Abstract.
Human sporotrichosis is an emerging disease caused by fungi of the genus Sporothrix, distributed worldwide, but mostly in tropical and subtropical regions. This disease is caused by traumatic inoculation of contaminated material (either animal or vegetal in origin) into the skin. Sporotrichosis cases caused by zoonotic transmission through felines have significantly increased over the last 20 years in Brazil. There is a spectrum of clinical outcomes, from classical lymphocutaneous and fixed forms to disseminated manifestations and extracutaneous lesions; however, hypersensitivity reactions related to sporotrichosis, including Sweet syndrome (acute febrile neutrophilic dermatoses), are uncommon. In Brazil, Sporothrix brasiliensis is repeatedly associated with feline infection and has consistently shown higher virulence, tendency to escalate to outbreaks or epidemics, and development of atypical forms. Therefore, the objective of the present study was to report the cases of 10 patients with sporotrichosis infected by S. brasiliensis species who developed Sweet syndrome to alert this association, especially in endemic areas.
INTRODUCTION
Sporotrichosis is a subacute or chronic disease caused by dimorphic fungi of the genus Sporothrix that are prevalent in tropical and subtropical areas worldwide.1 Infection usually occurs by traumatic inoculation of the fungus into cutaneous tissue, where polymorphic lesions on the skin and subcutaneous tissue develop, often affecting the underlying lymphatics. Involvement of other sites primarily occurs in immunocompromised patients.
In Brazil, there are two main routes of disease transmission: one is sapronotic, involving direct contact with soil and decaying organic matter, and the other is zoonotic, where felines play a key role in the chain of disease transmission to humans.2 Over the past 20 years, the incidence of feline-transmitted sporotrichosis has increased significantly, particularly in Southeastern Brazil and has gradually expanded to the northeast of the country.3,4 Outbreaks in these regions are characterized by clustered cases with occurrences of human and animal infections over a short period of time, sometimes involving several members of the same family; there are also atypical presentations of the disease.2,5–7 Unusual manifestations of sporotrichosis tend to be the disseminated forms of the disease, including mucosal infections and hypersensitivity reactions such as erythema nodosum, erythema multiforme, and Sweet syndrome.2,8
To date, there have been few reported cases of sporotrichosis causing Sweet syndrome.9,10 Therefore, we present a series of 10 cases of Sweet syndrome associated with sporotrichosis, catalogued at an outpatient clinic specialized in tropical dermatoses at the Clinical Hospital of Pernambuco, Northeast Brazil.
METHODS
Study design.
This was a retrospective cohort study based on 379 medical records of patients referred to the sporotrichosis outpatient clinic from March 2017 to July 2019 (28 months). The study was conducted in the dermatology sector of a tertiary hospital in Recife, Northeast Brazil. This study received ethics approval from the Ethical Review Board of the Federal University of Pernambuco, Brazil, under code CAAE 71105617.7.0000.5208.
Study population.
Of the 379 patients, sporotrichosis was confirmed by clinical and microbiological examination in 342. Of these, 13 had acute rash with papules and erythematous nodules consistent with Sweet syndrome. Three patients were excluded from the study because of lack of information on medical record analysis, totaling 10 patients included in this article. Data were collected regarding the age of presentation, gender, history of contact with cats, clinical form of sporotrichosis, distribution and morphology of lesions, associated symptoms and comorbidities, treatment instituted, and histopathological findings.
Mycological sampling and laboratory diagnosis.
Biological samples were taken from affected regions by swabbing secretions of ulcerative lesions. We performed direct microscopy using 20% potassium hydroxide and Giemsa staining of the secretions. Nodular secretions were seeded on potato dextrose agar plates and incubated for 7 days at 25°C, and isolate cultures were obtained. Slide cultures were stained with lactophenol and observed under a light microscope.
Molecular biology identification.
Sporothrix sp. genomic DNA was extracted from the mycelial phase, as previously described.11 Partial sequencing of the calmodulin gene12,13 was performed, and BLAST (www.ncbi.nlm.nih.gov/BLAST) analysis was carried out by searching for similar sequences deposited in GenBank.
Sweet syndrome definition and histopathology.
Sweet syndrome was defined as a sudden onset of nodular or plaque erythematous lesions, histopathological findings of dense neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, excellent clinical response to corticosteroids, with or without fever, and leukocytosis with neutrophilia.14 Patients with disseminated skin lesions suspected of having Sweet syndrome were subjected to 4-mm punch biopsy for histopathological processing. Tissue fragments for histopathological analysis were immediately fixed in 10% phosphate buffered saline-buffered formalin. Paraffin-embedded tissues were sectioned (4–6 μm), and slides were mounted and submitted separately to the staining techniques with hematoxylin and eosin.15
RESULTS
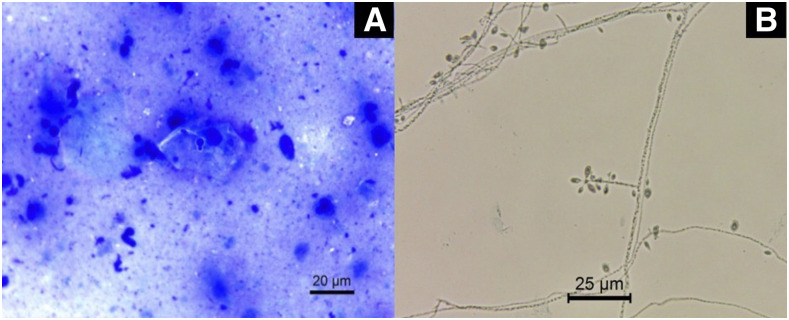
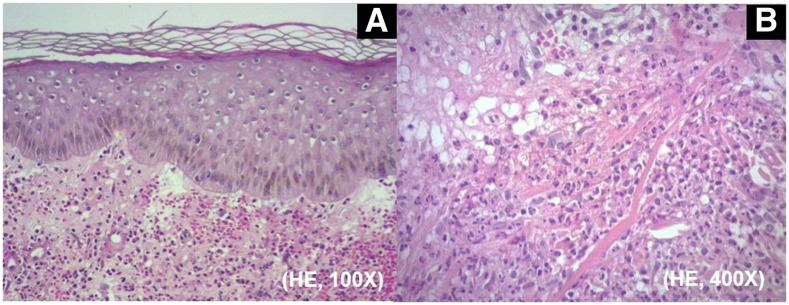
In the sporotrichosis-compatible lesions, direct mycological examination of Giemsa-stained tissue fragments and/or exudates revealed hyaline and elliptic yeast cells or cigar-shaped globular yeast cells (Figure 1A). Cultivation on Sabouraud agar for 7 days at 25°C promoted the development of grooved colonies with white coloration in the center with evident blackening at the edges. The micromorphology revealed multibranched, delicately septate hyaline mycelial filaments with terminal sympodial conidia, in fusiform or piriform round shape in floral arrangements (Figure 1B). Histopathology of biopsies in acute cutaneous lesions showed diffuse neutrophilic inflammatory infiltrate with dermal leukocytoclasia associated with marked edema characterizing Sweet syndrome (Figure 2).
Figure 1.
(A) Giemsa-stained direct microscopy showing the presence of rare and budding yeast cells (×630). (B) Micromorphology from the colony grown on potato dextrose agar for 7 days at 25°C showing septate mycelial filaments, delicate conidiophores, and conidia arranged sympodially with floral appearance. Lactophenol blue cotton staining (×630). This figure appears in color at www.ajtmh.org.
Figure 2.
(A) Histopathology of an erythematous cutaneous lesion from patient 6, compatible with Sweet syndrome. Diffuse inflammatory infiltrates associated with papillary dermal edema. (B) Close-up view of the infiltrates composed predominantly of neutrophils with leukocytoclasia (hematoxylin and eosin; original magnification: [A] ×100; [B] ×400). This figure appears in color at www.ajtmh.org.
All 10 patients selected for this series were urban dwellers with cutaneous sporotrichosis (confirmed by culture), and all met the diagnostic criteria for Sweet syndrome. The incidence of Sweet syndrome in the patients with sporotrichosis was 2.9%. We report 10 cases of patients who developed acute eruptions during their courses, all with clinical pictures compatible with Sweet syndrome at the point of first dermatological. In the disseminated lesions of these eruptions, the presence of neutrophilic dermatosis with substantial edema compatible with Sweet syndrome was demonstrated on histopathological study.
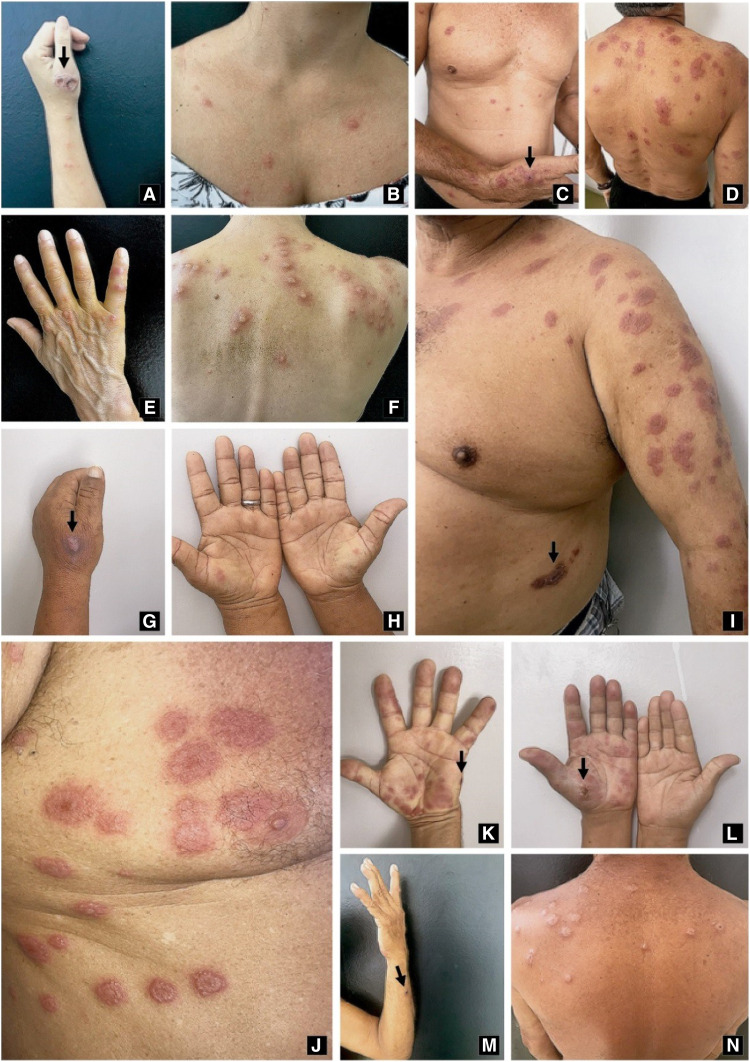
Patient 1 is 33-year-old female cashier without comorbidities. She reported a history of petting a stray cat with her left hand; subsequently, she developed ipsilateral upper limb injuries. Symptoms began 4 weeks before the consultation and showed no improvement with antibiotic therapy (amoxicillin and cephalexin). The patient presented to consultation with an ulcerated lesion on the left back (Figure 3A), accompanied by pseudovesicular lesions grouped in the upper limbs (Figure 3B) and erythematous plaques and nodules in the pretibial area and knees. We began treatment with itraconazole 100 mg/day for 12 weeks and prednisone 40 mg/day for 7 days. The patient was discharged only with left hand scarring.
Figure 3.
(A) Ulcerated lesion on the left back, accompanied by pseudovesicular lesions grouped in the upper limbs (B); sporotrichosis lesion on the left hand (C) with dorsal erythematous and violaceous plaques and papules (C and D); (E) pseudovesicular nodules and (F) papules on the hands and back; (G) ulcerated plaque on the left hand back and (H) erythematous papules on the palms; (I) ulcerated lesion on the trunk and target-like erythematous plaques on the chest and upper arm; (J) erythemato-edematous pseudovesicular plaques target like; (K and L) papules and nodules in hands associated with sporotrichosis ulcerated lesions; (M) ulcerated lesion in the left forearm; and (N) disseminated erythematous lesions on the back. Sporotrichosis skin lesions (arrows). This figure appears in color at www.ajtmh.org.
Patient 2 is a 54-year-old male caretaker without comorbidities. He had a history of contact with a domestic cat that had been diagnosed with sporotrichosis. The lesion appeared 1 week after the consultation on the back of his left hand (Figure 3C), with no improvement with treatment with cephalexin 2 g/day. He presented to our service with dorsal lesions and disseminated pseudovesicular erythematous lesions (Figure 3D). His leukocyte count was 9.85 × 109/L, with 82% neutrophils. Treatment started with itraconazole 100 mg/day for 12 weeks and prednisone 40 mg/day for 14 days, with excellent clinical outcome.
Patient 3 is 53-year-old female homemaker with a history of HIV infection, treated with regular antiviral therapy (lamivudine, tenofovir, and efavirenz) with undetectable viral load and 513 CD4 cells/mm3. She reported contact with domestic cats known to have sporotrichosis, one of which scratched her on the right hand, where she developed a small suppurative lesion (Figure 3E). She presented with fever and asthenia, as well as the appearance of disseminated nodular erythematous lesions on the trunk and back (Figure 3F). She was treated with itraconazole 100 mg/day for 10 weeks and prednisone 40 mg/day for 14 days. She was discharged with only residual dyschromic macules.
Patient 4 is 47-year-old female worker at a nongovernmental feline caring organization who reported treating approximately 40 domestic cats, some of which had been diagnosed with sporotrichosis. She referred to a skin lesion 8 weeks before that arose after a feline bite. An ulcerated lesion was observed on the left hand back (Figure 3G), in addition to disseminated erythematous lesions on the hands (Figure 3H) and trunk. She reported using itraconazole 200 mg/day, prescribed by another service 1 month before, with some improvement in the hand wound; however, the appearance of disseminated plaques motivated her to seek a referral. Prednisone 40 mg/day was given for 10 days, and we continued itraconazole at 100 mg/day until resolution of symptoms after 12 weeks.
Patient 5 is 56-year-old male dispatcher with systemic arterial hypertension using losartan 50 mg/day and hydrochlorothiazide 25 mg/day. He presented with an ulcerated skin lesion in the abdomen 4 weeks before the appointment. He was asymptomatic until contact with a domestic cat with skin wounds. He sought care for the appearance of pseudovesicular erythematous lesions in the upper limbs and cervical region (Figure 3I). His white blood cell count was 8.3 × 109/L, with 71% neutrophils. We treated him with itraconazole 100 mg/day for 16 weeks and prednisone 40 mg/day for 10 days. The lesion healed, leaving only slight residual macules on the nape of the neck and abdomen.
Patient 6 is a 66-year-old retired man. He had a history of alcoholism, systemic arterial hypertension, type II diabetes mellitus, hepatic steatosis, and benign prostatic hyperplasia. Medications included metformin 1.5 g/day, dapagliflozin 10 mg/day, rosuvastatin 10 mg/day, enalapril 10 mg/day, amlodipine 5 mg/day, and tamsulosin 0.4 mg/day. He reported a history of gardening activities and denied contact with cats. He had extensive pseudovesicular erythematous plaques on his trunk (Figure 3J) and lower limbs, an ulcerated right hand lesion (Figure 3K), and general symptoms of fever and chills that began 1 week before. His white blood cell count was 10.1 × 109/L, with 77% neutrophils. Treatment with terbinafine hydrochloride was chosen because of the risk of itraconazole drug interaction at 250 mg/day for 8 weeks and prednisone 60 mg/day for 30 days. After 4 weeks, the patient returned with significant improvement of the lesions and started weaning from prednisone, maintaining terbinafine for another 4 weeks.
Patient 7 is a 54-year-old unemployed woman with a history of epilepsy using chronic phenobarbital 100 mg/day and hydrochlorothiazide 25 mg/day for hypertension. She developed lesions on her right hand 2 weeks after being scratched by a cat known to have sporotrichosis. She presented with an ulcerated lesion on the thenar region of the right hand and erythematous lesions on the right hand and back (Figure 3L). Terbinafine hydrochloride treatment was initiated because of the risk of itraconazole drug interaction at a dose of 250 mg/day for 6 weeks and prednisone 40 mg/day for 10 days. Thirty days after the first consultation, the patient returned with edema and left ankle pain with reactive arthritis after a prednisone withdrawal. We prescribed a new 10-day cycle of prednisone 40 mg/day. After 8 weeks of treatment, the patient returned with complete resolution of the lesions.
Patient 8 is a 53-year-old male alcoholic. He reported contact with a cat known to have sporotrichosis. He presented with an ulcerated lesion in the left forearm (Figure 3M) and disseminated erythematous lesions on the face, back (Figure 3N), and lower limbs. His white blood cell count was 14.1 × 109/L, with 83% neutrophils. After treatment with prednisone 40 mg/day for 10 days and itraconazole 100 mg/day for 12 weeks, the patient returned with regression of his lesions.
Patient 9 is a 59-year-old retired woman with a history of hypertension and epilepsy but denied regular use of medications. She reported a history of contact with domestic cats with skin lesions as well as having participated in leisure activities in a garden. She reported onset of skin lesions on the face and spiking fevers within 10 days before the consultation. She had erythematous papulonodular lesions on the upper limbs, lower limbs, and back. Her white blood cell count was 4.7 × 109/L, with 46% neutrophils. We started treatment with itraconazole 100 mg/day and prednisone 40 mg/day for 7 days. After 10 weeks of treatment, the patient’s condition had resolved.
Patient 10 is a 51-year-old female homemaker with hypertension and type II diabetes mellitus. She was taking hydrochlorothiazide 25 mg/day, losartan 50 mg/day, and metformin 1 g/day. She reported having been bitten on the hand by a stray feline, with subsequent onset of injury 40 days before presentation. She presented ulcerated lesion in the right third finger and multiple papulonodular erythematous plaque lesions on the upper and lower limbs. She was treated with itraconazole 100 mg/day for 13 weeks and prednisone 40 mg/day for 14 days successfully.
DISCUSSION
In addition to the classic fixed and lymphocutaneous presentations of sporotrichosis that represents the vast majority of cases, atypical manifestations of Sporothrix schenckii complex fungal infections may occur. These presentations occur on a spectrum, at one end with both systemic dissemination of sporotrichosis, characterized by numerous integumentary and polymorphic lesions in immunosuppressed patients, at the other end with patients who develop hypersensitivity reactions such as erythema nodosum, reactive arthritis, erythema multiforme, and Sweet syndrome, with localized infection or even spontaneous healing without specific treatment.2 These peculiarities have been described in the recent epidemic of sporotrichosis in the state of Rio de Janeiro, where zoonotic transmission of the fungus Sporothrix brasiliensis from affected cats is epidemic8; this presentation has been spreading throughout Brazil, primarily affecting the northeast.
Contact with diseased cats was a common epidemiological antecedent in our series (90% of patients) (Table 1). This finding similar to those of other cases published in the literature, where immunoreactive forms such as erythema nodosum and erythema multiforme associated with sporotrichosis occurred in patients with histories of zoonotic transmission by cats with the same disease.16–19 These animals are thought to have a high parasitic burden; close and intimate contact in the home environment would favor repeated exposure to the fungus, allowing subclinical reinfections and hypersensitization of the human host, possibly leading to greater predisposition to atypical reactions.17
Table 1.
Epidemiological features, clinical manifestation, and laboratory findings of Sweet syndrome patients with sporotrichosis
| Case | Gender | Age (years) | Clinical features | Contact with cats | Treatment | Species |
|---|---|---|---|---|---|---|
| 1 | F | 33 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 12 weeks + prednisone 40 mg/day for 7 days | S. brasiliensis |
| 2 | M | 54 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 12 weeks + prednisone 40 mg/day for 14 days | S. brasiliensis |
| 3 | F | 53 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 10 weeks + prednisone 40 mg/day for 14 days | S. brasiliensis |
| 4 | F | 47 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 12 weeks + prednisone 40 mg/day for 10 days | S. brasiliensis |
| 5 | M | 56 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 16 weeks + prednisone 40 mg/day for 10 days | S. brasiliensis |
| 6 | M | 66 | Fixed cutaneous lesion | No | Terbinafine 250 mg/day for 8 weeks + prednisone 40 mg/day for 30 days | S. brasiliensis |
| 7 | F | 54 | Fixed cutaneous lesion | Yes | Terbinafine 250 mg/day for 8 weeks + prednisone 40 mg two cycles for 10 days | S. brasiliensis |
| 8 | M | 53 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 12 weeks + prednisone 40 mg/day for 10 days | S. brasiliensis |
| 9 | F | 59 | Multiple inoculation | Yes | Itraconazole 100 mg/day for 10 weeks + prednisone 40 mg/day for 7 days | S. brasiliensis |
| 10 | F | 51 | Fixed cutaneous lesion | Yes | Itraconazole 100 mg/day for 13 weeks + prednisone 40 mg/day for 14 days | S. brasiliensis |
F = female; M = male; S. brasiliensis = Sporothrix brasiliensis.
Early reports of Sweet syndrome associated with sporotrichosis were described by Freitas et al.9 These were three cases of fixed cutaneous sporotrichosis, with subsequent onset of the clinical–pathological picture compatible with Sweet syndrome; two of the patients were women. We also observed a higher frequency of immunoreactive cases in females (60%) (Table 1). This result apparently reflects the more urban profile of sporotrichosis in Brazil, where women are mostly responsible for household chores and establish greater contact with animals at home.2
Sweet syndrome is an acute neutrophilic dermatosis, described in 1964 by Robert D. Sweet. It is characterized by fever, predominantly polymorphonuclear leukocytosis; elevated and painful plaques on the face, neck, and extremities; and dense dermal infiltrates with mature neutrophils on histopathology.14 Its onset is associated with drug use, infections, autoimmune diseases, and neoplasms, suggesting that it results from cytokine-mediated hypersensitivity, followed by the characteristic neutrophilic infiltration. The presence of interleukins (ILs) 1 and 2 suggests a T helper type 1-type immune response.17
In classic Sweet syndrome, the condition may be associated with bacterial conditions such as streptococcal infections of the upper respiratory tract.14 In the case of fungal infections, cases associated with coccidioidomycosis have been previously described,20 and more recently sporotrichosis.9,10 In addition to meeting the clinical and histopathological criteria for the diagnosis of Sweet syndrome, the patients in the present study did not report the use of drugs commonly associated with the syndrome, including granulocyte colony-stimulating factor (G-CSF), tretinoin, trimethoprim–sulfamethoxazole, bortezomib, or azathioprine.14 In addition, the symptoms had no temporal relationship with the administration of medications and did not disappear with their discontinuation, ruling out the possibility of a drug-induced Sweet syndrome.
Individuals with Sweet syndrome usually show excellent response to systemic corticosteroids.14 The use of prednisone, given to all patients in the series for an average time of 14.6 days, was justified by the intensity of the symptoms and the exuberance of the eruptions, as well as its demonstrated efficacy; the excellent responses corroborated the diagnosis of Sweet syndrome. This management was endorsed by Freitas et al.9 in their case series of Sweet syndrome.
Despite the description of lymphocutaneous form as the most common clinical manifestation of sporotrichosis,5 in the present study, there was a predominance of the fixed cutaneous form of the disease (90% of cases) and rapid response to itraconazole, with an average of 11.3 weeks of treatment (Table 1). Intradermal reaction to sporotrichin could aid in the diagnosis of our reported cases. The sporotrichin skin test provides a fundamental tool to assess the patient’s cellular immune response and for epidemiological studies of sporotrichosis. According to Bonifaz et al.,21 the clinical form of sporotrichosis is dictated mainly by the immunity of the patient, and not just by virulence of the fungus. In their study, the researchers showed that most the normal positive responses were observed in the cutaneous lymphangitic form, whereas most hyperergic-positive responses occurred in the cutaneous fixed form. However, sporotrichin has fallen into disuse as a diagnostic test in several countries, such as Brazil and the United States, where it is not available, because there is in their opinion a lack of standardization.
Our data corroborate the findings of other studies that associated hypersensitivity reactions with more localized sporotrichosis.8,9,17 Barros et al.22 studied 645 patients with sporotrichosis treated with itraconazole and demonstrated in multivariate analysis that erythema nodosum was associated with shorter treatment time. Similarly, it can be inferred that the other immunoreactive forms may result from stimulation of immune system defenses, contributing to the local containment of fungal infection, manifested as fixed forms of sporotrichosis, and possibly shorter treatment times.9,15,23
It is noteworthy that itraconazole is primarily an antifungal agent. However, previous studies have identified its anti-inflammatory and immunomodulatory properties.24 Itraconazole has a marked immunosuppressive effect on human T-lymphocyte proliferations in vitro and enhances tumor necrosis factor production by cultured macrophages. An excellent review by Tsai and Tsai24 argues that itraconazole suppresses IL-4 and IL-5 production in anti-CD3 plus anti-CD28–stimulated T cells by reducing 3′,5′-cyclic adenosine monophosphate signaling. Furthermore, itraconazole has been proven to have anti-inflammatory effects in vivo. Thus, it is possible that the anti-inflammatory effects of itraconazole could help control the immunoreactive condition of our researched patients.
It is not yet clear whether the scenario of hypersensitivity reactions in sporotrichosis arises solely from the species involved in the host’s own infection and/or genetically determined immune responses. Several studies suggest that S. brasiliensis, the agent identified in all our cases, is the most virulent species of the S. schenckii complex. Different growth mechanisms, glycan cell wall compositions, adhesion to the host tissue, and immune system leakage have been described.25–27 Minor structural differences could modify the host’s inflammatory response and trigger production of distinct proinflammatory cytokines.25
Another characteristic of S. brasiliensis is its tendency to trigger outbreaks and epidemics among felines with high potential for zoonotic transmission. Infections with this species present higher fungal loads, invasiveness, and greater tissue damage compared with other agents of the Sporothrix complex.28 This increased virulence appears to be related to increased resistance to host oxidative stress,9 increased melanin production associated with increased resistance to phagocytosis,29 and the presence of Gp60 glycoprotein, the extracellular adhesion component of the fungus.
To date, it is not known which fungal structures have the greatest immunogenic potential and are therefore capable of inducing hypersensitivity reactions in individuals with sporotrichosis. It is possible that direct inoculation of yeast from affected felines leads to more complex host–pathogen interactions than in classical sapronotic transmission (from the filamentous form of the fungus found in soil and vegetables). This route of transmission, as well as the individual’s immune and inflammatory response to S. brasiliensis yeast, has yet to be further explored.
CONCLUSION
Sporotrichosis is a broad-clinical spectrum infectious disease that sometimes reveals a behavioral polymorphism. Atypical manifestations of the disease can be challenging for attending physicians, especially when S. brasiliensis is involved. Therefore, the present study suggests that sporotrichosis should be considered in the differential diagnosis of causes of Sweet syndrome. Despite the fact that it is little described in the medical literature, this association should be highlighted, especially in endemic areas or in the context of disease outbreaks.
REFERENCES
- 1.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S, 2015. Global epidemiology of sporotrichosis. Med Mycol 53: 3–14. [DOI] [PubMed] [Google Scholar]
- 2.Orofino-Costa R, de Macedo PM, Rodrigues AM, Bernardes-Engemann AR, 2017. Esporotricose: atualização epidemiológica, etiopatogênica, laboratorial e clínico-terapêutica. An Bras Dermatol 92: 606–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barros MB, Schubach AO, Schubach TM, Wanke B, Lambert-Passos SR, 2008. An epidemic of sporotrichosis in Rio de Janeiro, Brazil: epidemiological aspects of a series of cases. Epidemiol Infect 136: 1192–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gremião ID, Miranda LH, Reis EG, Rodrigues AM, Pereira SA, 2017. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog 13: e1006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barros MB, Schubach AO, do Valle AC, Gutierrez Galhardo MC, Conceição-Silva F, Schubach TM, Reis RS, Wanke B, Marzochi KB, Conceição MJ, 2004. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis 38: 529–535. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues AM, de Melo Teixeira M, de Hoog GS, Schubach TM, Pereira SA, Fernandes GF, Bezerra LM, Felipe MS, de Camargo ZP, 2013. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl Trop Dis 7: e2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues AM, de Hoog GS, de Camargo ZP, 2016. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog 12: e1005638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida-Paes R, de Oliveira MME, Freitas DFS, do Valle ACF, Zancope-Oliveira RM, Gutierrez-Galhardo MC, 2014. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis 8: e3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitas DF, Valle AC, Cuzzi T, Brandão LG, Zancope-Oliveira RM, Galhardo MC, 2012. Sweet syndrome associated with sporotrichosis. Br J Dermatol 166: 212–213. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Pyla V, 2014. Sweet’s syndrome-like sporotrichosis. Int J Dermatol 53: e324–e325. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira MM, Almeida-Paes R, Muniz MM, Gutierrez-Galhardo MC, Zancope-Oliveira RM, 2011. Phenotypic and molecular identification of Sporothrix isolates from an epidemic area of sporotrichosis in Brazil. Mycopathologia 172: 257–267. [DOI] [PubMed] [Google Scholar]
- 12.Marimon R, Serena C, Gené J, Cano J, Guarro J, 2007. Sporothrix brasiliensis, S. globosa, and S. mexicana three new Sporothrix species of clinical interest. J Clin Microbiol 45: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias NM, Oliveira MME, Portela MA, Santos C, Zancope-Oliveira RM, Lima N, 2011. Sporotrichosis caused by Sporotrhix mexicana. Emerg Infect Dis 17: 1975–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A, 2016. Sweet syndrome: a review and update. Actas Dermosifiliogr 107: 369–378. [DOI] [PubMed] [Google Scholar]
- 15.Melo-Júnior MR, Lima-Neto RG, Lacerda AM, Beltrão ECI, 2011. Comparative analysis of extracellular matrix and cellular carbohydrate expression in the sporotrichosis and chromoblastomycosis. Mycopathologia 171: 403–409. [DOI] [PubMed] [Google Scholar]
- 16.Xavier MO, Bittencourt LR, Silva CM, Vieira RS, Pereira HCP, 2013. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop 46: 116–118. [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez-Galhardo MC, Schubach AO, Barros MBL, Moita Blanco TC, Cuzzi TM, Schubach TMP, Lazéra MS, Valle ACF, 2005. Erythema multiforme associated with sporotrichosis. J Eur Acad Dermatol Venereol 19: 507–509. [DOI] [PubMed] [Google Scholar]
- 18.Galhardo MCG, Schubach AO, Barros MBL, Blanco TCM, Cuzzi-Maya T, Schubach TMP, Lazéra MS, Valle ACF, 2002. Erythema nodosum associated with sporotrichosis. Int J Dermatol 41: 114–116. [DOI] [PubMed] [Google Scholar]
- 19.Papaiordanou F, Silveira BRL, Abulafia LA, 2015. Hypersensitivity reaction to Sporothrix schenckii: erythema nodosum associated with sporotrichosis. Rev Soc Bras Med Trop 48: 504. [DOI] [PubMed] [Google Scholar]
- 20.DiCaudo DJ, 2006. Coccidioidomycosis: a review and update. J Am Acad Dermatol 55: 929–942. [DOI] [PubMed] [Google Scholar]
- 21.Bonifaz A, Toriello C, Araiza J, Ramírez-Soto MC, Tirado-Sanchez A, 2018. Sporotrichin skin test for the diagnosis of sporotrichosis. J Fungi 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros MBL, Schubach AO, Oliveira RVC, Martins EB, Teixeira JL, Wanke B, 2011. Treatment of cutaneous sporotrichosis with itraconazole-study of 645 patients. Clin Infect Dis 52: e200–e206. [DOI] [PubMed] [Google Scholar]
- 23.Almeida-Paes R, de Oliveira LC, Oliveira MME, Gutierrez-Galhardo MC, Nosanchuk JD, Zancopé-Oliveira RM, 2015. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. BioMed Res Int 2015: 212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai YC, Tsai TF, 2019. Itraconazole in the treatment of nonfungal cutaneous diseases: a review. Dermatol Ther (Heidelb) 9: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Marine M, Gene J, Cano J, Guarro J, 2009. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect 15: 651–655. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda MAK, de Almeida JRF, Jannuzzi GP, Cronemberger-Andrade A, Torrecilhas ACT, Moretti NS, da Cunha JPC, de Almeida SR, Ferreira KS, 2018. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front Microbiol 9: 2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossato L, 2017. Sporothrix brasiliensis: Aspectos Imunológicos e Virulência. Dissertation, Universidade de São Paulo, São Paulo, Brazil. [Google Scholar]
- 28.Fernandes GF, Santos PO, Rodrigues AM, Sasaki AA, Burger E, Camargo ZP, 2013. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 4: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida-Paes R, Figueiredo-Carvalho MHG, Brito-Santos F, Almeida-Silva F, Oliveira MME, Zancopé-Oliveira RM, 2016. Melanins protect Sporothrix brasiliensis and Sporothrix schenckii from the antifungal effects of terbinafine. PLoS One 11: e0152796. [DOI] [PMC free article] [PubMed] [Google Scholar]