Abstract.
Scrub typhus and Queensland tick typhus (QTT)—rickettsial infections endemic to tropical Australia—can cause life-threatening disease. This retrospective study examined the clinical course of all patients with laboratory-confirmed scrub typhus or QTT admitted to the intensive care unit (ICU) of a tertiary referral hospital in tropical Australia between 1997 and 2019. Of the 22 patients, 13 had scrub typhus and nine had QTT. The patients’ median (interquartile range [IQR]) age was 50 (38–67) years; 14/22 (64%) had no comorbidity. Patients presented a median (IQR) of seven (5–10) days after symptom onset. Median (IQR) Acute Physiology and Chronic Health Evaluation II scores were 13 (9–17) for scrub typhus and 13 (10–15) for QTT cases (P = 0.61). Following hospital admission, the median (IQR) time to ICU admission was five (2–19) hours. The median (IQR, range) length of ICU stay was 4.4 (2.9–15.9, 0.8–33.8) days. Multi-organ support was required in 11/22 (50%), 5/22 (22%) required only vasopressor support, 2/22 (9%) required only invasive ventilation, and 4/22 (18%) were admitted for monitoring. Patients were ventilated using protective lung strategies, and fluid management was conservative. Standard vasopressors were used, indications for renal replacement therapy were conventional, and blood product usage was restrictive; 9/22 (41%) received corticosteroids. One patient with QTT died, and two (8%) additional patients with QTT developed purpura fulminans requiring digital amputation. Death or permanent disability occurred in 3/9 (33%) QTT and 0/13 scrub typhus cases (P = 0.055). Queensland tick typhus and scrub typhus can cause multi-organ failure requiring ICU care in otherwise well individuals. Queensland tick typhus appears to have a more severe clinical phenotype than previously believed.
INTRODUCTION
Rickettsial infections are found on every continent, except Antarctica.1,2 In Australia, the most important rickettsial infections are caused by Orientia tsutsugamushi (which causes scrub typhus) and Rickettsia australis (which causes Queensland tick typhus [QTT]), and their incidence is increasing.1,3 Rickettsial infections were responsible for 6% of undifferentiated febrile illness in one inpatient series from tropical Australia.4
Scrub typhus—a common and well-described pathogen in Southeast Asia—often causes a mild, self-limiting illness, but it can also lead to multi-organ failure if untreated.5,6 There are fewer data describing the clinical course of QTT. It had been believed to cause only mild symptoms,7 but recent reports suggest that it can also cause disabling and lethal disease.3,8,9
Scrub typhus and QTT are uncommon causes of critical illness in Australia. However, a high index of suspicion for the infections is important, as diagnosis requires specific testing,1 and effective antibacterial therapy is often not included in empirical regimens for patients with sepsis.10–12
Although there are series from India,13 South Korea,14 Nepal,15 and China16 that have described the clinical course of rickettsial infection admitted to the ICU, none, to our knowledge, have been published from Australia.
MATERIALS AND METHODS
This retrospective study was performed at Cairns Hospital, a 531-bed tertiary referral center in Far North Queensland, tropical Australia. Patients were eligible for inclusion if they were admitted to the hospital’s ICU between January 1997 and December 2019 with a laboratory-confirmed diagnosis of scrub typhus or QTT. Definite infection was defined as a positive blood PCR or a 4-fold increase in titers of paired serological samples. Probable infection was defined as a single serological titer ≥ 128 with a clinically compatible syndrome (≥ 2 of fever, rash, eschar, myalgia, or headache). Patients were excluded if, on chart review, a non-rickettsial diagnosis was determined to be more likely.
Patients’ medical records were reviewed to collect demographic and epidemiological data, and details of their clinical presentation, comorbidities, and management. The patients’ Acute Physiology and Chronic Health Evaluation II (APACHE-II) and Sequential Organ Failure Assessment (SOFA) scores were calculated.17,18 Acute respiratory distress syndrome (ARDS) severity was graded using the Berlin definition.19 Appropriate anti-rickettsial therapy was defined as at least 7 days of doxycycline, or 5 days of azithromycin.11
Statistical analysis.
Data were de-identified, entered into an electronic database (Microsoft Excel 2016, Microsoft Corp., Redmond, WA), and analyzed with statistical software (Stata version 14.2, StataCorp, College Station, TX). Groups were compared with the Kruskal–Wallis test or Fisher’s exact test, where appropriate. Correlation coefficients were determined using Spearman’s method.
Ethics statement.
The Far North Queensland Human Research Ethics Committee provided ethical approval for the study (HREC/17/QCH/66–1148 QA). As the data were retrospective and de-identified, the committee waived the requirement for informed consent.
RESULTS
Twenty-two patients were admitted to the ICU with a laboratory-confirmed diagnosis of rickettsial infection during the study period. This included 13 (64%, five definite, eight probable) patients with scrub typhus and 9 (36%, four definite, five probable) patients with QTT. Almost two-thirds (14/22; 64%) had no comorbidity. No patient received anti-rickettsial antibiotics before their hospital admission. The patients’ other demographic and epidemiological characteristics are presented in Table 1.
Table 1.
Selected demographic and epidemiological characteristics of the 22 patients at the time of presentation to hospital
| Demographic | |
|---|---|
| Male gender | 12 (55%) |
| Age (years) | 51 (38–67, 22–77) |
| Interhospital transfer from a rural facility | 14 (64%) |
| Rural residential address | 16 (73%) |
| Duration of symptoms before presentation (days) | 7 (5–10, 0–14) |
| Significant comorbidity* | 8 (36%) |
All values represent the absolute number (%) and the median (interquartile range, range).
Significant comorbidity: chronic cardiovascular disease (receiving any ongoing treatment for a cardiovascular condition), chronic lung disease (receiving any ongoing treatment for a chronic lung condition), chronic renal disease (a serum creatinine ≥ 150 μmol/L documented before the presentation), immunosuppression (the use of immunosuppressive agents, including corticosteroids, chemotherapy, or immunomodulatory therapies), an active malignancy, or a diagnosis of diabetes mellitus.
Clinical presentation.
The median (interquartile range [IQR]) duration of symptoms before hospital presentation was 7 (5–10) days. Rickettsial infection was considered in the initial differential diagnosis of 9/22 (41%). The most common alternatively considered diagnoses were pneumonia (9/22 40%) and leptospirosis (9/22 40%). The patients’ symptoms and signs documented at the time of hospital presentation are detailed in Table 2.
Table 2.
Symptoms and signs documented in the 22 patients at the time of presentation to hospital
| Symptom | |
| Subjective fevers | 22 (100%) |
| Myalgia | 15 (68%) |
| Cough | 11 (50%) |
| Fatigue/lethargy | 10 (45%) |
| Rash | 10 (45%) |
| Headache | 10 (45%) |
| Nausea/vomiting | 10 (45%) |
| Rigors | 6 (27%) |
| Dyspnea | 6 (27%) |
| Confusion | 4 (18%) |
| Easy bleeding/bruising | 14 (5%) |
| Clinical findings | |
| Tachypnea (respiratory rate > 22 breaths/minute) | 19 (86%) |
| Hypoxia (oxygen saturations < 95% on room air) | 18 (82%) |
| Tachycardia (heart rate > 100 beats/minute) | 18 (82%) |
| Hypotension (mean arterial pressure < 65 mmHg) | 16 (72%) |
| Temperature > 38.0°C | 14 (63%) |
| Eschar | 6 (27%) |
| Glasgow Coma Scale score < 15 | 4 (18%) |
| Abnormal bleeding | 2 (9%) |
| Lymphadenopathy | 2 (9%) |
| Hepatomegaly | 2 (9%) |
| Splenomegaly | 1 (5%) |
ICU admission and management.
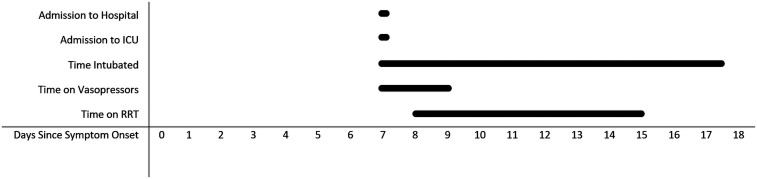
Following hospital admission, the median (IQR) time to ICU admission was five (2–19) hours; only 4/22 (18%) were admitted > 24 hours after presentation. The median (IQR) length of ICU stay was 4.4 (2.9–15.9) days (Figure 1). The reason for ICU admission was multi-organ support in 11/22 (50%), vasopressor support in 5/22 (22%), mechanical ventilation in 2/22 (9%), and continuous monitoring in 4/22 (18%). The patients’ median (IQR) APACHE-II score at ICU admission was 13 (9–16); their median (IQR) SOFA score on admission was eight (7–12).
Figure 1.
Median timing of initiation and duration of supportive therapies provided in the ICU.
Laboratory findings.
The patients’ laboratory findings at their hospital admission and during their hospitalization are presented in Table 3. There were 9/22 patients (40%) with simultaneously prolonged clotting times and low fibrinogen—consistent with disseminated intravascular coagulation,20 five (56%) of whom had abnormal bleeding. Two of these patients developed purpura fulminans.21
Table 3.
Laboratory findings at the time of hospital presentation of the 22 patients
| Variable | Reference range* | Value at hospital admission | Most deranged value (throughout hospitalization) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured | Median | IQR | Range | Abnormal* (%) | Measured† | Median | IQR | Range | Abnormal* (%) | ||
| Hemoglobin (g/L) | 135–180 | 22/22 | 134 | 122–145 | 91–180 | 14 | 22/22 | 88 | 74–108 | 66–139 | 82 |
| White cell count (×109/L) | 4.0–11.0 | 22/22 | 10.6 | 6.4–13.6 | 1.6–29.4 | 41 | 22/22 | 14 | 10.6–19.2 | 1.6–45.9 | 73 |
| Neutrophils (×109/L) | 2.00–8.00 | 21/22 | 8.4 | 4.6–11.8 | 1.1–26.5 | 52 | 22/22 | 11.4 | 8.2–16.9 | 1.1–39.9 | 77 |
| Lymphocytes (×109/L) | 1.00–4.00 | 21/22 | 0.63 | 0.36–1.1 | 0.15–4.1 | 62 | 22/22 | 0.54 | 0.36–0.95 | 0.05–11.2 | 77 |
| Platelets (×109/L) | 140–400 | 21/22 | 153 | 92–167 | 36–556 | 43 | 22/22 | 93 | 29–126 | 6–358 | 77 |
| Sodium (mmol/L) | 135–145 | 22/22 | 131 | 129–134 | 112–139 | 77 | 22/22 | 131 | 129–133 | 112–138 | 82 |
| Potassium (mmol/L) | 3.5–5.2 | 22/22 | 3.8 | 3.6–4.1 | 2.1–4.9 | 5 | 22/22 | 4.8 | 4.4–5.2 | 2.1–5.8 | 41 |
| Urea (mmol/L) | 2.1–7.1 | 22/22 | 8.0 | 5.1–16.9 | 3.8–31.1 | 50 | 22/22 | 14.1 | 7.4–20.0 | 4.8–34 | 64 |
| Creatinine (µmol/L) | 60–110 | 22/22 | 105 | 85–185 | 61–530 | 59 | 22/22 | 120 | 94–222 | 69–530 | 68 |
| Albumin (g/L) | 35–50 | 21/22 | 26 | 25–32 | 17–50 | 90 | 22/22 | 19 | 16–21 | 15–34 | 100 |
| Total bilirubin (µmol/L) | < 20 | 21/22 | 25 | 16–41 | 9–77 | 57 | 22/22 | 28 | 17–72 | 12–131 | 68 |
| Alkaline phosphatase (U/L) | 30–110 | 21/22 | 147 | 91–237 | 54–587 | 52 | 22/22 | 230 | 155–508 | 60–1,350 | 82 |
| Gamma glutamyl transpeptidase (U/L) | < 55 | 21/22 | 100 | 58–186 | 18–887 | 81 | 22/22 | 199 | 75–392 | 53–2,270 | 100 |
| Alanine transaminase (U/L) | < 45 | 21/22 | 89 | 74–206 | 25–404 | 95 | 22/22 | 133 | 81–241 | 37–3,880 | 100 |
| Aspartate transaminase (U/L) | < 35 | 21/22 | 143 | 99–309 | 24–864 | 95 | 22/22 | 183 | 131–356 | 60–18,100 | 100 |
| Lactate dehydrogenase (U/L) | 120–250 | 9/22 | 703 | 414–965 | 343–1,370 | 100 | 17/22 | 476 | 428–899 | 345–26,600 | 100 |
| Creatinine kinase (U/L) | 46–171 | 7/22 | 869 | 202–2,545 | 51–16,200 | 71 | 14/22 | 309 | 80–1,244 | 10–25,000 | 57 |
| Prothrombin time (seconds) | 9–13 | 12/22 | 13 | 12–16 | 11–23 | 42 | 22/22 | 16 | 13–20 | 11–100 | 73 |
| Activated partial thromboplastin time (seconds) | 24–39 | 12/22 | 36 | 32–40 | 24–70 | 33 | 22/22 | 40 | 36–61 | 28–150 | 68 |
| Fibrinogen (g/L) | 1.7–4.5 | 11/22 | 3.8 | 2.2–4.5 | 1.8–10 | 0 | 22/22 | 2.5 | 1.3–3.6 | 0.5–7.8 | 32 |
| C-reactive protein (mg/L) | < 5.0 | 11/22 | 206 | 179–289 | 2–498 | 82 | 15/22 | 219 | 179–302 | 5–500 | 87 |
IQR = interquartile range. The most deranged values (range) during the patient’s hospitalization are also presented.
Outside of the reference range provided by the reporting laboratory (Queensland Pathology).
Number of patients with this laboratory value measured on at least one occasion during their hospitalization.
Radiological findings.
All patients had at least one chest X-ray (CXR) performed: 4/22 (18%) showed single lobe consolidation, 6/22 (27%) had multi-lobar consolidation, 5/22 (22%) demonstrated interstitial changes, and in 1/22 (4%), bilateral pleural effusions were also present. The CXR was normal in 7/22 (31%) (Figure 2).
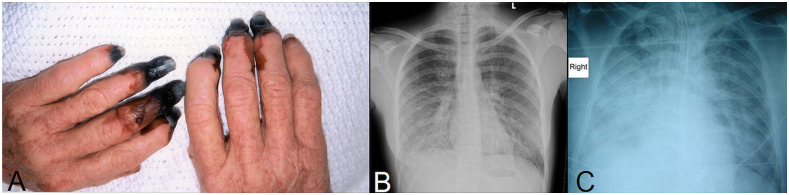
Figure 2.
(A) Digital necrosis complicating histologically proven purpura fulminans in a 69-year-old woman without comorbidity. She had a 4-fold increase in serology for Queensland tick typhus (QTT) infection; her fingers later required digital amputation. (B) Chest X-ray demonstrating bilateral patchy non-confluent alveolar opacification in a 26-year-old woman without comorbidities who presented with a maculopapular rash, deranged liver function tests, thrombocytopenia, and a prolonged prothrombin time; she had a single serological titer of 1:1,024 for QTT. She required ICU admission for vasopressor support but required only supplemental oxygen by a Hudson mask and made a complete recovery. (C) Chest X-ray demonstrating right middle and lower lobe pneumonia and early left lower lobe consolidation in a 45-year-old male patient with a history of tick bite and a 4-fold increase in serology for QTT infection. He required intubation and ventilation for 16 days but made a complete recovery. These images have been published previously.3 This figure appears in color at www.ajtmh.org.
Antibiotic therapy.
All patients received appropriate anti-rickettsial antibiotic therapy; 15/22 (68%) received doxycycline, 6/22 (27%) received both doxycycline and azithromycin, and 1/22 (4%) received azithromycin alone. In 17/22 (72%), anti-rickettsial therapy was administered within the first 24 hours of their hospitalization; the median (range) delay was 1 (1–4) days in the remainder.
Respiratory support.
A similar number of patients with scrub typhus and QTT required intubation (Table 4). A further 8/22 patients (36%) required high-flow oxygen; only 2/22 did not require supplemental oxygen. The median (IQR) PaO2/FiO2 ratio in the 20 patients in whom it could be calculated was 140 (106–190). There were four (18%) patients with severe and six (27%) with moderate ARDS.
Table 4.
Requirement of supportive therapies by type of rickettsial infection
| Disease | Scrub typhus (n = 13) | Queensland tick typhus (n = 9) | P-value |
|---|---|---|---|
| Mechanical ventilation | 8 (61%) | 4 (44%) | 0.67 |
| Vasopressor support | 9 (69%) | 6 (67%) | 1 |
| Renal replacement therapy | 2 (15%) | 3 (33%) | 0.61 |
The median (IQR) duration of intubation was 10.3 (4.5–15.4) days. Intubated patients had more severe disease than those not requiring intubation (median [IQR] APACHE-II score: 15 [13–18] versus nine [8–12], P = 0.0006). Patients requiring intubation had a greater fluid balance over the first 72 hours of their ICU stay than those who did not (median [IQR] 3,823 [3,061–6,344] mL versus −24 [−89 to 852] mL, P = 0.0002).
Other supportive therapies.
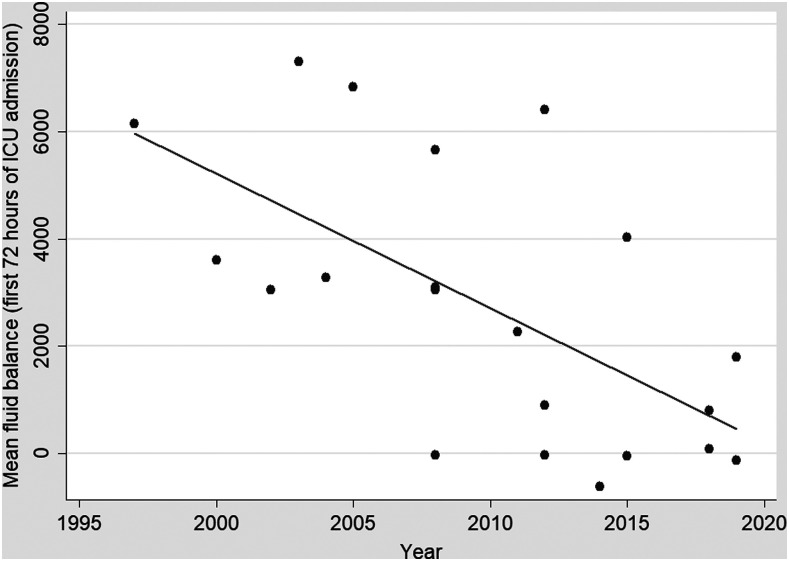
Vasopressor support was required in 15/22 (68%) for a median (IQR) duration of 2.1 (0.6–3.5) days. Noradrenaline (10/15; 67%) was the most frequently used vasopressor. Renal replacement therapy (RRT) was required in 5/22 (23%) and was initiated in all cases for oliguric or anuric renal failure with volume overload (Table 5). One patient requiring RRT died after 2.1 days; none of the remaining patients subsequently required long-term dialysis. Intravenous fluid therapy became more restrictive during the study period with the mean initial 72-hour fluid balance declining as the study proceeded (Spearman’s rho = −0.62, P = 0.003) (Figure 3).
Table 5.
Clinical and laboratory findings at the time of RRT commencement and subsequent duration of RRT
| Age (years), gender | pH | Urea (mmol/L) | Potassium (mmol/L | Base excess (mmol/L) | Urine output | Clinically volume overloaded? | Total positive fluid balance (mL) | Duration of RRT (days) |
|---|---|---|---|---|---|---|---|---|
| 53, Male | 7.07 | 11.2 | 4 | −16.7 | Oliguric | Yes | 20,483 | 3 |
| 44, Female | 7.26 | 16.8 | 3.6 | −12.3 | Oliguric | Yes | 16,995 | 3.9 |
| 69, Female | 7.36 | 18.9 | 4.3 | −3.4 | Anuric | Yes | 13,010 | 23 |
| 35, Male | 7.35 | 33.1 | 3.9 | −7.8 | Oliguric | Yes | 19,234 | 9.9 |
| 56, Male | 7.34 | 19.8 | 4.9 | −6.7 | Anuric | No | 4,937 | 2.1* |
RRT = renal replacement therapy.
Patient died at this point.
Figure 3.
Mean fluid balance over the first 3 days of each patient’s ICU admission over the course of the study.
Red blood cell transfusion was delivered to 5/22 (23%) and fresh frozen plasma to 4/22 (18%). Despite the ubiquity of thrombocytopenia, no platelet transfusions were administered. Only 2/22 (9%) had a clinically significant hemorrhage—both upper gastrointestinal bleeds—but neither had thrombocytopenia or coagulopathy at the time.
Corticosteroids were prescribed in 9/22 (41%) for a median (IQR) duration of 4 (1–5) days. Nasogastric feeding, deep venous thrombosis, and stress ulcer prophylaxis were provided to all patients, unless there was a contraindication.
Morbidity and mortality.
The single death occurred in a previously well 55-year-old man with QTT, who presented with established multi-organ failure 7 days after symptom onset. Two patients with QTT and purpura fulminans required surgical amputation of necrotic digits (Figure 2). The remaining 19 patients (86%) recovered without permanent disability.
DISCUSSION
Australian patients admitted to the ICU with rickettsial infections have excellent outcomes in the country’s well-resourced health system. The reported case fatality rate of ICU patients with scrub typhus in international studies varied from 10% to 24%,13–16 but there were no deaths from scrub typhus in 22 years at this center and just one (1/9; 11%) from QTT. This is despite the cohort having APACHE-II and SOFA scores that would have predicted an overall case fatality rate of approximately 20%.17,18
In this small series, it is not possible to define which components of care were responsible for these encouraging outcomes; however, prompt anti-rickettsial antibiotic therapy and early access to sophisticated multimodal ICU support are likely to have contributed. Patients were ventilated using protective lung strategies, and fluid management was generally conservative. Standard vasopressors were used, indications for RRT were conventional, and blood product usage was restrictive. Over 40% of the patients had severe thrombocytopenia (< 50 × 109/L) at some point during their hospitalization, but no platelet transfusions were administered, and no thrombocytopenic patient had major bleeding. Approximately 40% of the cohort received corticosteroids; however, this was usually delivered as only one of several concurrent interventions.
Pulmonary involvement heralded a more complicated course; despite this, 90% of patients with moderate to severe ARDS survived. Patients with a greater positive fluid balance were more likely to be intubated; it is therefore notable that fluid prescription became more restrictive over the course of the study, an approach which may have contributed to the good outcomes.22
Severe QTT has been thought to be rare.7 However, in this series, nine patients with QTT required ICU support, including one who died and two who required digital amputation. The clinical findings of patients with QTT in this cohort are consistent with the pathophysiology described in a murine model of the disease.23 Mice infected experimentally with R. australis developed a severe systemic vasculitis and multifocal hepatic necrosis and demonstrated extensive rickettsial invasion of the pulmonary alveolar septa, the renal glomeruli, and interstitium. Higher inoculums of R. australis were uniformly lethal.
Indeed, the pathophysiology of severe human QTT appears likely to be similar to that of scrub typhus and Rocky Mountain spotted fever, two rickettsial conditions whose pathological hallmark is also endothelial infection and inflammation.24,25 This endothelial inflammation leads to microvascular dysfunction, increasing vascular permeability, which in turn increases the risk of shock and lung injury.24,26 Endothelial activation may lead to widespread platelet adhesion and microvascular thrombosis, exacerbating the systemic microvascular dysfunction and increasing the likelihood of multi-organ failure.27,28
The patients in this cohort presented a median (IQR) of 7 (5–10) days after symptoms developed. Most had not sought medical advice before their presentation, and none had received anti-rickettsial antibiotic therapy, echoing findings from other studies that have shown that delayed antibiotic therapy increases the likelihood of severe rickettsial disease.29
It was notable that almost two-thirds of the patients in this ICU series had no comorbidity, highlighting the organisms’ pathogenic potential. The absence of any pediatric cases—the youngest patient was 22 years—was also striking; this is despite local children presumably having a similar exposure risk.30
This retrospective study has limitations, with patients’ symptoms and signs especially likely to be incompletely described. A minority had a PCR-confirmed diagnosis or a 4-fold increase in serology; although patients were only included if they satisfied prespecified criteria similar to those in the international literature. Patients with scrub typhus and QTT are presented together, and some may take issue with this approach. However, there are significant similarities in the infections’ pathophysiology and management, and hence, we felt that it was reasonable to present them together.3 The small sample precludes definitive conclusions about optimal management strategies; however, it provides hypothesis-generating data that might be tested in future prospective studies. The ability to deliver this care in the resource-limited settings—which bear the greatest global burden of rickettsial diseases—will be an essential consideration in this research.
In summary, rickettsial infections uncommonly require ICU admission in Australia but can cause critical illness in patients without comorbidity. Late presentation is likely to increase the risk of severe disease, but despite this, if patients can access prompt, multidisciplinary ICU support, their outcomes are usually excellent.
Acknowledgments:
We would like to thank all the healthcare workers involved in the care of the patients in this series. We would also like to acknowledge Markus Ott who provided valuable assistance with data retrieval.
REFERENCES
- 1.Abdad MY, Abou Abdallah R, Fournier PE, Stenos J, Vasoo S, 2018. A concise review of the epidemiology and diagnostics of rickettsioses: Rickettsia and Orientia spp. J Clin Microbiol 56: e01728-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parola P, et al. 2013. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26: 657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart AGA, Smith S, Binotto E, McBride WJH, Hanson J, 2019. The epidemiology and clinical features of rickettsial diseases in North Queensland, Australia: implications for patient identification and management. PLoS Negl Trop Dis 13: e0007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Susilawati TN, McBride WJ, 2014. Undiagnosed undifferentiated fever in Far North Queensland, Australia: a retrospective study. Int J Infect Dis 27: 59–64. [DOI] [PubMed] [Google Scholar]
- 5.Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH, 2017. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis 11: e0005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AJ, Paris DH, Newton PN, 2015. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis 9: e0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sexton DJ, Dwyer B, Kemp R, Graves S, 1991. Spotted fever group rickettsial infections in Australia. Rev Infect Dis 13: 876–886. [DOI] [PubMed] [Google Scholar]
- 8.McBride WJ, Hanson JP, Miller R, Wenck D, 2007. Severe spotted fever group rickettsiosis, Australia. Emerg Infect Dis 13: 1742–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart A, Armstrong M, Graves S, Hajkowicz K, 2017. Clinical manifestations and outcomes of Rickettsia australis infection: a 15-year retrospective study of hospitalized patients. Trop Med Infect Dis 2: 19 Available at: 10.3390/tropicalmed2020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YS, Yun HJ, Shim SK, Koo SH, Kim SY, Kim S, 2004. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin Infect Dis 39: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 11.Therapeutic Guidelines Limited , 2019. Antibiotic Guidelines Australia Electronic Therapeutic Guidelines Complete. Melbourne, Australia: Therapeutic Guidelines Limited. [Google Scholar]
- 12.Rhodes A, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 13.Griffith M, et al. 2014. Profile of organ dysfunction and predictors of mortality in severe scrub typhus infection requiring intensive care admission. Indian J Crit Care Med 18: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon KM, Han MS, Rim CB, Lee JH, Kang MS, Kim JH, Kim SI, Jung SY, Cho Y, 2016. Risk factors for mechanical ventilation in patients with scrub typhus admitted to intensive care unit at a University Hospital. Tuberc Respir Dis (Seoul) 79: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adhikari S, Poudel RS, Shrestha S, Lamichhane P, 2018. Predictors of mortality in scrub typhus infection requiring intensive care admission in Tertiary Healthcare Centre of Nepal. Interdiscip Perspect Infect Dis 2018: 4867958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N, et al. 2008. Risk factors associated with life-threatening rickettsial infections. Am J Trop Med Hyg 78: 973–978. [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE, 1985. Apache II: a severity of disease classification system. Crit Care Med 13: 818–829. [PubMed] [Google Scholar]
- 18.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S, 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 19.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, 2012. Acute respiratory distress syndrome: the Berlin definition. JAMA 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 20.Levi M, Ten Cate H, 1999. Disseminated intravascular coagulation. N Engl J Med 341: 586–592. [DOI] [PubMed] [Google Scholar]
- 21.Colling ME, Bendapudi PK, 2018. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev 32: 69–76. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network ; Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL, 2006. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575. [DOI] [PubMed] [Google Scholar]
- 23.Feng HM, Wen J, Walker DH, 1993. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol 142: 1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz FE, Abarca K, Kalergis AM, 2018. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev 31: e00076-17 Available at: 10.1128/CMR.00076-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker DH, Ismail N, 2008. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol 6: 375–386. [DOI] [PubMed] [Google Scholar]
- 26.Hsu YH, Chen HI, 2008. Pulmonary pathology in patients associated with scrub typhus. Pathology 40: 268–271. [DOI] [PubMed] [Google Scholar]
- 27.Chen LF, Sexton DJ, 2008. What’s new in Rocky Mountain spotted fever? Infect Dis Clin North Am 22: 415–432, vii–viii. [DOI] [PubMed] [Google Scholar]
- 28.Fine D, Mosher D, Yamada T, Burke D, Kenyon R, 1978. Coagulation and complement studies in Rocky Mountain spotted fever. Arch Intern Med 138: 735–738. [PubMed] [Google Scholar]
- 29.Kirkland KB, Wilkinson WE, Sexton DJ, 1995. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis 20: 1118–1121. [DOI] [PubMed] [Google Scholar]
- 30.Stewart AGA, Smith S, Binotto E, Hanson J, 2020. Clinical features of rickettsial infection in children in tropical Australia—a report of 15 cases. J Trop Pediatr (Epub ahead of print). Available at: https://pubmed.ncbi.nlm.nih.gov/32252063/. [DOI] [PubMed]