, Lopes RD, Alexander KP, et al. Age and outcomes in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: findings from the APEX-AMI trial. Arch Intern Med 2011;171:559–67. 10.1001/archinternmed.2011.36 [DOI] [PubMed] [Google Scholar]
- 4.Eagle KA, Lim MJ, Dabbous OH, et al. ; GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. [DOI] [PubMed] [Google Scholar]
- 5.Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000;101:2557–67. [DOI] [PubMed] [Google Scholar]
- 6.Devlin G, Gore JM, Elliott J, et al. ; GRACE Investigators. Management and 6-month outcomes in elderly and very elderly patients with high-risk non-ST-elevation acute coronary syndromes: The Global Registry of Acute Coronary Events. Eur Heart J 2008;29:1275–82. 10.1093/eurheartj/ehn124 [DOI] [PubMed] [Google Scholar]
- 7.Antonsen L, Jensen LO, Terkelsen CJ, et al. Outcomes after primary percutaneous coronary intervention in octogenarians and nonagenarians with ST-segment elevation myocardial infarction: from the Western Denmark Heart Registry. Catheter Cardiovasc Interv 2013;81:912–9. 10.1002/ccd.24591 [DOI] [PubMed] [Google Scholar]
- 8.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction—executive summary. J Am Coll Cardiol 2007;50:652–726. 10.1016/j.jacc.2007.02.028 [DOI] [Google Scholar]
- 9.Luo JG, Yang M, Han L, et al. Validity of the GRACE score for 6-month death or reinfarction after presentation with acute myocardial infarction in patients 80 years of age and older. Coron Artery Dis 2013;24:537–41. 10.1097/MCA.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 10.Lenderink T, Hernández AV, Boersma E, et al. Prediction of 30-day mortality in older patients with a first acute myocardial infarction. Cardiology. 2010;115:1–9. 10.1159/000243770 [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Konishi M, Akiyama E, et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2013;61:1964–72. 10.1016/j.jacc.2013.02.020 [DOI] [PubMed] [Google Scholar]
- 12.Gharacholou SM, Reid KJ, Arnold SV, et al. Cognitive impairment and outcomes in older adult survivors of acute myocardial infarction: findings from the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status registry. Am Heart J. 2011;162:860–869.e1. 10.1016/j.ahj.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich MW, Chyun DA, Skolnick AH, et al. ; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133:2103–22. 10.1161/CIR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 14.Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 15.Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the Comprehensive Evaluation of Risk Factors in Older Patients with AMI (SILVER-AMI) study. BMC Health Serv Res 2014;14:506 10.1186/s12913-014-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. 10.1016/j.jacc.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 17.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Arch Gen Psychiatry. 2007;64:966–74. [DOI] [PubMed] [Google Scholar]
- 18.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol 1988;1:111–7. [Google Scholar]
- 19.Rodriguez-Aranda C, Martinussen M. Age-related differences in performance of phonemic verbal fluency measured by Controlled Oral Word Association Task (COWAT): a meta-analytic study. Dev Neuropsychol. 2006;30:697–717. [DOI] [PubMed] [Google Scholar]
- 20.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. [DOI] [PubMed] [Google Scholar]
- 21.Wang CY, Chen LY. Grip strength in older adults: test-retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil 2010;91:1747–51. 10.1016/j.apmr.2010.07.225 [DOI] [PubMed] [Google Scholar]
- 22.Viccaro LJ, Perera S, Studenski SA. Is Timed Up and Go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc 2011;59:887–92. 10.1111/j.1532-5415.2011.03336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangione CM, Lee PP, Gutierrez PR, et al. ; National Eye Institute Visual Function Questionnaire Field Test Investigators. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–8. [DOI] [PubMed] [Google Scholar]
- 24.Hayman KJ, Kerse N, Dyall L, et al. Life and living in advanced age: a cohort study in New Zealand—e Puawaitanga o Nga Tapuwae Kia Ora Tonu, LiLACS NZ: study protocol. BMC Geriatr 2012;12:33 10.1186/1471-2318-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz S Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983;31:721–7. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 27.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243–52. 10.1161/CIRCOUTCOMES.110.957498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. [DOI] [PubMed] [Google Scholar]
- 29.Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397–405. [DOI] [PubMed] [Google Scholar]
- 30.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 31.Murphy TE, Tsang SW, Leo-Summers LS, et al. Bayesian model averaging for selection of a risk prediction model for death within thirty days of discharge: the SILVER-AMI study. Int J Stat Med Res 2019;8:1–7. 10.6000/1929-6029.2019.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: J Wiley; 2004. [Google Scholar]
- 33.Hoeting JA, Madigan D, Raftery AE, et al. Bayesian model averaging: a tutorial. Stat Sci 1999;14:382–417. [Google Scholar]
- 34.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72. [DOI] [PubMed] [Google Scholar]
- 35.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 36.Pepe MS, Feng Z, Huang Y, et al. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol 2008;167:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raftery A, Hoeting J, Volinsky C, et al. Bayesian Model Averaging, version 3.18.6. 2015. Accessed at https://mran.microsoft.com/snapshot/2017-02-04/web/packages/BMA/index.html on 28 October 2019.
- 38.Leening MJ, Vedder MM, Witteman JC, et al. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med 2014;160:122–31. [DOI] [PubMed] [Google Scholar]
- 39.Contrera KJ, Betz J, Genther DJ, et al. Association of hearing impairment and mortality in the National Health and Nutrition Examination Survey [Letter]. JAMA Otolaryngol Head Neck Surg 2015;141:944–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher D, Li CM, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES-Reykjavik Study. Age Ageing. 2014;43:69–76. 10.1093/ageing/aft122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donoghue OA, Savva GM, Cronin H, et al. Using Timed Up and Go and usual gait speed to predict incident disability in daily activities among community-dwelling adults aged 65 and older. Arch Phys Med Rehabil 2014;95:1954–61. 10.1016/j.apmr.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 42.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 43.Kulmala J, Nykänen I, Hartikainen S. Frailty as a predictor of all-cause mortality in older men and women. Geriatr Gerontol Int 2014;14:899–905. 10.1111/ggi.12190 [DOI] [PubMed] [Google Scholar]
- 44.Shaw RE, Anderson HV, Brindis RG, et al. Development of a risk adjustment mortality model using the American College of Cardiology–National Cardiovascular Data Registry (ACC-NCDR) experience: 1998–2000. J Am Coll Cardiol 2002;39:1104–12. [DOI] [PubMed] [Google Scholar]
- 45.Yueh B, Shapiro N, MacLean CH, et al. Screening and management of adult hearing loss in primary care: scientific review. JAMA. 2003;289:1976–85. [DOI] [PubMed] [Google Scholar]
- 46.Ryskulova A, Turczyn K, Makuc DM, et al. Self-reported age-related eye diseases and visual impairment in the United States: results of the 2002 National Health Interview Survey. Am J Public Health. 2008;98:454–61. 10.2105/AJPH.2006.098202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothnie KJ, Smeeth L, Pearce N, et al. Predicting mortality after acute coronary syndromes in people with chronic obstructive pulmonary disease. Heart. 2016;102:1442–8. 10.1136/heartjnl-2016-309359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopes RD, Gharacholou SM, Holmes DN, et al. Cumulative incidence of death and rehospitalization among the elderly in the first year after NSTEMI. Am J Med 2015;128:582–90. 10.1016/j.amjmed.2014.12.032 [DOI] [PubMed] [Google Scholar]
- 49.Krumholz HM, Normand ST, Wang Y. Twenty-year trends in outcomes for older adults with acute myocardial infarction in the United States. JAMA Netw Open. 2019;2:e191938 10.1001/jamanetworkopen.2019.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rashid M, Fischman DL, Gulati M, et al. Temporal trends and inequalities in coronary angiography utilization in the management of non-ST-elevation acute coronary syndromes in the U.S. Sci Rep 2019;9:240 10.1038/s41598-018-36504-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd J, Jahanpour E, Angell B, et al. Using national inpatient death rates as a benchmark to identify hospitals with inaccurate cause of death reporting – Missouri, 2009–2012. MMWR Morb Mortal Wkly Rep 2017;66:19–22. 10.15585/mmwr.mm6601a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med 1997;16:981–91. [DOI] [PubMed] [Google Scholar]
- 53.Chew PG, Frost F, Mullen L, et al. A direct comparison of decision rules for early discharge of suspected acute coronary syndromes in the era of high sensitivity troponin. Eur Heart J Acute Cardiovasc Care. 2019;8:421–31. 10.1177/2048872618755369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 2. Study Protocol
Supplement 1. Supplemental Materials