Abstract.
Since 2018, adolescents have been included as a target group for tuberculosis (TB) surveillance by the WHO. However, they are considered a neglected population, as there are considerable gaps in information about them. We aimed to analyze the risk factors for unfavorable TB treatment outcomes among adolescents in Rio de Janeiro, a Brazilian city with a high burden of TB. This is a retrospective study of adolescents (10–18 years) with TB notified in Rio de Janeiro, from four national database systems, covering 2014–2016. “Extreme vulnerability” was defined as adolescents who presented one of the following characteristics: homelessness, incarceration, tobacco use, illicit drug use, or alcohol abuse. Logistic regression analysis was used to identify factors associated with favorable (cure/completed treatment) and unfavorable outcomes (lost to follow-up, death, and treatment failure). A total of 725 adolescents with TB were included: 610 (84.1%) were cured, 94 (13%) were lost to follow-up, six (0.8%) died because of TB, 13 (1.8%) died because of other causes, and two (0.3%) failed treatment. Unfavorable outcomes were associated with retreatment (adjusted odds ratio [aOR]: 4.51; 95% CI: 2.23–9.17), TB–HIV coinfection (aOR: 10.15; 95% CI: 4.15–25.34), extreme vulnerability (aOR: 3.01; 95% CI: 1.70–5.33), and living in the two districts (3.1 and 3.3) with worst conditions: large population and rates of homicides and shantytowns (aOR: 4.11; 95% CI: 1.79–9.46 and aOR: 5.35; 95% CI: 2.20–13.03, respectively). Our findings underscore the need for strengthening early identification and interventions for adolescents at high risk of unfavorable outcomes, especially those living in shantytowns.
INTRODUCTION
Although the WHO has prioritized adolescents as an at-risk population for tuberculosis (TB), the definition of this age-group is not uniform among studies. This has contributed to a situation in which the specific needs of adolescents are not addressed in a standardized manner.1
Adolescents are very active, establishing many social interactions that may lead to increased transmission of TB, especially during the daytime. They exhibit pulmonary forms of TB resembling those of adults. Adolescents may develop bacilliferous pulmonary TB, therefore transmissible TB forms, and account for a considerable proportion of new cases.2–5 However, the rate of loss to follow-up of anti-TB treatment is high among adolescents compared with other age-groups.6,7 This increases morbidity and mortality rates and the risk for developing drug-resistant TB (DR-TB).8 Thus, adolescents represent an important link in the transmission chain of TB and DR-TB.
Brazilian adolescents exhibit high rates of social vulnerability. The homicide rate is high, with 3.65/1,000 adolescents > 12 years of age dying from homicide before reaching 19 years of age in municipalities with > 100,000 inhabitants such as Rio de Janeiro city.9 Moreover, between 2007 and 2017, there was a large increase in detection of AIDS among adolescents aged 15–19 years (from 258 to 614 cases), with increases also in the age-groups of 20–24 years (from 1,409 to 3,121 cases) and 25–29 years (from 3,258 to 4,395 cases), representing an increase of 137%, 171%, and 113%, respectively.10
According to the WHO, Brazil ranked 20th in terms of TB burden and 19th in TB–HIV coinfection.11 Diagnosing and treating TB are free of charge in Brazil and are mostly decentralized within primary care. Rio de Janeiro is the state capital with the second highest incidence of TB (66.3/100,000) in the country. It is also the national leader in informal settlements (otherwise known as “shantytowns”) with approximately 1.4 million inhabitants.12 Overall, Rio de Janeiro is the second most populous city in Brazil, with 6,320,446 inhabitants according to the most recent census figures, of whom 465,567 (14.7%) were adolescents aged 10–19 years.13 Since 1993, the city’s Municipal Health Department has planned its health care according to 10 city districts, all with different characteristics.14 The aim of the present study was to analyze the risk factors that lead to unfavorable TB treatment outcomes in adolescents residing in these districts of Rio de Janeiro.
METHODS
Study population and data sources.
The present study was a retrospective analysis of adolescents (age ≥ 10 and ≤ 18 years) with pulmonary TB alone, or in association with extrapulmonary TB, who were residents and notified in the city of Rio de Janeiro, Brazil, between August 1, 2014 and August 1, 2016. The study population included adolescents with a clinical diagnosis, those with clinical and microbiological diagnosis, and those with DR-TB. Patients without information regarding anti-TB treatment outcomes and those with extrapulmonary (i.e., no pulmonary involvement) forms of TB were excluded.
Data from four national computerized information systems developed by the Brazilian Ministry of Health were used. The reference system for data gathering was Sistema de Informação de Agravos de Notificação (SINAN-TB). This is a TB information system that notifies and investigates TB and houses demographic, clinical, laboratory, and follow-up data. Manual linkages were made with other information systems. Gerenciamento de Ambiente Laboratorial (GAL) is a laboratory information system used to monitor and control laboratory tests. Sistema de Informação de Tratamentos Especiais em Tuberculose (SITE-TB) is a DR-TB information system used for notification and follow-up of cases in which special treatment, mostly DR-TB, is indicated. Sistema de Informação em Mortalidade (SIM) is a mortality information system that records mortality information in Brazil.
The Brazilian National TB Program has published guidelines for diagnosis and treatment for ≥ 30 years, for all types of TB (pulmonary, extrapulmonary, and the association of both) for different populations (e.g., individuals living with HIV, comorbidities, and DR-TB) that are updated systematically, according to WHO recommendations.15
Xpert MTB/rifampicin (RIF) (Cepheid Inc., Sunnyvale, CA) was implemented in August 2014 in four municipal reference laboratories in this city, replacing sputum smear microscopy for adolescents and adults with suspected TB as the initial diagnostic test and was fully available during the study period.
Definitions.
Patients were classified according to their type of entry to the system: new cases and retreatment or transferred (patients who were transferred to follow their treatment in a different health unit).16 In addition, clinical presentation of TB, pulmonary TB alone or in association with extrapulmonary TB, was defined according to the notification in the SINAN-TB data system.16 The variables “homeless,” “illicit drug use,” “alcohol use,” and “tobacco use” are notified in SINAN-TB as self-reported.16 The variable “Inmate” is registered in SINAN-TB according to the Secretary of Penitentiary and/or Justice Administration.16
Treatment outcome was defined as cure/completed treatment, loss to follow-up, death due to TB or other cause of death (died of another cause during TB treatment), and treatment failure, in accordance with the data entry norms for SINAN.16 Cure or completed treatment was defined as a favorable outcome, whereas loss to follow-up, treatment failure, death due to TB, and other causes of death were defined as unfavorable outcomes. For all deaths (regardless of whether related to TB or not), the information was double-checked using the SIM database. The variable “extreme vulnerability” was defined as adolescents who exhibited at least one of the following characteristics: homelessness, inmate, tobacco use, illicit drug use, or alcohol abuse. The variable “comorbidities” was used to refer to diseases coexisting with TB. Adolescents with TB–HIV coinfection were analyzed separately.
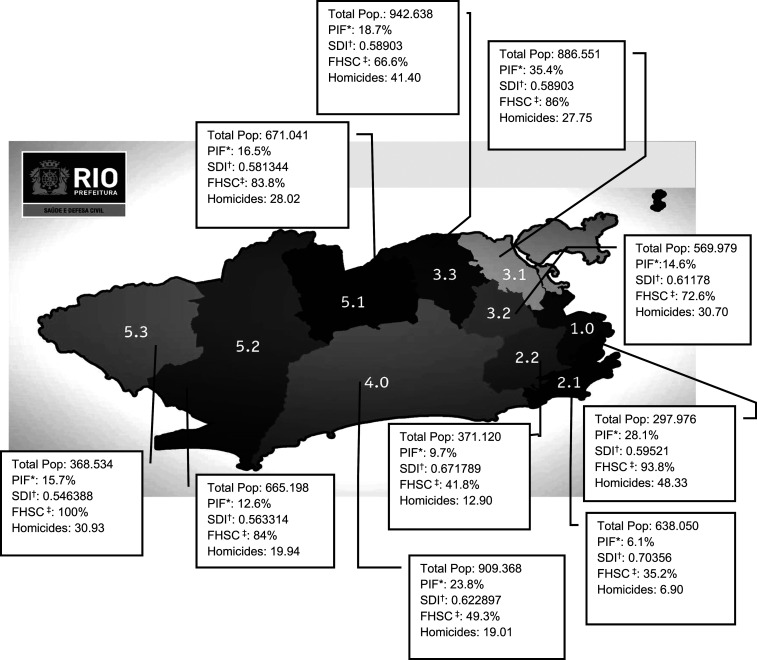
The city of Rio de Janeiro is divided into 10 healthcare-planning districts, as shown in Figure 1.17 From the sociodemographic characteristics of these 10 districts (total population, proportion of informal settlements, Family Health Strategy [FHS] coverage, homicide rate per 100,000 inhabitants per year, and Social Development Index [SDI]), four categories were defined: 1) district 2.0 (2.1 and 2.2), 2) district 3.1, 3) district 3.3, and 4) other districts.
Figure 1.
Rio de Janeiro healthcare-planning districts and their sociodemographic characteristics.14 *PIF = proportion of Informal Settlements (shantytowns). †SDI = Social Development Index. ‡FHSC = Family Health Strategy Coverage.
The term “informal settlements” was defined as groups of housing units that lacked essential public services and generally presenting the dense, disorganized layouts of shantytowns.12 The FHS is a national program instituted by the Brazilian Ministry of Health, with the aim of organizing primary care through a multiprofessional team comprising doctors, nurses, and community health agents who visit individuals’ homes. The percentage of the population covered by FHS teams is defined as the proportion of the Brazilian geographical area served by the FHS team.18 The degree of social development of a given geographical area compared with others of the same nature is measured according to the SDI. It is composed of four dimensions: access to basic sanitation, housing quality, schooling level, and income availability.19 The homicide rate is measured per 100,000 inhabitants per year.
Directly observed treatment (DOT) is characterized by the construction of ties with patients and observation of medication intake by healthcare professionals at the healthcare unit, the patient’s home, or even the patient’s workplace.15 Baseline drug susceptibility testing (DST) was defined as testing performed within 30 days of diagnosis. These methods included the proportion method and the automated method (mycobacterial growth indicator tube).15 The classification of DST was performed according to guidelines from the National TB Program.15
Ethics approval.
This study was approved by the Research Ethics Committees of the Instituto de Puericultura e Pediatria Martagão Gesteira of the Federal University of Rio de Janeiro, under reference no. 961452 on February 22, 2015, and of Rio de Janeiro Municipal Health Department under reference no. 1629126 on July 8, 2016.
Statistical analysis.
The data were initially summarized descriptively. Six adolescents were notified in the SINAN-TB database as transferred cases. These adolescents were cured and did not exhibit any risk factors. From the analysis, it was assumed that these were new cases that were not registered appropriately during the transfer from one health unit to another. Thus, these cases were reclassified as new cases for the univariate and multivariate analyses.
Missing information was handled as a category to analyze the profile of these adolescents without information and, thus, was maintained in the analysis. The data were analyzed using univariate logistic regression. Covariables for input to the multivariate model were selected from the univariate analyses on the study outcome according to their clinical relevance and statistical significance < 0.20; however, only those with statistical significance < 0.05 were maintained in the final model. A sensitivity analysis was performed including loss to follow-up as the only unfavorable outcome and the unfavorable outcomes as previously defined in this study (loss to follow-up, death, and treatment failure). Data were analyzed using SPSS version 20 (IBM Corporation, Armonk, NY).
RESULTS
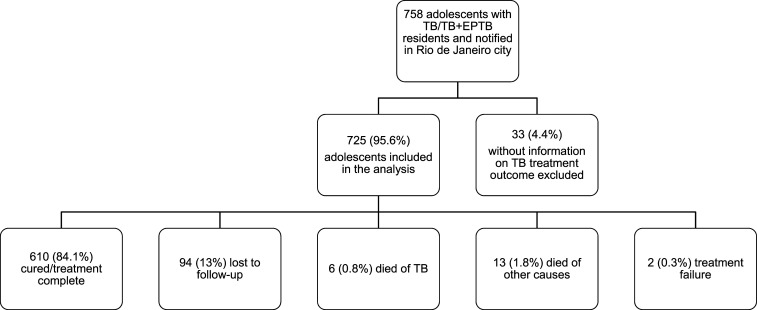
Between August 2014 and August 2016, 758 adolescents with TB who had been notified in the SINAN fulfilled the eligibility criteria. Among these cases, 33 (4.4%) were excluded because information regarding TB treatment outcome was unavailable. Thus, the study population consisted of 725 adolescents (Figure 2), of whom 610 (84.1%) were cured or completed treatment, 94 (13%) were lost to follow-up, six (0.8%) died because of TB, 13 (1.8%) died from other causes, and two (0.3%) experienced treatment failures. The 13 deaths due to other causes included seven from HIV (53.8%), three from violence (23.8%), one from alcoholism (7.7%), one from systemic lupus erythematosus (7.7%), and one from unknown cause (7.7%).
Figure 2.
Flowchart sampling.
As shown in Tables 1 and 2, 381 adolescents (52.6%) were aged 17–18 years and 388 (53.5%) were male. Pulmonary forms of TB accounted for 690 (95.2%) adolescents, of which 671 (92.6%) were new cases. Regarding geographical distribution, within a single district, district 2.0 accounted for the lowest (7.8%) and district 3.1 accounted for the highest (30.4%) proportion of adolescents that evolved unfavorably. However, the other six districts combined defined as “others” had an even higher proportion (39.2%). In 422 (58.2%) adolescents, treatment was directly observed. Xpert was used as a diagnostic tool in only 330 (45.5%) adolescents and was negative (i.e., not detectable) in 35 (10.6%). Among the 295 positive Xpert results, 11 (3.7% [95% CI: 1.9–6.3]) were resistant to RIF. Of these, eight (72.7%) were new TB adolescents and three (27.3%) were retreated TB adolescents.
Table 1.
Sociodemographic characteristics by treatment outcome in adolescents
| Tuberculosis treatment outcome, n (%) | Total, n (%) (N = 725) | ||
|---|---|---|---|
| Unfavorable (n = 115) | Favorable (n = 610) | ||
| Age (years) | |||
| 10–14 | 20 (17.4) | 107 (17.5) | 127 (17.5) |
| 15–16 | 23 (20) | 194 (31.8) | 217 (29.9) |
| 17–18 | 72 (62.6) | 309 (50.7) | 381 (52.6) |
| Gender | |||
| Male | 70 (60.9) | 318 (52.1) | 388 (53.5) |
| Female | 45 (39.1) | 292 (47.9) | 337 (46.5) |
| Residence area/districts | |||
| 2.0 | 9 (7.8) | 122 (20) | 131 (18.1) |
| 3.1 | 35 (30.4) | 118 (19.3) | 153 (21) |
| 3.3 | 26 (22.6) | 73 (12) | 99 (13.7) |
| Others | 45 (39.2) | 297 (48.7) | 342 (47.2) |
Table 2.
Clinical history characteristics by TB treatment outcome in adolescents
| TB treatment outcome, n (%) | Total, n (%) (N = 725) | ||
|---|---|---|---|
| Unfavorable (n = 115) | Favorable (n = 610) | ||
| TB form | |||
| Pulmonary | 107 (93) | 583 (95.6) | 690 (95.2) |
| Pulmonary + extrapulmonary | 8 (7) | 27 (4.4) | 35 (4.8) |
| Type of TB | |||
| Retreatment | 20 (17.4) | 28 (4.6) | 48 (6.6) |
| New case | 95 (82.6) | 576 (94.4) | 671 (92.6) |
| Transferred | 0 | 6 (1) | 6 (0.8) |
| TB–HIV coinfection | |||
| HIV negative | 79 (68.6) | 513 (84.1) | 592 (81.6) |
| HIV positive | 18 (15.7) | 10 (1.6) | 28 (3.9) |
| HIV not performed/missing data | 18 (15.7) | 87 (14.3) | 105 (14.5) |
| Extreme vulnerability | |||
| Yes | 28 (24.3) | 54 (8.9) | 82 (11.3) |
| No | 87 (75.7) | 556 (91.1) | 643 (88.7) |
| Direct observed treatment | |||
| Yes | 55 (47.8) | 367 (60.2) | 422 (58.2) |
| No | 26 (22.6) | 132 (21.6) | 158 (21.8) |
| Missing data | 34 (29.6) | 111 (18.2) | 145 (20) |
| Homeless | |||
| Yes | 7 (6.1) | 4 (0.7) | 11 (1.5) |
| No | 83 (72.2) | 468 (76.7) | 551 (76) |
| Missing data | 25 (21.7) | 138 (22.6) | 163 (22.5) |
| Inmates | |||
| Yes | 2 (1.7) | 10 (1.6) | 12 (1.6) |
| No | 90 (78.3) | 463 (75.9) | 553 (76.2) |
| Missing data | 23 (20) | 137 (22.5) | 160 (2.2) |
| Illicit drug use | |||
| Yes | 23 (20) | 34 (5.6) | 57 (7.8) |
| No | 71 (61.7) | 466 (76.4) | 537 (74.1) |
| Missing data | 21 (18.3) | 110 (18) | 131 (18.1) |
| Alcohol abuse | |||
| Yes | 6 (5.2) | 6 (1) | 12 (1.7) |
| No | 99 (86.1) | 564 (92.5) | 663 (91.4) |
| Missing data | 10 (8.7) | 40 (6.5) | 50 (6.9) |
| Tobacco use | |||
| Yes | 10 (8.7) | 24 (3.9) | 34 (4.7) |
| No | 82 (71.3) | 480 (78.7) | 562 (77.5) |
| Missing data | 23 (20) | 106 (17.4) | 129 (17.8) |
| Comorbidities | |||
| Yes | 19 (3.1) | 1 (0.9) | 20 (2.8) |
| No | 591 (96.9) | 114 (99.1) | 705 (97.2) |
| Xpert testing | |||
| Yes | 54 (47) | 276 (45.2) | 330 (45.5) |
| No | 61 (53) | 334 (54.8) | 395 (54.5) |
| Xpert testing results | |||
| RIF sensitive | 44 (38.3) | 240 (39.3) | 284 (39.2) |
| RIF resistant | 3 (2.6) | 8 (1.3) | 11 (1.5) |
| Not detectable | 7 (6.1) | 28 (4.6) | 35 (4.8) |
| Not performed | 61 (53) | 334 (54.8) | 395 (54.5) |
| Baseline DST performed | |||
| Yes | 41 (35.7) | 187 (30.7) | 228 (31.4) |
| No | 74 (64.3) | 423 (69.3) | 497 (68.6) |
| Baseline DST results | |||
| First-line drug susceptible | 37 (32.2) | 169 (27.7) | 206 (28.4) |
| INH resistant | 1 (0.9) | 10 (1.6) | 11 (1.5) |
| RIF and INH resistant | 3 (2.6) | 4 (0.7) | 7 (1) |
| RIF resistant | 0 | 4 (0.7) | 4 (0.5) |
| Not performed | 74 (64.3) | 423 (69.3) | 497 (68.6) |
DST = drug susceptibility testing; INH = isoniazid; RIF = rifampicin; TB = tuberculosis.
Among the 11 Xpert RIF-resistant adolescents, baseline DST revealed that seven (63.6%) were RIF- and isoniazid-resistant and four (36.4%) were RIF-resistant (Table 2). First-line treatment was initially administered in three (27.2%) of these adolescents, and the other eight (72.7%) were started on treatment for multidrug resistance (MDR) as the initial treatment option, and TB–HIV coinfection was present in 28 (3.9%) adolescents. Exposure to social vulnerability and risky behavior, when observed individually, ranged from 1.5% (homeless) to 7.9% (illicit drug use). However, given that these factors were regarded to coexist among the same individuals, 11.3% of these adolescents were extremely vulnerable. Most of the adolescents were healthy (97.2%), with comorbidity identified in only 20 adolescents (2.8%), most often of a hematological nature (four adolescents).The types of comorbidities are described in online Supplemental Appendix 1.
Results of the univariate and multivariate analyses are summarized in Table 3. Unfavorable outcomes were more likely in the following situations: retreatment (adjusted odds ratio [aOR]: 4.51; 95% CI: 2.23–9.17; P < 0.001), living in districts 3.1 (aOR: 4.11; 95% CI: 1.79–9.46; P < 0.001) and 3.3 (aOR: 5.35; 95% CI: 2.20–13.03; P < 0.001), TB–HIV coinfection (aOR: 10.15; 95% CI: 4.15–25.34; P < 0.001), extreme vulnerability (aOR: 3.01; 95% CI: 1.70–5.33; P < 0.001), and lack of information regarding DOT (aOR: 2.48; 95% CI: 1.31–4.96; P = 0.006). Sensitivity analysis yielded similar findings between all unfavorable outcomes (loss to follow-up, death, and treatment failure) and loss to follow-up, except for the variables TB–HIV coinfection and retreatment (Table 4). Retreatment had a stronger association with unfavorable outcomes (aOR: 4.51) than with loss to follow-up (aOR: 3.90). The same was observed with TB–HIV coinfection (aOR: 10.15 versus aOR: 6.09, respectively).
Table 3.
Univariate and multivariable associations between adolescent characteristics and TB treatment unfavorable outcome
| Characteristic | Univariate logistic model | Multivariate logistic model | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Age (years) | ||||
| 10–14 | Reference | – | – | – |
| 15–16 | 0.63 (0.33–1.21) | 0.166 | 0.54 (0.26–1.10) | 0.091 |
| 17–18 | 1.25 (0.72–2.14) | 0.425 | 1.20 (0.65–2.21) | 0.552 |
| Type of TB | ||||
| New case | Reference | – | – | – |
| Retreatment | 4.38 (2.37–8.08) | < 0.001 | 4.51 (2.23–9.17) | < 0.001 |
| Residence area/districts | ||||
| 2.0 | Reference | – | – | – |
| 3.1 | 4.02 (1.85–8.72) | < 0.001 | 4.11 (1.79–9.46) | < 0.001 |
| 3.3 | 4.82 (2.14–10.87) | < 0.001 | 5.35 (2.20–13.03) | < 0.001 |
| Other | 2.05 (0.97–4.33) | < 0.059 | 1.77 (0.81–3.87) | 1.159 |
| TB–HIV coinfection | ||||
| HIV negative | Reference | – | – | – |
| HIV positive | 11.69 (5.21–26.24) | < 0.001 | 10.15 (4.15–25.34) | < 0.001 |
| Not performed/missing data | 1.34 (0.77–2.35) | 0.301 | 1.02 (0.54–1.89) | 0.970 |
| Extreme vulnerability | ||||
| No | Reference | – | – | – |
| Yes | 3.31 (1.99–5.51) | < 0.001 | 3.01 (1.70–5.33) | < 0.001 |
| Direct observed treatment | ||||
| No | Reference | – | – | – |
| Yes | 0.76 (0.46–1.26) | 0.291 | 1.10 (0.61–1.97) | 0.749 |
| Missing data | 1.55 (0.88–2.75) | 0.129 | 2.48 (1.31–4.96) | 0.006 |
| Gender | ||||
| Female | Reference | – | – | – |
| Male | 0.70 (0.47–1.05) | 0.086 | – | – |
| TB form | ||||
| Pulmonary | Reference | – | – | – |
| Pulmonary + extrapulmonary | 0.62 (2.74–1.40) | 0.25 | – | – |
| Homeless | ||||
| No | Reference | – | – | – |
| Yes | 9.88 (2.82–34.45) | < 0.001 | – | – |
| Inmates | ||||
| No | Reference | – | – | – |
| Yes | 1.02 (0.22–4.77) | 0.607 | – | – |
| Illicit drug use | ||||
| No | Reference | – | – | – |
| Yes | 4.44 (2.47–7.97) | < 0.001 | – | – |
| Alcohol abuse | ||||
| No | Reference | – | – | – |
| Yes | 5.7 (1.80–18.02) | 0.005 | – | – |
| Comorbidities | ||||
| No | Reference | – | – | – |
| Yes | 3.66 (0.49–27.65) | 0.208 | – | – |
| Tobacco use | ||||
| No | Reference | – | – | – |
| Yes | 2.44 (1.12–5.29) | 0.028 | – | – |
| Xpert testing | ||||
| Yes | Reference | – | – | – |
| No | 0.73 (0.63–1.39) | 0.735 | – | – |
| Xpert testing result | ||||
| RIF sensitive | Reference | – | – | – |
| RIF resistant | 2.06 (0.38–11.30) | 0.404 | – | – |
| Baseline drug susceptibility testing performed | ||||
| Yes | Reference | – | – | – |
| No | 1.25 (0.82–1.92) | 0.291 | – | – |
OR = odds ratio; RIF = rifampicin; TB = tuberculosis.
Table 4.
Comparison between loss to follow-up exclusively and unfavorable outcomes (loss to follow-up, deaths, and failure) in the multivariate regression
| Characteristic | Loss to follow-up | Unfavorable outcomes | ||
|---|---|---|---|---|
| aOR (95% CI) | P-value | aOR (95% CI) | P-value | |
| Age (years) | ||||
| 10–14 | Reference | – | – | – |
| 15–16 | 0.53 (2.49–1.14) | 0.105 | 0.54 (0.26–1.10) | 0.091 |
| 17–18 | 1.15 (0.61–2.19) | 0.666 | 1.20 (0.65–2.21) | 0.552 |
| Type of TB | ||||
| New case | Reference | – | – | – |
| Retreatment | 3.90 (1.80–8.47) | 0.001 | 4.51 (2.23–9.17) | < 0.001 |
| Residence area/districts | ||||
| 2.0 | Reference | – | – | – |
| 3.1 | 4.73 (1.91–11.69) | 0.001 | 4.11 (1.79–9.46) | < 0.001 |
| 3.3 | 5.83 (2.21–15.38) | < 0.001 | 5.35 (2.20–13.03) | < 0.001 |
| Other | 1.85 (0.78–4.41) | 0.160 | 1.77 (0.81–3.87) | 1.159 |
| TB–HIV coinfection | ||||
| HIV negative | Reference | – | – | – |
| HIV positive | 6.09 (2.12–17.49) | 0.001 | 10.15 (4.15–25.34) | < 0.001 |
| Not performed/missing data | 1.10 (0.58–2.08) | 0.781 | 1.02 (0.54–1.89) | 0.970 |
| Extreme vulnerability | ||||
| No | Reference | – | – | – |
| Yes | 3.00 (1.65–5.4) | < 0.001 | 3.01 (1.70–5.33) | < 0.001 |
| Direct observed treatment | ||||
| No | Reference | – | – | – |
| Yes | 1.15 (0.60–2.19) | 0.656 | 1.10 (0.61–1.97) | 0.749 |
| Missing data | 2.40 (1.17–4.90) | 0.016 | 2.48 (1.31–4.96) | 0.006 |
aOR = adjusted odds ratio; TB = tuberculosis.
DISCUSSION
In this study, we observed an association between adolescents with unfavorable outcomes and socioeconomic and organizational aspects of health services and disease-related factors, mainly TB–HIV coinfection, in the context of implementation of Xpert in Rio de Janeiro as the initial diagnostic test for pulmonary TB, which replaced sputum smear microscopy. Although 725 (84.1%) adolescents evolved favorably, 94 (81.7%) of the 115 (15.9%) with unfavorable outcomes were lost to follow-up. This unfavorable outcome is often observed among adolescents. In other countries with a high burden of TB–HIV coinfection, such as South Africa and Haiti, adolescents aged 15–19 years exhibited a higher risk for loss to follow-up than adults or younger adolescents (10–15 years), particularly within the context of TB–HIV coinfection.6,7 In our study, in addition to TB–HIV coinfection, other risk factors such as retreatment, residence district, extreme vulnerability, and lack of information regarding DOT, were also risk factors for unfavorable outcomes in different age-groups.
Reports in the literature show that although most cases of retreatment achieve cure, a notable percentage experience treatment failure or loss to follow-up.20,21 This emphasizes the importance of retreatment as an early marker of risk, for contributing to the transmissibility chain of DR-TB in groups that tend to engage in many social interactions such as adolescents.
Regarding the role of the districts in our study, residing in district 2 appeared to protect against TB. Although FHS coverage in district 2 was low, living conditions were better, and this was the only district with an SDI ≥ 0.67. An ecological study in Rio de Janeiro reported that SDI scores were inversely associated with nonadherence to TB treatment, with a stronger association than the Human Development Index (HDI).22 This was probably because of the broader composition of the SDI, with a greater number of indicators than the HDI, including access to basic sanitation and housing quality (absent from the HDI).22 We also observed that districts 3.1 and 3.3 presented worse living conditions. District 3.1 had the third largest total population, the largest proportion of informal settlements, and the sixth highest homicide rate. District 3.3 had the largest total population, the fourth largest proportion of informal settlements, and the second highest homicide rate. The FHS coverage (> 60% in these districts) appeared not to have been sufficient to compensate for the effects of worse quality of life on control over TB in these districts. These districts are central in the municipality and in close proximity to shopping centers and other residential areas, which highlights the importance of local TB control in mitigating the TB transmission chain. In fact, Dowdy et al.23 reported that places considered to be “hot spots” in Rio de Janeiro, more specifically those with high disease transmission burdens but accounting for only 6% of the resident population, generated 35.3% of all disease transmission events in the city.
Although DOT was observed in more than one-half of these adolescents, it was not shown to be a protective factor against unfavorable outcomes. Two issues may have influenced our results: the missing information rate was 20%, which may have biased the data, and the SINAN database does not provide additional information regarding where the treatment was implemented. Kibuule et al.24 compared the effectiveness of community versus facility-based DOT and found that although the former significantly increased the success rate, this strategy was insufficient to attain a 95% success rate. Similarly, other studies have indicated that DOT combined with other strategies (e.g., psychoemotional and socioeconomic) yields a greater chance of beneficial effects.8,25 We also observed that lack of information regarding DOT demonstrated an aOR of 2.48 (95% CI: 1.31–4.96), thus leading to an unfavorable outcome in the multivariate analysis. This may be an indicator of organizational fragility in the healthcare unit. Possible explanations include different understandings that health professionals may have regarding DOT, and that, at times, it was not completely followed through, as observed by other authors.6 This, in turn, may have led health professionals to be unsure as to whether to qualify the treatment as DOT. Nevertheless, this represents a challenge for local TB control.
Tuberculosis–HIV coinfection exhibited a greater aOR (10.15 [95% CI: 4.15–25.34]) for an unfavorable treatment outcome. In our study, seven of 13 (53.8%) deaths were among TB–HIV–coinfected patients. Studies in populations without DR-TB have confirmed that these coinfected individuals experience higher mortality.26,27 In fact, sensitivity analysis revealed that TB–HIV coinfection and retreatment had a stronger association with unfavorable outcomes as a group, thus demonstrating their association with death and failure of TB treatment, besides loss to follow-up, as pointed out by other authors.6,7 This is worrisome, given the notable increase in detection of AIDS among Brazilian male adolescents: from 3% to 7% between 2007 and 2017 among 15- to 19-year- olds.10 This situation is very delicate in the context of adolescents already being exposed to multiple deprivations (monetary and nonmonetary).28
In addition to TB–HIV coinfection, we analyzed the cumulative effect of risk factors on the variable of “extreme vulnerability,” which is very important for planning TB control at the local level. Models for social determinants of TB use multiple interactions between factors precisely because individuals may exhibit associated risky behavior.29,30 However, it should be noted that individuals with TB–HIV coinfection were excluded from this variable; thus, only the tip of the iceberg regarding social vulnerability among these adolescents was observed.
Brazil is a low-burden MDR TB country, with an estimated rate of MDR/RIF-resistant TB of 1.2 (0.89–1.5) per 100,000 inhabitants, with an estimated proportion of 1.5% for new cases and 8% for retreatment.31 Although, only 45.4% of Xpert testing were performed, the proportion (3.7%) of RIF-resistant TB detected by Xpert in this study was similar to that found in two other Brazilian studies (3.6% and 3.8%).32,33
Xpert testing did not have any impact on treatment outcomes (Table 3), as observed in recent meta-analyses.34,35 Furthermore, we did not observe any association between unfavorable outcomes and DST or Xpert RIF-resistant results. One possible explanation for this finding is that Brazil is a low-burden MDR TB country, and other factors such as retreatment, TB–HIV coinfection, residence area, and extreme vulnerability were thus more important risk factors for unfavorable outcomes. Moreover, in countries with high TB burden, such as Brazil, empirical TB treatment may mask the beneficial effects of Xpert.32
In addition, we observed that 27.2% of adolescents, with an Xpert RIF-resistant result, initiated first-line TB treatment, despite the Xpert result. Considering that our study’s time frame was during the implementation of Xpert as the initial diagnostic test for suspected TB (replacing sputum smear microscopy), this finding may reflect health professionals’ unfamiliarity with Xpert during this transition period.
Despite the importance of the panorama provided by our data, among adolescents with TB in a country with high TB burden and TB–HIV coinfection, its limitations and strengths need to be considered. One limitation was the small number of adolescents, some with missing information, which may have biased the risk analysis. This is always an issue when secondary data are used. Another limitation was the lack of clinical details regarding how the diagnosis of TB was made because the data system (SINAN-TB) did not provide this information. However, these data were extracted from four national databases used by the Brazilian Ministry of Health for decision-making, which further supports the reliability of our data.
Thus, through analysis of healthcare-planning districts in Rio de Janeiro, worse living conditions (high homicide rate, large population, and high proportion of informal settlements), TB–HIV coinfection, cases of retreatment, extreme vulnerability, and lack of information regarding DOT were risk factors for unfavorable outcomes in this city with high TB incidence. Moreover, FHS coverage ≥ 60% in districts with worse living conditions and use of DOT appear not to have been sufficient to promote favorable outcomes in our study. Further studies with the specific aim of exploring these associations are, therefore, warranted.
Supplemental Appendices
Note: Supplemental appendices appear at www.ajtmh.org.
REFERENCES
- 1.Waddington C, Sambo C, 2013. Financing health care for adolescents: a necessary part of universal health coverage. Bull World Health Organ 93: 57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow KJ, Sismanidis C, Denholm J, Sawyer SM, Graham SM, 2018. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 51: 1702352. [DOI] [PubMed] [Google Scholar]
- 3.Paterson B, Morrow CD, Kohls D, Deignan C, Ginsburg S, Wood R, 2017. Mapping sites of high TB transmission risk: integrating the shared air social behavior of TB cases and adolescents in South Africa township. Sci Total Environ 583: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sant’Anna CC, Schmidt CM, March MFBP, Pereira SM, Barreto ML, 2013. Tuberculose em adolescentes em duas capitais brasileiras. Cad Saúde Pública 29: 111–116. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization , 2018. Roadmap towards Ending TB in Children and Adolescents, 2nd edition Geneva, Switzerland: WHO; Available at: https://apps.who.int/iris/bitstream/handle/10665/275422/9789241514798-eng.pdf?ua=1. Accessed November 4, 2019. [Google Scholar]
- 6.Berry KM, Rodriguez CA, Berhanu RH, Ismail N, Mvusi I, Long L, Evans D, 2019. Treatment outcomes in children, adolescents, and adults on treatment for tuberculosis in two metropolitan municipalities in Gauteng province, South Africa. BMC Public Health 19: 973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reif LK, et al. 2018. Outcomes across the tuberculosis care continuum among adolescents in Haiti. Public Health Action 8: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, Nahid P, 2018. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med 15: e1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melo DLB, Cano I, eds., 2017. Índice de Homicídios na Adolescência: IHA 2014. Rio de Janeiro, Brazil: Observatório de Favelas. [Google Scholar]
- 10.Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde , 2018. Boletim Epidemiológico HIV AIDS. Brasília, Brazil: Ministério da Saúde; Available at: http://www.aids.gov.br/pt-br/pub/2018/boletim-epidemiologico-hivaids-2018. Accessed December 6, 2019. [Google Scholar]
- 11.World Health Organization , 2018. Global Tuberculosis Report. Geneva, Switzerland: WHO; Available at: https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1&ua=1. Accessed November 12, 2019. [Google Scholar]
- 12.Cavalieiri F, Vidal A, 2012. Favelas na cidade do Rio de Janeiro: o quadro populacional com base no Censo 2010. Coleção Estudos Cariocas. Rio de Janeiro, Brazil: Instituto Pereira Passos; Report number 20120501. Available at: http://portalgeo.rio.rj.gov.br/estudoscariocas/download%5C3190_FavelasnacidadedoRiodeJaneiro_Censo_2010.PDF. Accessed November 21, 2019. [Google Scholar]
- 13.Instituto Brasileiro de Geografia e Estatística (IBGE) , 2020. Panorama do município do Rio de Janeiro. Available at: https://cidades.ibge.gov.br/brasil/rj/rio-de-janeiro/panorama. Accessed January 10, 2020. [Google Scholar]
- 14.Secretaria Municipal de Saúde do Rio de Janeiro , 2013. Plano Municipal de Saúde 2014–2017 Rio de Janeiro. Available at: http://www.rio.rj.gov.br/dlstatic/10112/3700816/4128745/PMS_20142017.pdf. Accessed November 28, 2019. [Google Scholar]
- 15.Brazil, Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis , 2018. Manual de Recomendações para o Controle da tuberculose no Brasil. Brasília, Brazil: Ministério da Saúde. [Google Scholar]
- 16.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, GT-SINAN , 2019. Sistema de Informação de Agravos de Notificação. Dicionário de Dados – SINAN-NET – Versão 5.0. Available at: https://portalsinan.saude.gov.br/images/documentos/Agravos/Tuberculose/DICI_DADOS_Tuberculose.pdf. Accessed June 1, 2020. [Google Scholar]
- 17.Pio JE, Durovni P, Piller R, 2019. Tuberculose: perfil epidemiológico do município do Rio de Janeiro 2015–2017. REVSF Rev Saude em Foco (Rio de Janeiro) 4: 03–62. [Google Scholar]
- 18.Ministério da Saúde, Sala de Apoio à Gestão Estratégica- SGEP , 2016. Guia de Apoio à Gestão Estadual do SUS, 2016. Available at: https://www.conass.org.br/guiainformacao/notas_tecnicas/NT6-Cobertura-ESF-e-ESB.pdf. Accessed November 23, 2019. [Google Scholar]
- 19.Instituto Pereira Passos, Prefeitura da Cidade do Rio de Janeiro , 2008. Índice de Desenvolvimento Social-IDS: Comparando as realidades microurbanas da cidade do Rio de Janeiro. No. 20080401; April, 2008. Rio de Janeiro, Brazil: IPP; Available at: http://portalgeo.rio.rj.gov.br/estudoscariocas/download/2394_%C3%8Dndice%20de%20Desenvolvimento%20Social_IDS.pdf. Accessed November 12, 2019. [Google Scholar]
- 20.Mulongeni P, Heramns S, Cladwell J, Bekker LG, Wood R, Kaplan R, 2019. HIV prevalence and determinants of loss-to-follow-up in adolescents and young adults with tuberculosis in Cape Town. PLoS One 14: e0210937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genet F, Sileshi H, SEifu W, Yrga S, Akeny AS, 2017. Do retreatment tuberculosis patients need special treatment response follow-up beyond the standard regimen? Finding of five-year retrospective study in pastoralist setting. BMC Infect Dis 17: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maciel EMGS, Amancio JS, Castro DB, Braga JU, 2018. Social determinants of pulmonary tuberculosis treatment non-adherence in Rio de Janeiro, Brazil. PLoS One 13: e0190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowdy DW, Golub JE, Chaisson RE, Saraceni V, 2012. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci USA 109: 9557–9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kibuule D, Rennie TW, Ruswa N, Mavhunga F, Thomas A, Amutenya R, Verbeeck RK, 2019. Effectiveness of community-based DOTS strategy on tuberculosis treatment success rates in Namibia. Int J Tuberc Lung Dis 23: 441–449. [DOI] [PubMed] [Google Scholar]
- 25.Van Hoorn R, Jaramillo E, Collins D, Gebhard A, van den Hof S, 2016. The effects of psycho-emotional and socio-economic support for tuberculosis patients on treatment adherence and treatment outcomes – a systematic review and meta-analysis. PLoS One 11: e0154095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enane LA, Lowenthal ED, Arscott-Mills T, Matlhare M, Smallcomb LS, Kgwaadira B, Coffin SE, Steenhoof AP, 2016. Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis 20: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 27.Snow K, Hesseling AC, Naidoo P, Graham SM, Denholm J, Du Preez K, 2017. Tuberculosis in adolescents and young adults: epidemiology and treatment outcomes in the Western Cape. Int J Tuberc Lung Dis 21: 651–657. [DOI] [PubMed] [Google Scholar]
- 28.UNICEF , 2019. Well-Being and Multiple Deprivations in Childhood and Adolescence in Brazil. Brasília, Brazil: UNICEF; Available at: https://www.unicef.org/brazil/media/4541/file/Well-being-and-multiple-deprivations-in-childhood-and-adolescence-in-brazil.pdf.pdf. Accessed November 28, 2019. [Google Scholar]
- 29.Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH, 2011. The social determinants of tuberculosis: from evidence to action. Am J Public Health 101: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedrazzoli D, Boccia D, Dodd PJ, Lonnroth K, Dowdy DW, Siroka A, Kimerling ME, White RG, Houben RMGJ, 2017. Modelling the social and structural determinants of tuberculosis: opportunities and challenges. Int J Tuberc Lung Dis 21: 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization , 2019. Global Tuberculosis Report 2019. Annex 2 – Country Profiles for 30 High TB Burden Countries. Geneva, Switzerland: WHO; Available at: https://www.who.int/tb/publications/global_report/tb19_Report_country_profiles_15October2019.pdf?ua=1. Accessed June 5, 2019. [Google Scholar]
- 32.Durovni B, Saraceni V, van den Hof S, Trajman A, Cordeiro-Santos M, Menezes A, Cobelens F, 2014. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS Med 11: e1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira GR, Barbosa MS, Dias NJD, de Almeida CPB, Silva DR. (2018) Impact of introduction of Xpert MTB/RIF test on tuberculosis (TB) diagnosis in a city with high TB incidence in Brazil. PLoS One 13: e0193988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agizew T, Boyd R, Auld AF, Payton L, Pals SL, Lekone P, Chihota V, Finlay A, 2019. Treatment outcomes, diagnostic and therapeutic impact: Xpert vs. smear. A systematic review and meta-analysis. Int J Tuberc Lung Dis 23: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Tanna GL, et al. 2019. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Health 7: e191–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.