Abstract.
In epidemic-prone areas of the western highlands, the Kenya Ministry of Health conducted campaigns of indoor residual spraying (IRS) of households, followed by mass distribution of insecticide-treated bed nets (ITNs), as part of the National Malaria Strategy. We previously reported that in the highland areas of Kipsamoite and Kapsisiywa, widespread IRS coverage in 2007, after lower but substantial coverage in 2005 and 2006, contributed to possible local interruption of malaria transmission between 2007 and 2008. Indoor residual spraying campaigns in the area ended in 2010, succeeded by a mass ITN distribution campaign in 2011 and 2012 targeting universal coverage. Insecticide-treated bed net use in the area increased from 17.1% pre-campaign in 2011 to 51.7% post-campaign in 2012, but decreased to 35.8% in 2013. The ITN campaign did not reduce malaria incidence in the population as a whole (odds ratio [OR] after ITN distribution versus before, 1.29, 95% CI: 1.00–1.66, P = 0.049). However, in 2011–2013, individuals who stated that they slept under ITNs as compared with those who did not had a decrease in malaria incidence that approached statistical significance (OR 0.74, 95% CI: 0.52–1.04, P = 0.08). Mass ITN distribution after previous annual IRS campaigns was insufficient to further reduce malaria transmission in this area of low and highly seasonal transmission possibly because of low ITN use despite the mass campaign.
INTRODUCTION
Malaria is a leading cause of mortality among children younger than 5 years and is second to acute respiratory illnesses in terms of outpatient morbidity, accounting for up to 30% of attendance at and 19% of admissions to health facilities in Kenya.1 In unstable transmission settings such as western Kenya’s highland areas, malaria occurs among individuals of all ages because immunity is not built or sustained with limited exposure.2 About 20% of estimated 45.9 million Kenyans live in highland settings.1,3,4 With less immunity than populations of high malaria transmission, these populations are susceptible to epidemics.5
The Kenya Ministry of Health (MOH) first formalized the National Malaria Strategy (NMS) in 2001 and revised it in 2009.1,6 The approaches for reaching many objectives are specific to the country's defined epidemiologic zones, one of which is the “epidemic-prone areas of the western highlands,” which is where our study areas of Kipsamoite (Kip) and Kapsisiywa (Kap) are located. In line with the NMS, the MOH conducted a campaign of annual indoor residual spraying (IRS) of households from 2005 to 2010, followed by a mass distribution of insecticide-treated bed nets (ITNs) to individuals in the study area from 2011 to 2012. Both are effective prevention tools that have been shown to reduce morbidity and mortality in various transmission settings.7–10 The class of insecticides used in the MOH IRS campaigns was pyrethroids, which have residual effects typically lasting 2–3 months.11,12 We have previously reported that widespread (> 85%) IRS coverage contributed to possible interruption of malaria transmission in the study area from April 2007 to April 2008.13 Malaria transmission resumed in these areas in 2008, but with lower seasonal peak incidence than in the years before the presumed interruption of transmission. Indoor residual spraying ceased in the area after 2010 following the guidelines set forth in the revised NMS, which called for 2 years of IRS in this epidemiologic zone, transitioning to focused IRS in response to epidemics thereafter.1 Mass ITN distribution campaigns targeting 100% population coverage were carried out by the MOH in Kip in November 2011 and Kap in June 2012, using the WHO universal coverage definition of one net for every two household members as an operational target.14 Before this, government-led bed net distributions were targeted to the high-risk population of pregnant mothers and infants.6
Our research studies have conducted ongoing malaria surveillance in Kip and Kap since April 2003. In the present study, we assessed ITN intervention coverage levels during the mass distribution campaign and then evaluated malaria incidence before the ITN distribution campaign (since the period of possible interruption, during which period IRS was conducted annually) and after the ITN distribution campaign to determine whether the ITN mass distribution campaign reduced malaria incidence in 1) the population of the study area as a whole after the earlier IRS campaigns and 2) among individuals in the study area who specifically said they used ITNs. With prospective data collected on the study population before, during, and after the implementation of the MOH interventions, we were well positioned to evaluate and provide feedback on the effectiveness of these efforts specific to this epidemic-prone highland Kenya setting.
MATERIALS AND METHODS
Study population and location.
The study population consists of men, women, and children of all ages from the Kip and Kap areas of Nandi County in western Kenya. The study population grew from 6,752 in April 2003 to 9,186 in December 2013, with the total number of households increasing from 1,309 to 1,799. The median household size was 5 in both April 2003 (interquartile range [IQR], 4–7) and December 2013 (IQR, 2–7). Kipsamoite comprises seven villages, and Kap comprises 10 villages. The study sites are located approximately 20 km apart and share similar ranges of altitudes (Kip: 1,941–2,108 m; Kap: 1,887–2,065 m).15,16 Both sites are in Kenya’s “epidemic-prone areas of the western highlands” epidemiologic zone, experience unstable highly seasonal malaria transmission patterns, and are at risk for malaria epidemics. Two MOH dispensaries, Kip Health Center and Kap Health Center, are the only healthcare facilities in the study area; community health workers do not operate in this area. Residents can purchase antimalarials at local stores, but receive free malaria evaluation and treatment at the health dispensaries, so report primarily coming to the dispensaries for malaria treatment.
Data sources.
Demography and climate surveillance.
During the study period, field assistants enumerated all households in the entire study area and recorded data on births, deaths, and migrations of occupants. Surveillance was carried out by the study and not as part of MOH activities and was conducted with the consent of study participants (see the Study Ethical Review section). At the time of initial enrollment, the field assistants collected household data including roof material and number of rooms and also used global positioning systems to map household coordinates and elevation. In 2005, the data collection instrument was modified to add assessment of individual household member travel, use of ITNs (“Do you sleep under a bednet?” [yes/no]. “If yes, is the bednet treated with insecticide?” [yes/no]), and IRS treatment of houses. These data were collected every 3–4 months (2003–2005), every 4–6 months (2006–2008), or annually (2009–2013; in March 2009; July during years 2010, 2011, and 2013; and in October 2012). Daily cumulative rainfall and minimum and maximum temperatures in Kip and Kap were collected throughout the study period.
Passive malaria surveillance.
Clinical malaria was evaluated through ongoing passive surveillance at the Kip Health Center and Kap Health Center, which are MOH dispensaries and are the only healthcare facilities in the study area. Study participants who presented with symptoms of malaria (primarily fever) were evaluated for malaria infection by microscopy of peripheral blood smears.13 Microscopy was performed independently by two trained microscopists, with a third and final assessment by a third microscopist if the first two results were discordant. A diagnosis of clinical malaria was given to any person with symptoms of malaria who had a positive blood smear for any Plasmodium species. Free diagnosis and treatment of malaria was provided as per MOH guidelines. As noted in the Introduction, mass ITN distribution was conducted by the MOH in November 2011 in Kip and June 2012 in Kap. Distribution of ITN took place over the course of 3 days where free nets were distributed at the health facilities.
Statistical analysis.
To evaluate the impact of the mass ITN campaign on malaria incidence in the study area, we used generalized estimating equations (GEEs) on a dataset with monthly individual malaria incidence data. We created a dichotomous variable to indicate whether the record was before or after the mass ITN campaign (predictor), taking into account the different time frames of distribution for Kip and Kap (Kip: before May 2008–October 2011 and after November 2011–December 2013; Kap: before May 2008–May 2012 and after June 2012–December 2013).
We used GEEs with a binomial distribution and logit link, with clusters being individuals and an autoregressive of order one (AR-1) working correlation; the analysis used robust standard errors. An alternative GEE defined clusters to be households and used an exchangeable (EXC) working correlation.
To evaluate whether an individual’s reported ITN use affected their risk of clinical malaria, we estimated odds ratios (OR) using multilevel mixed effects logistic regression, including two random effects for households and individuals within households. The time period for this analysis covered January 2011–December 2013, after any potential effects from the last round of IRS conducted in the study area in 2010 had worn off. Individual ITN use as reported during each annual demography survey was considered the status for the respective calendar year.
We considered the potential confounding effects of age; village; average monthly rainfall; average monthly minimum and maximum temperatures; household elevation; roof material; and distances to nearest clinic, forest, and swamp, which were selected a priori.
We used the method of purposeful selection of covariates, with consideration of the correlated nature of these data, to determine the final adjusted models; used fractional polynomials to evaluate the linearity in the logit of each continuous variable, transforming variables where analysis indicated nonlinearity; and tested for statistical interaction of ITN use with age, and of elevation with the weather patterns of rainfall and temperatures.17–19
Statistical analyses were performed using Stata SE version 14,20 and P < 0.05 was considered statistically significant.
Study ethical review.
Written consent was obtained from heads of households during initial enrollment for participation in demography and for passive clinical malaria surveillance. For blood sample collection, informed consent from adults and parents or guardians of all children, plus assent from children aged 13–17 years, was obtained from participants presenting to the clinics with symptoms of malaria. The studies were approved by the Institutional Review Boards of Case Western Reserve University and the University of Minnesota, and by the Kenya Medical Research Institute Ethical Review Committee.
RESULTS
Indoor residual spraying.
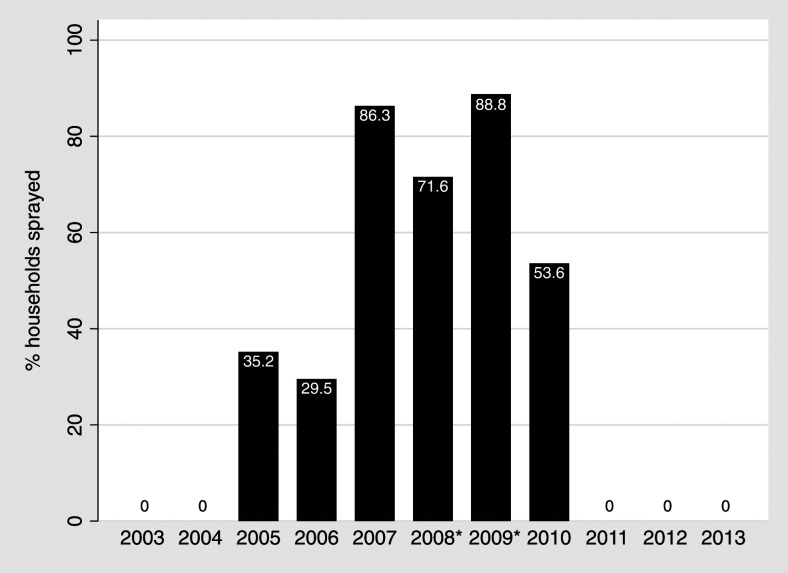
The MOH-led IRS campaigns that started in 2005 increased to widespread (> 85%) coverage in 2007, which contributed to possible local transmission interruption in the study area (Figure 1).13 In subsequent years, the MOH targeted fewer study area homes and focused efforts near potential breeding sites in surrounding areas (2008, 71.6% household coverage; 2009, 88.8%; 2010; 53.6%; Figure 1). Indoor residual spraying was not conducted in the study area after 2010.
Figure 1.
Percentages of households reporting indoor residual insecticide spraying, by year 2003–2013. *In 2008 and 2009, spraying commenced after malaria cases were reported.
Insecticide-treated bed nets.
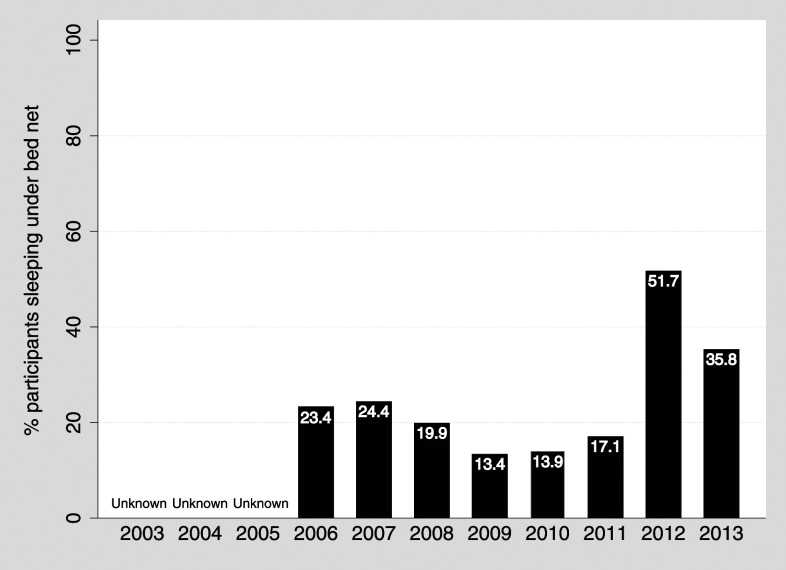
Reported ITN use by study participants was consistently low (< 25%) for years 2006–2011, but increased from 17.1% in 2011 (pre-campaign) to 51.7% in 2012 (post-campaign) and decreased to 35.8% in 2013 (Figure 2). Bed net use by villages was highly variable, ranging from 1.6% to 69.6% before the campaign, and 2.9% to 98.4% after the campaign (Table 1).
Figure 2.
Percentages of study participants reporting sleeping under bed nets during demography surveillance, by year 2003–2013.
Table 1.
Percentages of study participants reporting sleeping under bed nets during July 2011 and October 2012 demography surveillance, respectively, pre- and post-mass bed net distribution campaign (Kip: November 2011, Kap: June 2012), by village
| Village | 2011 (%) | 2012 (%) |
|---|---|---|
| Kwindich* | 6.3 | 2.9 |
| Birei† | 1.6 | 22.1 |
| Kipsaget* | 9.6 | 28.3 |
| Morongen* | 13.3 | 29.7 |
| Chepyewet* | 27.5 | 39.6 |
| Emgwen† | 4.9 | 45.6 |
| Kabuson* | 6.7 | 47.5 |
| Chepkober† | 17.7 | 53.9 |
| Kip* | 69.6 | 57.1 |
| Tiriin† | 11.3 | 64.2 |
| Mataget† | 13.2 | 66.6 |
| Kapkwenio* | 1.8 | 68.6 |
| Kamagande† | 17.6 | 78.9 |
| Kabasgei† | 31.4 | 80.3 |
| Kimondi† | 32.4 | 82.4 |
| Chemase† | 10.5 | 93.6 |
| Kapsile† | 15.5 | 98.4 |
Kap = Kapsisiywa; Kip = Kipsamoite. Villages are listed in the order of increasing bed net use as reported in 2012.
* Village in Kip.
† Village in Kap.
Malaria incidence.
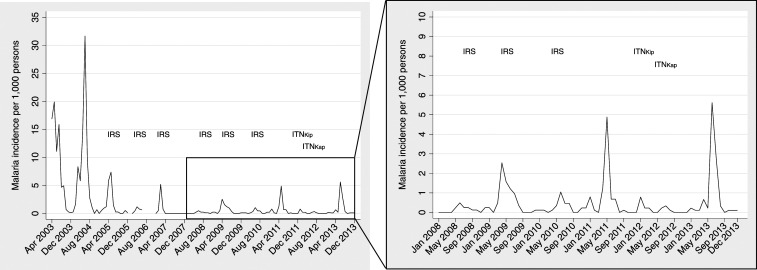
Post-interruption of malaria transmission, incidence of clinical malaria was low, but malaria was reported each year, particularly during peak seasons after long rainy seasons (Figure 3). From the period of interruption (May 2008) to the ITN distribution campaign (November 2011 in Kip, June 2012 in Kap), 183 cases of malaria were reported, whereas 105 cases were reported after ITN distribution through the end of 2013 (eighty-seven cases were reported from October 2010, after the residual effects of the last IRS campaign had worn off, to the ITN distribution campaign). In May 2011, before mass ITN distribution, incidence peaked at 4.9 per 1,000 persons, and in June 2013, after mass ITN distribution, incidence reached 5.6 per 1,000 persons.
Figure 3.
Malaria incidence per 1,000 persons and Ministry of Health indoor residual spraying (IRS) and insecticide-treated bed net (ITN) campaigns in Kipsamoite (Kip) and Kapsisiywa (Kap), April 2003–December 2013.
Impact of mass ITN campaign after repeated annual IRS campaigns.
In the crude GEE model that accounts for the correlation of individuals within households, during the study period (May 2008–December 2013), the odds of developing clinical malaria did not differ after the ITN campaign versus before the campaign (OR = 1.07, 95% CI: 0.82–1.39, P = 0.61). The estimated working correlation in the EXC model with clustering by households is 0.00052, suggesting the correlation within households is small, which justifies using traditional logistic regression models under the independence assumption. A similar small working correlation starting at −0.00049 was estimated for the AR-1 model with clustering by individuals.19 Subsequent adjusted GEE models did not converge because of these small working correlations; hence, we report crude and adjusted results from traditional logistic regression models alongside the crude GEE model estimates in Table 2 for comparison. In the adjusted model, we evaluated risk factors for malaria as potential confounders. The final model adjusted for age, elevation, and average monthly maximum temperature. No statistical interactions were observed. Results of the adjusted model did not show a reduction in incidence of malaria after the mass ITN campaign versus before in this study population (OR = 1.29, 95% CI: 1.00–1.66, P = 0.049); instead, the period after the mass ITN campaign had statistically significant greater risk of malaria than the period before the mass ITN campaign when IRS was conducted annually.
Table 2.
Risk (OR) of malaria incidence in the highland areas of Kipsamoite and Kapsisiywa after mass ITN distribution (Kip: November 2011–December 2013; Kap: June 2012–December 2013) compared with before ITN distribution (Kip: May 2008–October 2011; Kap: May 2008–May 2012)
| Method | Cluster | Working | Crude | Adjusted* | ||
|---|---|---|---|---|---|---|
| Correlation | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| GEE | Individuals | AR-1 | 1.07 (0.84, 1.37) | 0.57 | † | – |
| GEE | Households | EXC | 1.07 (0.82, 1.39) | 0.61 | † | – |
| LR | None | IND | 1.06 (0.83, 1.35) | 0.63 | 1.29 (1.00, 1.66) | 0.049 |
AR-1 = autoregressive of order 1; EXC = exchangeable; GEEs = generalized estimating equations; IND = independent; ITN = insecticide-treated bed net; Kap = Kapsisiywa; Kip = Kipsamoite; LR = logistic regression; OR = odds ratio.
* Adjusted for age, elevation, and average monthly maximum temperature. Two transformation terms for age were included, . Two transformation terms for average monthly maximum temperature were included, .
† Odds ratios are not reported as the models did not converge because of near-zero working correlations.
Impact of individual ITN use.
An adjusted multilevel mixed effects logistic regression model, which included random effects for households and individuals within households, showed that individuals who slept under ITNs in Kip and Kap from January 2011 to December 2013 had a nonsignificant reduction in malaria incidence compared with study area individuals who did not sleep under ITNs (OR = 0.74, 95% CI: 0.52–1.04, P = 0.08, Table 3). The adjusted model controlled for age, village, elevation, distance to the nearest clinic, average monthly total rainfall, average monthly maximum temperature, and the significant interaction terms (the statistical interaction between elevation and average monthly rainfall was significant).
Table 3.
Association between individual insecticide-treated bed net use and malaria incidence in Kipsamoite and Kapsisiywa, January 2011–December 2013
| Method | Levels | Crude | Adjusted* | ||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Multilevel mixed effects logistic regression | Households, Individuals within households | 0.88 (0.64, 1.21) | 0.415 | 0.74 (0.52, 1.04) | 0.084 |
OR = odds ratio.
* Adjusted for age, village, elevation, distance to the nearest clinic, average monthly total rainfall, average monthly maximum temperature, and the interactions between elevation and transformed rainfall terms. Transformed terms for age , average total monthly rainfall , and average monthly maximum temperature were included in the model.
Approximately 3% of monthly individual-level ITN data were missing in the dataset because of missed demography surveys or people who left or entered the study area before or after the demography survey took place in a given year.
DISCUSSION
In this highland study site, a mass ITN distribution campaign after 6 years of annual IRS campaigns did not further reduce area-wide malaria incidence after cessation of the IRS campaigns. Interruption of malaria in areas of low transmission will likely require a high level of IRS or ITN coverage. The WHO recommends high coverage of one or the other core intervention (IRS or ITN) and delivering it at a high standard, rather than both concurrently to compensate for implementation deficiencies.21 High ITN coverage has never been reported in this study area (see Figure 2), and ITN use only peaked 50% once in the first demography survey post-campaign before decreasing again. Insecticide-treated bed net coverage ≥ 50% is expected to provide a community-level effect with protection for nonusers.21
The MOH's change in strategy outlined in 2009, phasing out routine IRS and implementing mass ITN distribution campaigns, with IRS reserved solely in response to epidemics, may not be sufficient to interrupt local transmission in this epidemiologic zone. Universal coverage of ITN usage was targeted in the mass ITN distribution campaign. Because we did not assess ITN ownership, we cannot comment definitively on the extent of ITN coverage (ownership) achieved in this study site. However, the low rates of ITN use suggest that universal coverage was unlikely, so it is unknown whether truly complete coverage and usage of ITNs in the area would have led to a greater decrease in transmission or interruption. Other evaluations of bed net usage after mass ITN distribution campaigns in sub-Saharan Africa share this finding.22–27 In Tanzania, after a universal coverage campaign, only 58.4% of households reported owning enough ITNs to cover all of their sleeping places.23 In a separate survey in western Kenya among respondents from several locations in various transmission settings, almost 41% of those at risk for malaria needed additional ITNs to achieve universal coverage.24 Insecticide-treated net usage was lower in our study area both before and after the mass distribution campaign than these studies.22–27
As suggested elsewhere, “hang-up campaigns” where assistance is provided to physically hang nets in households should complement mass distribution campaigns to ultimately alleviate barriers to use due to difficultly in hanging them.22 Having a net hung has been shown to be associated with higher ITN use.22,28 In addition, round nets as opposed to rectangular nets may be more appropriate in settings where round thatched roof homes are common and should be considered in planning future campaigns.
A proposed recommendation from the Kenya MOH to increase ITN coverage is to conduct a “follow-up/mop-up campaign” after initial mass distributions, which would target households that did not achieve universal coverage.14 However, the WHO recently recommended against this type of “top-up” campaign because of concerns regarding feasibility and costs.21 Another strategy to increase ITN coverage after a mass campaign is to leave behind nets at a health facility or with community leaders and implement community-based distribution to households who were, for example, absent during the initial campaign, do not have one net for every two household members, have a bed net that is damaged and needs replacement, or where a birth occurred outside an antenatal clinic.21,29
In this study, ITN use status collected during annual demography surveys may be misclassified because of a self-reporting social desirability bias: ITNs are known to protect against malaria, so people may self-report their use if they own them, even when they do not truly sleep under them. Underreporting of ITN use is also a possibility if a participant thought they might receive an ITN by responding “no” to current use. We do not suspect this is the case, however, as ITN distribution has never been a part of the study protocols.
Insecticide-treated net use data collected during cross-sectional demography surveys also may not reflect variability of use among individuals throughout the year. This has been reported in other studies in highland areas where unstable transmission occurs.27 The higher reported bed net usage among the entire study population in the 2012 demography survey could be attributed to more people sleeping under nets during rainy seasons as the survey was conducted in October in 2012, whereas the 2010, 2011, and 2013 surveys were conducted in July. In the study area, October is a wetter month than July, and rain increases the number of vector-breeding sites.30 People may sleep under bed nets during times of the year when they perceive more risk of malaria.27,31–33 However, ITN usage reported in the 2013 demography survey was still higher than all years before the mass ITN distribution campaign, so even when potential climate-driven behavior is taken into consideration, the campaign likely influenced the increased usage reported after the mass campaign.
A limitation of passive surveillance for diagnosing clinical malaria is that case status can be misclassified if individuals sought care outside of the two study health facilities. We believe this is unlikely as the Kip and Kap Health Centers are the only MOH clinics in the study area where free diagnostics and treatment have always been provided as per the study protocols. We are also unaware of any community health workers trained to test and treat for malaria in the study area, and we know travel is infrequent for our study cohort members.
The trend toward decreased malaria risk in individuals who reported ITN use suggests that truly universal coverage might lead to a decrease in transmission. The goal of universal coverage might be improved with supplemental educational, “follow-up/mop up” or community-based distribution, and “hang-up” campaigns to help alleviate potential barriers to ITN use post-distribution. However, universal coverage and use in areas of low transmission, with prolonged periods with few cases of malaria and few mosquitoes, may be difficult to achieve even with these campaigns. Further research is required to determine how additional measures such as IRS campaigns, mass treatment, identification and treatment of asymptomatic carriers, or vaccination may contribute to achieving malaria elimination in areas of low and unstable transmission.
Acknowledgments:
We would like to thank the motivated study participants from the 17 villages comprising our study area in western highland Kenya; the field assistants, clinicians, and laboratory staff at Kipsamoite and Kapsisiywa Health Centers; and the KEMRI-UMN Malaria Research Project team in Kisumu, Kenya. Some of the work was performed while K. E. S. H. and C. C. J. were at the University of Minnesota. This work was published with the permission of the director, Kenya Medical Research Institute.
REFERENCES
- 1.Kenya Ministry of Public Health and Sanitation , 2009. National Malaria Strategy 2009–2017. Nairobi, Kenya: Division of Malaria Control, 96. [Google Scholar]
- 2.Doolan DL, Dobano C, Baird JK, 2009. Acquired immunity to malaria. Clin Microbiol Rev 22: 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Agency for International Development , 2011. President’s Malaria Initiative Malaria Operational Plan Kenya FY 2011. Washington, DC: USAID. [Google Scholar]
- 4.Central Intelligence Agency , 2015. The World Factbook. Africa: Kenya 2015. Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/ke.html. Accessed September 14, 2015. [Google Scholar]
- 5.Noland GS, Hendel-Paterson B, Min XM, Moormann AM, Vulule JM, Narum DL, Lanar DE, Kazura JW, John CC, 2008. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun 76: 5721–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenya Ministry of Health , 2001. National malaria strategy 2001–2010. Nairobi, Kenya: Division of Malaria Control, 48. [Google Scholar]
- 7.Hamel MJ, Otieno P, Bayoh N, Kariuki S, Were V, Marwanga D, Laserson KF, Williamson J, Slutsker L, Gimnig J, 2011. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am J Trop Med Hyg 85: 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lengeler C, 2004. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews, 2. [DOI] [PubMed] [Google Scholar]
- 9.Payne D, Grab B, Fontaine RE, Hempel JHG, 1976. Impact of control measures on malaria transmission and general mortality. Bull World Health Organ 54: 369–377. [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis CF, Mnzava AEP, 2000. Comparison of house spraying and insecticide-treated nets for malaria control. Bull World Health Organ 78: 1389–1400. [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization , 1997. House-spraying with residual insecticides. Rozendaal JA, ed. Vector Control: Methods for Use by Individuals and Communities. Geneva, Switzerland: WHO. [Google Scholar]
- 12.Kenya Ministry of Health , 2007. Implementation of IRS Campaign in Malaria Epidemic Prone Districts in Kenya. Nairobi, Kenya: Division of Malaria Control, Ministry of Public Health and Sanitation. [Google Scholar]
- 13.John CC, Riedesel MA, Magak NG, Lindblade KA, Menge DM, Hodges JS, Vulule JM, Akhwale W, 2009. Possible interruption of malaria transmission, highland Kenya, 2007–2008. Emerg Infect Dis 15: 1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Public Health and Sanitation , 2012. Evaluation of the 2011 Mass Long-Lasting Insecticide Treated Net (LLIN) Distribution Campaign: Phase 1 and 2 Report. Nairobi, Kenya: Division of Malaria Control, Ministry of Public Health and Sanitation.
- 15.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC, 2006. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J 5: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst KC, Lindblade KA, Koech D, Sumba PO, Kuwuor DO, John CC, Wilson ML, 2009. Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study. Trop Med Int Health 14: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S, Sturdivant RX, 2013. Model-building strategies and methods for logistic regression. Applied Logistic Regression, 3rd edition Hoboken, NJ: John Wiley & Sons, 89–151. [Google Scholar]
- 18.Hosmer DW, Lemeshow S, Sturdivant RX, 2013. Logistic regression for matched case-control studies. Applied Logistic Regression. 3rd edition Hoboken, NJ: John Wiley & Sons, 243–268. [Google Scholar]
- 19.Hosmer DW, Lemeshow S, Sturdivant RX, 2013. Logistic regression models for the analysis of correlated data. Applied Logistic Regression. 3rd edition Hoboken, NJ: John Wiley & Sons, 313–376. [Google Scholar]
- 20.StataCorp , 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 21.World Health Organization , 2019. Guidelines for Malaria Vector Control. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 22.Bennett A, Smith SJ, Yambasu S, Jambai A, Alemu W, Kabano A, Eisele TP, 2012. Household possession and use of insecticide-treated mosquito nets in Sierra Leone 6 months after a national mass-distribution campaign. PLoS One 7: e37927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West PA, Protopopoff N, Rowland MW, Kirby MJ, Oxborough RM, Mosha FW, Malima R, Kleinschmidt I, 2012. Evaluation of a national universal coverage campaign of long-lasting insecticidal nets in a rural district in north-west Tanzania. Malar J 11: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou G, Li JS, Ototo EN, Atieli HE, Githeko AK, Yan G, 2014. Evaluation of universal coverage of insecticide-treated nets in western Kenya: field surveys. Malar J 13: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens ER, Aldridge A, Degbey Y, Pignandi A, Dorkenoo MA, Hugelen-Padin J, 2013. Evaluation of the 2011 long-lasting, insecticide-treated net distribution for universal coverage in Togo. Malar J 12: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plucinski MM, Chicuecue S, Macete E, Colborn J, Yoon SS, Kachur SP, Aide P, Alonso P, Guinovart C, Morgan J, 2014. Evaluation of a universal coverage bed net distribution campaign in four districts in Sofala Province, Mozambique. Malar J 13: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atieli HE, Zhou G, Afrane Y, Lee MC, Mwanzo I, Githeko AK, Yan G, 2011. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasit Vectors 4: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macintyre K, Littrell M, Keating J, Hamainza B, Miller J, Eisele TP, 2012. Determinants of hanging and use of ITNs in the context of near universal coverage in Zambia. Health Policy Plan 27: 316–325. [DOI] [PubMed] [Google Scholar]
- 29.Killian A, et al. 2017. Evaluation of a continuous community-based ITN distribution pilot in Lainya County, South Sudan 2012–2013. Malar J 16: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snow RW, Gilles HM, 2002. The epidemiology of malaria. Warrell DA, Gilles HM, ed. Essential Malariology. 4th edition London, United Kingdom: Arnold, 85–106. [Google Scholar]
- 31.Winch PJ, Makemba AM, Kamazima SR, Lwihula GK, Lubega P, Minjas JN, Shiff CJ, 1994. Seasonal variation in the perceived risk of malaria: implications for the promotion of insecticide-impregnated bed nets. Soc Sci Med 39: 63–75. [DOI] [PubMed] [Google Scholar]
- 32.Binka FN, Adongo P, 1997. Acceptability and use of insecticide impregnated bednets in northern Ghana. Trop Med Int Health 2: 499–507. [PubMed] [Google Scholar]
- 33.Alaii JA, Hawley WA, Kolczak MS, ter Kuile FO, Gimnig JE, Vulule JM, Odhacha A, Oloo AJ, Nahlen BL, Phillips-Howard PA, 2003. Factors affecting use of permethrin-treated bed nets during a randomized controlled trial in western Kenya. Am J Trop Med Hyg 68 (Suppl 4): 137–141. [PubMed] [Google Scholar]