Abstract
As an essential lipid, cholesterol is of great value in keeping cell homeostasis, being the precursor of bile acid and steroid hormones, and stabilizing membrane lipid rafts. As a kind of cholesterol metabolite produced by enzymatic or radical process, oxysterols have drawn much attention in the last decades. Among which, the role of 25-hydroxycholesterol (25-HC) in cholesterol and bile acid metabolism, antivirus process, and inflammatory response has been largely disclosed. This review is aimed at revealing these functions and underlying mechanisms of 25-HC.
1. Introduction
Cholesterol is a member of the sterol family that plays essential roles in a variety of biological processes [1]. Under physiological state, cholesterol is mainly metabolized into bile acids and steroid hormones such as estrogens and androgens. In addition, cholesterol is also the crucial component of membrane lipid rafts [2]. In the past years, the function of this basic and pleiotropic molecule has been deeply studied. It is then realized that the derivatives of this precursor are implicated in a broad of physiological processes, such as cholesterol metabolism, antivirus process, and inflammatory and immune response, and are involved in a series of diseases development, such as atherosclerosis, neurodegeneration disease, inflammatory bowel disease, and nonalcoholic liver disease [3–7]. Among these cholesterol metabolites, oxysterols are 27-carbon molecules that are formed via enzymatic or radical process adding an epoxide or ketone or an additional hydroxyl group in the sterol nucleus and/or a hydroxyl group in the side chain [8]. These compounds are much more chemically reactive than cholesterol and are involved in a wider range of physiological processes.
In the past decades, side-chain oxysterols including 24S-, 25-, and 27-HC have drawn much attention [9–12]. Both 24S-HC and 27-HC are responsible for excessive intracellular cholesterol efflux in extrahepatic tissues like brain and macrophages. When compared with cholesterol, 24S-HC and 27-HC have greater polarity, thus can be transported to liver for further metabolism [9]. 24S-HC and 27-HC are considered to be the major players in mediating cholesterol efflux from extrahepatic organs to liver [9]. Specifically, 24S-HC is merely produced in the brain, owing to the exclusively expression of cholesterol 24-hydroxylase (CYP46A1) [11].
When compared to 24S-HC and 27-HC, 25-HC is a minor side-chain oxysterol formed by cholesterol 25-hydroxylase (CH25H) [8]. As like other oxysterols, it was firstly thought that 25-HC had a potent ability to mediate cholesterol homeostasis. However, this hypothesis came into question when the cholesterol homeostasis was not affected on the condition of CH25H deficiency [13–15]. With the deep and broad investigations of this molecule, the veil of the involvement of 25-HC in antivirus process and inflammatory and immune response has been disclosed [12]. Over the past decades, the roles of 25-HC in cholesterol and bile acid metabolism, antivirus process, inflammatory and immune response, and survival signaling pathway have been widely investigated, and this review will depict the functions of 25-HC in these processes as comprehensive as possible.
2. 25-HC Production
25-HC is synthesized from cholesterol by the addition of a hydroxyl group at position 25-carbon. This reaction is catalyzed by CH25H, which is a member of a small family of enzymes that use oxygen and a di-iron cofactor to catalyze hydroxylation reaction [16]. CH25H is located in endoplasmic reticulum and is ubiquitously expressed in tissues, especially in macrophages [17]. There is recent study indicating that CH25H is highly expressed in mouse liver and peritoneal macrophages [18], although it was once considered that this protein was poorly expressed in healthy liver. In addition, a few of cytochromes (CYP3A4, CYP27A1, and CYP46A1) and even reactive oxygen and nitrogen species (ROS/RNS) can catalyze cholesterol to form this oxysterol [15, 19, 20] (Figure 1). However, the effect of these enzymes on 25-HC production in vivo is poorly investigated.
Figure 1.
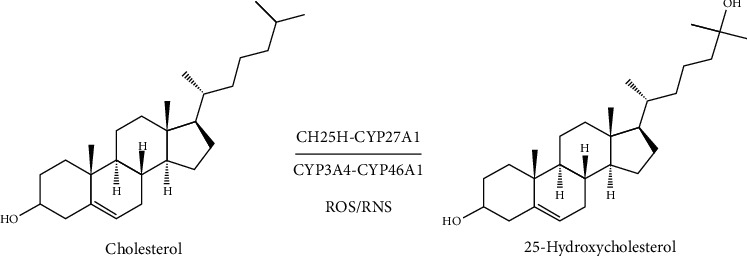
The production of 25-HC. Cholesterol can be catalyzed by enzymes CH25H, CYP3A4, CYP27A1, and CYP46A1 and reactive oxygen and nitrogen species (ROS/RNS) to 25-hydroxycholesterol.
3. The Regulation of CH25H
Intracellular 25-HC content is mainly determined by CH25H activity; thus, regulation of CH25H is of great importance in 25-HC production. CH25H is a highly dynamically regulated enzyme and especially so in inflammatory conditions. Firstly, it was unexpectedly found that CH25H was strongly upregulated in lipopolysaccharide (LPS; endotoxin) stimulated macrophages in vitro, and this increased CH25H expression was independent of myeloid differentiation protein 88 (Myd88) signaling but dependent on toll-like receptor 4 (TLR4) signaling [21]. On the basis of this study, dendritic cells and macrophages were verified to be significant source of CH25H, and the TLR-mediated expression of CH25H was dependent on TIR-domain-containing adapter-inducing interferon-β (TRIF), production of type I interferons (IFNs), and signaling through the interferon production regulator (IFNR)/Janus kinase(JAK)/signal transducer and activator of transcription 1 (STAT1) pathway [17]. Subsequent study further validated the Ch25h as an INF-stimulated gene (ISG) via STAT1 pathway and 25-HC as the only macrophage synthesized and secreted oxysterol [22]. In addition, a cholesterol oxidation and efflux-related gene, krüppel-like factor 4 (KLF4) was recognized to be able to transactivate Ch25h in vascular endothelial cells and macrophages [23]. Recently, a study that is aimed at investigating the mechanisms regulating CH25H expression found that 25-HC itself was able to activate CH25H expression, thus forming a positive feedback loop, and this effect was dependent on liver X receptors (LXRs), which were receptors of 25-HC [18]. Furthermore, inflammatory cytokine interleukin-1β (IL-1β), tumor necrosis factor-α (TNFα), and IL-6 can also promote CH25H expression through the STAT1 transcription factor in virus-infected human macrophages [24]. On the contrary, activating transcription factor 3 (ATF3) was reported to be a negative regulator of Ch25h gene by directly binding to the promoter of Ch25h and epigenetically repressing Ch25h expression [25] (Figure 2).
Figure 2.
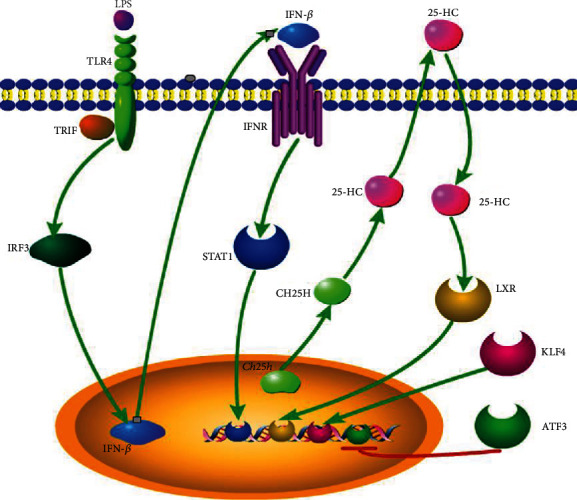
The regulation of CH25H expression. In LPS-stimulated macrophages, Ch25h is induced via a TLR4/IRF3/IFN-β/STAT1 signaling pathway. In addition, anti-inflammatory transcriptional factor KLF4 transactivates Ch25h in vascular endothelial cells. On the contrary, transcriptional factor ATF3 represses Ch25h transcription via directly binding to the Ch25h promotor. Furthermore, in hepatocytes and peritoneal macrophages, 25-HC induces CH25H expression in an LXR-dependent manner. Inflammatory cytokines IL-1β, TNFα, and IL-6 can also promote CH25H expression through the STAT1 transcription factor in virus-infected human macrophages (not shown).
4. The Receptors and Binding Proteins of 25-HC
The past years' investigations have shown that 25-HC is far more than just a kind of cholesterol metabolite. This molecule is an active mediator in a variety of physiological process. As an endogenous ligand, 25-HC binds to a strand of receptors [8, 26], including nuclear receptors LXRs [27, 28], retinoic acid receptor- (RAR-) related orphan receptors (ROR) [29, 30] and the estrogen receptor α (ERα) [31], and membrane receptor G protein-coupled receptor 183 (GPR183, also known as EBI2 for Epstein Barr virus-induced G protein-coupled receptor 2) [32, 33](Table 1). As the most broadly studied receptors, LXRs consist of two isoforms, LXRα (NR1H3) and LXRβ (NR1H2). LXRα is expressed mainly in adipose tissue, liver, and intestine, with the highest in liver, while LXRβ is ubiquitously expressed [34, 35]. 25-HC-activating LXRs are involved in a broad spectrum of physiological processes, such as cholesterol homeostasis and inflammatory response [35]. RORs are another family of nuclear receptor with three subtypes, RORα (NR1F1), RORβ (NR1F2), and RORγ (NR1F3), with RORγ having two isoforms, RORγ1 and RORγt [36, 37]. 25-HC has been described as an inverse agonist of RORα and RORγ [38, 39]. However, there is also a study indicating that 25-HC may have some agonistic activity to RORγ [30]. 25-HC-activating RORγt is an essential transcription factor of T helper 17 (Th17) cell differentiation [30]. In addition, 25-HC was also shown to be an agonist of ERα-mediating gene expression changes and growth responses in breast and ovarian cancer cells [31]. Oxysterol-activating membrane receptor GPR183 directs immune cell migration [40], and 25-HC was demonstrated to be one of its agonists [32, 33]. In fact, the most potent GPR183 endogenous agonist is 7α,25-hydroxycholesterol (7α,25-HC), which is a 25-HC metabolite, catalyzed by oxysterol 7α-hydroxylase (CYP7B1) [32, 33].
Table 1.
The receptors and binding proteins of 25-HC.
| Receptors | Role | Functions |
|---|---|---|
| Liver X receptors (LXRα, LXRβ) | LXRα agonist LXRβ agonist |
1. Negatively regulates cholesterol biosynthesis and promotes cholesterol efflux [27, 28, 34, 35] 2. Inflammatory regulation [35] 3. Promotes pyroptosis [83] |
| Retinoic-related orphan receptors (RORα, RORβ, RORγ) | RORα ligand RORγ agonist |
Th17 cell differentiation [30, 36–39] |
| Estrogen receptor α (ERα) | Agonist | Mediates gene expression changes and growth responses in breast and ovarian cancer cells [31] |
| G protein-coupled receptor 183 (GPR183, EBI2) | Agonist | Directs immune cell migration [32, 33] |
| Proteins binding oxysterols | ||
| Insulin-induced gene protein (INSIG) | Ligand | Maintains SREBP in the ER and inhibits cholesterol biosynthesis [41] |
| Niemann-Pick protein C1 (NPC1) | Ligand | Cholesterol clearance in lysosomal [42–44] |
| Oxysterol-binding protein family 8 (ORP8) | Ligand | 1. Cholesterol efflux in macrophages [46] 2. Induces apoptosis of the hepatoma cell lines [47] |
| Steroidogenic acute regulatory-related lipid transfer (START) domain proteins | Ligand | Maintenance of cellular cholesterol homeostasis [48–50] |
In addition to receptors, 25-HC is able to bind some proteins binding oxysterols, including the insulin-induced gene protein (INSIG), Niemann-Pick protein (NPC), the oxysterol-binding protein family (OSBP related, OSBPL, or ORPs), and steroidogenic acute regulatory-related lipid transfer (START) domain proteins [8] (Table 1). INSIG is a regulatory protein for the sterol regulatory element-binding protein (SREBP), which regulates the expression of enzymes involved in cholesterol biosynthesis [41]. NPC1 is a membrane glycoprotein which resides primarily in the late endosomes and transiently in lysosomes [42], and 25-HC treatment was found to recover cholesterol clearance in lysosomal induced by NPC1 deficiency [43, 44]. OSBP and its related proteins are a family of lipid transfer proteins (LTPs), involving in lipid metabolism and signal transduction [45]. 25-HC-activating ORP8 suppresses ATP-binding cassette transporter (ABCA1), which mediates phospholipid and cholesterol efflux and inhibits macrophage cholesterol efflux [46]. In addition, ORP8 may be implicated in 25-HC-induced apoptosis of the hepatoma cell lines, HepG2 and Huh7, via the endoplasmic reticulum (ER) stress response pathway [47]. START domain is a protein module of approximately 210 residues that binds lipids, including sterols [48]. Fifteen mammalian proteins, STARD1-STARD15, possess a START domain. 25-HC can bind to STARD4 and STARD5 [49, 50], indicating their role in the maintenance of cellular cholesterol homeostasis.
5. Cholesterol and Bile Acid Metabolism
As a primary cholesterol metabolite, 25-HC mediates cholesterol biosynthesis, uptake, and efflux (Figure 3). The cholesterol homeostasis is controlled by a negative feedback loop by cholesterol itself and its derivatives, oxysterols, with the latter ones having more potent ability to suppress cholesterol biosynthesis [51, 52]. Transcriptional factor SREBP mediates the expression of cholesterol biosynthesis rate-limiting enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) and many other related enzymes [53, 54]. SREBPs are retained in the ER in its inactive form. To become active, SREBPs must move from the ER to the Golgi by the multitransmembrane SREBP cleavage-activating protein (SCAP) [54]. The ER membrane protein, INSIG, with the interaction of SCAP keeps the SREBP-SCAP complex remaining in the ER [41]. 25-HC can bind to INSIG, thus keeping SREBPs inactive and inhibiting cholesterol synthesis [55]. In addition, 25-HC can directly reduce the level of the rate-limiting enzyme, HMGCR, by promoting its ubiquitylation and proteasomal degradation [56].
Figure 3.
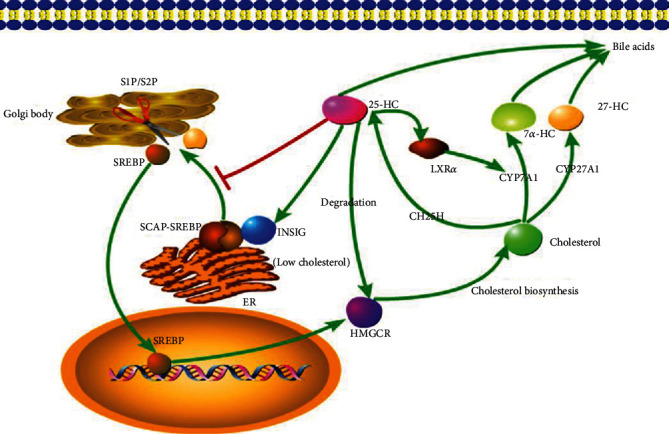
The regulation of 25-HC in cholesterol metabolism. Transcriptional factor SREBP controls the rate-limiting enzyme HMGCR. When the cholesterol level is low, the SREBP is escorted by SCAP from ER to Golgi body, in which this complex is cleaved by site-1 protease (S1P) and site-2 protease (S2P), and then the SREBP is released. 25-HC retains SREBP in ER via binds to an anchor protein INSIG in ER and suppresses SREBP translocation. Furthermore, 25-HC promotes HMGCR ubiquitylation and proteasomal degradation. 25-HC can also promote the CYP7A1 expression via activating transcriptional factor LXRα.
In addition to suppressing biosynthesis, 25-HC can also promote the cholesterol to be catalyzed to bile acids and intracellular cholesterol efflux. These effects are mainly dependent on LXRs [57]. 25-HC-activating LXRα in hepatocytes induces the rate-limiting enzyme in the classic bile acid synthetic pathway, cholesterol 7α-hydroxylase (CYP7A1) [57], promoting cholesterol to be converted to bile acids. In macrophages, LXRs can induce the expression of ATP-binding cassette subfamily members A1 (ABCA1) and G1 (ABCG1), which are responsible for reverse cholesterol transport, eliminating excessive intracellular cholesterol [57, 58].
Above all, it seems clear that 25-HC suppresses the cholesterol biosynthesis and promotes the intracellular cholesterol efflux through a variety of mechanisms. However, in vivo study using Ch25h knockout mice showed that CH25H and 25-HC deficiency did not affect whole cholesterol metabolism, which questioned the mediation of 25-HC in cholesterol homeostasis [13, 14]. These inconsistent conclusions led to the hypothesis that 25-HC might play a role in cholesterol catabolism in a districted area and limited cells, not affecting the whole cholesterol homeostasis [12].
Bile acids are exclusively synthesized in liver through two distinct routes. In addition to the classical one initiated by CYP7A1, the so-called alternative pathway started with cholesterol 27-hydroxylase (CYP27A1) followed by oxysterols 7α-hydroxylase (CYP7B1) [13, 59]. 25-HC is also a precursor of bile acids, although it is not so impressive as 27-hydroxycholesterol (27-HC). The bile acids derived from 25-HC count less than 5% in total per day [60]. And as like cholesterol metabolism, CH25H and 25-HC deficiency does not affect the whole bile acids homeostasis [13].
6. Antivirus Effects
Oxysterols link the bridge between lipid metabolism and innate and adaptive immune response [61]. As an ISG, Ch25h is highly induced in virus infection, and 25-HC is impressive for its potent ability to inhibit virus invasion through a strand of mechanisms [62].
25-HC has a broad antivirus spectrum, including enveloped viruses and nonenveloped viruses [22, 63–72]. The enveloped viruses mainly consist of murine cytomegalovirus (MCMV), vesicular stomatitis virus (VSV), West Nile virus (WNV), the human immunodeficiency viruses (HIV), influenza virus, murid herpesvirus 68 (MHV68) and Ebola virus, Rift Valley fever virus (RVFV), Russian spring-summer encephalitis virus (RSSEV), Nipah virus, herpes simplex virus 1 (HSV-1), varicella-zoster virus (VZV), hepatitis B virus (HBV), hepatitis C virus (HCV), and the recently epidemic coronavirus disease 2019 (COVID-19) [22, 63–68]. The nonenveloped viruses include poliovirus, the encephalomyocarditis virus (EMCV), human papillomavirus type 16 (HPV-16), human rotavirus (HRoV), and human rhinovirus (HRhV) [69–72].
25-HC exhibits its antivirus function via a variety of mechanisms. Cholesterol metabolism is of great significance for viruses invading into the cells, the adsorption, entry, assembly, budding, and release of some viruses preferentially occurring in cholesterol-enriched microdomains (“lipid rafts”) of the cell membrane, especially the enveloped viruses [73]. 25-HC can directly change the position, orientation, and solvent accessibility of cholesterol in membrane, thus blocking the virus entry. Furthermore, 25-HC may insert into the cell membrane, changing the stability and integrity of cholesterol-enriched cytomembranes, inhibiting the fusion of virus and host cell membrane [74]. In addition to mediate the cell membrane status, 25-HC can also directly inhibit the virus replication. For example, the nonstructural protein 1 alpha (nsp1α) is an essential protein for porcine reproductive and respiratory syndrome virus (PRRSV) replication. CH25H/CH25H-M could degrade nsp1α through the ubiquitin-proteasome pathway [75]. Furthermore, high micromolar amounts of 25-HC-induced integrated stress response in host cells were also demonstrated to suppress the virus replication [63]. Lastly, Ch25h and 25-HC are crucial mediators in innate and adaptive immune response, and 25-HC treatment-induced inflammatory factors' release and the mediation in immune response are reported to inhibit virus invasion as well [22, 76].
7. Inflammatory Response
Does 25-HC amplify inflammatory response? It is a question. On the one hand, 25-HC is able to suppress interleukin-1 (IL-1) family cytokine production, such as IL-1α, IL-1β, and IL-18 [77, 78]. Via using Ch25h knockout mice, it was reported that 25-HC acted by antagonizing SREBP processing to reduce IL-1β transcription and to broadly repress IL-1-activating inflammasomes [79]. However, the specific mechanism by which the SREBPs promote IL-1β transcription is not clear. The authors speculated that it might be induced by cellular lipid content alteration caused by altered SREBP activity [12]. Another study verified the effect of cellular lipid content on NLR family pyrin domain containing 3 (NLRP3) inflammasome activation. This study showed that reduced synthesis of 25-HC resulting from the lysosomal acid lipase (LIPA) inhibition, which hydrolyzes cholesteryl esters to free cholesterol for 25-HC synthesis in macrophages, contributed to defective mitochondria-associated membrane (MAM) leading to mitochondrial oxidative stress-induced NLRP3 inflammasome activation [80]. In addition, SCAP escorts both NLRP3 and SREBP2 by forming a ternary complex, and 25-HC inhibited NLRP3 inflammasome formation via maintaining SCAP in ER [81]. Furthermore, 25-HC was found to suppress another common inflammasome activation. It was reported that high cholesterol content in macrophages was enough to activate the DNA sensor protein absent in melanoma 2 (AIM2) inflammasome by inducing impaired mitochondrial metabolism and mtDNA release, and 25-HC was able to maintain mitochondrial integrity and prevent AIM2 inflammasome activation in activated macrophages, in which the CH25H was upregulated [82] (Figure 4(a)). However, there is a study showing that 25-HC promotes the caspase-1-dependent cell death of colon cancer cells via activating LXRβ, but not LXRα [83]. And subsequent study found that 25-HC promoted robust NLRP3 inflammasome assembly and activation via potassium efflux, mitochondrial reactive oxygen species (ROS), and LXR-mediated pathways in X-linked adrenoleukodystrophy (X-ALD) [84] (Figure 4(b)). These controversial conclusions are hard to explain. It might be speculated that the function of 25-HC in NLRP3 activation may follow a tissue and cell-dependent manner.
Figure 4.
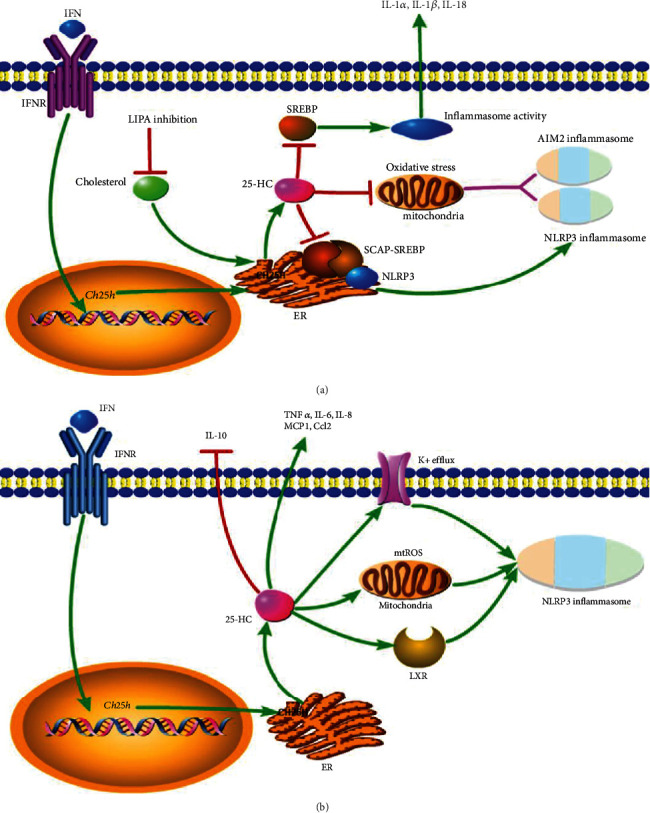
(a) The anti-inflammatory effect of 25-HC. 25-HC reduces IL-1 family (IL-1α, IL-1β, and IL-18) release and inflammasome activity via inhibiting SREBP. 25-HC decreases NLRP3 and AIM2 inflammasome formation by reducing mitochondria oxidative stress. Furthermore, NLRP3 associates with SCAP and SREBP2 to form a ternary complex which translocated to the Golgi apparatus adjacent to a mitochondrial cluster for optimal inflammasome assembly, and 25-HC inhibits this process. (b) Proinflammatory effect of 25-HC. 25-HC amplifies the expression of proinflammatory factors (TNFα, IL-6, IL-8, MCP1, and Ccl2) and reduces the anti-inflammatory factor IL-10. 25-HC promotes robust NLRP3 inflammasome assembly and activation via potassium efflux, mitochondrial ROS, and LXR-mediated pathways in brain.
Except for activating inflammasome, 25-HC promotes proinflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP1), and C-C motif chemokine ligand 2 (Ccl2) [85–90]. In addition, it is also able to suppress the secretion and production of anti-inflammatory factor interleukin-10 (IL-10). In human CD4 T cells, 25-HC reduces IL-10 production via decreasing the master transcriptional regulator of IL-10, c-Maf. In IL-27-induced type 1 regulatory T (TR1) cells, 25-HC acts as a negative regulator of TR1 cells in particular of IL-10 secretion via LXR signaling [91, 92].
Two transcriptional factors, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Activator protein 1 (AP-1) are downstream effectors while 25-HC exerting its proinflammatory function [88, 93, 94]. And a variety of downstream signaling pathways are involved in this process. In mouse peritoneal macrophages and human umbilical cord vein endothelial cells, 25-HC induces retinoic inducible gene I (RIG-I). RIG-I transduces the signal to downstream molecules, mitochondrial antiviral-signaling protein (MAVS), transforming growth factor-β-activated kinase 1 (TAK-1), and mitogen-activated protein kinase (MAPK/ERK/P38/JNK), leading to the activation of NF-κB and AP-1, inducing IL-8 production [95]. Furthermore, 25-OH triggers the activation/phosphorylation of the AP-1 component c-Jun and, consistently, increases the transcriptional activity of AP-1 [96].
8. 25-HC and Cell Survival
8.1. Autophagy
Autophagy is an evolutionarily ancient process whereby eukaryotic cells eliminate disposable or potentially dangerous cytoplasmic material to support bioenergetic metabolism and adapt to stress. It remains controversial of the function of 25-HC in mediating autophagy which is dependent on cell types. Lysosomal cholesterol accumulation sensitizes hepatocytes to acetaminophen toxicity by impairing mitophagy, and 25-HC recovers hepatocyte mitophagy by decreasing lysosomal cholesterol accumulation [97]. However, in human glioblastoma cell line (U87-MG), 25-HC is ineffective to restore autophagy flux and to decrease apoptosis levels [98]. In non-small-cell lung cancer cells (H1299), 25-HC is reported to induce cell death via attenuating autophagy [99].
8.2. Apoptosis
25-HC is reported to induce cell apoptosis in dose-dependent manner; however, the underlying mechanisms are poorly understood. Endoplasmic reticulum (ER) stress resulting from 25-HC seems to play a key role in this oxysterol-mediated proapoptotic effect. In macrophages, oxysterol-binding protein-related protein 4L (ORP4L) coexpresses and forms a complex with Gαq/11 and phospholipase C- (PLC-) β3. ORP4L facilitates PLCβ3 activation, IP3 production, and Ca2+ release from the endoplasmic reticulum. Through this mechanism, ORP4L sustains antiapoptotic Bcl-XL expression through Ca2+-mediated c-AMP responsive element-binding protein transcriptional regulation and thus protects macrophages from apoptosis. However, excessive 25-HC disassembles these ORP4L/Gαq/11/PLCβ3 complexes, reducing PLCβ3 activity, IP3 production, and Ca2+ release, resulting in macrophage apoptosis [100, 101]. In hepatic cell HepG2 and Huh7, 25-HC facilitates apoptosis via enhancing endoplasmic reticulum (ER) stress, and oxysterol-binding protein-related protein 8 (ORP8) is involved in 25-HC-mediated ER stress and hepatic cell apoptosis [47]. ORP8 knockdown rescues this effect. Neutral cholesterol ester hydrolase 1 (Nceh1) is a hydrolysis enzyme that dissolves 25-HC ester to free 25-HC. Incubating Nceh1-deficient thioglycollate-elicited peritoneal macrophages (TGEMs) with 25-HC caused massive accumulation of 25-HC ester in the endoplasmic reticulum (ER) due to its defective hydrolysis, thereby activating ER stress signaling and subsequent apoptosis [102]. In addition, 25-HC is able to induce apoptosis of vascular smooth muscle cells (VSMC) by controlling mitochondrial Bax translocation and ROS formation in a soluble adenylyl cyclase (sAC)/protein kinase A- (PKA-) dependent pathway [103].
9. Conclusions and Perspective
This review depicts the function of a kind of oxysterol, 25-HC, in cholesterol and bile acid metabolism, antivirus process, inflammatory response, and cell survival, especially in autophagy and apoptosis. It is astonishing that such a small molecule is involved in so broad variety of physiological processes. The only difference between 25-HC and another two common primary oxysterols, 27-HC and 24S-HC, lies in the carbon position, to which a hydroxyl group is added. However, 25-HC has much more potent antivirus ability than the other two. The reason is not clear yet. Owing to the broad antivirus spectrum and potent antivirus effect, there is a high possibility that 25-HC will be used as a drug in antivirus treatment. And studies exploring the specific mechanisms of 25-HC antivirus effect can be anticipated. Furthermore, the dual effects of 25-HC in proinflammatory and anti-inflammatory lead to the hypothesis that 25-HC may not be a simple positive or negative regulator in inflammatory response, but a mediator keeping inflammatory response in an accepted degree. In addition, the final phenotype in inflammatory response may be 25-HC amount and tissue dependent. Thus, more in vivo studies are needed to tell us the whole story.
Acknowledgments
This study was supported by the Medical Science Advancement Program (Clinical Medicine) of Wuhan University (no. TFLC2018003, grant to Qifa Ye) and the Joint Foundation for Translational Medicine and Interdisciplinary Research in Zhongnan Hospital (no. ZNLH201903, grant to Qifa Ye).
Conflicts of Interest
The authors declare that they have no conflict of interests.
References
- 1.Sanchez L. D., Pontini L., Marinozzi M., Sanchez-Aranguren L. C., Reis A., Dias I. H. K. Cholesterol and oxysterol sulfates: pathophysiological roles and analytical challenges. British Journal of Pharmacology. 2020 doi: 10.1111/bph.15227. [DOI] [PubMed] [Google Scholar]
- 2.Sviridov D., Mukhamedova N., Miller Y. I. Lipid rafts as a therapeutic target. Journal of Lipid Research. 2020;61(5):687–695. doi: 10.1194/jlr.TR120000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand P. K. Lipids, inflammasomes, metabolism, and disease. Immunological Reviews. 2020;297(1):108–122. doi: 10.1111/imr.12891. [DOI] [PubMed] [Google Scholar]
- 4.Kloudova A., Guengerich F. P., Soucek P. The role of oxysterols in human cancer. Trends in Endocrinology & Metabolism. 2017;28(7):485–496. doi: 10.1016/j.tem.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duc D., Vigne S., Pot C. Oxysterols in autoimmunity. International Journal of Molecular Sciences. 2019;20(18, article 4522) doi: 10.3390/ijms20184522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willinger T. Oxysterols in intestinal immunity and inflammation. Journal of Internal Medicine. 2019;285(4):367–380. doi: 10.1111/joim.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Testa G., Rossin D., Poli G., Biasi F., Leonarduzzi G. Implication of oxysterols in chronic inflammatory human diseases. Biochimie. 2018;153:220–231. doi: 10.1016/j.biochi.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Mutemberezi V., Guillemot-Legris O., Muccioli G. G. Oxysterols: from cholesterol metabolites to key mediators. Progress in Lipid Research. 2016;64:152–169. doi: 10.1016/j.plipres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Crosignani A., Zuin M., Allocca M., Del Puppo M. Oxysterols in bile acid metabolism. Clinica Chimica Acta. 2011;412(23-24):2037–2045. doi: 10.1016/j.cca.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Vurusaner B., Leonarduzzi G., Gamba P., Poli G., Basaga H. Oxysterols and mechanisms of survival signaling. Molecular Aspects of Medicine. 2016;49:8–22. doi: 10.1016/j.mam.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Moutinho M., Nunes M. J., Rodrigues E. Cholesterol 24-hydroxylase: brain cholesterol metabolism and beyond. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2016;1861(12):1911–1920. doi: 10.1016/j.bbalip.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Cyster J. G., Dang E. V., Reboldi A., Yi T. 25-Hydroxycholesterols in innate and adaptive immunity. Nature Reviews Immunology. 2014;14(11):731–743. doi: 10.1038/nri3755. [DOI] [PubMed] [Google Scholar]
- 13.Russell D. W. The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry. 2003;72(1):137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 14.Björkhem I. Are side-chain oxidized oxysterols regulators also in vivo? Journal of Lipid Research. 2009;50(Supplement):S213–S218. doi: 10.1194/jlr.R800025-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diczfalusy U. On the formation and possible biological role of 25-hydroxycholesterol. Biochimie. 2013;95(3):455–460. doi: 10.1016/j.biochi.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Lund E. G., Kerr T. A., Sakai J., Li W. P., Russell D. W. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. The Journal of Biological Chemistry. 1998;273(51):34316–34327. doi: 10.1074/jbc.273.51.34316. [DOI] [PubMed] [Google Scholar]
- 17.Park K., Scott A. L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. Journal of Leukocyte Biology. 2010;88(6):1081–1087. doi: 10.1189/jlb.0610318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Wei Z., Ma X., et al. 25-Hydroxycholesterol activates the expression of cholesterol 25-hydroxylase in an LXR-dependent mechanism. Journal of Lipid Research. 2018;59(3):439–451. doi: 10.1194/jlr.M080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda A., Miyazaki T., Ikegami T., et al. Cholesterol 25-hydroxylation activity of CYP3A. Journal of Lipid Research. 2011;52(8):1509–1516. doi: 10.1194/jlr.M014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diczfalusy U., Björkhem I. Still another activity by the highly promiscuous enzyme CYP3A4: 25-hydroxylation of cholesterol. Journal of Lipid Research. 2011;52(8):1447–1449. doi: 10.1194/jlr.E017806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diczfalusy U., Olofsson K. E., Carlsson A. M., et al. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. Journal of Lipid Research. 2009;50(11):2258–2264. doi: 10.1194/jlr.M900107-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanc M., Hsieh W. Y., Robertson K. A., et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38(1):106–118. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Martin M., Zhang J., et al. Krüppel-like factor 4 regulation of cholesterol-25-hydroxylase and liver X receptor mitigates atherosclerosis susceptibility. Circulation. 2017;136(14):1315–1330. doi: 10.1161/CIRCULATIONAHA.117.027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magoro T., Dandekar A., Jennelle L. T., et al. IL-1β/TNF-α/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. The Journal of Biological Chemistry. 2019;294(40):14591–14602. doi: 10.1074/jbc.RA119.007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold E. S., Ramsey S. A., Sartain M. J., et al. ATF3 protects against atherosclerosis by suppressing 25-hydroxycholesterol-induced lipid body formation. The Journal of Experimental Medicine. 2012;209(4):807–817. doi: 10.1084/jem.20111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L., Nelson E. R. Oxysterols and nuclear receptors. Molecular and Cellular Endocrinology. 2019;484:42–51. doi: 10.1016/j.mce.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Janowski B. A., Grogan M. J., Jones S. A., et al. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(1):266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Wei Z., Zhang Y., et al. Activation of liver X receptor plays a central role in antiviral actions of 25-hydroxycholesterol. Journal of Lipid Research. 2018;59(12):2287–2296. doi: 10.1194/jlr.M084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jetten A. M., Takeda Y., Slominski A., Kang H. S. Retinoic acid-related orphan receptor γ (RORγ): connecting sterol metabolism to regulation of the immune system and autoimmune disease. Current Opinion in Toxicology. 2018;8:66–80. doi: 10.1016/j.cotox.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soroosh P., Wu J., Xue X., et al. Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12163–12168. doi: 10.1073/pnas.1322807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappano R., Recchia A. G., de Francesco E. M., et al. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor α-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6(1, article e16631) doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannedouche S., Zhang J., Yi T., et al. Oxysterols direct immune cell migration via EBI2. Nature. 2011;475(7357):524–527. doi: 10.1038/nature10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C., Yang X. V., Wu J., et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475(7357):519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 34.Endo-Umeda K., Makishima M. Liver X receptors regulate cholesterol metabolism and immunity in hepatic nonparenchymal cells. International Journal of Molecular Sciences. 2019;20(20, article 5045) doi: 10.3390/ijms20205045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leussink S., Aranda-Pardos I., A-Gonzalez N. Lipid metabolism as a mechanism of immunomodulation in macrophages: the role of liver X receptors. Current Opinion in Pharmacology. 2020;53:18–26. doi: 10.1016/j.coph.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Dzhagalov I., Zhang N., He Y. W. The roles of orphan nuclear receptors in the development and function of the immune system. Cellular & Molecular Immunology. 2004;1(6):401–407. [PubMed] [Google Scholar]
- 37.Zhao L., Zhou S., Gustafsson J. Å. Nuclear receptors: recent drug discovery for cancer therapies. Endocrine Reviews. 2019;40(5):1207–1249. doi: 10.1210/er.2018-00222. [DOI] [PubMed] [Google Scholar]
- 38.Kumar N., Solt L. A., Conkright J. J., et al. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Molecular Pharmacology. 2010;77(2):228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin L., Martynowski D., Zheng S., Wada T., Xie W., Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Molecular Endocrinology. 2010;24(5):923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daugvilaite V., Arfelt K. N., Benned-Jensen T., Sailer A. W., Rosenkilde M. M. Oxysterol-EBI2 signaling in immune regulation and viral infection. European Journal of Immunology. 2014;44(7):1904–1912. doi: 10.1002/eji.201444493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang S., Mo Z., Sun S., Yin K., Lv Y. Emerging role of Insig-1 in lipid metabolism and lipid disorders. Clinica Chimica Acta. 2020;508:206–212. doi: 10.1016/j.cca.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler S., Sillence D. J. Niemann-Pick type C disease: cellular pathology and pharmacotherapy. Journal of Neurochemistry. 2020;153(6):674–692. doi: 10.1111/jnc.14895. [DOI] [PubMed] [Google Scholar]
- 43.Feng X., Cozma C., Pantoom S., et al. Determination of the pathological features of NPC1 variants in a cellular complementation test. International Journal of Molecular Sciences. 2019;20(20, article 5185) doi: 10.3390/ijms20205185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frolov A., Zielinski S. E., Crowley J. R., Dudley-Rucker N., Schaffer J. E., Ory D. S. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. The Journal of Biological Chemistry. 2003;278(28):25517–25525. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- 45.Pietrangelo A., Ridgway N. D. Bridging the molecular and biological functions of the oxysterol-binding protein family. Cellular and Molecular Life Sciences. 2018;75(17):3079–3098. doi: 10.1007/s00018-018-2795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan D., Mäyränpää M. I., Wong J., et al. OSBP-related protein 8 (ORP8) suppresses ABCA1 expression and cholesterol efflux from macrophages. The Journal of Biological Chemistry. 2008;283(1):332–340. doi: 10.1074/jbc.M705313200. [DOI] [PubMed] [Google Scholar]
- 47.Li J., Zheng X., Lou N., Zhong W., Yan D. Oxysterol binding protein-related protein 8 mediates the cytotoxicity of 25-hydroxycholesterol. Journal of Lipid Research. 2016;57(10):1845–1853. doi: 10.1194/jlr.M069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alpy F., Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. Journal of Cell Science. 2005;118(13):2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Agudo D., Ren S., Hylemon P. B., et al. Human StarD5, a cytosolic StAR-related lipid binding protein. Journal of Lipid Research. 2005;46(8):1615–1623. doi: 10.1194/jlr.M400501-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Agudo D., Ren S., Hylemon P. B., et al. Localization of StarD5 cholesterol binding protein. Journal of Lipid Research. 2006;47(6):1168–1175. doi: 10.1194/jlr.M500447-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Brown M. S., Goldstein J. L. Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. Journal of Lipid Research. 2009;50(Supplement):S15–S27. doi: 10.1194/jlr.R800054-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griffiths W. J., Wang Y. Oxysterol research: a brief review. Biochemical Society Transactions. 2019;47(2):517–526. doi: 10.1042/BST20180135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RA D. B.-B., Ye J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends in Biochemical Sciences. 2018;43(5):358–368. doi: 10.1016/j.tibs.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng X., Li J., Guo D. SCAP/SREBPs are central players in lipid metabolism and novel metabolic targets in cancer therapy. Current Topics in Medicinal Chemistry. 2018;18(6):484–493. doi: 10.2174/1568026618666180523104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radhakrishnan A., Ikeda Y., Kwon H. J., Brown M. S., Goldstein J. L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(16):6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong Y., Lee J. N., Lee P. C. W., Goldstein J. L., Brown M. S., Ye J. Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metabolism. 2006;3(1):15–24. doi: 10.1016/j.cmet.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Wang B., Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nature Reviews. Endocrinology. 2018;14(8):452–463. doi: 10.1038/s41574-018-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sparrow C. P., Baffic J., Lam M. H., et al. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. The Journal of Biological Chemistry. 2002;277(12):10021–10027. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 59.Worthmann A., John C., Rühlemann M. C., et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nature Medicine. 2017;23(7):839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 60.Javitt N. B. Cholesterol, hydroxycholesterols, and bile acids. Biochemical and Biophysical Research Communications. 2002;292(5):1147–1153. doi: 10.1006/bbrc.2001.2013. [DOI] [PubMed] [Google Scholar]
- 61.Hubler M. J., Kennedy A. J. Role of lipids in the metabolism and activation of immune cells. The Journal of Nutritional Biochemistry. 2016;34:1–7. doi: 10.1016/j.jnutbio.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J., Chen J., Li M., Chen M., Sun C. Multifaceted functions of CH25H and 25HC to modulate the lipid metabolism, immune responses, and broadly antiviral activities. Viruses. 2020;12(7):p. 727. doi: 10.3390/v12070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibata N., Carlin A. F., Spann N. J., et al. 25-Hydroxycholesterol activates the integrated stress response to reprogram transcription and translation in macrophages. The Journal of Biological Chemistry. 2013;288(50):35812–35823. doi: 10.1074/jbc.M113.519637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu S. Y., Aliyari R., Chikere K., et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38(1):92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cagno V., Civra A., Rossin D., et al. Inhibition of herpes simplex-1 virus replication by 25-hydroxycholesterol and 27-hydroxycholesterol. Redox Biology. 2017;12:522–527. doi: 10.1016/j.redox.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwamoto M., Watashi K., Tsukuda S., et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochemical and Biophysical Research Communications. 2014;443(3):808–813. doi: 10.1016/j.bbrc.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 67.Sagan S. M., Rouleau Y., Leggiadro C., et al. The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication. Biochemistry and Cell Biology. 2006;84(1):67–79. doi: 10.1139/o05-149. [DOI] [PubMed] [Google Scholar]
- 68.Yuan S., Chan C. C. Y., Chik K. K. H., et al. Broad-spectrum host-based antivirals targeting the interferon and lipogenesis pathways as potential treatment options for the pandemic coronavirus disease 2019 (COVID-19) Viruses. 2020;12(6):p. 628. doi: 10.3390/v12060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arita M., Kojima H., Nagano T., Okabe T., Wakita T., Shimizu H. Oxysterol-binding protein family I is the target of minor enviroxime-like compounds. Journal of Virology. 2013;87(8):4252–4260. doi: 10.1128/JVI.03546-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S., Li L., Zhu H., et al. Cholesterol 25-hydroxylase inhibits encephalomyocarditis virus replication through enzyme activity-dependent and independent mechanisms. Veterinary Microbiology. 2020;245, article 108658 doi: 10.1016/j.vetmic.2020.108658. [DOI] [PubMed] [Google Scholar]
- 71.Civra A., Cagno V., Donalisio M., et al. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Scientific Reports. 2015;4(1, article 7487) doi: 10.1038/srep07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Civra A., Francese R., Gamba P., et al. 25-Hydroxycholesterol and 27-hydroxycholesterol inhibit human rotavirus infection by sequestering viral particles into late endosomes. Redox Biology. 2018;19:318–330. doi: 10.1016/j.redox.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pombo J. P., Sanyal S. Perturbation of intracellular cholesterol and fatty acid homeostasis during flavivirus infections. Frontiers in Immunology. 2018;9, article 1276 doi: 10.3389/fimmu.2018.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bielska A. A., Olsen B. N., Gale S. E., et al. Side-chain oxysterols modulate cholesterol accessibility through membrane remodeling. Biochemistry. 2014;53(18):3042–3051. doi: 10.1021/bi5000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ke W., Fang L., Jing H., et al. Cholesterol 25-hydroxylase inhibits porcine reproductive and respiratory syndrome virus replication through enzyme activity-dependent and -independent mechanisms. Journal of Virology. 2017;91(19, article e00827) doi: 10.1128/JVI.00827-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu T., Ma F., Ma X., et al. Regulating innate and adaptive immunity for controlling SIV infection by 25-hydroxycholesterol. Frontiers in Immunology. 2018;9, article 2686 doi: 10.3389/fimmu.2018.02686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ludigs K., Parfenov V., Du Pasquier R. A., Guarda G. Type I IFN-mediated regulation of IL-1 production in inflammatory disorders. Cellular and Molecular Life Sciences. 2012;69(20):3395–3418. doi: 10.1007/s00018-012-0989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guarda G., Braun M., Staehli F., et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Reboldi A., Dang E. V., McDonald J. G., Liang G., Russell D. W., Cyster J. G. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345(6197):679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viaud M., Ivanov S., Vujic N., et al. Lysosomal cholesterol hydrolysis couples efferocytosis to anti-inflammatory oxysterol production. Circulation Research. 2018;122(10):1369–1384. doi: 10.1161/CIRCRESAHA.117.312333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo C., Chi Z., Jiang D., et al. Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 inflammasome activation and cholesterol biosynthetic signaling in macrophages. Immunity. 2018;49(5):842–856.e7. doi: 10.1016/j.immuni.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 82.Dang E. V., McDonald J. G., Russell D. W., Cyster J. G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell. 2017;171(5):1057–1071.e11. doi: 10.1016/j.cell.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Derangère V., Chevriaux A., Courtaut F., et al. Liver X receptor β activation induces pyroptosis of human and murine colon cancer cells. Cell Death and Differentiation. 2014;21(12):1914–1924. doi: 10.1038/cdd.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang J., Park S., Jin Hur H., et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nature Communications. 2016;7(1, article 13129) doi: 10.1038/ncomms13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong M. Y., Lewis M., Doherty J. J., et al. 25-Hydroxycholesterol amplifies microglial IL-1β production in an apoE isoform-dependent manner. Journal of Neuroinflammation. 2020;17(1):p. 192. doi: 10.1186/s12974-020-01869-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pokharel S. M., Shil N. K., GC J. B., et al. Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nature Communications. 2019;10(1, article 1482) doi: 10.1038/s41467-019-09453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu H., Spieler F., Großmann J., et al. Interleukin-1 potently contributes to 25-hydroxycholesterol-induced synergistic cytokine production in smooth muscle cell-monocyte interactions. Atherosclerosis. 2014;237(2):443–452. doi: 10.1016/j.atherosclerosis.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Palozza P., Simone R., Catalano A., et al. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. The Journal of Nutritional Biochemistry. 2011;22(3):259–268. doi: 10.1016/j.jnutbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Dugas B., Charbonnier S., Baarine M., et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: cytoprotective effects and prevention of VEGF secretion by resveratrol. European Journal of Nutrition. 2010;49(7):435–446. doi: 10.1007/s00394-010-0102-2. [DOI] [PubMed] [Google Scholar]
- 90.Prunet C., Montange T., Véjux A., et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry Part A. 2006;69A(5):359–373. doi: 10.1002/cyto.a.20272. [DOI] [PubMed] [Google Scholar]
- 91.Perucha E., Melchiotti R., Bibby J. A., et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nature Communications. 2019;10(1):p. 498. doi: 10.1038/s41467-019-08332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vigne S., Chalmin F., Duc D., et al. IL-27-induced type 1 regulatory T-cells produce oxysterols that constrain IL-10 production. Frontiers in Immunology. 2017;8, article 1184 doi: 10.3389/fimmu.2017.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu L., Shen S., Ma Y., et al. 25-Hydroxycholesterol-3-sulfate attenuates inflammatory response via PPARγ signaling in human THP-1 macrophages. American Journal of Physiology. Endocrinology and Metabolism. 2012;302(7):E788–E799. doi: 10.1152/ajpendo.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aye I. L. M. H., Waddell B. J., Mark P. J., Keelan J. A. Oxysterols exert proinflammatory effects in placental trophoblasts via TLR4-dependent, cholesterol-sensitive activation of NF-κB. Molecular Human Reproduction. 2012;18(7):341–353. doi: 10.1093/molehr/gas001. [DOI] [PubMed] [Google Scholar]
- 95.Wang F., Xia W., Liu F., Li J., Wang G., Gu J. Interferon regulator factor 1/retinoic inducible gene I (IRF1/RIG-I) axis mediates 25-hydroxycholesterol-induced interleukin-8 production in atherosclerosis. Cardiovascular Research. 2012;93(1):190–199. doi: 10.1093/cvr/cvr260. [DOI] [PubMed] [Google Scholar]
- 96.Lemaire-Ewing S., Berthier A., Royer M. C., et al. 7β-Hydroxycholesterol and 25-hydroxycholesterol-induced interleukin-8 secretion involves a calcium-dependent activation of c-fos via the ERK1/2 signaling pathway in THP-1 cells. Cell Biology and Toxicology. 2009;25(2):127–139. doi: 10.1007/s10565-008-9063-0. [DOI] [PubMed] [Google Scholar]
- 97.Baulies A., Ribas V., Núñez S., et al. Lysosomal cholesterol accumulation sensitizes to acetaminophen hepatotoxicity by impairing mitophagy. Scientific Reports. 2016;5(1, article 18017) doi: 10.1038/srep18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tricarico P. M., Gratton R., Braga L., Celsi F., Crovella S. 25-Hydroxycholesterol and inflammation in Lovastatin-deregulated mevalonate pathway. The International Journal of Biochemistry & Cell Biology. 2017;92:26–33. doi: 10.1016/j.biocel.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 99.Kim H., Choi S. Y., Lim J., Lindroth A. M., Park Y. J. EHMT2 inhibition induces cell death in human non-small cell lung cancer by altering the cholesterol biosynthesis pathway. International Journal of Molecular Sciences. 2020;21(3, article 1002) doi: 10.3390/ijms21031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charman M., Colbourne T. R., Pietrangelo A., Kreplak L., Ridgway N. D. Oxysterol-binding protein (OSBP)-related protein 4 (ORP4) is essential for cell proliferation and survival. The Journal of Biological Chemistry. 2014;289(22):15705–15717. doi: 10.1074/jbc.M114.571216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhong W., Pan G., Wang L., et al. ORP4L facilitates macrophage survival via G-protein-coupled signaling: ORP4L-/- mice display a reduction of atherosclerosis. Circulation Research. 2016;119(12):1296–1312. doi: 10.1161/CIRCRESAHA.116.309603. [DOI] [PubMed] [Google Scholar]
- 102.Sekiya M., Yamamuro D., Ohshiro T., et al. Absence of Nceh1 augments 25-hydroxycholesterol-induced ER stress and apoptosis in macrophages. Journal of Lipid Research. 2014;55(10):2082–2092. doi: 10.1194/jlr.M050864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong Q., Chen Y., Liu W., et al. 25-Hydroxycholesterol promotes vascular calcification via activation of endoplasmic reticulum stress. European Journal of Pharmacology. 2020;880, article 173165 doi: 10.1016/j.ejphar.2020.173165. [DOI] [PubMed] [Google Scholar]


