Abstract.
Despite myriad improvements in the care of COVID-19 patients, atypical manifestations are least appreciated during the current pandemic. Because COVID-19 is primarily manifesting as an acute respiratory illness with interstitial and alveolar pneumonia, the possibility of viral invasions into the other organs cannot be disregarded. Acute kidney injury (AKI) has been associated with various viral infections including dengue, chikungunya, Zika, and HIV. The prevalence and risks of AKI during the course of COVID-19 have been described in few studies. However, the existing literature demonstrate great disparity across findings amid variations in methodology and population. This article underscores the propensity of AKI among COVID-19 patients, limitations of the exiting evidence, and importance of timely identification during the case management. The prevalence of AKI is variable across the studies ranging from 4.7% to 81%. Evidence suggest old age, comorbidities, ventilator support, use of vasopressors, black race, severe infection, and elevated levels of baseline serum creatinine and d-dimers are independent risk factors of COVID-19 associated with AKI. COVID-19 patients with AKI also showed unsatisfactory renal recovery and higher mortality rate as compared with patients without AKI. These findings underscore that AKI frequently occurs during the course of COVID-19 infection and requires early stratification and management.
Recent research advances on COVID-19 have explicitly underscored the classical respiratory symptoms associated with the disease. However, the growing body of evidence describes numerous atypical manifestations in association with the disease course. Acute kidney injury (AKI) is recently emerged as a potential complication during the course of COVID-19.1 However, current investigations demonstrate wide disparities in the prevalence and mechanisms of COVID-19 associated with AKI. A recent case series by Wang et al.2 reported that AKI is uncommon among COVID-19 patients and even not seen in preexisting chronic kidney disease (CKD). Given the differences across findings, we felt inclined to share our point of view regarding the propensity of AKI during the course of COVID-19. We believe that odds of AKI occurrence among COVID-19 patients are substantially higher and must be considered during the case management. Because of unprecedented COVID-19 cases, clinicians are primarily engaged in the case management along with virus containment to avoid the disease spillover, and there is high propensity that healthcare professionals may overlook a slight rise in serum creatinine (SCr) among patients.
Because COVID-19 is primarily manifested as an acute respiratory illness with interstitial and alveolar pneumonia, the possibility of viral invasions into other organs cannot be disregarded. It is pertinent to mention that AKI remained neglected intricacy during various viral infections and was later emerged as highly morbid and fatal complication.3,4 Dengue-associated AKI is such an example which was initially least appreciated during various outbreaks and was subsequently evolved as a serious complication.5 Although Wang and others did not observe any substantial rise in SCr among patients, a firm conclusion cannot be drawn amid small sample size (N = 116) in their study.2 Recent investigations accentuate the involvement of multiple organs in COVID-196,7 and report AKI in 6–36.6% of cases.8–10 Subsequent studies reported that COVID-19 presents with massive albuminuria (34%), elevated blood urea nitrogen (27%), proteinuria along with hematuria (44%), and increased SCr (15.5%),11–13 either on admission and or during hospitalization. A Chinese study indicated abnormal urine dipstick in 75.4% hospitalized patients.14 A large cohort study from New York showed that 52.2% patients receiving mechanical ventilation developed AKI within 24 hours following intubation, suggesting mechanical ventilation as a significant predictor of AKI.10 Furthermore, serious concerns have also been raised about the impending shortage of renal replacement therapy for COVID-19 patients.15 Moreover, AKI has also been estimated as an independent predictor of in-hospital mortality among COVID-19 patients.13,16 On the other hand, a Japanese study showed that COVID-19 portends higher mortality (16.2%) among dialysis patients than the general population (5.3%), suggesting hospitalization of these patients.1 Table 1 describes the descriptive summary of studies illustrating renal involvement during the course of COVID-19.
Table 1.
Summary of studies describing the renal abnormalities and prevalence of AKI during COVID-19 infection
| Authors, year | Country | Sample size | Demographics | Type of renal abnormalities | Prevalence of AKI | Outcomes/remarks |
|---|---|---|---|---|---|---|
| Chen et al.25 | China | N = 274 (dead: 113, recovered: 161) | Median age: 60 years, 73% males | AKI (11%), proteinuria (60%), hematuria (50.6%) | AKI in total cohort: 11% | The renal anomalies were common in dead cases as compared with recovered patients |
| AKI in died cases: 25% | ||||||
| AKI in recovered cases: 1% | ||||||
| Cheng et al.11 | China | N = 701 | Median age: 63 years, 52.4% males | OA elevated SCr (14.4%), OA elevated BUN (13.1%), glomerular filtration rate ≪ 60 mL/minute per 1.73 m2 (13.1%), proteinuria (43.9%), hematuria (26.7%) | AKI: 5.1% during hospital stay; prevalence of AKI was higher among patients with elevated SCr on hospital admission | Elevated SCr, BUN, hematuria, proteinuria, and AKI (stage 2) were associated with mortality. |
| Pei et al.14 | China | N = 333 | Mean age: 56.3 years, 54.7% males | Overall renal involvements (75.4%), proteinuria (65.8%), hematuria (41.7%) | 4.7% by KDIGO criteria and 7.5% by expanded KDIGO criteria | Overall mortality: 29 (20 cases had AKI defined by either criteria), out of 16/35 AKI cases (defined by expanded criteria) showed renal recovery |
| Taher et al.24 | Bahrain | N = 73 | Mean age: 54 years, 60.3% males | Hematuria (20.5%), proteinuria (52.1%) | AKI: 29/73, 39.7% (stage 1: 11%, stage 2: 15.1%, stage 3: 13.7%) | 7 cases required RRT, 12/13 died cases had AKI, renal recovery was observed in 16 cases, one patient was discharged on dialysis |
| Hirsch et al.10 | USA | N = 5,449 | Median age: 64 years, 60.9% males | Hematuria (36.5%), leukocytouria (40.9%), protein 3+ (78 cases) | AKI: 1993/5,449, 36.6% (stage 1: 46.5%, stage 2: 22.4%, stage 3: 31.1%) | AKI was substantially associated with mortality, risk factors of AKI included were old age, cardiovascular disease and mechanical ventilation |
| Joseph et al.22 | France | N = 100 | Median age: 59 years, 70% males | Only AKI | AKI: 81/100, 81% (stage 1: 44 cases, stage 2: 10 cases, stage 3: 27 cases) | Overall 29 death cases, 28 cases had AKI |
| Ng et al.26 | USA | N = 9,657 | Median age: 62 years, 58% males | Only AKI | AKI: 3,854/9,657 | Among AKI patients without RRT, 74% showed renal recovery at discharge, |
| (3,116 cases had non-kidney replacement therapy required AKI, 638 cases had stage 3 AKI requiring replacement therapy) | Among patients with AKI requiring replacement therapy, 30.6% remained on dialysis on discharge | |||||
| Cui et al.23 | China | N = 116 | Mean age: 59 years, 56.9% males | Only AKI | AKI: 21/116, 18.1% | Overall mortality rate was 15.5%, patients with AKI had higher mortality rate than those without AKI |
| Early AKI (developed within 72 hours): 13 cases | ||||||
| Late AKI (developed after 72 hours of admission): eight cases |
AKI = acute kidney injury; BUN = blood urea nitrogen; SCr = serum creatinine; KDIGO = kidney disease: improving global outcomes; RRT = renal replacement therapy.
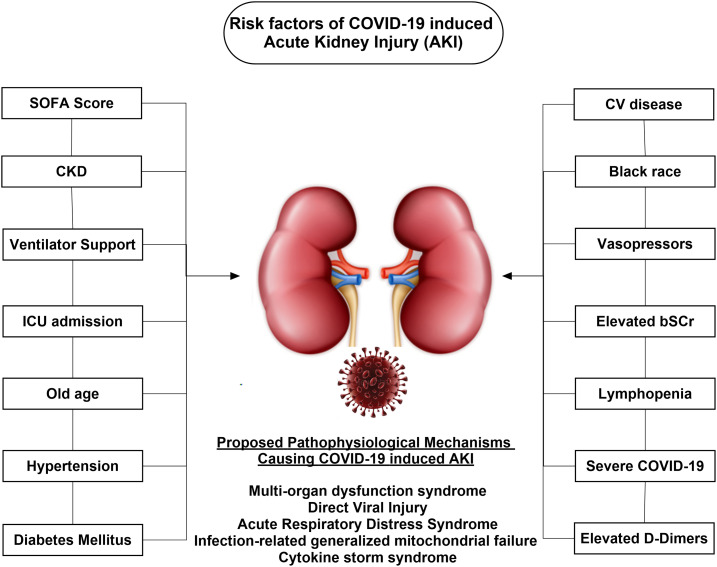
The renal injury during the course of COVID-19 is multifaceted, involving various triggers. Renal hypoperfusion–related acute tubular necrosis or cellular damage, dysregulated inflammatory response, microcirculatory dysfunction, metabolic reprogramming, cytokine storm syndrome precipitated by sepsis, and direct viral injury are the possible etiopathological mechanisms (Figure 1).13 Moreover, the presence of angiotensin-converting enzyme 2 receptors in renal tissues could also facilitate viral invasion, resulting in direct injury.17,18 Viral RNA has been isolated from the urine sample of one patient (6.9% of 58 cases), suggesting kidney as a vulnerable target for infection.19 Viral particles have also been detected in the postmortem kidney samples of COVID-19 fatal cases.20 These findings underscore the capability of the virus to cause renal damage either by direct invasion into the renal parenchyma or through other secondary mechanisms.
Figure 1.
Risk factors for the development of acute kidney injury (AKI) during COVID-19 infection.
Some additional studies suggest association of COVID-19–induced AKI with advance age and preexisting comorbidities.21 Available studies10,11,22–24 have reported various risk factors for the development of AKI during the course of COVID-19 as described in Figure 1. Taken together, in contrast to the results of Wang et al.,2 these findings underscore that AKI is frequently encountered10 highly morbid and fatal complication10,14,22,23 during COVID-19 and warrant the dire need of nephrology consultation. However, these studies are accompanied by potential limitations including the less number of patients, unavailability of baseline SCr, disparity in AKI diagnostic criteria, and lack of follow-up to ascertain the renal recovery. Future studies must be conducted in light of these limitations, so findings could be appropriately incorporated into the clinical practice. The use of baseline SCr is subjected to wide variations and depends on the individual clinical settings. Moreover, diagnostic criteria of AKI must be followed to ascertain the true estimate. Clinicians must adhere to the kidney disease: improving global outcomes criteria on classification and treatment of AKI to establish the uniformity across the literature.
Before the era of CKD staging, AKI was considered as reversible with satisfactory renal outcomes. However, with the recent advancements in renal care, it is widely accepted that patients who survive an episode of AKI might recover adequate renal functions, but still such patients are at risk of developing CKD.3 Considering the widespread nature of the disease and substantial number of patients with atypical manifestations, subsequent renal deterioration following an episode of AKI may pose substantial risks of CKD among survivors. We urge early stratification of AKI during COVID-19 case management which would provide opportunity to clinicians for appropriate management in a timely manner. Moreover, the follow-up of patients to ascertain the renal recovery will be of paramount importance to design and implement intervention strategies for high-risk patients.
Acknowledgment:
Publication charges for this article were waived due to the ongoing pandemic of COVID-19.
REFERENCES
- 1.Kikuchi K, et al. 2020. COVID-19 in dialysis patients in Japan: current status and guidance on preventive measures . Ther Apher Dial 24: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Li X, Chen H, Yan S, Li D, Li Y, Gong Z, 2020. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China . Am J Nephrol 24: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallhi TH, Khan AH, Adnan AS, Sarriff A, Khan YH, Jummaat F, 2015, Incidence, characteristics and risk factors of acute kidney injury among dengue patients: a retrospective analysis . PLoS One 10: e0138465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallhi TH, Khan AH, Adnan AS, Sarriff A, Khan YH, Gan SH, 2018. Short-term renal outcomes following acute kidney injury among dengue patients: a follow-up analysis from large prospective cohort . PLoS One 13: e0192510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallhi TH, Khan AH, Sarriff A, Adnan AS, Khan AH, Jummaat F, 2016. Defining acute kidney injury in dengue viral infection by conventional and novel classification systems (AKIN and RIFLE): a comparative analysis . Postgrad Med J 92: 78–86. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, et al. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China . JAMA 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, et al. 2020. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study . Lancet Respir Med 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Wj, et al. 2020. Clinical characteristics of 2019 novel coronavirus infection in China . MedRxiv. [Google Scholar]
- 9.Chen N, et al. 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study . Lancet 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch JS, et al. 2020. Acute kidney injury in patients hospitalized with COVID-19 . Kidney Int 98: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, et al. 2020. Kidney impairment is associated with in-hospital death of COVID-19 patients. MedRxiv. [Google Scholar]
- 12.Wang T, et al. 2020. Caution on kidney dysfunctions of 2019-nCoV patients . MedRxiv. [Google Scholar]
- 13.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V, 2020. The Novel Coronavirus 2019 epidemic and kidneys . Kidney Int 97: 824–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei G, et al. 2020. Renal involvement and early prognosis in patients with COVID-19 pneumonia . J Am Soc Nephrol 31: 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldfarb DS, Benstein JA, Zhdanova O, Hammer E, Block CA, Caplin NJ, Thompson N, Charytan DM, 2020. Impending shortages of kidney replacement therapy for COVID-19 patients . Clin J Am Soc Nephrol 15: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study . Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuiri S, Ohashi Y, 2015. ACE and ACE2 in kidney disease . World J Nephrol 4: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA, 2020. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic . Nat Rev Nephrol 95: 305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling Y, et al. 2020. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients . Chin Med J 133: 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su H, et al. 2020. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China . Kidney Int 98: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, Castellano G, 2020. Acute kidney injury in SARS-CoV-2 infected patients . Crit Care 24: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph A, Zafrani L, Mabrouki A, Azoulay E, Darmon M, 2020. Acute kidney injury in patients with SARS-CoV-2 infection . Ann Intensive Care 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, et al. 2020. Acute kidney injury in patients with the coronavirus disease 2019: a multicenter study . Kidney Blood Press Res 45: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taher A, Alalwan AA, Naser N, Alsegai O, Alaradi A, 2020. Acute kidney injury in COVID-19 pneumonia: a single-center experience in Bahrain . Cureus 12: e9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, et al. 2020. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj 2020 Mar 26: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng JH, et al. 2020. Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis 2020 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]