Abstract.
Immune correlates of protection against clinical malaria are difficult to ascertain in low-transmission areas because of the limited number of malaria cases. We collected blood samples from 5,753 individuals in a Kenyan highland area, ascertained malaria incidence in this population over the next 6 years, and then compared antibody responses to 11 Plasmodium falciparum vaccine candidate antigens in individuals who did versus did not develop clinical malaria in a nested case–control study (154 cases and 462 controls). Individuals were matched by age and village. Antigens tested included circumsporozoite protein (CSP), liver-stage antigen (LSA)-1, apical membrane antigen-1 FVO and 3D7 strains, erythrocyte-binding antigen-175, erythrocyte-binding protein-2, merozoite surface protein (MSP)-1 FVO and 3D7 strains, MSP-3, and glutamate-rich protein (GLURP) N-terminal non-repetitive (R0) and C-terminal repetitive (R2) regions. After adjustment for potential confounding factors, the presence of antibodies to LSA-1, GLURP-R2, or GLURP-R0 was associated with decreased odds of developing clinical malaria (odds ratio [OR], [95% CI] 0.56 [0.36–0.89], 0.56 [0.36–0.87], and 0.77 [0.43–1.02], respectively). Levels of antibodies to LSA-1, GLURP-R2, and CSP were associated with decreased odds of developing clinical malaria (OR [95% CI]; 0.61 [0.41–0.89], 0.60 [0.43–0.84], and 0.49 [0.24–0.99], for every 10-fold increase in antibody levels, respectively). The presence of antibodies to CSP, GLURP-R0, GLURP-R2, and LSA-1 combined best-predicted protection from clinical malaria. Antibodies to CSP, GLURP-R0, GLURP-R2, and LSA-1 are associated with protection against clinical malaria in a low-transmission setting. Vaccines containing these antigens should be evaluated in low malaria transmission areas.
INTRODUCTION
In malaria-endemic settings, repeated exposure to bites from mosquitoes infected with human Plasmodium species leads to a level of naturally acquired immunity to clinical disease.1,2 Lack of clinical immunity is an important contributor to the development of severe disease, and it is one of the reasons why 69% of the 429,000 deaths from malaria worldwide occurred in children younger than 5 years.3 Older children and adults living in stable transmission settings have acquired a degree of immunity to Plasmodium species that prevents symptoms despite infection, and this immunity appears to be maintained with continuous inoculations.3 Although it is known that partial immunity can develop with increased malaria exposure, the mechanisms by which it does so remain poorly understood. Multiple studies have suggested that antibodies play a role in protection from clinical malaria, which has in turn triggered vaccine development research.4–8 Current vaccine candidates largely target Plasmodium falciparum, which is responsible for most morbidity and mortality worldwide.3,9,10 Efficacy in trials of the most advanced vaccine candidates has been low and of limited duration, prompting calls to explore an expanded pool of candidate antigens in varying malaria transmission settings, as well as multiple antigens in combination, including antigens from different stages of the parasite life cycle.10–12
In unstable, low-transmission settings, symptomatic disease may be seen in older individuals because limited exposure leads to slower acquisition of clinical immunity. With the use of prevention measures such as bed nets and indoor residual spraying, along with improved testing with rapid diagnostic tests and better treatment with artemisinin combination therapy, increasing numbers of people will be living in areas of unstable transmission and have less naturally acquired immunity than when the areas had stable transmission. However, few studies have assessed whether antibody-associated protection from clinical malaria occurs in settings of low and unstable transmission, primarily because the low incidence of malaria in these areas requires testing for antibodies and follow-up for clinical malaria in a large cohort. Using a novel nested case–control design matched on age and village, with prolonged follow-up for malaria over 6 years in a large study population, we were able to assess the risk of clinical malaria in a population with highly seasonal malaria transmission that experienced interruption of clinical malaria for 13 months just before the study sample collection and maintained low transmission during the subsequent follow-up period.13 We hypothesized that correlates of protection against clinical malaria identified in this low-transmission setting may be different from those identified in higher transmission settings, and would be important in considerations for vaccine development for low-transmission settings.
MATERIALS AND METHODS
Study site, participants, and data and sample collection.
We performed a nested case–control study of men, women, and children from the highland Kipsamoite and Kapsisiywa areas of Nandi County in western highland Kenya (altitude, 1,887–2,108 m), which experience unstable malaria transmission.14 Field assistants (FA) enumerated households in the entire study area to participate in surveillance for clinical malaria at the Kipsamoite Health Center (starting in 2001) and at the Kapsisiywa Health Center (starting in 2003), both of which are Kenya Ministry of Health dispensaries and the only health-care facilities in the study area. Study participants presenting to the clinics with fever, or a history of fever or other symptoms consistent with malaria, were tested for malaria. In 2007, we requested all individuals resident at the study sites to participate in a blood sample collection. Of the approximately 7,975 individuals who lived in the study sites, 5,753 were present and agreed to provide a blood sample for study testing. A single sample was collected from each individual in the period from April to June 2007, and only these samples were tested in the present study.
The present study examines subjects who participated in this blood sample collection who subsequently developed clinical malaria (cases) versus those who did not (controls), during malaria surveillance from June 2007 to June 2013. In prior studies, we documented that measured fever was attributable to malaria even at the lowest levels of parasitemia14 and that asymptomatic parasitemia was rare in this population,13 so a positive blood smear for Plasmodium species at any density, in conjunction with measured fever (T ≥ 37.5°C) or a history of fever or headache, was defined as clinical malaria. Headache was included as a criterion because we previously documented that screening persons presenting with fever or headache increased sensitivity of detection of clinical malaria in the present study setting.15 Three controls per case were selected from among participants who had the same follow-up time as cases but in whom clinical malaria was not detected during the follow-up period; controls were matched with cases on village and age. Controls in age categories < 5, 5–14, and 15–64 years were matched to cases in the same categories and differing in age by no more than 2 years and controls of age 65 years and older were matched to cases in the same category and differing in age by no more than 5 years because of low numbers in this age group.
At the time of initial enrollment, the FA collected household data, including roof material and number of rooms, and used GPS to map household coordinates and elevation. The study area’s health centers, forest edge, and swamps were also mapped using GPS.16 The Euclidean distance from each subject’s household at the time of the site-wide blood collection to the nearest of each of these study area attributes was calculated in ArcGIS version 10.1 (ESRI, Redlands, CA).17 Individual use of bed nets (yes or no), individual travel outside the study area (yes or no), and household treatment by indoor residual spraying (yes or no) were collected during demography surveys, which were conducted every 4–6 months during 2007 and 2008 and annually starting in 2009. Bed net use was also ascertained at the time of the site-wide blood collection. We considered as covariates bed net use, travel, and indoor residual spraying defined for each subject as a fraction, the number of yes responses divided by the total number of responses from 2007 to the year corresponding to the case date, which for controls was the year of the case to which they were matched. We also considered as covariates roof material, distance to the nearest forest, elevation, and potential residual confounding on age.
Human subjects protection.
Written consent was obtained from heads of households during initial enrollment for participation in demography and passive clinical malaria surveillance, from individual participants in the site-wide blood collection, and from individual participants presenting to the clinics with symptoms of malaria for blood collection. The study was approved by the University of Minnesota Institutional Review Board and the Kenya Medical Research Institute Ethical Review Committee.
Antibody testing.
Stored plasma samples from the site-wide blood collection were used to test human IgG antibody responses to 11 antigens, including two preerythrocytic-stage antigens: circumsporozoite protein (CSP) (NANP)5 repeat peptide and liver-stage antigen 1 (LSA-1) C-terminal region 3D7 strain; and nine blood-stage antigens: apical membrane antigen 1 (AMA-1) FVO strain, AMA-1 3D7 strain, erythrocyte-binding antigen 175 (EBA-175), erythrocyte-binding protein-2 (EBP-2), merozoite surface protein 1 (MSP-142) FVO strain, MSP-142 3D7 strain, MSP-3 FVO strain, glutamate-rich protein N-terminal non-repetitive region (GLURP-R0), and glutamate-rich protein C-terminal repetitive region (GLURP-R2). Antigen expression and testing of antibodies to the recombinant antigens by a multiplex cytometric bead assay (CBA) was performed as previously described,18,19 with the additional inclusion of EBP-2 (coating concentration of 2 μg for 612,500 beads). IgG antibodies to CSP (NANP)5 peptide were measured by ELISA, as previously described.20 To evaluate consistency of testing across plates, each plate also included negative controls consisting of nine samples tested in duplicate from North Americans never exposed to malaria, as well as two positive control samples consisting of a plasma pool from 30 Kenyans living in a lowland area where malaria is endemic, and four blank sample wells containing phosphate-buffered saline diluents. Optical density (OD) or median fluorescence intensity (MFI) values were calculated after subtracting values from blank wells. A total of 616 plasma samples were tested (154 cases and 462 controls) on 10 plates run over 4 days. Cases and controls were tested on the same plate. The same bead coupling set was used for all CBA testing.
Statistical analysis.
Antibody levels were expressed in arbitrary units (AUs). For ELISA, these were calculated for a particular plate as the test sample OD value divided by a quantity derived from the ODs for the North American controls on the same plate, namely, the mean OD plus 3 SDs. For CBA, AUs were calculated using MFI values. The antibody levels were used in two ways in the analyses: first, dichotomizing them as positive or negative, where AU ≥ 1 was considered a positive response; and second, using the common log (log to base 10) of the antibody level as a measurement on a continuous scale. AU values for controls were consistent across plates (coefficient of variation [CV] for different antibodies: CV, 10.1–25.3%).
We evaluated the balance of age between cases and controls after matching using the standardized difference in means. To assess whether associations exist between antibody responses to the antigens tested and clinical malaria, we estimated odds ratios (ORs) using conditional logistic regression, taking into account the matching of age and village in the study design. Crude associations were used to compare cases and controls in antibody responses dichotomized as positive or negative, and median antibody levels (AU) as a continuous measure, as we used a one case to three controls matching design. During final model selection, we considered the potential confounding effects of household elevation; treatment by indoor residual spraying; roof material; number of rooms; distances to the nearest forest, swamp, and health clinic; individual bed net use; and travel, identified a priori.21 To determine the final adjusted models, we used the purposeful selection of covariates method; used fractional polynomials to evaluate the linearity in the logit of each continuous variable; tested for statistical interaction of both antibody response with age and bed net use with age; calculated diagnostic statistics of leverage, lack of fit (change in Pearson’s chi-square), and influence (Cook’s distance) for the matched data; and evaluated the sensitivity of the model fits when excluding data for the matched groups that were potentially poorly fit or influential.22,23
To evaluate whether antibodies in combination predicted clinical malaria more accurately than antibodies alone, we used the predicted probability generated from the adjusted conditional logistic regression models for single antibodies as well as antibodies in combination and compared their respective areas under the receiver operating characteristic curve (AUROC). Single antibody responses that predicted protection at P < 0.1 were explored in all possible combinations.
Statistical analyses were performed using Stata SE version 12 (Stata Corp., College Station, TX).24 P < 0.05 was considered statistically significant.
RESULTS
Malaria incidence in the study site.
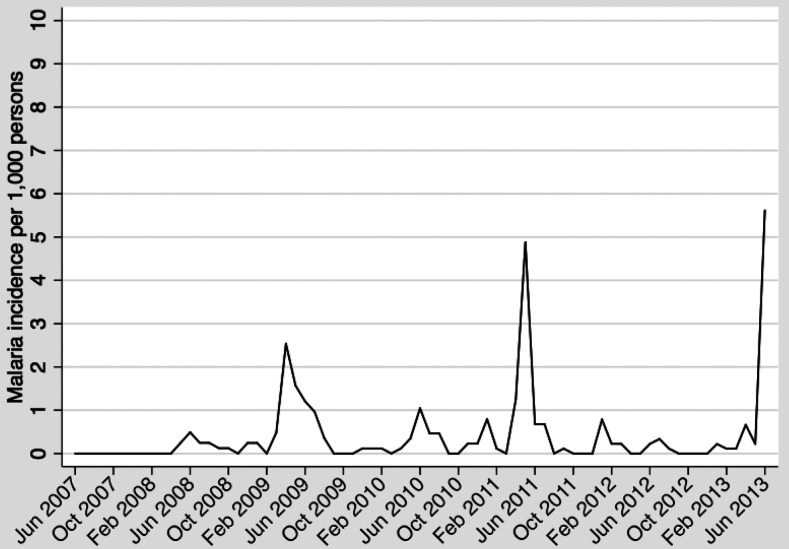
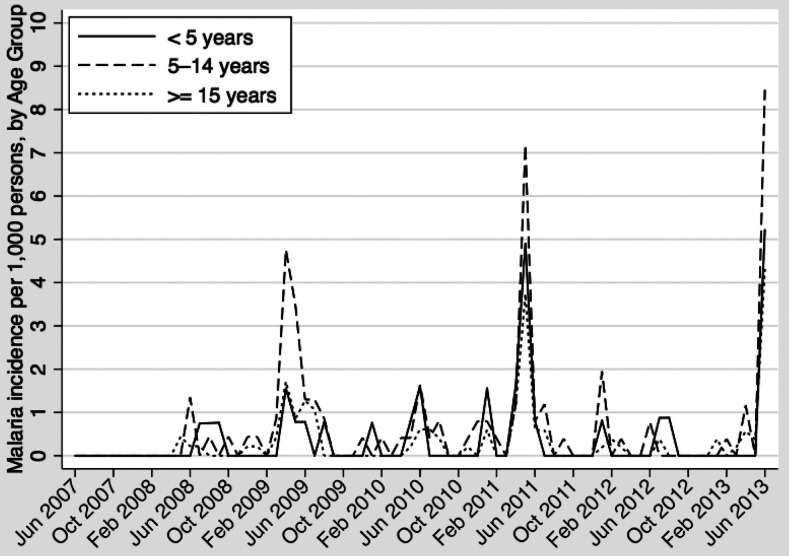
Monthly incidence of clinical malaria in the entire study site from June 2007 to June 2013 is shown in Figure 1, which illustrates the highly seasonal nature of the disease in the study area. Incidence of clinical malaria was variable during the study period, with an annual incidence per 1,000 person-years ranging from 1.7 in 2008 to 10.3 in 2013. During periods of peak transmission, incidence was highest among children ages 5–14 years (Figure 2).
Figure 1.
Clinical malaria incidence per 1,000 persons in Kipsamoite and Kapsisiywa, Kenya, June 2007–June 2013.
Figure 2.
Clinical malaria incidence per 1,000 persons in Kipsamoite and Kapsisiywa, Kenya, June 2007–June 2013, by age group.
Study population characteristics.
Of 257 persons with clinical malaria from June 2007 to June 2013, 154 (59.9%) lived in the study area between April and June 2007, participated in the site-wide blood collection during that time period, and had a stored plasma sample to test. The mean age of case subjects at the time of the site-wide blood collection was 15.3 years (SD, 14.7 years). The mean temperature of these subjects at case detection was 38.2°C (SD, 1.2°C) and the median parasite density was 19,220/µL (interquartile range [IQR], 3,840/µL–53,860/µL). Table 1 shows the mean ages of cases and controls, and the standardized differences, by the age categories in which they were matched. The standardized difference in mean ages overall in cases and controls was 0.006, which indicates that the selection of age ranges for matching within each age category produced a balanced distribution of age between cases and controls after matching. However, among children younger than 5 years at the time of the site-wide blood collection, the mean age of cases was 3.0 years (SD, 1.3 years), and of controls was 2.7 years (SD, 1.2 years), with a standardized difference in means of 0.257, which suggests a potential for residual confounding in age even after matching.
Table 1.
Evaluation of balance of age between cases and controls after matching, overall and by age category
| Age category (years) | Cases | Controls | Standardized difference* | ||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| < 5 | 43 | 3.0 (1.3) | 129 | 2.7 (1.2) | 0.257 |
| 5–14 | 61 | 9.3 (2.5) | 183 | 9.4 (2.5) | −0.034 |
| 15–64 | 49 | 32.3 (11.5) | 147 | 32.2 (11.6) | 0.006 |
| 65+ | 1 | 72.4† | 3 | 71.7 (4.1) | 0.053 |
| All ages | 154 | 15.3 (14.7) | 462 | 15.2 (14.7) | 0.006 |
* .
† Age of the single case in this age category.
Frequencies and levels of antibodies, and association of antibody responses or levels with protection against clinical malaria.
A higher frequency of antibody responses was seen in controls as compared with cases for all antigens except EBP-2, with frequencies ranging from 34.0% to 92.2% in controls and 38.3% to 89.0% in cases, but significant differences were seen only for GLURP-R2 and LSA-1 in unadjusted analysis (Table 2). Antibody levels were also higher for most antigens in controls than cases, but were again significantly different only for GLURP-R2 and LSA-1 in unadjusted analysis (Table 3). Antibody frequencies and levels for all antigens increase with age (Supplemental Tables 1, (2a, and 2b).
Table 2.
Associations between dichotomized antibody responses to Plasmodium falciparum antigens and developing clinical malaria over a 6-year time period (June 2007–June 2013)
| Antigen | Dichotomized response* | |||||
|---|---|---|---|---|---|---|
| Cases (N = 154), n (%) | Controls (N = 462) n (%) | OR (95% CI) crude | P-value† | OR (95% CI) adjusted‡ | P-value† | |
| AMA-1 3D7 | 137 (89.0) | 426 (92.2) | 0.63 (0.32–1.24) | 0.179 | 0.61 (0.31–1.21) | 0.159 |
| AMA-1 FVO | 134 (87.0) | 413 (89.4) | 0.73 (0.38–1.41) | 0.352 | 0.75 (0.39–1.45) | 0.389 |
| Erythrocyte-binding antigen 175 | 81 (52.6) | 243 (52.6) | 1.00 (0.63–1.57) | 0.999 | 0.93 (0.58–1.49) | 0.775 |
| Erythrocyte-binding protein-2 | 59 (38.3) | 157 (34.0) | 1.32 (0.83–2.10) | 0.236 | 1.29 (0.80–2.07) | 0.291 |
| GLURP-N-terminal non-repetitive | 83 (53.9) | 285 (61.7) | 0.67 (0.44–1.01) | 0.056 | 0.67 (0.43–1.02) | 0.060 |
| GLURP-C-terminal repetitive | 77 (50.0) | 275 (59.5) | 0.58 (0.37–0.90) | 0.016 | 0.56 (0.36–0.89) | 0.013 |
| Liver-stage antigen-1 | 87 (56.5) | 308 (66.7) | 0.58 (0.38–0.88) | 0.011 | 0.56 (0.36–0.87) | 0.010 |
| MSP-142 3D7 | 108 (70.1) | 326 (70.6) | 0.97 (0.57–1.62) | 0.894 | 0.86 (0.50–1.47) | 0.584 |
| MSP-142 FVO | 117 (76.0) | 369 (79.9) | 0.69 (0.39, 1.21) | 0.193 | 0.61 (0.34–1.10) | 0.101 |
| MSP-3 | 123 (79.9) | 384 (83.1) | 0.79 (0.49–1.28) | 0.341 | 0.75 (0.45–1.23) | 0.252 |
| Circumsporozoite protein§ | 78 (50.6) | 252 (55.4) | 0.77 (0.51–1.15) | 0.203 | 0.75 (0.49–1.14) | 0.184 |
AMA = apical membrane antigen; GLURP = glutamate-rich protein; MSP = merozoite surface protein; OR = odds ratio. Text in bold font indicate results are statistically significant at P = 0.05. Text in italics indicate results approach statistical significance.
* Association between case–control status and dichotomized antibody response where an antibody response of arbitrary unit ≥ 1 was considered a positive response.
† All analyses used conditional logistic regression that implicitly adjusted for age and village by the matched case–control design that was used.
‡ Adjusted for bed net use, household treatment by indoor residual spraying, roof material, distance to nearest forest, elevation, and potential residual confounding on age.
§ N = 455 controls measured by ELISA.
Table 3.
Associations between continuous antibody levels to Plasmodium falciparum antigens and developing clinical malaria over a 6-year time period (June 2007–June 2013)
| Antigen | Continuous response* | |||||
|---|---|---|---|---|---|---|
| Cases (N = 154) median (IQR) | Controls (N = 462) median (IQR) | OR (95% CI) crude | P-value† | OR (95% CI) adjusted‡ | P-value† | |
| AMA-1 3D7 | 12.3 (2.4–42.6) | 11.5 (3.2–43.1) | 0.83 (0.57–1.22) | 0.351 | 0.73 (0.49–1.09) | 0.129 |
| AMA-1 FVO | 10.1 (2.7–37.1) | 9.6 (2.4–33.2) | 1.02 (0.69–1.50) | 0.922 | 0.92 (0.62–1.37) | 0.681 |
| Erythrocyte-binding antigen 175 | 1.1 (0.4–9.9) | 1.1 (0.4–8.8) | 0.88 (0.61–1.27) | 0.498 | 0.83 (0.57–1.21) | 0.336 |
| Erythrocyte-binding protein-2 | 0.4 (0.2–2.1) | 0.5 (0.2–2.0) | 0.83 (0.56–1.24) | 0.362 | 0.78 (0.52–1.17) | 0.237 |
| GLURP-N-terminal non-repetitive | 1.1 (0.7–3.7) | 1.4 (0.7–4.0) | 0.72 (0.46–1.10) | 0.127 | 0.70 (0.45–1.09) | 0.115 |
| GLURP-C-terminal repetitive | 0.9 (0.4–6.2) | 1.6 (0.5–8.0) | 0.62 (0.45–0.86) | 0.004 | 0.60 (0.43–0.84) | 0.003 |
| Liver-stage antigen-1 | 1.5 (0.5–4.3) | 1.8 (0.7–4.9) | 0.60 (0.41–0.89) | 0.010 | 0.61 (0.41–0.89) | 0.011 |
| MSP-142 3D7 | 5.6 (0.8–16.2) | 3.6 (0.8–15.1) | 1.11 (0.77–1.60) | 0.588 | 1.02 (0.70–1.50) | 0.915 |
| MSP-142 FVO | 18.7 (1.2–55.9) | 15.4 (1.4–54.6) | 0.98 (0.73–1.32) | 0.907 | 0.90 (0.66–1.24) | 0.528 |
| MSP-3 | 2.4 (1.3–5.4) | 2.3 (1.2–6.1) | 0.92 (0.63–1.36) | 0.681 | 0.89 (0.60–1.33) | 0.576 |
| Circumsporozoite protein§ | 1.1 (0.6–1.8) | 1.1 (0.7–1.7) | 0.51 (0.26–1.02) | 0.058 | 0.49 (0.24–0.99) | 0.046 |
AMA = apical membrane antigen; GLURP = glutamate-rich protein; IQR = interquartile range; MSP = merozoite surface protein; OR = odds ratio. Text in bold font indicate results are statistically significant at P = 0.05. Text in italics indicate results approach statistical significance.
* Association between case–control status and antibody response as a continuous measure in arbitrary units; the OR is for a 10-fold increase in the antibody level.
† All analyses used conditional logistic regression that implicitly adjusted for age and village by the matched case–control design that was used.
‡ Adjusted for bed net use, household treatment by indoor residual spraying, roof material, distance to nearest forest, elevation, and potential residual confounding on age.
§ N = 455 controls measured by ELISA.
Fractional polynomial transformation models were not better than the linear models for elevation and age, and these covariates were treated as linear in the logit in the final models. No statistically significant interactions were found between antibody response and age or bed net use and age. The sensitivity analyses performed did not identify influential matched groups; all matched groups were included in final models. After adjusting for bed net use, household treatment by indoor residual spraying, roof material, distance to the nearest forest, elevation, and potential residual confounding by age, individuals who had antibodies to GLURP-R2 (AU ≥ 1) in the baseline period from April to June 2007 had a 44% decrease in odds of developing clinical malaria between June 2007 and June 2013 (the follow-up period), compared with subjects without antibodies to GLURP-R2 during the baseline period (OR = 0.56, 95% CI: 0.36–0.89, P = 0.013). Similarly, individuals with antibodies to LSA-1 had a 44% decrease in odds of developing clinical malaria (OR = 0.56, 95% CI: 0.36–0.87, P = 0.01). Individuals with antibodies to GLURP-R0 had a 33% decrease in odds of developing clinical malaria, which approached statistical significance (OR = 0.67, 95% CI: 0.43–1.02, P = 0.06).
Increases in antibody levels to three antigens, LSA-1, GLURP-R2, and CSP, were associated with protection against clinical malaria during follow-up: for every 10-fold increase in antibody response levels, a 39% (OR = 0.61, 95% CI: 0.41–0.89, P = 0.011), 40% (OR = 0.60, 95% CI: 0.43–0.84, P = 0.003), and 51% (OR = 0.49, 95% CI: 0.24–0.99, P = 0.046) decrease in odds of clinical malaria, respectively, was found (Table 3). Trends for protection were similar for 3-year follow-up as for 6-year follow-up for antibodies associated with protection, with the exception of GLURP-R0 (Supplemental data).
Combinations of antibody responses.
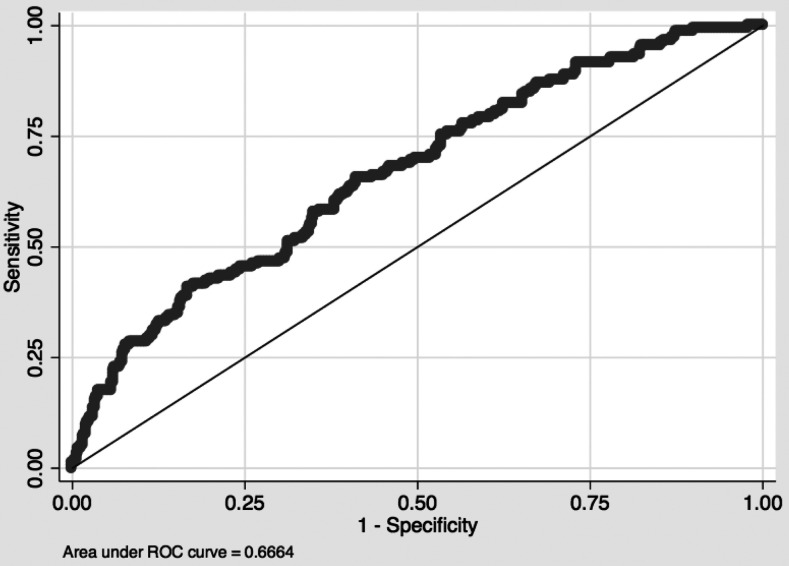
Predictive accuracy of the correlation of antibody responses to individual versus multiple antigens with protection from clinical malaria was measured by AUROC analysis (Table 4).25 GLURP-R2 and LSA-1 were the best single predictors of protection, considering antibody response as dichotomous or continuous measures (Table 4). In general, treating antibody response as a continuous measure provided better predictions than treating it as dichotomous. Nine different combinations of antibody responses to CSP, GLURP-R0, GLURP-R2, and LSA-1, each with predicted protection at P < 0.1, performed better than any single predictor, with the combination of all four doing best (Table 4 and Figure 3, AUROC, 0.6664). The AUROC of this combination was significantly different from the AUROC of the individual antibody responses to each of AMA-1 3D7, AMA-1 FVO, EBA-175, EBP-2, GLURP-R0, MSP-142 3D7, MSP-142 FVO, and MSP-3 (P < 0.03), and approached significance compared with CSP (P < 0.06).
Table 4.
Area under the receiver operating characteristic curve of predicted probabilities of developing clinical malaria for single antibody responses to 11 Plasmodium falciparum antigens and combination antibody responses to select antigens CSP, GLURP-R0, GLURP-R2, and LSA-1
| Antigens | AUROC (95% CI) dichotomous | AUROC (95% CI) continuous |
|---|---|---|
| CSP, GLURP-R0, GLURP-R2, and LSA-1 | 0.6605 (0.6117–0.7093) | 0.6664 (0.6173–0.7155) |
| CSP, GLURP-R2, and LSA-1 | 0.6586 (0.6095–0.7078) | 0.6655 (0.6162–0.7148) |
| GLURP-R0, GLURP-R2, and LSA-1 | 0.6528 (0.6049–0.7007) | 0.6617 (0.6137–0.7097) |
| CSP, GLURP-R0, and GLURP-R2 | 0.6505 (0.6017–0.6993) | 0.6610 (0.6117–0.7104) |
| CSP and GLURP-R2 | 0.6468 (0.5976–0.6960) | 0.6600 (0.6106–0.7095) |
| GLURP-R2 and LSA-1 | 0.6531 (0.6052–0.7011) | 0.6592 (0.6112–0.7071) |
| CSP, GLURP-R0, and LSA-1 | 0.6551 (0.6057–0.7046) | 0.6548 (0.6050–0.7046) |
| CSP and LSA-1 | 0.6495 (0.5998–0.6992) | 0.6548 (0.6050–0.7046) |
| GLURP-R0 and GLURP-R2 | 0.6417 (0.5936–0.6898) | 0.6527 (0.6044–0.7010) |
| GLURP-R2 | 0.6385 (0.5904–0.6867) | 0.6521 (0.6038–0.7003) |
| LSA-1 | 0.6428 (0.5943–0.6912) | 0.6472 (0.5991–0.6953) |
| GLURP-R0 and LSA-1 | 0.6451 (0.5969–0.6933) | 0.6463 (0.5982–0.6944) |
| CSP and GLURP-R0 | 0.6373 (0.5875–0.6872) | 0.6396 (0.5891–0.6901) |
| CSP | 0.6281 (0.5779–0.6784) | 0.6351 (0.5842–0.6860) |
| GLURP-R0 | 0.6296 (0.5804–0.6787) | 0.6294 (0.5805–0.6784) |
| AMA-1 3D7 | 0.6254 (0.5763–0.6745) | 0.6287 (0.5793–0.6781) |
| Erythrocyte-binding protein-2 | 0.6297 (0.5807–0.6786) | 0.6223 (0.5727–0.6718) |
| MSP-142 FVO | 0.6307 (0.5807–0.6806) | 0.6206 (0.5711–0.6702) |
| AMA-1 FVO | 0.6228 (0.5736–0.6719) | 0.6204 (0.5710–0.6699) |
| Erythrocyte-binding antigen-175 | 0.6183 (0.5686–0.6679) | 0.6203 (0.5708–0.6697) |
| MSP-3 | 0.6244 (0.5757–0.6731) | 0.6197 (0.5704–0.6691) |
| MSP-142 3D7 | 0.6208 (0.5711–0.6704) | 0.6194 (0.5699–0.6690) |
AMA = apical membrane antigen; AUROC = area under the receiver operating characteristic curve; CSP = circumsporozoite protein; GLURP-R0 = glutamate-rich protein N-terminal non-repetitive region; GLURP-R2 = glutamate-rich protein C-terminal repetitive region; LSA = liver-stage antigen; MSP = merozoite surface protein.
Rows are sorted in decreasing order of AUROC, treating antibody response as a continuous measure.
Figure 3.
Area under the receiver operating characteristic curve; 0.6664 for the combination of circumsporozoite protein, glutamate-rich protein N-terminal non-repetitive region, glutamate-rich protein C-terminal repetitive region, and liver-stage antigen-1 as continuous measures; n = 616 (154 cases and 462 controls).
DISCUSSION
With the introduction of more effective measures to prevent malaria, a number of areas are experiencing substantially reduced malaria transmission.26–29 In areas of low transmission, older children and adults may remain at risk for clinical malaria because their malaria exposure is lower during childhood than that of individuals in areas of high transmission. As more areas reach low levels of malaria transmission, it is particularly important to understand how antigen-specific immunity relates to protection in low-transmission areas, but studies of immune correlates of protection are difficult to do in these areas because the low incidence of the primary outcome, clinical malaria requires recruitment and follow-up of a large cohort. The nested case–control design of our study, along with prolonged follow-up, allowed us to detect associations of specific antibodies with P. falciparum antigens with protection from clinical malaria in an area of very low transmission (EIR < 1 infectious bite per year,14 incidence throughout the study < 6 cases per 1,000 persons per month). We found that increasing antibody levels to the P. falciparum preerythrocytic antigens CSP and LSA-1, and the blood-stage antigens GLURP-R2 and GLURP-R0 were associated with protection from clinical malaria, and the association with protection did not differ by age. Our findings show that acquisition of antibodies associated with protection against clinical malaria occurs even in areas of very low malaria transmission, that protection is associated with antibodies to specific antigens (CSP, LSA-1, GLURP-R2, and GLURP-R0), that the association with protection occurs over a prolonged period of time, and that it occurs across all age levels. The findings have important implications for assessment of population-level risk of malaria in areas of low transmission and for vaccine strategies for areas of low or decreasing transmission.
Antibody correlates of protection from clinical malaria frequently differ across study sites, so although multiple previous studies in areas of higher malaria transmission have also found that antibody responses to CSP, GLURP-R0, GLURP-R2, and LSA-1 correlate with protection from clinical malaria, either alone or in combination with other antigens,20,30–42 other previous studies have not.36,43–50 Antibody responses to the other blood-stage antigens we tested, which did not show protection in our study, have also produced conflicting results in the literature.51–56 Differences in study results could be due to a number of factors, including the assessment of antibodies to a single antigen or two or three antigens rather than multiple antigens, the specific forms of antigens used and the method used for testing antibody levels, assessment of associations in cohorts with different age ranges or with different follow-up times (almost always shorter than that of the present study), and, importantly, the malaria transmission level of the study area. Most studies tested samples collected from populations inhabiting malaria-endemic settings where transmission is year-round,20,30,31,41–43,48,52,57–62 whereas our study tested antibodies in individuals living in a highly seasonal, very low-transmission area.
A few studies have tested associations of antibodies with protection from clinical malaria in areas of highly seasonal transmission.32,44,49,50 These studies found protection associated with AMA-1, GLURP-R0, MSP-1, MSP-2 (not tested in this study), and MSP-3. However, even in these studies, rates of clinical malaria were much higher than those in the present study (e.g., in Pang et al.,50 monthly malaria incidence was noted to vary from 50 to 250 malaria episodes per 1,000 persons, considerably higher than the monthly incidence of 0.21 to 5.8 per 1,000 persons noted in the present study area). Thus, the present study provides important new information showing that immune correlates of protection from clinical malaria are present even in very low-transmission areas. The association of clinical protection only with antibodies to particular antigens strengthens the case that these associations are not nonspecific markers of exposure to malaria. The presence of antibodies (AU ≥ 1 versus < 1, dichotomous variable) was used to assess correlations with protection. We used this cutoff because < 0.3% of nonexposed individuals would be expected to have “positive” antibody responses with this cutoff. Continuous antibody levels were also compared with protection and showed only modest improvement in prediction of protection over seropositivity, suggesting that the use of the AU ≥ 1 as a consistent cutoff value was valid in this population. Association with protection did not differ by age but with only small percentages of children in the area younger than 5 years having antibodies to antigens such as LSA-1 and CSP; additional study is required to determine whether antibody-associated protection in this age group is truly similar to that in older age groups. Future studies that assess the loss of antibodies to CSP, LSA-1, GLURP-R2, and GLURP-R0, in conjunction with assessment of antibodies that provide information primarily about recent malaria exposure, may provide the most useful information about the potential risk of malaria outbreaks in populations living in areas of low malaria transmission.
In the present study, as in earlier studies in areas of higher malaria transmission, combinations of antibody responses predicted protection from malaria better than a single response27,28 and combinations of antibody levels treated as continuous measures predicted protection better than combinations of dichotomized antibody responses.28 The results lend support to the idea that an efficacious vaccine may require a combination of preerythrocytic- and blood-stage antigens, the former to prevent clinical disease altogether and the latter to reduce disease severity.10,12,27
Vaccines incorporating CSP, LSA-1, and GLURP-R0 have been tested in human clinical trials. RTS,S is the most advanced malaria vaccine with Phase III trials completed.63 The European Medicine Agency recently supported implementing its use in malaria-endemic settings in combination with continued use of established control measures, such as bed net use.64 RTS,S, branded as Mosquirix™, incorporates CSP but is not long-lasting; efficacy after 4 years was 16.8%.65 Improving the vaccine’s efficacy is a goal that could be approached by developing a new vaccine based on RTS,S that includes other antigens, such as LSA-1.66 A vaccine incorporating LSA-1 proved safe but did not elicit protection from infection.67 Phase I trials of vaccines incorporating GLURP-R0 alone and in combination with MSP-3 proved safe and well tolerated.68–72 The latter combination vaccine, GMZ2, has progressed to a multicenter Phase IIB trial in Africa.73 To our knowledge, GLURP-R2 has not yet been tested in any human vaccine. The present study’s results support the further and continued use of CSP, LSA-1, GLURP-R2, and GLURP-R0 as vaccine candidate antigens, including vaccines for use in areas of low transmission.
The nested case–control study design was not only a particular strength of the study but also introduced some limitations. The prolonged follow-up period introduced a number of other factors that could influence protection from clinical malaria, including changes in the antibody level over time. However, antibodies to most antigens tested, including GLURP-R0, GLURP-R2, and LSA-1, were highly correlated over a 3-year period of study in this area, although they typically decreased over this period (Ondigo et al., manuscript in preparation). Overall, the strength of association with protection and the increased association after controlling for many known factors associated with risk of clinical malaria provide reassurance that the associations are likely accurate. Ages of cases and controls overall were well balanced, although in the younger than 5 years category, after matching controls to cases within 2 years, controls were slightly younger than cases. The adjusted analyses controlled for potential residual confounding of age. Clinic-based surveillance can misclassify individuals as controls if they sought care outside the study clinics. However, this is unlikely as the Kipsamoite and Kapsisiywa Health Centers are the only Ministry of Health clinics in the study area, where free diagnostics and treatment have always been provided as per study protocols, and travel is infrequent for members of the study cohort. The prospective collection of exposure and covariate data protected these data from recall bias.
In conclusion, in the present study, we show that immune correlates of protection (antibodies to CSP, LSA-1, GLURP-R2, and possibly GLURP-R0) are present in a setting of very low transmission, correlate with protection across the range of age groups, and are associated with protection over a prolonged time period (at least 6 years). We also showed that antibodies to all four of these preerythrocytic- and blood-stage antigens were associated with the greatest level of protection. Identifying markers of protection in less-immune individuals is important for future vaccine development because as malaria control efforts increase with renewed calls to eradicate malaria worldwide, transmission will continue to decrease, leaving many populations living in transmission settings similar to our highland Kenya cohort. The present study provides a “proof of principle” of the utility of nested case–control studies in populations with low transmission. The study findings require validation in studies in other areas of low and seasonal malaria transmission. Future studies will focus on assessing how immunity changes with altered transmission, particularly with prolonged low transmission, modeling outbreak risk with changes in antibody levels, and assessing the potential for vaccines as a tool in malaria control and elimination programs in areas of low transmission.
Supplemental materials
Acknowledgments:
We would like to thank the motivated study participants from the 17 villages comprising our study area in western highland Kenya; the field assistants, clinicians, and laboratory staff at Kipsamoite and Kapsisiywa Health Centers; the KEMRI-UMN Malaria Research Project team in Kisumu, Kenya; David L. Narum from the Laboratory of Malaria Immunology and Vaccinology; National Institute of Allergy and Infectious Diseases; National Institutes of Health, Bethesda, MD; and David E. Lanar from the Malaria Vaccine Branch at Walter Reed Army Institute for Research, Silver Spring, MD, for provision of recombinant antigens used for the testing.
Note: Supplemental tables appear at www.ajtmh.org.
References
- 1.Marsh K, Kinyanjui S, 2006. Immune effector mechanisms in malaria. Parasite Immunol 28: 51–60. [DOI] [PubMed] [Google Scholar]
- 2.Doolan DL, Dobano C, Baird JK, 2009. Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO , 2016. World Malaria Report 2016. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Cohen S, Mc GI, Carrington S, 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192: 733–737. [DOI] [PubMed] [Google Scholar]
- 5.McGregor IA, 1964. The passive transfer of human malarial immunity. Am J Trop Med Hyg 13: 237–239. [DOI] [PubMed] [Google Scholar]
- 6.Clyde DF, Most H, McCarthy VC, Vanderberg JP, 1973. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci 266: 169–177. [DOI] [PubMed] [Google Scholar]
- 7.Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, Foucault C, Chongsuphajaisiddhi T, Druilhe P, 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45: 297–308. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Butcher GA, Crandall RB, 1969. Action of malarial antibody in vitro. Nature 223: 368–371. [DOI] [PubMed] [Google Scholar]
- 9.Olotu A, et al. 2011. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis 11: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards JS, Beeson JG, 2009. The future for blood-stage vaccines against malaria. Immunol Cell Biol 87: 377–390. [DOI] [PubMed] [Google Scholar]
- 11.Bejon P, et al. 2013. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of Phase 2 data. Lancet Infect Dis 13: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeson JG, Fowkes FJ, Reiling L, Osier FH, Drew DR, Brown GV, 2014. Correlates of protection for Plasmodium falciparum malaria vaccine development: current knowledge and future research. Engers H, Corradin G, eds. Malaria Vaccine Development: Over 40 Years of Trials and Tribulations: Future Medicine. London, UK: Giampietro Corradin & Howard Engers; 81–104. [Google Scholar]
- 13.John CC, Riedesel MA, Magak NG, Lindblade KA, Menge DM, Hodges JS, Vulule JM, Akhwale W, 2009. Possible interruption of malaria transmission, highland Kenya, 2007–2008. Emerg Infect Dis 15: 1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolfes MA, McCarra M, Magak NG, Ernst KC, Dent AE, Lindblade KA, John CC, 2012. Development of clinical immunity to malaria in highland areas of low and unstable transmission. Am J Trop Med Hyg 87: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutanda AL, Cheruiyot P, Hodges JS, Ayodo G, Odero W, John CC, 2014. Sensitivity of fever for diagnosis of clinical malaria in a Kenyan area of unstable, low malaria transmission. Malar J 13: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC, 2006. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ESRI , 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- 18.Ondigo BN, Park GS, Gose SO, Ho BM, Ochola LA, Ayodo GO, Ofulla AV, John CC, 2012. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar J 11: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, Narum DL, Park GS, Ofulla AV, John CC, 2014. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis 210: 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW, 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 73: 222–228. [PubMed] [Google Scholar]
- 21.Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, John CC, Wilson ML, 2009. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study. Trop Med Int Health 14: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Lemeshow S, Sturdivant RX, 2013. Model-building strategies and methods for logistic regression. Applied Logistic Regression, 3rd edition Hoboken, NJ: John Wiley & Sons, Inc., 89–151. [Google Scholar]
- 23.Hosmer DW, Lemeshow S, Sturdivant RX, 2013. Logistic regression for matched case-control studies. Applied Logistic Regression, 3rd edition Hoboken, NJ: John Wiley & Sons, Inc., 243–268. [Google Scholar]
- 24.StataCorp , 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. [Google Scholar]
- 25.Greiner M, Pfeiffer D, Smith RD, 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med 45: 23–41. [DOI] [PubMed] [Google Scholar]
- 26.Barnes KI, et al. 2005. Effect of artemether-lumefantrine policy and improved vector control on malaria burden in KwaZulu-Natal, South Africa. PLoS Med 2: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattarai A, et al. 2007. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med 4: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steketee RW, Campbell CC, 2010. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malar J 9: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinschmidt I, Sharp B, Benavente LE, Schwabe C, Torrez M, Kuklinski J, Morris N, Raman J, Carter J, 2006. Reduction in infection with Plasmodium falciparum one year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg 74: 972–978. [PubMed] [Google Scholar]
- 30.John CC, Tande AJ, Moormann AM, Sumba PO, Lanar DE, Min XM, Kazura JW, 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis 197: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migot-Nabias F, Deloron P, Ringwald P, Dubois B, Mayombo J, Minh TN, Fievet N, Millet P, Luty A, 2000. Immune response to Plasmodium falciparum liver stage antigen-1: geographical variations within Central Africa and their relationship with protection from clinical malaria. Trans R Soc Trop Med Hyg 94: 557–562. [DOI] [PubMed] [Google Scholar]
- 32.Iriemenam NC, et al. 2009. Antibody responses to a panel of Plasmodium falciparum malaria blood-stage antigens in relation to clinical disease outcome in Sudan. Vaccine 27: 62–71. [DOI] [PubMed] [Google Scholar]
- 33.Nebie I, et al. 2008. Do antibody responses to malaria vaccine candidates influenced by the level of malaria transmission protect from malaria? Trop Med Int Health 13: 229–237. [DOI] [PubMed] [Google Scholar]
- 34.Dodoo D, et al. 2008. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar J 7: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meraldi V, Nebie I, Tiono AB, Diallo D, Sanogo E, Theisen M, Druilhe P, Corradin G, Moret R, Sirima BS, 2004. Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol 26: 265–272. [DOI] [PubMed] [Google Scholar]
- 36.Dodoo D, Theisen M, Kurtzhals JA, Akanmori BD, Koram KA, Jepsen S, Nkrumah FK, Theander TG, Hviid L, 2000. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis 181: 1202–1205. [DOI] [PubMed] [Google Scholar]
- 37.Dziegiel M, Rowe P, Bennett S, Allen SJ, Olerup O, Gottschau A, Borre M, Riley EM, 1993. Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: correlation with clinical immunity in Gambian children. Infect Immun 61: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soe S, Theisen M, Roussilhon C, Aye KS, Druilhe P, 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun 72: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nebie I, et al. 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect Immun 76: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theisen M, et al. 2001. Selection of glutamate-rich protein long synthetic peptides for vaccine development: antigenicity and relationship with clinical protection and immunogenicity. Infect Immun 69: 5223–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogh B, Petersen E, Dziegiel M, David K, Hanson A, Borre M, Holm A, Vuust J, Jepsen S, 1992. Antibodies to a recombinant glutamate-rich Plasmodium falciparum protein: evidence for protection of individuals living in a holoendemic area of Liberia. Am J Trop Med Hyg 46: 307–313. [DOI] [PubMed] [Google Scholar]
- 42.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P, 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun 68: 2617–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lusingu JP, Vestergaard LS, Alifrangis M, Mmbando BP, Theisen M, Kitua AY, Lemnge MM, Theander TG, 2005. Cytophilic antibodies to Plasmodium falciparum glutamate rich protein are associated with malaria protection in an area of holoendemic transmission. Malar J 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, Lanar DE, Dutta S, Rosenthal PJ, Dorsey G, John CC, 2011. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J Infect Dis 204: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Z, Xiao L, Branch OH, Kariuki S, Nahlen BL, Lal AA, 2002. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of western Kenya. XIII. Asembo Bay Cohort Project. Am J Trop Med Hyg 66: 7–12. [DOI] [PubMed] [Google Scholar]
- 46.Wongsrichanalai C, Webster HK, Permpanich B, Chuanak N, Ketrangsri S, 1991. Naturally acquired circumsporozoite antibodies and their role in protection in endemic falciparum and vivax malaria. Am J Trop Med Hyg 44: 201–204. [DOI] [PubMed] [Google Scholar]
- 47.Webster HK, Brown AE, Chuenchitra C, Permpanich B, Pipithkul J, 1988. Characterization of antibodies to sporozoites in Plasmodium falciparum malaria and correlation with protection. J Clin Microbiol 26: 923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman SL, Oster CN, Plowe CV, Woollett GR, Beier JC, Chulay JD, Wirtz RA, Hollingdale MR, Mugambi M, 1987. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science 237: 639–642. [DOI] [PubMed] [Google Scholar]
- 49.Marsh K, Hayes RH, Carson DC, Otoo L, Shenton F, Byass P, Zavala F, Greenwood BM, 1988. Anti-sporozoite antibodies and immunity to malaria in a rural Gambian population. Trans R Soc Trop Med Hyg 82: 532–537. [DOI] [PubMed] [Google Scholar]
- 50.Pang LW, Limsomwong N, Karwacki J, Webster HK, 1988. Circumsporozoite antibodies and falciparum malaria incidence in children living in a malaria endemic area. Bull World Health Organ 66: 359–363. [PMC free article] [PubMed] [Google Scholar]
- 51.Fowkes FJ, Richards JS, Simpson JA, Beeson JG, 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. Plos Med 7: e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarra MB, Ayodo G, Sumba PO, Kazura JW, Moormann AM, Narum DL, John CC, 2011. Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr Infect Dis J 30: 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polley SD, Tetteh KK, Lloyd JM, Akpogheneta OJ, Greenwood BM, Bojang KA, Conway DJ, 2007. Plasmodium falciparum merozoite surface protein 3 is a target of allele-specific immunity and alleles are maintained by natural selection. J Infect Dis 195: 279–287. [DOI] [PubMed] [Google Scholar]
- 54.Gray JC, et al. 2007. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin Chem 53: 1244–1253. [DOI] [PubMed] [Google Scholar]
- 55.Okenu DM, Riley EM, Bickle QD, Agomo PU, Barbosa A, Daugherty JR, Lanar DE, Conway DJ, 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect Immun 68: 5559–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osier FH, et al. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanisic DI, et al. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richards JS, et al. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51: e50–e60. [DOI] [PubMed] [Google Scholar]
- 59.Al-Yaman F, Genton B, Kramer KJ, Chang SP, Hui GS, Baisor M, Alpers MP, 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg 54: 443–448. [DOI] [PubMed] [Google Scholar]
- 60.Migot-Nabias F, et al. 1999. Immune responses against Plasmodium falciparum asexual blood-stage antigens and disease susceptibility in Gabonese and Cameroonian children. Am J Trop Med Hyg 61: 488–494. [DOI] [PubMed] [Google Scholar]
- 61.Osier FH, Polley SD, Mwangi T, Lowe B, Conway DJ, Marsh K, 2007. Naturally acquired antibodies to polymorphic and conserved epitopes of Plasmodium falciparum merozoite surface protein 3. Parasite Immunol 29: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polley SD, et al. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23: 718–728. [DOI] [PubMed] [Google Scholar]
- 63.RTS,S Clinical Trials Partnership , 2015. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. European Medicines Agency , 2015. First malaria vaccine receives positive scientific opinion from EMA. London.
- 65.Olotu A, et al. 2013. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med 368: 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heppner DG, et al. 2005. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine 23: 2243–2250. [DOI] [PubMed] [Google Scholar]
- 67.Cummings JF, et al. 2010. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-γ/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine 28: 5135–5144. [DOI] [PubMed] [Google Scholar]
- 68.Hermsen CC, et al. 2007. Glutamate-rich protein (GLURP) induces antibodies that inhibit in vitro growth of Plasmodium falciparum in a phase 1 malaria vaccine trial. Vaccine 25: 2930–2940. [DOI] [PubMed] [Google Scholar]
- 69.Mordmuller B, et al. 2010. Safety and immunogenicity of the malaria vaccine candidate GMZ2 in malaria-exposed, adult individuals from Lambarene, Gabon. Vaccine 28: 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esen M, et al. 2009. Safety and immunogenicity of GMZ2—a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine 27: 6862–6868. [DOI] [PubMed] [Google Scholar]
- 71.Belard S, et al. 2011. A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PLoS One 6: e22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jepsen MP, et al. 2013. The malaria vaccine candidate GMZ2 elicits functional antibodies in individuals from malaria endemic and non-endemic areas. J Infect Dis 208: 479–488. [DOI] [PubMed] [Google Scholar]
- 73.Noor RA, et al. 2010. Design of a phase IIb, randomized, controlled, double-blind, multi-centre study to evalute the efficacy, safety, and immunogenicity of the GMZ2 candidate malaria vaccine in Ugandan, Ghanian, Burkinabe and Gabonese children aged 12–60 months. Malar J 9 (Suppl 2): P26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.