Abstract.
Prevalence and levels of antibodies to multiple Plasmodium falciparum antigens show promise as tools for estimating malaria exposure. In a highland area of Kenya with unstable transmission, we assessed the presence and levels of antibodies to 12 pre-erythrocytic and blood-stage P. falciparum antigens by multiplex cytometric bead assay or ELISA in 604 individuals in August 2007, with follow-up testing in this cohort in April 2008, April 2009, and May 2010. Four hundred individuals were tested at all four time points. During this period, the only substantial malaria incidence occurred from April to August 2009. Antibody prevalence in adults was high at all time points (> 70%) for apical membrane antigen 1, erythrocyte-binding antigen 175, erythrocyte-binding protein-2, glutamate rich protein (GLURP)-R2, merozoite surface protein (MSP) 1 (19), MSP-1 (42), and liver-stage antigen-1; moderate (30–70%) for GLURP-R0, MSP-3, and thrombospondin-related adhesive protein; and low (< 30%) for SE and circumsporozoite protein (CSP). Changes in community-wide malaria exposure were best reflected in decreasing antibody levels overtime for highly immunogenic antigens, and in antibody seroprevalence overtime for the less-immunogenic antigens. Over the 3 years, antibody levels to all antigens except CSP and schizont extract (SE) decreased in an age-dependent manner. Prevalence and levels of antibodies to all antigens except CSP and SE increased with age. Increases in antibody prevalence and levels to CSP and SE coincided with increases in community-wide malaria incidence. Antibody levels to multiple P. falciparum antigens decrease in the absence of consistent transmission. Multiplex assays that assess both the presence and level of antibodies to multiple pre-erythrocytic and blood-stage P. falciparum antigens may provide the most useful estimates of past and recent malaria transmission in areas of unstable transmission and could be useful tools in malaria control and elimination campaigns.
INTRODUCTION
Malaria remains a major cause of death and illness with an estimated 3.3 billion people at risk of infection and an estimated 219 million cases reported globally.1 There is a recognized need for an accurate picture of malaria incidence overtime and space to support elimination.2 Tracking antibody concentration and reactivity to Plasmodium falciparum antigens is an attractive option for monitoring P. falciparum malaria transmission and elimination to inform decision-making in public health.3–11 In an earlier study, we assessed antibodies to multiple pre-erythrocytic and blood-stage P. falciparum antigens in 1,000 individuals over a 14-month period of very low malaria transmission in the highland areas of Kipsamoite and Kapsisiywa, Kenya.8 We showed that half-lives of antibody seropositivity varied by age, antigen, and time since last P. falciparum exposure and that by assessing antibodies to multiple antigens, estimates of long-term and recent exposure could be obtained.
Mathematical models have frequently been used to bridge the gap between immunological theory and population-level data on malaria infection and immune responses. Models using serological survey data have been used in a number of infectious diseases.12–19 Serological and parasitological data in conjunction with mathematical models have been used to provide estimates of malaria transmission dynamics and epidemiological patterns overtime.20–23 It is important to validate model predictions against appropriate well-characterized epidemiological cohorts with at least one serological survey.
Here, we expand on our earlier study8 by using two different models to analyze antibody changes overtime and assessing data collected over multiple time points. The models are applied to data on antibodies to multiple P. falciparum antigens in a cohort of 604 individuals living in the same highland area over a period of 33 months, to obtain new estimates of antibody half-lives over a longer time period. In addition, we describe the effects of increased malaria exposure on antibody concentration and determine whether assessment of antibody levels, in addition to seropositivity, provides additional information about past and recent malaria transmission in areas of low and unstable transmission.
MATERIALS AND METHODS
Study site, cohort enrollment, and surveillance for clinical malaria.
A cohort of 604 individuals from Kapsisiywa and Kipsamoite, highland areas of Kenya with low and unstable transmission, were randomly selected from a total population of ∼8,000 individuals for active surveillance of malaria episodes starting in July 2007 and continuing through May 2010. A computer-generated algorithm randomly selected households from a demography database, comprising a total of 700 individuals. Individuals in these households were requested to participate in the study, and the households agreeing to participate were included until a total of approximately 600 individuals were reached (active cohort). The main rainy season typically occurs between March and June, with an occasional second rainy season in October–November. Trained village health workers conducted weekly active surveillance to monitor for malaria symptoms among participants. Participants with malaria symptoms (fever, chills, headache, or severe malaise) were referred to the local dispensary for further evaluation and treatment. Clinical malaria was defined as microscopy testing positive for any human Plasmodium species in the presence of symptoms consistent with malaria.24 Individuals with microscopy positive malaria were treated with artemether–lumefantrine, according to Kenya Ministry of Health guidelines. Concurrent passive surveillance for malaria was carried out for all other consenting individuals living in the area, who were included in yearly demography surveys, and these individuals were requested to go to the health center if they had any symptoms consistent with malaria. There they were evaluated and treated in the same way as individuals in the active cohort.
Ethical approval for the study was obtained from Kenya Medical Research Institute National Ethical Review Committee and the Institutional Review Boards for Human Studies at the University of Minnesota. Informed consent was obtained from study individuals or, in the case of minors, from their parents or guardians.
Microscopy and polymerase chain reaction (PCR) testing for Plasmodium species infection.
Microscopy testing for the presence of Plasmodium species was performed by Giemsa-stained thick and thin peripheral blood smears. Smears were examined independently by two microscopists, with a third reading performed for slides with discordant results.25 Nested PCR testing for P. falciparum infection was performed on filter paper blood spot samples as previously described.26,27 In brief, P. falciparum PCR was performed with nest-one (genus-specific primers) and nest-two primers (species-specific primers). The merozoite surface protein (MSP)-2 PCRs were performed using two sets of primers that detected two major allelic families, FC27 and ICI for the MSP-25, and scored positive for P. falciparum if there was a PCR product for both FC27 and ICI or for anyone of these alleles.
Preparation of P. falciparum crude schizont extract (SE).
Plasmodium falciparum–infected red blood cells of the 3D7 line (obtained from MR4) were maintained in vitro at pH 7.4 in sealable flasks using human group O+ erythrocytes, at 3% hematocrit, in RPMI–HEPES medium supplemented with 50 μg/mL hypoxanthine, 25 mM NaHCO3, 20 μg/mL gentamicin, 5% (vol/vol) heat-inactivated pooled human sera from donor residents in North America, and 0.3% Albumax II®) (Gibco BRL, Grand Island, NY) maintained in an atmosphere of 1% O2, 4% CO2, and 95% N2 at 37°C. Cultures were synchronized three times per week by resuspending culture pellets in 5% D-sorbitol (Sigma, St. Louis, MO) in water to lyse schizont-infected erythrocytes and vortexed for homogenization. The bicinchonic acid (BCA) assay was performed for protein estimation, and the extract was frozen without centrifugation at −80°C in working aliquots.
Testing for IgG antibodies to P. falciparum antigens.
Antibody responses to recombinant proteins of the P. falciparum blood-stage antigens, such as apical membrane antigen 1 (AMA1, FVO variant), erythrocyte-binding antigen 175 (EBA-175), erythrocyte-binding protein-2 (EBP-2), glutamate rich protein R0 and R2 (GLURP-R0 and -R2), MSP-1 (19), MSP-1 (42), and MSP-3 (FVO variants), and to the pre-erythrocytic antigens, such as liver-stage antigen-1 (LSA-1) and thrombospondin-related adhesive protein (TRAP), were assessed by multiplex cytometric bead assay (CBA), as previously described,28 whereas antibodies to the NANP repeated peptide of circumsporozoite protein (CSP) and heterogeneous crude P. falciparum SE were assessed by ELISA, as previously described.29
Antibody levels were expressed in arbitrary units (AUs), which were calculated by dividing the median fluorescence intensity (MFI) (for CBA) or optical density (OD) (for ELISA) from the test plasma sample by the mean MFI or OD plus 3 SDs from plasma samples from North American individuals never exposed to malaria. Arbitrary units are used to standardize antibody values across plates. Values from nine nonexposed individuals were run on each plate, and two duplicate positive pooled controls from adults’ plasma samples (n = 30) living in a highly malaria-endemic area were used to confirm standard MFI or OD responses across plates from all time points (i.e., all positive control values were required to be within 20% of the mean value for all plates). An AU > 1.0 was considered seropositive.30 The distribution and correlations of measured antibody levels are shown in Supplemental Figures 1 and 2.
Statistical model for reduction in antibody levels.
We assume that for a given antibody for person i, we have measurements of antibody level Aij at times tj. We assume that person i's initial antibody levels (during the first collection, August 2007) is described as an independent draw from a normal distribution (N) with mean loge (α0) and SD σα. Person i’s rate of antibody decay on the log scale is assumed to be described by a draw from a normal distribution with mean r0 and SD σr, which is drawn independently of other people’s rates and of person i’s initial level. These assumptions imply a mixed-effects linear regression model of the following form:
| (1) |
Note that this method provides estimates of the duration of the long-lived component of the antibody response, as samples were not frequent enough to investigate short-term antibody kinetics.22 We also investigate age-dependent variation in antibody levels and antibody decay rates by fitting a variant of the previous model with age incorporated as a categorical variable. In particular, we assume three age categories corresponding to the age groups 1–5 years (n = 91), 5–15 years (n = 177), and > 15 years (n = 337). Episodes of clinical malaria were detected in four individuals; antibody measurements after clinical episodes were censored from the analysis. Participants younger than 1 year were not included because of the possible confounding effects of maternal antibodies.
Statistical model for reduction in proportion of antibody seropositivity.
Here, we consider how the seropositive proportion of the sampled population changes between each cross-sectional survey. We assume that the initial proportion seropositive (in August 2007) is P0 and that seropositive individuals revert to seronegative at rate ρ per year. The model assumes that individuals do not seroconvert. Data from the four individuals in the active surveillance who had clinical malaria during the 33-month period were censored at the time of their diagnosis. However, the population as a whole did experience an increased incidence of clinical malaria from April–August 2009 (Figure 1), and some individuals in the active study cohort, particularly older individuals, may have had asymptomatic seroconversion during this period, so the model may not accurately reflect these changes. The seroprevalence at time tj years after the first sample at time t1 can be modeled as follows:
| (2) |
Let xij denote the serostatus of the individual i at time tj: seropositive (xij = 1) or seronegative (xij = 0). With this model, the likelihood function of the parameters P0 and ρ arising from the data D is given by the following equation:
| (3) |
This model is fit to the data in a Bayesian analysis with vague uniform priors, using Markov chain Monte Carlo (MCMC) methods.
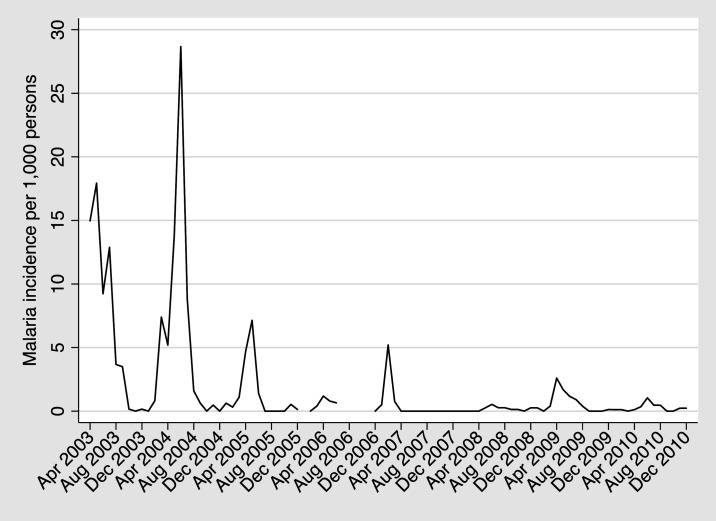
Figure 1.
Clinical malaria incidence overtime per 1,000 persons in Kipsamoite and Kapsisiywa, Kenya. A spike in malaria incidence occurred between April 2009 and May 2010, a period within our blood collection time points and antibody testing.
RESULTS
Study cohort characteristics and incidence of clinical malaria.
Blood samples were collected in August 2007 (n = 604), April 2008 (n = 533), April 2009 (n = 513), and May 2010 (n = 437). Four hundred individuals (66%) had blood samples collected at all four visits. Reasons for not collecting samples during follow-up visits included participants not being available at home during sample collection by either moving out of the study area or declining further participation.
Table 1 summarizes the number of participants who had samples collected at each time point, the age stratification of those participants, and the presence of P. falciparum by microscopy or PCR. Age stratification did not differ overtime, and only 0–0.4% of participants were asymptomatically parasitemic by blood smear or PCR at any of the four time points. Malaria incidence had a substantial off-season peak from January–March 2007 (total of 51 cases in the entire community), and there were no clinical cases of malaria from April 2007–April 2008. From August 2007 to May 2010, there was only one period in which there was substantially increased malaria incidence (more than one case/1,000 persons/month), which was from April–August 2009 when a total of 52 cases occurred (Figure 1).
Table 1.
Samples collected, by age, and presence of P. falciparum, by microscopy or PCR, at each time point
| Sample time | N | N (%) by age | Microscopy positive, P. falciparum, N (%) | PCR positive, P. falciparum, N (%) | ||
|---|---|---|---|---|---|---|
| 0–5 years | 5–15 years | > 15 years | ||||
| August 2007 | 604 | 91 (15) | 177 (29) | 336 (56) | 0 (0.0) | 1 (0.2) |
| April 2008 | 533 | 79 (15) | 162 (30) | 292 (55) | 1 (0.2) | 0 (0.0) |
| April 2009 | 513 | 80 (16) | 155 (30) | 278 (54) | 2 (0.4) | 2 (0.4) |
| May 2010 | 437 | 73 (17) | 138 (32) | 226 (52) | 0 (0.0) | 0 (0.0) |
PCR = polymerase chain reaction; P. falciparum = Plasmodium falciparum.
Antibody seropositivity and level to P. falciparum antigens differ by antigen and age.
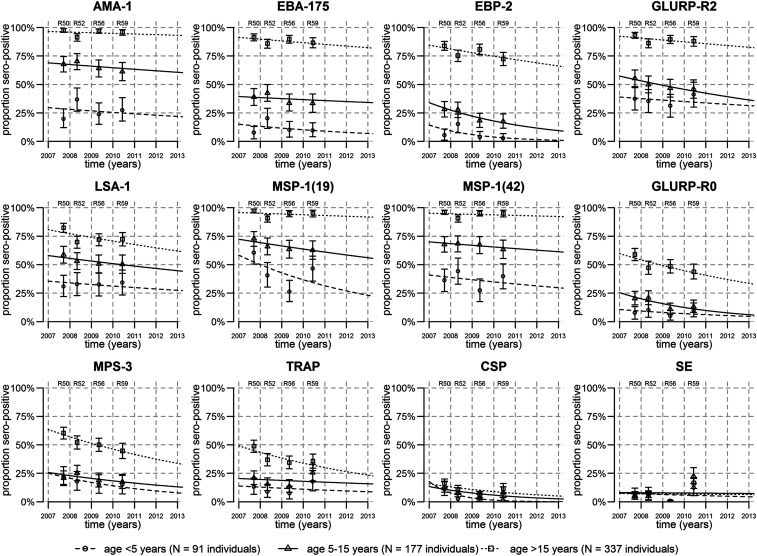
Three types of antigen immunogenicity were observed (Figure 2). The first type was antibodies to highly immunogenic antigens (seroprevalence > 70% for all four time points in adults) including antibodies to the blood-stage antigens AMA-1, EBA-175, EBP-2, GLURP-R2, MSP-1 (19), and MSP-1 (42), and the pre-erythrocytic antigen LSA-1. The second type was antibodies to moderately immunogenic antigens (seroprevalence 30–70% at all four time points in adults) including the blood-stage antigens GLURP-R0, MSP-3 and the pre-erythrocytic antigen TRAP. The third type was antibodies to poorly immunogenic antigens (seroprevalence < 30% at all four time points) including the blood-stage SE combination of antigens and the pre-erythrocytic antigen CSP (Figure 2).
Figure 2.
Waning of seroprevalence overtime. In 4 cross-sectional cohorts with repeatedly sampled individuals, seroprevalence to 12 Plasmodium falciparum antigens reduced overtime. Points represent the proportion seropositive in three age-groups from each of the four cross sections, with 95% CIs represented by vertical bars. Lines represent the fit of models of waning seroprevalence.
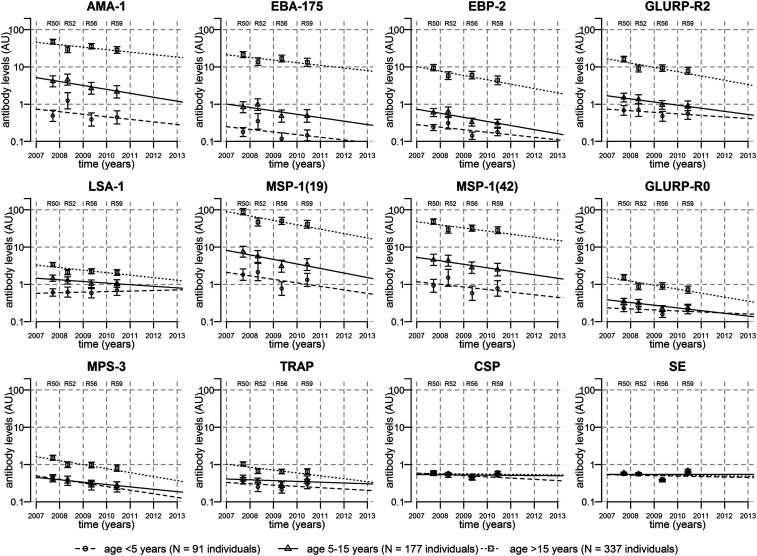
Antibody levels showed similar patterns as antibody seroprevalence: the most immunogenic had mean levels of antibodies > 5 AU, moderately immunogenic antigens had mean antibody levels in borderline of 1 AU, and poorly immunogenic antigens SE and CSP had mean levels < 1 AU (Figure 3). Antibody levels to LSA-1 were an exception, as levels were lower than those for other antigens classified as highly immunogenic (Figures 2 and 3). A strong age-dependent variation in antibody seroprevalence and levels was also observed, with older individuals having higher antibody seropositivity and levels for all antigens except CSP and SE (Figures 2 and 3).
Figure 3.
Waning of antibody levels overtime. In four cross-sectional cohorts with repeatedly sampled individuals, antibody levels to 12 Plasmodium falciparum antigens reduced overtime. Points represent the geometric mean antibody levels in three age groups from each of the four cross sections, with 95% CIs represented by vertical bars. Lines represent the fit of mixed-effects linear regression models to the data.
With low-level malaria exposure, antibody seropositivity and levels provide different information overtime, according to antigen immunogenicity.
Antibody seropositivity did not differ significantly overtime for most immunogenic antigens (AMA-1, EBA-175, GLURP-R2, MSP-1 [19], and MSP-1 [42]), as shown by the relatively flat seroprevalence lines at all ages (Figure 2) and the overlapping curves of seropositivity at all time points (Supplemental Figure 3). By contrast, there were notable decreases in antibody seropositivity overtime for the less-immunogenic antigens (GLURP-R0, MSP-3, and TRAP) for all ages. Antibody seropositivity to CSP showed a modest decrease overtime, whereas antibodies to SE did not change overtime (Figure 2, Supplemental Figure 3).
Antibody levels had similar patterns of increasing levels with age (Figure 3), with the exception of CSP and SE, which did not show any increase of antibody levels with age (Supplemental Figure 4). Antibody levels to all antigens except CSP and SE decreased overtime, although decreases for the less-immunogenic antigens GLURP-R0, MSP-3, and TRAP were primarily in those younger than 15 years and were less pronounced than the decreases in seropositivity (Figures 2 and 3).
Thus, antibody levels and seropositivity to different antigens provided different kinds of information: antibody levels were more sensitive to change overtime for highly immunogenic antigens, and antibody seropositivity rates were more sensitive to change for less-immunogenic antigens. The two least immunogenic antigens (CSP and SE) had no strong associations with age or time, but seropositivity rates provided a clearer signal of waning overtime for CSP.
Antibodies to CSP and SE in the overall population, and antibodies to CSP, SE, and MSP-1 (19) in children younger than 5 years, increased after a resurgence of malaria incidence in 2009.
We compared antibody seropositivity and levels from the collections in April 2009 (just before the peak malaria incidence period of April–August 2009) and in May 2010 (after the spike in malaria incidence) to determine which antibodies best detected changes in incidence and whether seropositivity or levels were most useful for detecting this change. In the population as a whole, antibodies to the least immunogenic antigens (CSP and SE) increased following the period of increased malaria incidence during April–August 2009, whereas antibodies to the more-immunogenic antigens did not increase (Table 2). However, in children younger than < 5 years, antibody seropositivity and levels to CSP, SE, and MSP-1 (19) increased significantly, and antibody seropositivity and levels to GLURP-R0, GLURP-R2, MSP-1 (42), and TRAP also increased although not significantly (Supplemental Table 1). The findings highlight the importance of antigen and age in the acquisition, development, and persistence of antibodies. In younger children, the lower proportions of antibodies present, and the lower antibody levels for some moderately and poorly immunogenic antigens, appear to make them suitable for detection of an increase in malaria exposure, but only in this age-group. Specific antigens were also important because the same increase was not seen for other highly immunogenic antigens (AMA-1 and EBA-175) in children younger than 5 years. The results suggest that antibody levels and seroprevalence to SE are sensitive and specific for the detection of recent malaria infection or exposure.
Table 2.
Changes in antibody seropositivity and levels between collections in April 2009 (before spike in malaria incidence) and May 2010 (after spike in malaria incidence)
| Antigen | Geometric mean antibody level (arbitrary unit) | Proportion seropositive | ||||
|---|---|---|---|---|---|---|
| April 2009 | May 2010 | P-value | April 2009 | May 2010 | P-value | |
| Strongly immunogenic | ||||||
| Apical membrane antigen 1 | 8.09 (6.52, 10.04) | 6.26 (4.96, 7.90) | 0.968 | 0.76 (0.72, 0.79) | 0.73 (0.69, 0.77) | 0.971 |
| Erythrocyte-binding antigen 175 | 2.64 (2.07, 3.38) | 2.19 (1.69, 2.83) | 0.968 | 0.61 (0.56, 0.65) | 0.57 (0.52, 0.62) | 0.971 |
| Erythrocyte-binding protein-2 | 1.40 (1.14, 1.72) | 1.11 (0.90, 1.37) | 0.968 | 0.50 (0.46, 0.54) | 0.44 (0.39, 0.48) | 0.971 |
| GLURP-R2 | 2.96 (2.47, 3.54) | 2.54 (2.10, 3.08) | 0.968 | 0.68 (0.63, 0.72) | 0.67 (0.62, 0.71) | 0.971 |
| Liver-stage antigen-1 | 1.43 (1.26, 1.62) | 1.38 (1.21, 1.58) | 0.968 | 0.59 (0.55, 0.63) | 0.59 (0.54, 0.63) | 0.971 |
| MSP-1 (19) | 11.34 (9.02, 14.25) | 10.52 (8.39, 13.19) | 0.968 | 0.75 (0.71, 0.78) | 0.77 (0.72, 0.80) | 0.852 |
| MSP-1 (42) | 8.29 (6.71, 10.24) | 7.24 (5.78, 9.07) | 0.968 | 0.76 (0.73, 0.80) | 0.76 (0.72, 0.80) | 0.971 |
| Moderately immunogenic | ||||||
| GLURP-R0 | 0.45 (0.39, 0.52) | 0.41 (0.35, 0.48) | 0.968 | 0.31 (0.27, 0.35) | 0.28 (0.24, 0.33) | 0.971 |
| MSP-3 | 0.56 (0.49, 0.64) | 0.47 (0.41, 0.54) | 0.968 | 0.35 (0.31, 0.39) | 0.31 (0.27, 0.35) | 0.971 |
| Thrombospondin-related adhesive protein | 0.43 (0.38, 0.47) | 0.48 (0.42, 0.55) | 0.295 | 0.24 (0.20, 0.28) | 0.29 (0.25, 0.34) | 0.151 |
| Poorly immunogenic | ||||||
| Circumsporozoite protein | 0.46 (0.45, 0.48) | 0.58 (0.55, 0.61) | < 0.0001 | 0.05 (0.03, 0.07) | 0.09 (0.07, 0.12) | 0.056 |
| Schizont extract | 0.38 (0.38, 0.39) | 0.64 (0.61, 0.66) | < 0.0001 | 0.00 (0.00, 0.01) | 0.18 (0.15, 0.22) | < 0.0001 |
GLURP = Glutamate rich protein; MSP = Merozoite surface protein. P-values for antibody levels are calculated using a one-sided t-test for an increase between April 2009 and May 2010. P-values for proportion seropositive are calculated using a one-sided two-proportions Z-test for an increase between April 2009 and May 2010. P-values have been corrected for multiple hypothesis testing using the Benjamini–Hochberg procedure. Note: this procedure does not allow adjusted P-values to take a value greater than the maximum unadjusted P-value.
Changes in antibody levels in individuals with clinical malaria vary by antigen and are less robust than population serological responses.
We also assessed changes in antibody levels in the four individuals in the cohort with known clinical malaria during the 33-month period to gain further insight into the utility of antibodies to multiple antigens as biomarkers of malaria exposure in individual persons (Supplemental Figure 5). One person (P3) did not have assessment of antibodies after the episode of clinical malaria. Among the three others, a 38-year-old (P1) had increases in antibody levels only to SE, an 8-year-old (P2) had increases in antibody level to all antigens except, surprisingly, CSP and SE, and a 35-year-old (P4) did not have substantial increases in antibodies to any antigen despite two episodes of clinical malaria. Although the data from the three individuals represent a fraction of the studied cohort, the results suggest that antibody changes may provide some information on an individual basis, particularly in children, but the high degree of variation in individual response makes them most useful for evaluation of population-wide changes.
Shorter half-lives by antibody levels and longer half-lives by antibody seropositivity to P. falciparum antigens.
In an exponential decay pattern, the concentration of a biological substance decreases at a constant rate. The antibody level and seropositivity half-lives were calculated for each antigen, assessing changes from August 2007–May 2010 (Table 3). The antibody level half-life (the time to a 50% decrease in antibody levels) was similar for most antigens and was generally shorter (range, 2.55–47.62 years) than seropositivity half-life (the time to a 50% decrease in proportion seropositive) (range, 2.75–68.55 years) (Supplemental Figure 6). Seropositivity half-life estimates were typically longer than those reported previously, when two time points (May 2007 and July 2008) were used, possibly because there was essentially no malaria transmission between these time points, whereas there was increased transmission from April–August 2009 before the last sample collection for these study participants. Supporting this contention, antibody seropositivity half-lives calculated for the period from August 2007–April 2009 were closer to those previously calculated (Supplemental Table 2). Consistent with the previous study, antibody seropositivity half-lives increased with age (Supplemental Table 3).
Table 3.
Antibody level and seropositivity half-life estimates (changes in antibodies from August 2007–May 2010)
| AUs | Seropositivity | |||
|---|---|---|---|---|
| Antigen | Geometric mean AU in August 2007 | AU half-life (years) | Proportion seropositive in August 2007 | Seropositivity half-life (years) |
| Strongly immunogenic* | ||||
| Apical membrane antigen 1 | 11.43 (9.51, 13.75) | 3.71 (3.00, 4.85) | 0.77 (0.75, 0.80) | 49.75 (17.17, 1,099.96) |
| Erythrocyte-binding antigen 175 | 3.92 (3.19, 4.81) | 3.79 (3.07, 4.98) | 0.63 (0.60, 0.66) | 24.54 (11.49, 226.94) |
| Erythrocyte-binding protein-2 | 2.29 (1.92, 2.74) | 2.76 (2.39, 3.27) | 0.56 (0.53, 0.59) | 8.02 (5.33, 14.70) |
| GLURP-R2 | 4.53 (3.83, 5.34) | 3.13 (2.67, 3.78) | 0.72 (0.69, 0.75) | 18.35 (10.97, 57.97) |
| Liver-stage antigen-1 | 1.84 (1.65, 2.06) | 6.30 (4.88, 8.90) | 0.65 (0.61, 0.68) | 16.74 (9.19, 76.57) |
| MSP-1 (19) | 21.00 (17.28, 25.53) | 2.55 (2.19, 3.06) | 0.82 (0.79, 0.84) | 19.06 (11.58, 48.58) |
| MSP-1 (42) | 12.54 (10.42, 15.10) | 3.49 (2.87, 4.43) | 0.79 (0.76, 0.81) | 44.25 (18.93, 442.43) |
| Moderately immunogenic† | ||||
| GLURPR0 | 0.68 (0.59, 0.77) | 3.53 (2.98, 4.35) | 0.39 (0.36, 0.42) | 5.33 (3.61, 9.80) |
| MSP-3 | 0.80 (0.71, 0.90) | 3.25 (2.83, 3.81) | 0.43 (0.40, 0.46) | 5.68 (3.94, 11.07) |
| Thrombospondin-related adhesive protein | 0.60 (0.54, 0.66) | 5.55 (4.35, 7.69) | 0.32 (0.29, 0.35) | 8.35 (4.70, 37.32) |
| Poorly immunogenic‡ | ||||
| Circumsporozoite protein | 0.56 (0.54, 0.58) | 26.59 (14.39, 174.75) | 0.12 (0.10, 0.15) | 2.75 (1.72, 5.91) |
| Schizont extract | 0.54 (0.52, 0.55) | 47.62 (21.51, +inf) | 0.07 (0.06, 0.08) | 68.55 (14.66, 2,614.44) |
AU = antibody level. GLURP = Glutamate rich protein; MSP = Merozoite surface protein. Estimates are shown with 95% CIs. +inf corresponds to no reduction in antibody levels.
* Seroprevalence in individuals older than 15 years, > 50% at all time points.
† Seroprevalence in individuals older than 15 years, > 30% but < 70% at all time points.
‡ Seroprevalence in individuals older than 15 years, < 30% at all time points.
For the poorly immunogenic antigens CSP and SE, the half-life of antibody levels and half-life of seropositivity were estimated to be substantially larger than for other antigens (Table 3). Rather than representing a long-lived antibody response that is maintained overtime, these estimates reflect that mean antibody levels to these antigens are low, so samples with values just above or below the cutoff for seropositivity contribute to variability, and that these antibodies are sensitive to changes in malaria incidence, and so were affected in this analysis by the increase in malaria incidence from April–August 2009. The antibody level and seropositivity half-lives were considerably shorter for CSP and SE if only data from 2007 to 2009 were used (Supplemental Table 2). Some individuals may also have had asymptomatic infections that led to the CSP or SE level or seropositivity. Overall, the concept of “antibody half-life” and calculation of this value was less valuable than the categorization of antibodies by immunogenicity, decrease by level or seropositivity overtime, and increase with recent malaria exposure.
DISCUSSION
In the present study, we show that antibodies to multiple P. falciparum antigens of differing immunogenicity can provide valuable information about long-term and recent malaria exposure, that antibody levels and seropositivity provide different but complementary information, depending on antigen immunogenicity and age, and that antibodies to CSP or SE in the population as a whole, or antibodies to CSP, SE, or MSP-1 (19) in children younger than 5 years, may provide evidence of recent malaria exposure. The study adds to past studies showing the value of antibodies to multiple P. falciparum antigens by studying changes in antibodies over an almost 3-year period, which allowed more accurate estimation of antibody longevity and the effects of malaria exposure in a population in an area of very low transmission. In combination with information about protection from clinical malaria associated with specific antibodies (GLURP-R0, GLURP-R2, CSP, and LSA-1),31 the study findings provide further impetus for the use of multiplex platforms assessing antibodies to provide information about long-term and recent malaria exposure in a population and potentially about age-specific risk of clinical malaria in the population. It also demonstrates that antibody levels to blood-stage antigens decrease consistently overtime in the absence of continued malaria exposure, a phenomenon that may contribute to population risk for malaria epidemics.
A recent review by Greenhouse et al.3 suggested the use of standardized multiplex antibody testing to P. falciparum antigens could be an important new tool in malaria control and elimination efforts, by supplementing malaria surveillance information or, perhaps more importantly, where such information is not available, providing estimates of malaria exposure. The present study supports this contention and suggests that information on both less- and more-immunogenic antigens and level and seropositivity could provide optimal estimates. Assessments of seropositivity to antigens such as CSP and SE that are less immunogenic but more likely to increase with recent exposure, combined with assessments of seropositivity and levels to highly immunogenic antigens such as AMA-1, MSP-1, and EBA-175, could give the best estimation of malaria exposure overtime for both long- and short-term exposure. This work demonstrates how combining multiple serological models can be used to answer critical questions in malaria epidemiology. Our results also support an association between anti-CSP antibodies and recent malaria exposure, consistent with some prior studies,32 suggesting antibodies to CSP may be particularly useful to measure when assessing for recent exposure.
Because serological data integrate exposure overtime, they have the potential to reveal changes in transmission (e.g., recent resurgence and subsidence).9,33,34 Cross-sectional studies that include all ages might provide the most useful information: because seropositivity and levels for most antigens are highest in older individuals, and antibody seropositivity half-lives are shortest in children younger than 5 years, information from all age groups might provide the best way to estimate recency of exposure and long-term exposure in the population. In addition, the study suggests that assessment of changes in antibody seropositivity in children younger than 5 years may be a particularly useful way to determine recent malaria exposure because antibodies to multiple antigens, including CSP and SE and also some more immunogenic antigens such as MSP-1 and TRAP, appeared to increase after increased malaria incidence in this population. A hybrid approach where machine-learning tools combine these complementary information streams may best allow for detection of changes in malaria exposure.
Schizont extract poses a problem in that it is a soluble extract of blood-stage schizont antigens, and so not a purified or recombinant antigen that can be reliably used across studies with the same known composition and concentration. Variability in prevalence of antibodies to SE in different studies is likely because of differences in transmission intensity and because of formulation of SE and testing methods by ELISA. In addition, because SE comprises multiple antigens, it likely cannot be reproducibly used in a multiplex assay. A further limitation is the potential for systematic differences between the ELISA and multiplex assays, particularly given that the least immunogenic antigens were measured using ELISA. Future work will assess the comparability of Luminex and ELISA testing for CSP, using recombinant CSP antigen. However, the high utility of antibodies to SE for detection of recent malaria exposure in our studies and others34,35 suggests that either an alternate purified antigen with similar characteristics should be identified or that a standardized preparation of SE should be made to use across multiple studies.
On an individual basis, an increase in antibody levels to some or most P. falciparum antigens, in particular SE, was seen in two of the three individuals in whom antibody testing was obtained at a point after the clinical episode. The number of individuals with clinical malaria was too small to make any firm conclusion on how reliable antibody assessment after clinical malaria might be on an individual basis. Additional well-designed follow-up studies of individuals with clinical malaria are needed to confirm changes in serological responses to multiple P. falciparum antigens, similar to those that have been undertaken in non-malaria–endemic countries.36
In the same area, we have recently documented that over a prolonged period of follow-up (up to 6 years), the combination of antibodies to GLURP-R0, GLURP-R2, LSA-1, and CSP were associated with protection from malaria.31 Whereas this finding is specific to this area and may not apply to other areas with differing transmission patterns, knowledge of antibody-associated protection could allow for estimates of population-level risk of clinical malaria, based on changes in antibody levels to these antigens. Such models would be complex and would need to take a number of additional factors into account but could be very useful in considering follow-up and risk levels in subnational malaria campaigns. For example, knowledge of the much lower levels of GLURP-R0 and LSA-1 in children younger than 15 years than in individuals older than 15 years in this study might suggest a need for particular protection measures for children, whether younger than 5 years or 5–15 years.
So how can we use the results to guide future studies working toward malaria elimination? The first and most critical need is for standardization of antibody testing, using standard antigens, concentrations, and methods of testing and assessment, as suggested in the recent commentary by Greenhouse et al.3 on multiplex antibody testing for surveillance. This is challenging, as each research group currently uses their own antigens and methods, and standardization will require agreement across groups on these components, and central resources need to be adequately funded to supply reagents and testing methods or to do the testing for multiple groups. However, with agreement on the importance of such standardization, a basic set of antigens and methodology could be devised, and this would be a major step forward in making this testing a practical tool for malaria surveillance. With standardized testing and greater consistency, the multiplex assay could be used in a number of ways: 1) to determine the level of overall malaria exposure, recent and long-term, in communities without active or accurate malaria surveillance programs; 2) to assess geographic disparities in recent and long-term malaria exposure, to allow for targeted interventions; 3) to evaluate which areas and age-groups might be at highest risk for clinical malaria during peak transmission periods, particularly if specific antibodies are associated in that area with protection from clinical malaria; 4) to guide subnational malaria elimination campaigns in all of these areas, and to provide post-elimination evaluation of exposure and potentially of area-wide risk if malaria is reintroduced through vector or human travel.
In summary, the present study provides new evidence that antibodies to multiple P. falciparum antigens of differing immunogenicity can be used to estimate long-term and recent malaria exposure, that antibody levels and seropositivity provide different but complementary information, depending on antigen immunogenicity and age, and that antibodies to TRAP, CSP, and especially SE may provide evidence of recent exposure. The study findings provide data that support development of a standardized multiplex assay of antibodies to P. falciparum antigens that can ultimately be used in malaria control and elimination efforts and in pre- and post-elimination malaria surveillance.
Supplemental tables and figures
Acknowledgments:
We thank the study participants and their families and the study team, including clinical officers, nurses, laboratory technologists, and data entry personnel. Jim Hodges of the University of Minnesota, Division of Biostatistics made helpful comments on drafts of this work. We thank David L. Narum (National Institutes of Health), Sheetij Dutta, David E. Lanar (Walter Reed Army Institute for Research), Michael Theisen (Statens Serum Instut), and the Malaria Research and Reference Reagent Resource Center for provision of P. falciparum antigens and 3D7 parasite clone. B. N. O. was a Fogarty Global Health research fellow at the time of this work. This work was published with the permission of the director, Kenya Medical Research Institute.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2018. World Malaria Report. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Mercado CE, Ekapirat N, Dondorp AM, Maude RJ, 2017. An assessment of national surveillance systems for malaria elimination in the Asia Pacific. Malar J 16: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenhouse B, Smith DL, Rodriguez-Barraquer I, Mueller I, Drakeley CJ, 2018. Taking sharper pictures of malaria with CAMERAs: combined antibodies to measure exposure recency assays. Am J Trop Med Hyg 99: 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franca CT, Li Wai Suen CSN, Carmagnac A, Lin E, Kiniboro B, Siba P, Schofield L, Mueller I, 2017. IgG antibodies to synthetic GPI are biomarkers of immune-status to both Plasmodium falciparum and Plasmodium vivax malaria in young children. Malar J 16: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewasurendra RL, Dias JN, Sepulveda N, Gunawardena GS, Chandrasekharan N, Drakeley C, Karunaweera ND, 2017. Effectiveness of a serological tool to predict malaria transmission intensity in an elimination setting. BMC Infect Dis 17: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson JC, Stresman GH, Baidjoe A, Okoth A, Oriango R, Owaga C, Marube E, Bousema T, Cox J, Drakeley C, 2015. Use of different transmission metrics to describe malaria epidemiology in the highlands of western Kenya. Malar J 14: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha MG, Silva ES, Sepulveda N, Costa SP, Saboia TC, Guerreiro JF, Povoa MM, Corran PH, Riley E, Drakeley CJ, 2014. Serologically defined variations in malaria endemicity in Para state, Brazil. PLoS One 9: e113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, Narum DL, Park GS, Ofulla AV, John CC, 2014. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis 210: 1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema T, Youssef RM, Cook J, Cox J, Alegana VA, Amran J, Noor AM, Snow RW, Drakeley C, 2010. Serologic markers for detecting malaria in areas of low endemicity, Somalia, 2008. Emerg Infect Dis 16: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson S, Booth M, Jones FM, Mwatha JK, Kimani G, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW, 2007. Age-adjusted Plasmodium falciparum antibody levels in school-aged children are a stable marker of microgeographical variations in exposure to Plasmodium infection. BMC Infect Dis 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drakeley CJ, et al. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA 102: 5108–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsent A, et al. 2018. The utility of serology for elimination surveillance of trachoma. Nat Commun 9: 5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold BF, van der Laan MJ, Hubbard AE, Steel C, Kubofcik J, Hamlin KL, Moss DM, Nutman TB, Priest JW, Lammie PJ, 2017. Measuring changes in transmission of neglected tropical diseases, malaria, and enteric pathogens from quantitative antibody levels. PLoS Negl Trop Dis 11: e0005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber GE, et al. 2017. Sero-catalytic and antibody acquisition models to estimate differing malaria transmission intensities in western Kenya. Sci Rep 7: 16821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pothin E, Ferguson NM, Drakeley CJ, Ghani AC, 2016. Estimating malaria transmission intensity from Plasmodium falciparum serological data using antibody density models. Malar J 15: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yman V, White MT, Rono J, Arca B, Osier FH, Troye-Blomberg M, Bostrom S, Ronca R, Rooth I, Farnert A, 2016. Antibody acquisition models: a new tool for serological surveillance of malaria transmission intensity. Sci Rep 6: 19472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf CJE, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, Ferguson NM, Burke DS, Grenfell BT, 2016. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet 388: 728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellriegel B, 2001. Immunoepidemiology–bridging the gap between immunology and epidemiology. Trends Parasitol 17: 102–106. [DOI] [PubMed] [Google Scholar]
- 19.Chan MS, Isham VS, 1998. A stochastic model of schistosomiasis immuno-epidemiology. Math Biosci 151: 179–198. [DOI] [PubMed] [Google Scholar]
- 20.Helb DA, et al. 2015. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci USA 112: E4438–E4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Hoogen LL, et al. 2015. Serology describes a profile of declining malaria transmission in Farafenni, The Gambia. Malar J 14: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC, 2014. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis 210: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 23.Corran P, Coleman P, Riley E, Drakeley C, 2007. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol 23: 575–582. [DOI] [PubMed] [Google Scholar]
- 24.Mutanda AL, Cheruiyot P, Hodges JS, Ayodo G, Odero W, John CC, 2014. Sensitivity of fever for diagnosis of clinical malaria in a Kenyan area of unstable, low malaria transmission. Malar J 13: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV, 2005. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg 99: 780–786. [DOI] [PubMed] [Google Scholar]
- 26.Menge DM, Ernst KC, Vulule JM, Zimmerman PA, Guo H, John CC, 2008. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg 79: 173–177. [PMC free article] [PubMed] [Google Scholar]
- 27.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN, 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61: 315–320. [DOI] [PubMed] [Google Scholar]
- 28.Ondigo BN, Park GS, Gose SO, Ho BM, Ochola LA, Ayodo GO, Ofulla AV, John CC, 2012. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar J 11: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John CC, Moormann AM, Pregibon DC, Sumba PO, McHugh MM, Narum DL, Lanar DE, Schluchter MD, Kazura JW, 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg 73: 222–228. [PubMed] [Google Scholar]
- 30.Noland GS, Hendel-Paterson B, Min XM, Moormann AM, Vulule JM, Narum DL, Lanar DE, Kazura JW, John CC, 2008. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun 76: 5721–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamre KE, Ondigo BN, Hodges JS, Dutta S, Theisen M, Ayodo G, John CC, 2020. Antibody correlates of protection from clinical Plasmodium falciparum malaria in an area of low and unstable malaria transmission. Am J Trop Med Hyg 103: 2174–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kremsner PG, Neifer S, Zotter GM, Bienzle U, Rocha RM, Maracic M, Clavijo P, Nussenzweig RS, Cochrane AH, 1992. Prevalence and level of antibodies to the circumsporozoite proteins of human malaria parasites, including a variant of Plasmodium vivax, in the population of two epidemiologically distinct areas in the state of Acre, Brazil. Trans R Soc Trop Med Hyg 86: 23–27. [DOI] [PubMed] [Google Scholar]
- 33.Stewart L, et al. 2009. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One 4: e6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook J, Reid H, Iavro J, Kuwahata M, Taleo G, Clements A, McCarthy J, Vallely A, Drakeley C, 2010. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneko A, et al. 2014. Characteristic age distribution of Plasmodium vivax infections after malaria elimination on Aneityum island, Vanuatu. Infect Immun 82: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yman V, White MT, Asghar M, Sundling C, Sonden K, Draper SJ, Osier FHA, Farnert A, 2019. Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med 17: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.