Abstract.
Independent evaluations of XEh Rapid®, an IgG4-based rapid dipstick test, were performed to assess its diagnostic performance to detect amebic liver abscess (ALA) using 405 samples at seven laboratories in four countries. The test showed high diagnostic specificity (97–100%) when tested with samples from healthy individuals (n = 100) and patients with other diseases (n = 151). The diagnostic sensitivity was tested with a total of 154 samples, and the results were variable. It was high in three laboratories (89–94%), and moderate (72%) and low (38%) in two other laboratories. Challenges and issues faced in the evaluation process are discussed. Nevertheless, XEh Rapid is promising to be developed into a point-of-care test in particular for resource-limited settings, and thus merits further confirmation of its diagnostic sensitivity.
INTRODUCTION
Amoebiasis, caused by Entamoeba histolytica, is estimated to affect 50 million people worldwide and causes 40,000–100,000 deaths annually.1 In terms of mortality due to protozoan parasites, it ranks second place after malaria.2,3 Of these, 90% are asymptomatic carriers, and about 10% of infected individuals show clinical symptoms such as colitis, dysentery, and extraintestinal amoebiasis.2 The most common extraintestinal clinical manifestation is amebic liver abscess (ALA), and delays in diagnosis and treatment can be fatal.4,5
After clinical suspicion of ALA and history of travel or residence in an amoebiasis-endemic area, the diagnosis is initiated using imaging techniques such as ultrasound, computed tomography, and magnetic resonance to detect the presence of an abscess in the liver. If indicated, aspiration of pus may follow. The pus may be used for microscopy, culture, or detection of the protozoan DNA.2,6 However, pus aspiration is an invasive procedure and not suitable for many suspected patients. Highly sensitive imaging methods detect the presence of an abscess in the liver, but they are unable to distinguish the various causes of the liver lesion, for example, ALA, pyogenic abscesses, or necrotic tumors.7,8 DNA-based assays such as real-time PCR have been used to detect E. histolytica DNA in the pus aspirates with high sensitivity and specificity.9–12 However, the cost of performing this technique is quite high, thus limiting its use only to reference laboratories in poorly resourced countries. A laboratory test that can be concurrently performed to detect anti-amebic antibody in serum/plasma samples can avoid a costly and an invasive surgical procedure.
Serology is an essential laboratory diagnostic test for ALA to complement the imaging findings. Currently, indirect hemagglutination assay (IHA) and ELISA are still the most common tests, with the latter being more popular in routine diagnosis of ALA.2 Some of the commercially available antibody detection ELISA tests include NovaLisa™ Entamoeba IgG (NovaTec Immunodiagnostica GmbH, Dietzenbatch, Germany), Novagnost® E. histolytica IgG (Siemens Novagnost, München, Germany), E. histolytica IgG ELISA (Creative Diagnostics, Shirley, NY), anti–E. histolytica IgG human ELISA (Abcam, Cambridge, United Kingdom), E. histolytica IgG ELISA (GenWay Biotech, San Diego, CA), E. histolytica Antibody Detection Test Kit (SCIMEDX, Dover, NJ), E. histolytica IgG ELISA (Bordier, Suisse, Switzerland), DRG E. histolytica IgG (DRG International Inc., Springfield, NJ), E. histolytica IgG (Amoebiasis) ELISA (Diagnostic Automation Inc., Calabasas, CA), Amebiasis Serology Microwell EIA (LMD Laboratories, Inc., Carlsbad, CA), and RIDASCREEN® Entamoeba IgG (R-Biopharma AG, Darmstadt, Germany). However, some ELISAs have lower sensitivity and specificity when used in low-to-medium endemic areas, especially in developing and underdeveloped countries.2,13–15 Therefore, a highly sensitive and specific diagnostic tool is necessary to detect the disease, especially at the early stage of infection, for the purpose of administering prompt treatment to prevent complications.
In low-income communities, clinical laboratories are often faced with financial and human resource constraints. Therefore, the diagnosis of infectious diseases depends mostly on the availability and accessibility of point-of-care tests.16 To date, there is no serological test using a recombinant antigen-based test on the market for the diagnosis of extraintestinal amoebiasis. The use of a defined recombinant antigen is likely to increase the diagnostic sensitivity and specificity in detecting ALA.
Previously, we have shown the promising performance of E. histolytica pyruvate phosphate dikinase (PPDK) as a diagnostic marker for the detection of ALA.17 Pyruvate phosphate dikinase is an excretory-secretory protein produced by E. histolytica and a key enzyme in its anaerobic metabolism via pyrophosphate-dependent glycolysis.18 A proof of concept of a lateral flow dipstick test for rapid detection of ALA known as XEh Rapid has been developed. The test is based on the detection of the anti-PPDK IgG4 antibody in infected patients. The initial “in-house” evaluation of the rapid test showed 87% diagnostic sensitivity and 100% specificity.19 The test is simple to perform, the results are easy to interpret, and the method does not require any specialized equipment, and has the potential to assist in the management of amoebiasis. Thus, this study aims to further evaluate the diagnostic sensitivity and specificity of this rapid test independently at seven laboratories in four different countries.
MATERIALS AND METHODS
Serum samples.
The samples were grouped into three categories, that is, ALA, healthy individuals’ serum samples, and samples from patients with other infections. All ALA samples were diagnosed based on relevant clinical presentations, radiology imaging, and serology results. The details of the serum samples from each laboratory are given in Table 1. A total of 405 serum samples from seven collaborating laboratories were used in this study, that is, Universiti Sains Malaysia, Health Campus, Kelantan, Malaysia (USM, n = 83); Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia (UKM, n = 77); University of Malaya, Kuala Lumpur, Malaysia (UM, n = 45); Institute for Medical Research, Kuala Lumpur, Malaysia (IMR, n = 64); Pasteur Institute of Iran, Tehran, Iran (PII, n = 64); NSW Health Pathology, Sydney, Australia (NSW HP, n = 54); and Parasitology Department, National Microbiology Center, Instituto de Salud Carlos III, Madrid, Spain (ISC, n = 18). Previously, serum samples from the USM, UM, and NSW HP were tested with IHA for detection of anti-IgG amebic antibodies, whereas samples from the ISC were tested using NovaLisa E. histolytica IgG ELISA (Szabo-Scandic, Vienna, Austria). Before the present evaluation study, ELISA using RIDASCREEN E. histolytica IgG (R-Biopharm AG) was performed on serum samples at all laboratories, except at the ISC and PII. Only serum samples from the ISC, Spain, had accompanying positive real-time PCR results of liver abscess samples, which targeted SSU rRNA gene of E. histolytica.20,21
Table 1.
Details on the kinds and number of serum samples from collaborating laboratories
| No | Collaborating laboratories | Positive sera from individuals with amebic liver abscess | Sera from individuals with other infections* | Sera from healthy individuals* | Total number of serum samples |
|---|---|---|---|---|---|
| 1 | Universiti Sains Malaysia, Health Campus, Kelantan, Malaysia | n = 23 IHA (+) and IgG ELISAs (+) | n = 29 Toxoplasmosis = 7, pyogenic liver abscess = 4, salmonellosis = 3, hepatoma = 2, urinary tract infections = 2, leptospirosis = 2 and one sample each from patient with the following diseases/infections: shigellosis, ascariasis, Escherichia coli, Stenotrophomonas maltophilia, advanced ovarian cancer, choledocholithiasis, Gram-positive cocci, recurrent liver abscess with cellulitis, and unknown non-liquified liver abscess | n = 31 | 83 |
| 2 | University of Malaya, Kuala Lumpur, Malaysia | n = 33 27 were IHA (+) and IgG ELISAs, and 6 were IgG ELISAs (+) | n = 12 Cysticercosis = 5, schistosomiasis = 3, strongyloidiasis = 2, echinococcosis = 1, and leishmaniasis = 1 | – | 45 |
| 3 | Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia | – | n = 65 Toxoplasmosis = 36, HIV = 26, and malaria = 3 | n = 12 | 77 |
| 4 | Institute for Medical Research, Kuala Lumpur, Malaysia | n = 64 IgG ELISAs (+) | – | – | 64 |
| 5 | NSW Health Pathology, Sydney, Australia | n = 16 IHA (+) and IgG ELISAs (+) | n = 26 Influenza A = 20, hydatidosis = 3, and strongyloidiasis = 3 | n = 12 | 54 |
| 6 | Instituto de Salud Carlos III, Madrid, Spain | n = 18 PCR of liver abscess (+) and IgG ELISAs (+) | – | – | 18 |
| 7 | Pasteur Institute of Iran, Tehran, Iran | – | n = 19 Toxoplasmosis = 9, hydatidosis = 5, fascioliasis = 3, and toxocariasis = 2 | n = 45 | 64 |
| Total = 154 | Total = 151 | Total = 100 | 405 |
IHA = indirect hemagglutination assay.
All sera were negative when tested with commercial anti-amebic IgG-ELISA, except for serum samples from the PII. Iran is not endemic for amoebiasis; thus, the serum samples at PII were presumed to be negative for anti-amebic IgG antibodies.
Lateral flow dipstick test (XEh Rapid).
The XEh Rapid tests were individually pouched with a desiccant and sent to the seven laboratories, together with detailed instructions on how to conduct the test, including a video. The USM laboratory performing the evaluation was located on a different campus away from the laboratory where the tests were prepared, thus maintaining independence of the evaluation at the USM.
The XEh Rapid was produced as previously described by Saidin et al.,19 with modifications. In brief, a recombinant PPDK (test line) and a goat anti-mouse IgG (control line) (Invitrogen, Carlsbad, CA) at 3 mg/mL and 0.25 mg/mL, respectively, were absorbed onto a Hi-Flow Plus 90 nitrocellulose membrane card (Millipore Corporation, Bedford, MA). The lined card was dried at 37°C for 2 hours and coated with a blocking solution (Roche Diagnostics, Mannheim, Germany). After 2 hours of incubation, an absorbent pad was attached on the top part of the nitrocellulose membrane and then cut into 5-mm-wide dipstick strips.
The gold conjugation of the mouse anti-human IgG4 (Calbiochem, EMD Millipore, Billerica, MA) was performed according to the protocol by Makhsin et al.22 The gold conjugate was mixed with a drying buffer (0.05 M Na2HPO4, 1% w/v bovine serum albumin, 0.1% w/v NaN3) and 5% v/v trehalose to a final optical density (OD)8, and then added to the middle well of a triple-well flat-bottomed ELISA microplate. The conjugate–buffer mix was allowed to dry at 35°C for 8 hours.
The XEh Rapid was performed by adding three drops of buffer 1 (Reszon Diagnostics International, Selangor, Malaysia) into well C. Then, 35 µL of buffer 2 (phosphate buffered saline) was pipetted into well B to reconstitute the dried conjugate; 10 µL of buffer 2 was added to well A and mixed with an equal volume of a serum sample. The strip was placed into the well A containing the diluted serum sample, and the sample was allowed to migrate up the strip. The strip was then transferred into well B until all the conjugate was fully absorbed onto the strip before moving into well C to wash the excess unbound gold conjugate. The result was interpreted as positive when reddish lines appeared at both the control and the test line (recombinant PPDK) position, whereas a negative result showed only the control line.
RESULTS
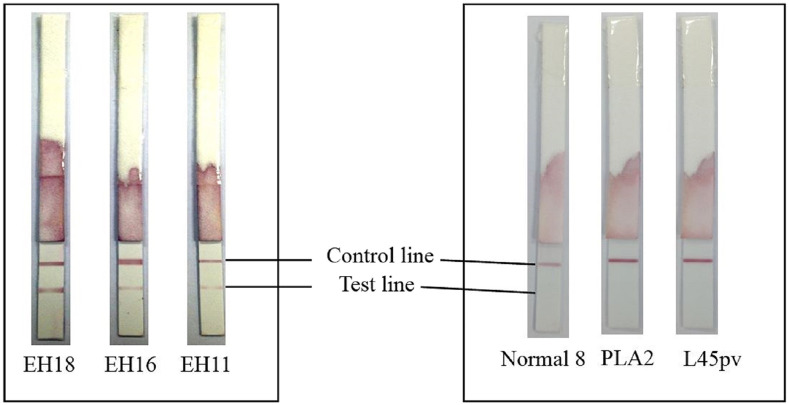
Figure 1 shows the appearance of XEh Rapid dipsticks after testing was completed. The difference in test line intensities of the dipsticks is due to the varying levels of anti-amebic antibodies in the serum samples. The diagnostic specificity and sensitivity of the test reported by each laboratory are presented in Table 2. The diagnostic specificity of XEh Rapid was consistently high (97–100%), with an average of 98%. Of note, the overall specificity of sera from healthy individuals and sera from other infections were almost the same, that is, 97% and 98%, respectively. The serum samples of other diseases which were positive by XEh Rapid were from patients with HIV (UKM), leptospirosis (USM), and choledocholithiasis (USM).
Figure 1.
Appearance of XEh Rapid dipsticks after testing was completed. Strips labeled as EH18, EH16, and EH11 were tested with serum samples from patients with amebic liver abscess. Strips labeled as Normal 8, PLA2, and L45pv were tested with serum samples from healthy individuals, with pyogenic liver abscess and malaria, respectively. This figure appears in color at www.ajtmh.org.
Table 2.
Summary of the results of the evaluation of diagnostic sensitivity and specificity of XEh Rapid
| Laboratory | Number of positive | Number of negative | Diagnostic sensitivity (%) | Diagnostic specificity (%) | |
|---|---|---|---|---|---|
| Amebic liver abscess | Healthy | Other infections | |||
| Universiti Sains Malaysia, Health Campus, Kelantan, Malaysia | 21/23 | 31/31 | 27/29 | 91 | 97 |
| Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia | – | 12/12 | 64/65 | – | 99 |
| University of Malaya, Kuala Lumpur, Malaysia | 31/33 | – | 12/12 | 94 | 100 |
| Institute for Medical Research, Kuala Lumpur, Malaysia | 46/64 | – | – | 72 | – |
| Pasteur Institute of Iran, Tehran, Iran | – | 43/45 | 19/19 | – | 97 |
| NSW Health Pathology, Sydney, Australia | 6/16 | 11/12 | 26/26 | 38 | 97 |
| Malaria and Emerging Parasitic Diseases Laboratory, National Microbiology Center, Instituto de Salud Carlos III, Madrid, Spain | 16/18 | – | – | 89 | – |
However, some variations were observed in diagnostic sensitivities obtained by the different laboratories. Three of the laboratories gave high diagnostic sensitivity, that is, 94% at the UM, 91% at the USM, and 89% at the ISC. However, evaluation of the test at the IMR and the NSW HP showed moderate and low sensitivity of 72% and 38%, respectively.
DISCUSSION
Currently, most commercial kits for amoebiasis serology are in the form of IgG ELISA. Amebiasis Serology Microwell EIA (LMD Laboratories) showed 92.5–97.9% sensitivity and 91.3–94.8% specificity for the detection of anti–E. histolytica IgG antibodies in ALA patients.23,24 Novagnost E. histolytica IgG (Siemens Novagnost) demonstrated 95% sensitivity and 98% specificity in a study of confirmed liver abscess cases,25 whereas in another study, it showed 89% sensitivity and 56% specificity.26 Meanwhile, E. histolytica Antibody Detection Test Kit (SCIMEDX) demonstrated 100% sensitivity and specificity when tested on serum samples of 22 patients with liver aspirates positive for E. histolytica by PCR.27 Entamoeba histolytica IgG ELISA (Bordier) performed on biobank serum samples at a laboratory in Grenoble, France, showed a sensitivity of 95% (61/64) and a specificity of 94% (63/67) as compared with previously available indirect fluorescent antibody test (IFAT) results.28
A prototype lateral flow test, XEh Rapid, an IgG4-based assay using PPDK recombinant protein, has been developed for the detection of ALA.19 Here, we present the results of an independent evaluation of the performance of XEh Rapid in seven laboratories in four countries, that is, Malaysia, Iran, Australia, and Spain. The test showed high diagnostic sensitivity of 89–91% at three of the five laboratories, thus almost comparable to the diagnostic sensitivity reports of commercial IgG-ELISA kits described earlier, with the additional benefit of being a lateral flow rapid test. The results were also consistent with the initial sensitivity data of the rapid test that we reported earlier.19 Our previous study used wet colloidal gold-conjugated IgG4 at OD5 and 1.25 mg/mL rPPDK as the test line. Meanwhile, the present study used dried gold-conjugated IgG4 which required higher concentrations of both conjugate (OD 8) and rPPDK (3.0 mg/mL).
However, the evaluation at the NSW HP showed a surprisingly low sensitivity of 38%, with only six of 16 positive samples giving positive results. The reduced sensitivity might be due to factors related to international transport and customs clearance affecting the test quality, leading to its suboptimal performance during the evaluation in Australia. Although the prototype tests were individually pouched, they were likely not as robust as the commercial rapid tests because the former were not processed and packaged in an optimum condition like that of the latter.
The diagnostic sensitivity at the IMR displayed a moderate sensitivity of 72%, with 46 positives of 64 serum samples. The Parasitology Unit at the IMR receives samples of patients from various government and private hospitals clinically diagnosed as ALA and/or samples that were visualized by imaging diagnostic methods presumed to characterize lesions due to extraintestinal amoebiasis.
Inconsistent sensitivity results of RIDASCREEN E. histolytica IgG ELISA kit have also been reported. In the year 2018, the kit was reported to be positive among 78.5% (690/879) samples of ALA patients diagnosed based on clinical manifestations, and radiological and microbiological examinations.29 In the year 2005, using a panel of serum from 239 individuals, the kit was shown to be highly sensitive (97.7%) and specific (100%, n = 18) for detecting antibodies in ALA patients.30 However, in 2016, the ELISA kit showed 56.4% sensitivity and 92.1% specificity among confirmed cases of liver abscess (n = 157).31 In yet another study, the sensitivity and specificity of this kit was 69% (n = 100) and 90% (n = 100), respectively, when tested with ALA patients, other parasitic and liver disease patients, and healthy individuals.32 Thus, when all of the aforementioned information is taken into consideration, the diagnostic sensitivity of XEh Rapid at 72% obtained at the IMR in this study may not be surprising.
Ideally, positive serum samples for XEh Rapid evaluation should be obtained from individuals clinically diagnosed as ALA, and the presence of amoeba in the pus confirmed by PCR or equivalent method, or by microscopy (although this method has very low sensitivity). However, pus aspiration of liver abscess is rarely performed because of the invasiveness of the procedure.12,33 Thus, most of the time, we rely on the definition of positive samples as those with a clinical diagnosis of ALA, along with positive radiological (ultrasound) findings and serological results. As mentioned earlier, only the ISC in Spain had serum samples from patients whose liver abscess samples were PCR positive. Because of the different kinds of positive samples among the multi-laboratories, this can be considered as an immature evaluation of XEh Rapid.
It should also be noted that IgG ELISAs detect not only antibodies to ALA (and other extraintestinal amebic diseases) but also about 75% intestinal infections and about 10% asymptomatic infections.34 Obaid35 reported that Novagnost IgG ELISA (Siemens Novagnost) showed 27.6% (24/87) positivity among patients who suffered abdominal pain and diarrhea (n = 97) and were confirmed of being infected with E. histolytica using a stool antigen detection test, TechLab E. histolytica II Test (TechLab, Blackburg, VA). Another study, using DRG E. histolytica serum IgG ELISA (DRG International Inc.), detected serum IgG antibody against E. histolytica in 36.2% (n = 34/94) patients who complained of diarrhea and/or abdominal discomfort.36 Among asymptomatic preschool children in an urban slum of Dhaka, Bangladesh, 32.7% (n = 76/232) were seropositive with E. histolytica serum IgG when tested with anti-lectin IgG ELISA (TechLab Inc.).37 Meanwhile, in a cross-sectional study in rural Mexico using E. histolytica IgG (Amebiasis) ELISA kit (Diagnostic Automation Inc.), 41.8% (n = 118/282) of subjects had anti–E. histolytica IgG antibodies.38
After an intestinal invasion by the amoeba, specific IgG antibodies rise soon after and remain for some time after the infection is cured. IgG antibodies may be detectable for years after treatment; therefore, they may not be reliable to distinguish between active and past infection.1 Meanwhile, as shown in helminthic infections, specific IgG4 antibodies are detected slightly later and disappear relatively sooner after the disease has resolved.39,40 Because XEh Rapid test is an IgG4 test, we can expect the overall detection rate to be lower than that of an IgG test. This may explain the lower sensitivity by the XEh Rapid at IMR and NSW HP as well as the two “false-negative” samples at each USM and UM. Furthermore, there is a possibility of IgG4 antibodies degrading naturally on long-term storage. It is unknown whether IgG4 antibodies may be more prone to degrade faster than other subclasses of antibodies.
The diagnostic specificity of the XEh Rapid test was evaluated with serum samples from healthy individuals and a variety of other diseases. The result showed that the test has high diagnostic specificity, with an average specificity of 97.6% (245/251), and ranged from 96.7% to 100%. Thus, at all laboratories, the diagnostic specificity of the XEh Rapid test was consistently high. In this regard, as described earlier, most commercial IgG-ELISA kits were reported to achieve at least 90% diagnostic specificity.
In conclusion, the present independent evaluation study conducted at seven laboratories demonstrated that XEh Rapid has excellent specificity and variable sensitivity. XEh Rapid has advantages of being convenient, accessible, and easy to use for the detection of ALA, in particular for resource-limited settings. Thus, further validation studies to confirm the diagnostic sensitivity of XEh Rapid is merited. In future evaluations, there should be a more stringent selection of positive serum samples and more attention paid to transportation challenges.
Acknowledgments:
We would like to thank the following individuals who have helped with the testing of the samples: Vishal Ahuja at the NSW HP; Wong Weng Kin, Nik Zairi Zakaria, and Zulnuhisham Suhaimi at the USM; and Sam Khanbabaei at the PII.
REFERENCES
- 1.Shirley DAT, Farr L, Watanabe K, Moonah S, 2018. A review of the global burden, new diagnostics, and current therapeutics for amebiasis. Open Forum Infect Dis 5: ofy161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fotedar R, Stark D, Beebe N, Marriott D, Ellis J, Harkness J, 2007. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev 20: 511–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley SL, Jr., 2003. Amoebiasis. Lancet 361: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 4.Zlobl TL, 2001. Amebiasis. Prim Care Update Ob Gyns 8: 65–68. [DOI] [PubMed] [Google Scholar]
- 5.Prakash V, Bhimji SS, 2017. Abscess, amebic liver. StatPearls (Internet) Treasure Island, FL: StatPearls Publishing. [Google Scholar]
- 6.Tanyuksel M, Petri WA, 2003. Laboratory diagnosis of amebiasis. Clin Microbiol Rev 16: 713–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aucott JN, Ravdin JI, 1993. Amebiasis and “nonpathogenic” intestinal protozoa. Infect Dis Clin North Am 7: 467–485. [PubMed] [Google Scholar]
- 8.Sharma MP, Ahuja V, 2003. Amoebic liver abscess. J Indian Acad Clin Med 4: 107–111. [Google Scholar]
- 9.Ahmad N, Khan M, Hoque MI, Haque R, Mondol D, 2007. Detection of Entamoeba histolytica DNA from liver abscess aspirate using polymerase chain reaction (PCR): a diagnostic tool for amoebic liver abscess. Bangladesh Med Res Counc Bull 33: 13–20. [PubMed] [Google Scholar]
- 10.Othman N, Mohamed Z, Verweij JJ, Huat LB, Olivos-Garcia A, Yeng C, Noordin R, 2010. Application of real-time polymerase chain reaction in detection of Entamoeba histolytica in pus aspirates of liver abscess patients. Foodborne Pathog Dis 7: 637–641. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Kabir M, Mondal D, Ali IK, Petri WA, Jr., Haque R, 2005. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J Clin Microbiol 43: 2168–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haque R, Kabir M, Noor Z, Rahman SM, Mondal D, Alam F, Rahman I, Al Mahmood A, Ahmed N, Petri WA, 2010. Diagnosis of amebic liver abscess and amebic colitis by detection of Entamoeba histolytica DNA in blood, urine, and saliva by a real-time PCR assay. J Clin Microbiol 48: 2798–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parija SC, Mandal J, Ponnambath DK, 2014. Laboratory methods of identification of Entamoeba histolytica and its differentiation from look-alike Entamoeba spp. Trop Parasitol 4: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeehaida M, Wan Nor Amilah WA, Amry AR, Hassan S, Sarimah A, Rahmah N, 2008. A study on the usefulness of Techlab Entamoeba histolytica II antigen detection ELISA in the diagnosis of amoebic liver abscess (ALA) at Hospital Universiti Sains Malaysia (HUSM), Kelantan, Malaysia. Trop Biomed 25: 209–216. [PubMed] [Google Scholar]
- 15.Zengzhu G, Bracha R, Nuchamowitz Y, Cheng IW, Mirelman D, 1999. Analysis by enzyme-linked immunosorbent assay and PCR of human liver abscess aspirates from patients in China for Entamoeba histolytica. J Clin Microbiol 37: 3034–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peeling RW, Mabey D, 2010. Point-of-care tests for diagnosing infections in the developing world. Clin Microbiol Infect 16: 1062–1069. [DOI] [PubMed] [Google Scholar]
- 17.Wong WK, Tan ZN, Othman N, Lim BH, Mohamed Z, Olivos Garcia A, Noordin R, 2011. Analysis of Entamoeba histolytica excretory-secretory antigen and identification of a new potential diagnostic marker. Clin Vaccine Immunol 18: 1913–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saavedra-Lira E, Ramirez-Silva L, Perez-Montfort R, 1998. Expression and characterization of recombinant pyruvate phosphate dikinase from Entamoeba histolytica. Biochim Biophys Acta 1382: 47–54. [DOI] [PubMed] [Google Scholar]
- 19.Saidin S, Yunus MH, Zakaria ND, Razak KA, Huat LB, Othman N, Noordin R, 2014. Production of recombinant Entamoeba histolytica pyruvate phosphate dikinase and its application in a lateral flow dipstick test for amoebic liver abscess. BMC Infect Dis 14: 182. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Verweij JJ, Oostvogel F, Brienen EA, Nang-Beifubah A, Ziem J, Polderman AM, 2003. Prevalence of Entamoeba histolytica and Entamoeba dispar in northern Ghana. Trop Med Int Health 8: 1153–1156. [DOI] [PubMed] [Google Scholar]
- 21.Gutiérrez-Cisneros MJ, Cogollos R, López-Vélez R, Martín-Rabadán P, Martínez-Ruiz R, Subirats M, Merino FJ, Fuentes I, 2010. Application of real-time PCR for the differentiation of Entamoeba histolytica and E. dispar in cyst-positive faecal samples from 130 immigrants living in Spain. Ann Trop Med Parasitol 104: 145–149. [DOI] [PubMed] [Google Scholar]
- 22.Makhsin SR, Razak KA, Noordin R, Zakaria ND, Chun TS, 2012. The effects of size and synthesis methods of gold nanoparticle-conjugated MalphaHIgG4 for use in an immunochromatographic strip test to detect brugian filariasis. Nanotechnology 23: 495719. [DOI] [PubMed] [Google Scholar]
- 23.Shenai BR, Komalam BL, Arvind AS, Krishnaswamy PR, Rao PV, 1996. Recombinant antigen-based avidin-biotin microtiter enzyme-linked immunosorbent assay for serodiagnosis of invasive amebiasis. J Clinc Microbiol 34: 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hira PR, Iqbal J, Al-Ali F, Philip R, Grover S, D’Almeida E, Al-Eneizi AA, 2001. Invasive amebiasis: challenges in diagnosis in a non-endemic country (Kuwait). Am J Trop Med Hyg 65: 341–345. [DOI] [PubMed] [Google Scholar]
- 25.van Hal SJ, Stark DJ, Fotedar R, Marriott D, Ellis JT, Harkness JL, 2007. Amoebiasis: current status in Australia. Med J Aust 186: 412–416. [DOI] [PubMed] [Google Scholar]
- 26.Jaiswal V, Ghoshal U, Baijal SS, Mittal B, Dhole TN, Ghoshal UC, 2012. Evaluation of antigen detection and polymerase chain reaction for diagnosis of amoebic liver abscess in patients on anti-amoebic treatment. BMC Res Notes 5: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morshed M, Cherian SS, Lo T, Lee MK, Wong Q, Hoang L, 2017. Superiority of PCR against microscopy for diagnosing Entamoeba histolytica in liver abscess samples. Can J Infect Dis Med Microbiol 2: 1–3. [Google Scholar]
- 28.Beyls N, Cognet O, Stahl JP, Rogeaux O, Pelloux H, 2018. Serodiagnosis of extraintestinal amebiasis: retrospective evaluation of the diagnostic performance of the Bordier® ELISA kit. Korean J Parasitol 56: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal S, Verma N, Perumalla S, Mirdha BR, 2018. Decreasing trend of seroprevalence of hepatic amoebiasis in tertiary care hospital of north India: 2010–2015. J Lab Physicians 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knappik M, Borner U, Jelinek T, 2005. Sensitivity and specificity of a new commercial enzyme-linked immunoassay kit for detecting Entamoeba histolytica IgG antibodies in serum samples. Eur J Clin Microbiol Infect Dis 24: 701–703. [DOI] [PubMed] [Google Scholar]
- 31.Dhanalakshmi S, Parija SC, 2016. Seroprevalence of Entamoeba histolytica from a tertiary care hospital, south India. Trop Parasitol 6: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhanalakshmi S, Meenachi C, Parija SC, 2016. Indirect haemagglutination test in comparison with ELISA for detection of antibodies against invasive amoebiasis. J Clin Diagn Res 10: DC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhawan VK, 2008. Current diagnosis and treatment of amoebiasis. US Infect Dis 4: 59–61. [Google Scholar]
- 34.Espinosa-Cantellano M, Martinez-Palomo A, 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev 13: 318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obaid HM, 2016. Detection and differentiation of Entamoeba histolytica and Entamoeba dispar by enzyme linked immune sorbent assay. Kirkuk Univ J Sci Stud 11: 263–277. [Google Scholar]
- 36.Kadir MA, Daoud IS, Al-Bayati Z, 2013. Differentiation between Entamoeba histolytica and E. dispar using enzyme-linke immunosorbent assay and wet mount method. Tirkit J Pharm Sci 9: 122–129. [Google Scholar]
- 37.Haque R, Mollah NU, Ali IKM, Alam K, Eubanks A, Lyerly D, Petri WA, 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clinc Microbiol 38: 3235–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarado-Esquivel C, Hernandez-Tinoco J, Sanchez-Anguiano LF, 2015. Seroepidemiology of Entamoeba histolytica infection in general population in rural Durango, Mexico. J Clin Med Res 7: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arifin N, Hanafiah KM, Ahmad H, Noordin R, 2019. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol 52: 371–378. [DOI] [PubMed] [Google Scholar]
- 40.Atmadja AK, Atkinson R, Sartono E, Partono F, Yazdanbakhsh M, Maizels RM, 1995. Differential decline in filaria-specific IgG1, IgG4, and IgE antibodies in Brugia malayi-infected patients after diethylcarbamazine chemotherapy. J Infect Dis 172: 1567–1572. [DOI] [PubMed] [Google Scholar]