Abstract
Objectives
A seroprevalence study of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was conducted in a high-incidence area located in northeastern Italy.
Methods
All citizens above 10 years of age resident in five municipalities of the Autonomous Province of Trento, with the highest incidence of coronavirus disease 2019 (COVID-19) cases, were invited to participate in the study. Among 6098 participants, 6075 sera and a standardized questionnaire administered face-to-face were collected between 5 May and 15 May 2020 and examined. Symptomatic individuals and their family contacts were tested by RT-PCR. Anti-SARS-CoV-2 antibodies were detected using an Abbott SARS-CoV-2 IgG assay, which was performed on the Abbott Architect i2000SR automated analyser. Seroprevalence was calculated as the proportion of positive results among the total number tested. A multivariable logistic regression model was performed to assess the relationship between seropositive versus seronegative individuals for a set of explanatory variables.
Results
A total of 1402 participants were positive for IgG antibodies against SARS-CoV-2, with a prevalence of 23.1% (1402/6075). The highest prevalence was found in the age class 40–49 years. Overall, 34.4% (2096/6098) of the participants reported at least one symptom. The ratio between reported cases identified by molecular test and those with seropositive results was 1:3, with a maximum ratio of about 1:7 in the age group <20 years and a minimum around 1:1 in those >70 years old. The infection fatality rate was 2.5% (35/1402). Among the symptoms, anosmia and ageusia were strongly associated with seropositivity.
Conclusions
The estimated seroprevalence of 23% was three-fold higher than the number of cases reported in the COVID-19 Integrated Surveillance data in the study area. This may be explained in part by a relatively high number of individuals presenting mild or no illness, especially those of younger age, and people who did not seek medical care or testing, but who may contribute to virus transmission in the community.
Keywords: IgG, Infection fatality rate, Population-based study, SARS-CoV-2, Seroprevalence
Introduction
It is widely known that the number of cases of coronavirus disease 2019 (COVID-19) that is reported to the surveillance systems largely underestimates the impact of the disease, because a large proportion of cases are not recognized because they are asymptomatic, paucisymptomatic, or just not tested. Seroprevalence studies are needed to estimate the number of people who have been exposed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a specific area, to provide information on symptoms associated with infection, disease severity and infection fatality rate.
As reported by Havers et al. [1] from several geographic sites in the USA, between 6 and 24 times more infections were estimated per site using seroprevalence than with COVID-19 case report data. A recent nationwide seroprevalence study in Spain showed remarkable geographical variation [2].
In the Autonomous Province (AP) of Trento, in northeastern Italy, a large number of people visited ski resorts between February and March 2020. This contributed to the introduction and autochthonous spread of the infection among the resident population. In particular, five localities reported a high incidence of COVID-19. After the national lockdown, which was implemented on 11 March 2020, the number of cases started to decrease; the incidence in the study area, reported as 23 per 1000 inhabitants in March, was 0.3 per 1000 in June (source: COVID-19 Integrated surveillance in Italy, Istituto Superiore di Sanità, unpublished data). Overall, 412 COVID-19 cases (cumulative incidence 52.2 cases per 1000 inhabitants, source: COVID-19 Integrated surveillance in Italy, Istituto Superiore di Sanità, unpublished data) and 35 deaths were reported, since the beginning of the pandemic, in the five municipalities involved in the seroprevalence study (see Supplementary material, Fig. S1).
To evaluate the spread of SARS-CoV-2 infection in the five municipalities of the AP of Trento a seroprevalence study was conducted to estimate the proportion of SARS-CoV-2 seropositive individuals. The association of antibody positivity with self-reported symptoms and secondary transmission to close contacts of individuals with COVID-19 were also investigated.
Methods
Study population and design
The study was conducted in five municipalities of the AP of Trento with the highest incidence of COVID-19 confirmed cases (23.0 per 1000 inhabitants by 31 March 2020): Borgo Chiese (cumulative incidence rate: 25.6/1000), Campitello di Fassa (24.0/1000), Canazei (27.6/1000), Pieve di Bono-Prezzo (19.4/1000) and Vermiglio (18.7/1000).
All the COVID-19 cases confirmed by real-time PCR are reported to the local surveillance system and to the Integrated Surveillance data of the Istituto Superiore di Sanità, (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard).
To the purpose of the study, the Azienda Provinciale per i Servizi Sanitari (APSS), Department of Prevention, sent a letter of invitation to all citizens resident in the five municipalities who were at least 10 years old. Individuals with severe disease or who were institutionalized in nursing homes were excluded. The serosurvey was conducted between 5 May and 15 May 2020.
The study consisted of a serological test performed on venous blood and the administration of a standardized questionnaire face-to-face. Within the study, individuals presenting symptoms since 1 April 2020 suggestive of COVID-19 were tested by real-time PCR, and their family contacts were traced and laboratory confirmed for SARS-CoV-2.
Serum preparation and storage
Blood samples (5 mL) were collected in serum separator tubes (BD Diagnostic Systems, Franklin Lakes, NJ, USA) and centrifuged at room temperature at 1100g for 10 min. Aliquots were transferred to 2-mL polypropylene, screw-cap cryo tubes (Sorfa, Zhejiang, China) and immediately frozen at –20°C. Frozen sera were then shipped to the Istituto Superiore di Sanità laboratory in dry ice following biosafety shipment conditions. Upon arrival, serum samples were immediately stored at –80°C.
SARS-CoV-2 IgG immunoassay
Abbott SARS-CoV-2 IgG assays were performed on the Abbott Architect i2000SR automated analyser (Abbott Diagnostics, Chicago, IL, USA) according to the manufacturer's instructions. Before the assay, sera were thawed and a volume of 600 μL was transferred to analysis tubes (BD Vacutainer No Additive (Z) Plus Tube, BD Diagnostic Systems). The assay is a chemiluminescent immunoassay that detects IgG directed against the SARS-CoV-2 nucleocapsid protein, measured as a relative light unit (RLU). There is a direct relationship between the concentration of IgG antibodies to SARS-CoV-2 in the sample and the RLU. The result for the SARS-CoV-2 IgG assay is given as the index (S/C), i.e. the ratio between sample RLU and the calibrator mean chemiluminescent signal from three calibrator replicates. An index of ≥1.4 is interpreted as positive and an index of <1.4 as negative.
Real-time PCR on swab samples
The assay for molecular detection of SARS-CoV-2 on swabs was performed using the Abbott RealTime SARS-CoV-2 assay, on the Abbott m2000System. The SARS-CoV-2 primer and probe sets are designed to detect RNA from SARS-CoV-2 in naso/oropharyngeal swabs. A sample volume of 0.5 mL was extracted and a volume of 40 μL was used in the reaction.
Statistical analysis
Seroprevalence data were presented as proportions with 95% CI. Data were also analysed by age group (<20, 20–29, 30–39, 40–49, 50–59, 60–69, 70+ years), gender and geographical area (municipality). Differences among percentages of individuals with seropositive results were assessed by χ2 or Fisher exact tests. The infection fatality rate was estimated using the number of deaths as numerator and the number of infected individuals (calculated using seroprevalence and population size) as denominator. To establish the association between SARS-CoV-2 antibody (IgG) prevalence and symptoms, we used logistic regression models and the odds ratio (OR) as the measure of association.
A multivariable logistic regression model was used to determine the relationship between seroprevalence (positive versus negative) and a set of explanatory variables. The following variables that were significantly associated (p < 0.10) in the univariate analysis were included in the multivariable model: gender, age group, geographical area, presence of symptoms, working in contact with the public and household size. The likelihood ratio test was used to compare different models. In all analyses, a p value < 0.05 was considered statistically significant. Statistical analysis was performed by STATA version 16.1 (STATA Corp., College Station, TX, USA).
Ethical approval
Informed consensus for blood collection was obtained from all the participants. The study was approved by the Ethics Committee of the Istituto Superiore di Sanità (Prot. PRE BIO CE n.15997, 04.05.2020).
Results
Study plan and demographic characteristics
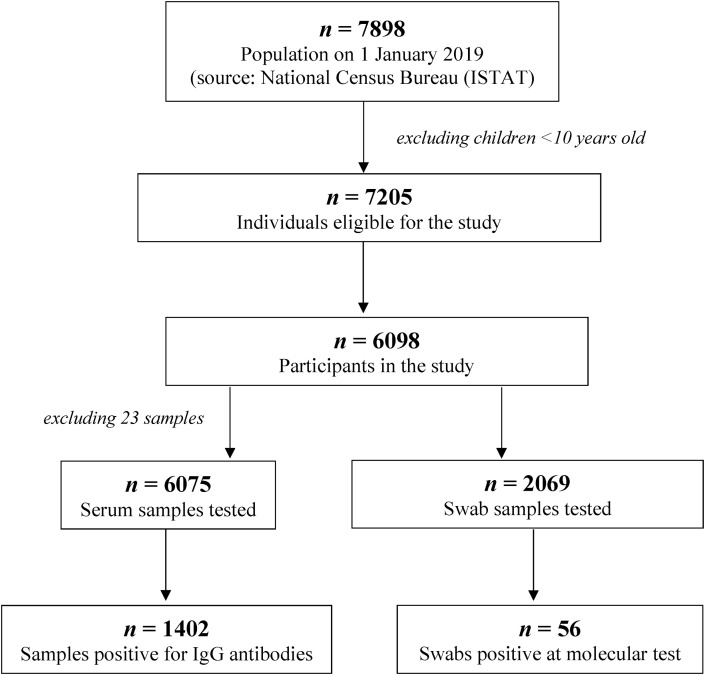
Fig. 1 shows the flow chart describing the study plan and sample collection. Based on the census data, 7898 individuals resident in the municipalities were involved in the study (population on 1 January 2019; source: National Census Bureau, ISTAT). After excluding children <10 years old, 7205 individuals remained eligible for the study. Overall, 84.6% (6098/7205) of individuals participated in the serosurvey from 5 to 15 May 2020 (range 74.6% Borgo Chiese to 89.6% Campitello di Fassa). The participation of eligible individuals in the study was homogeneous among the different age groups (see Supplementary material, Table S1).
Fig. 1.
Flow chart of population and samples analysed in the seroprevalence study.
The median age of the participants was 50 years (range 10–98 years) with differences among the municipalities (range from 48 years in the municipality of Canazei to 51 years in the municipality of Pieve di Bono, Borgo Chiese and Campitello; p 0.007). No difference in participation was found between males and females (51.3% of the participants were female).
Seroprevalence
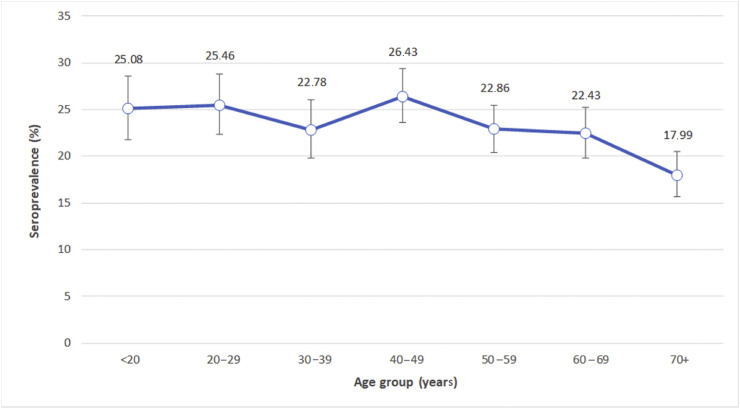
Overall, 6075 serum samples were tested. Twenty-three sera were not analysed because the subsample was not sufficient or was inadequate for the analysis. A total of 1402 participants were positive for IgG antibodies against SARS-CoV-2, with a prevalence of 23.1% (95% CI 22.0%–24.1%. Seroprevalence data stratified by municipality of residence ranged from 17.8% to 27.7% (see Supplementary material, Table S2). After stratifying by age class, the highest seroprevalence was found in the age class 40–49 years and appeared to decrease with increasing age, being as low as 18.0% in the ≥70 years group (Fig. 2 ). Higher seropositivity was observed among male participants compared with female (24.4% versus 21.8%, respectively; p 0.018).
Fig. 2.
Seroprevalence (%) of SARS-CoV-2 by age; bars indicate 95% confidence intervals.
Factors associated with seropositivity
The multivariable logistic regression model showed that age group, gender, municipality of residence, presence of symptoms, and working in contact with the public were associated with seropositivity (Table 1 ). The number of individuals in the household was not included in the multivariable logistic regression model because it was reported for only 4778 participants. However, the seroprevalence by household size is shown in Table 2 . The highest seroprevalence was observed in large households, ranging from 21.2% (95% CI 19.8%–22.6%) for household size of three or less to 30.0% (95% CI 27.7%–32.5%) for household size of more than three.
Table 1.
Factors associated with seropositivity (multivariable logistic regression model)
| Variables | OR | 95% CI |
|---|---|---|
| Municipalities | ||
| Pieve di Bono | Ref | |
| Borgo Chiese | 1.23 | 1.01–1.51 |
| Campitello | 1.51 | 1.18–1.93 |
| Canazei | 1.74 | 1.43–2.12 |
| Vermiglio | 1.40 | 1.15–1.70 |
| Gender | ||
| Female | Ref | |
| Male | 1.18 | 1.04–1.33 |
| Age group (years) | ||
| 70+ | Ref | |
| <20 | 1.46 | 1.14–1.87 |
| 20–29 | 1.44 | 1.13–1.85 |
| 30–39 | 1.22 | 0.95–1.57 |
| 40–49 | 1.47 | 1.16–1.86 |
| 50–59 | 1.22 | 0.97–1.54 |
| 60–69 | 1.27 | 1.01–1.60 |
| Presence of symptoms | ||
| No | Ref | |
| Yes | 1.39 | 1.23–1.58 |
| Working in contact with the public | ||
| No | Ref | |
| Yes | 1.17 | 1.01–1.34 |
Table 2.
Seroprevalence (%) by household size
| Household size | Number of households | Number of individuals | Number of positive individuals | Percentage of positive individuals | 95% CI |
|---|---|---|---|---|---|
| 1 | 172 | 171 | 33 | 19.30 | 13.67–26.02 |
| 2 | 973 | 1943 | 400 | 20.59 | 18.81–22.45 |
| 3 | 410 | 1229 | 276 | 22.46 | 20.15–24.90 |
| 4 | 283 | 1127 | 335 | 29.72 | 27.07–32.49 |
| 5 | 48 | 238 | 70 | 29.41 | 23.70–35.64 |
| 6 | 9 | 54 | 19 | 35.19 | 22.68–49.38 |
| 7 | 0 | 0 | 0 | NA | NA |
| 8 | 2 | 16 | 7 | 43.75 | 19.75–70.12 |
| Any | 1897 | 4778 | 1140 | 23.86 | 22.66–25.09 |
| 1 to 3 | 1555 | 3343 | 709 | 21.21 | 19.83–22.63 |
| >3 | 342 | 1435 | 431 | 30.03 | 27.67–32.48 |
Abbreviation: NA, not available.
Symptoms associated with seropositivity
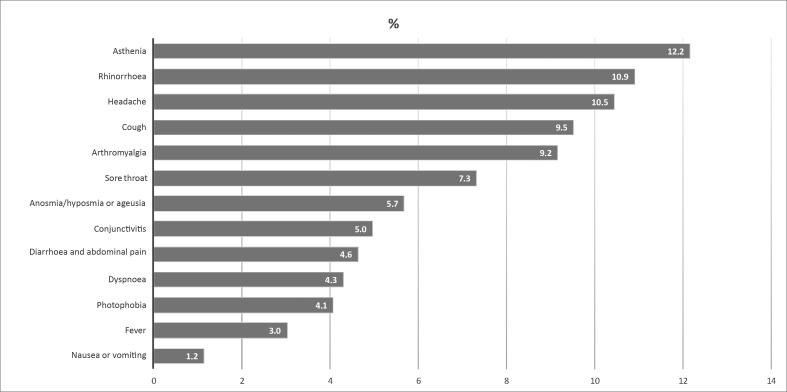
Since 1 April 2020, 34.4% (2096/6098) of the study participants reported at least one symptom. In particular, 15% (916/6098) presented one symptom, 6.9% (421/6098) two symptoms, and the remaining 12.5% (759/6098) three or more symptoms. Fever was reported by 175 individuals (2.9%).
Fig. 3 shows the distribution of the main symptoms reported by population. Asthenia, rhinorrhoea and headache were the most frequent symptoms (>10%).
Fig. 3.
Percentage of symptoms reported by the participants analysed in the study.
Seropositive participants were more likely to report at least one symptom: 26.3% (548/2085) versus 21.4% (854/3990) of seropositive participants without symptoms (p < 0.001).
Olfactory and gustatory dysfunctions, anosmia and/or ageusia, were more likely to be reported by seropositive participants compared with seronegative participants (OR 7.9; p < 0.001). Fever was also strongly associated with IgG positivity (OR 3.3; p < 0.001). Weakness (OR 1.6; p < 0.001), cough (OR 1.4; p 0.001), dyspnoea (OR 1.4; p < 0.009), arthralgia (OR 1.3; p 0.006), diarrhoea and abdominal pain (OR 1.4; p 0.009) and vomiting (OR 1.8; p 0.022) were also associated with positive serology. Other symptoms, such as pharyngodynia, headache, rhinorrhoea, photophobia and conjunctivitis were not associated with seropositivity, (see Supplementary material, Table S3).
Molecular analysis on swab samples
Overall, 2069 samples were tested and 56 produced RT-PCR positives (2.7%). Among these 56 participants, only seven resulted negative for IgG (of them, five reported at least one symptom) and 49 were positive for IgG. Finally, 575 individuals with negative swabs had anti-SARS-CoV-2 IgG antibodies, suggesting past exposure to the virus.
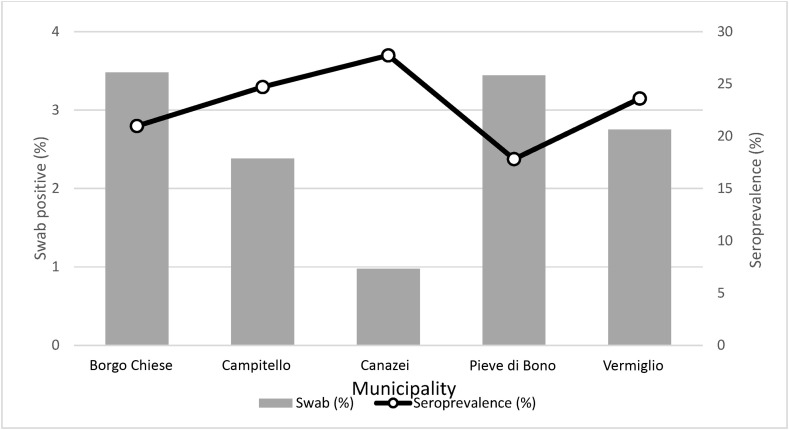
A lower proportion of positive swabs in the municipalities with higher seroprevalence was observed, (e,g, in Canazei municipality; Fig. 4 ).
Fig. 4.
Comparison between seroprevalence (%) and the proportion of positivity on swabs in each Municipality.
Comparison between seroprevalence results and COVID-19 cases reported by the integrated surveillance data
The number of individuals with COVID-19 reported by the Integrated surveillance data was underestimated, especially in the younger age groups (Table 3 ). Overall, a ratio of 3.4 cases identified by serology test versus 1 by molecular test was observed, with a maximum rate of about 7:1 in the age group <20 years and a minimum of about 1:1 in the age group >70 years. Overall, the infection fatality rate was 2.5%, ranging from 0 in those less than 20 years of age to 16.6% in those >70 years old (data not shown).
Table 3.
Comparison between SARS-CoV-2 IgG positives identified in the study and COVID-19 cases reported (last update, 29 July 2020) by the Integrated National Surveillance data in the same area by age
| Age (years) | SARS-CoV-2 IgG positives | COVID-19 cases by NSS | Ratio |
|---|---|---|---|
| <20 | 163 | 23 | 7.1 |
| 20-29 | 179 | 31 | 5.8 |
| 30-39 | 164 | 39 | 4.2 |
| 40-49 | 241 | 50 | 4.8 |
| 50-59 | 262 | 51 | 5.1 |
| 60-69 | 216 | 66 | 3.3 |
| 70+ | 175 | 147 | 1.2 |
| Total | 1400 | 407 | 3.4 |
Abbreviations: COVID-19, coronavirus disease 2019; NSS, Integrated National Surveillance; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
We investigated the prevalence of anti-SARS-CoV-2 antibodies at the beginning of the reopening phase (the so-called ‘phase 2’), after the 2-month lockdown period, in the population of five municipalities with a high incidence of COVID-19 located in an area of northeast Italy.
Overall, the results indicate that almost one-fifth of the population developed an antibody response, suggesting that they acquired the infection during the initial pandemic wave. The Abbott SARS-CoV-2 IgG test is reported to have a specificity of 100% (95% CI 97.1%–100.0%) and a sensitivity of 99.6% (95% CI 99.0%–99.9%) [3]. Independent evaluation found a sensitivity of 100% and a specificity of 99.9% 17 days after appearance of symptoms [4]. However, analysis based on stored blood samples from PCR-positive individuals found a lower sensitivity [5]. Moreover, test sensitivity may depend on infection severity, with lower antibody titres generally associated with younger age, and milder infections [6], and sensitivity varying depending on time from onset of symptoms [7,8].
When we recalculated estimates of prevalence adjusted for a test sensitivity of 90%, the prevalence increased homogeneously in each age group, without any specific and additional difference (see Supplementary material, Table S4).
Serological surveys are the best tool to determine the spread of an infectious disease, particularly in the presence of asymptomatic cases or incomplete ascertainment of those with symptoms. Other SARS-CoV-2 seroprevalence studies have been conducted in different areas of the world [1,2,[9], [10], [11]] showing that for every reported case the real number of infections in the community is higher. The relatively low seroprevalence observed in the context of an intense epidemic [1,2,[9], [10], [11]], with the consequence of a large susceptible population, emphasizes the need to maintain public health measures to avoid a new epidemic wave [[12], [13], [14]].
In this study, geographical variation within the considered area was observed, with prevalence ranging between 18% and 25%. We also found that the youngest age groups had higher seroprevalence, suggesting greater exposure to the virus but less susceptibility to the disease. However, the lower proportion of seropositive individuals in the age group >70 years can be partially explained by the fact that some elderly people with laboratory-confirmed results on swab died or were hospitalized, so they did not participate in the study. In fact, 35 deaths have been registered among elderly individuals during the study period. However, older people may have fewer social contacts due to more prudent behaviour. Male gender was associated with higher seroprevalence, which, however, does not completely explain the higher risk of developing full-blown, or even more severe disease.
Anosmia and ageusia were strongly associated with the presence of antibodies. As expected, other symptoms, such as fever and cough, were also more likely to occur among seropositive individuals compared with seronegative individuals; however, the association was weaker, perhaps because of the concomitant occurrence of other viral illnesses, which may cause similar complaints.
The high estimated number of seropositive individuals compared with reported cases may be the consequence of the high number of persons who have mild or no illness or who do not seek medical care or testing, but who still contribute to virus transmission in the community.
When combined with local surveillance data, age-specific seroprevalence estimates can lead to robust estimates of the infection risk, which is crucial for the post-lockdown strategies. Differences in the antibody positivity among the five municipalities were probably the result of different times of sampling from the beginning of the spread of COVID-19 cases. Interestingly, seropositivity levels were higher in families with more members, suggesting a higher chance of becoming infected in overcrowded settings or higher probability of multiple introductions into the household. Findings regarding the association between seroprevalence level and household size or the presence of specific symptoms were consistent with those reported by other studies [15,16].
Before drawing conclusions, strengths and limits should be mentioned. First, the refusal rate was low, so the possibility of a selection bias was minimized. The information regarding the presence of symptoms eventually associated with the presence of antibodies was retrospectively collected, so a recall bias cannot be excluded. Moreover, other viruses might have co-circulated in the study area in the same period. Third, reported symptoms may be underestimated because they referred only to the month of April. This may have led to conservative estimates of the association between specific symptoms and seropositivity. However, the results suggest a high predictive value for specific symptoms such as anosmia. Finally, although the serological assay we used is assumed to have high sensitivity and specificity, the occurrence of some false-positive results could not be completely ruled out.
In conclusion, we found a relatively high SARS-CoV-2 seroprevalence and an infection fatality rate of 2.5% in a community with high incidence of COVID-19, with ski resort venues, located close to the border with the Lombardy Region, where a devastating outbreak of COVID-19 had been reported. Our study confirms that reported cases of COVID-19 underestimate the prevalence of SARS-CoV-2 infection in the affected community, though they also show that herd immunity is far from being reached. Serological studies are crucial to provide fundamental information for understanding the extent of past transmission and the current immunological state of the population. Repeated seroprevalence studies could provide further evidence on the transmission dynamics of SARS-CoV-2 in the population [14].
Acknowledgements
The authors would like to thank the study's participants. They also thank the Serosurvey Study Group for COVID-19 in AP of Trento: Mattevi Elisabetta, Dalla Valle Lorenza, Endrizzi Luca, Pecoraro Lucia, Varesco Andrea, Brida Elisa, Daldoss Alessia, Sebis Claudia, Zanon Bruno, Bandera Mauro, Laboratory Dept., APSSTrento; Riccardo Flavia and Pezzotti Patrizio, Dept. Infectious Diseases, Istituto Superiore di Sanità; Pocher Massimo, Sannicolò Renzo, Manica Andrea, Fogarolli Angela, E-healthcare Solution Service, APSS Trento; Maroni Veronica, Chizzola Elisa, Privacy Office, APSS Trento; Sforzin Simona, Primary Care Director APSS Trento, and the entire staff of primary care involved in the study.
Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.11.013.
Funding
APSS sustained the expenses for the IgG assays on collected sera.
Transparency declaration
The authors declare no conflict of interest related to this study.
Author contributions
PS, AB together with AF were responsible for the conception and design of the study; GF and SP coordinated the analysis on sera; PL, PV, AN, AC, CF, MS, IS, EB, SF and CF performed the analysis on sera; MGZ, GB, RM and PPB organized the samples and data collection; SM performed the statistical analysis together with AB; SB and GR helped in the discussions of data; GR critically revised the manuscript; PS wrote the manuscript. All the authors revised and approved the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Map of the five municipalities of the Autonomous Province of Trento involved in the study
References
- 1.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.F., Hall A.J. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4130. published online. [DOI] [PubMed] [Google Scholar]
- 2.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance
- 4.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naaber Paul, Hunt Kaidi, Pesukova Jaana. Evaluation of SARS-CoV-2 IgG antibody response in PCR positive patients: Comparison of nine tests in relation to clinical data. Plos One. 2020 doi: 10.1371/journal.pone.0237548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F., Liu M., Wang A. Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicol T., Lefeuvre C., Serri O. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J Clin Virology. 2020 doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chew K.L., Tan S.S., Saw S., Pajarillaga A., Zaine S., Khoo C. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.036. published online: 9 June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biggs H.M., Harris J.B., Breakwell L., Dahlgren F.S., Abedi G.R., Szablewski C.M. Estimated community seroprevalence of SARS-CoV-2 antibodies — two Georgia Counties. MMWR. 2020;69:965–970. doi: 10.15585/mmwr.mm6929e2. April 28–May 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Sun J., Nie S., Li H., Kong Y., Liang M. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 11.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention . 2020. Large-scale geographic seroprevalence surveys.https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/geographic-seroprevalence-surveys.html Available from: [Google Scholar]
- 13.Wu X., Fu B., Chen L., Feng Y. Serological tests facilitate identification of asymptomatic SARS-CoV-2 infection in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25904. published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metcalf C.J.E., Farrar J., Cutts F.T., Basta N.E., Graham A.L., Lessler J. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;88:728–730. doi: 10.1016/S0140-6736(16)30164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward H., Atchison C.J., Whitaker M., Ainslie K.E., Elliott J., Okell L.C. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv. 2020 doi: 10.1101/2020.08.12.20173690. [DOI] [Google Scholar]
- 16.Martin C.A., Jenkins D.R., Minhas J.S., Gray L.J., Tang J., Williams C. Socio-demographic heterogeneity in the prevalence of COVID-19 during lockdown is associated with ethnicity and household size: results from an observational cohort study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Map of the five municipalities of the Autonomous Province of Trento involved in the study