Abstract
Background
Improved imaging techniques have increased the incidence of subsegmental pulmonary embolism (ssPE). Indirect evidence is suggesting that ssPE may represent a more benign presentation of venous thromboembolism not necessarily requiring anticoagulant treatment. However, correctly diagnosing ssPE is challenging with reported low interobserver agreement, partly due to the lack of widely accepted diagnostic criteria.
Objectives
We sought to derive uniform diagnostic criteria for ssPE, guided by expert consensus.
Methods
Based on an extensive literature review and expert opinion of a Delphi steering committee, two surveys including statements regarding diagnostic criteria and management options for ssPE were established. These surveys were conducted electronically among two panels, respectively: expert thoracic radiologists and clinical venous thromboembolism specialists. The Delphi method was used to achieve consensus after multiple survey rounds. Consensus was defined as a level of agreement >70%.
Results
Twenty‐nine of 40 invited radiologists (73%) and 40 of 51 clinicians (78%) participated. Following two survey rounds by the expert radiologists, consensus was achieved on 15 of 16 statements, including on the established diagnostic criteria for ssPE (96% agreement): a contrast defect in a subsegmental artery, that is, the first arterial branch division of any segmental artery independent of artery diameter, visible in at least two subsequent axial slices, using a computed tomography scanner with a desired maximum collimator width of ≤1 mm. These criteria were approved by 83% of the clinical venous thromboembolism (VTE) specialists. The clinical expert panel favored anticoagulant treatment in case of prior VTE, antiphospholipid syndrome, pregnancy, cancer, and proximal deep vein thrombosis.
Conclusion
The results of this analysis provide standard radiological criteria for ssPE that may be applicable in both clinical trials and practice.
Keywords: anticoagulant treatment, Delphi analysis, diagnosis, pulmonary embolism, subsegmental
Essentials.
Establishing a subsegmental pulmonary embolism diagnosis is challenging.
Surveys were conducted among expert thoracic radiologists and clinical thrombosis specialists.
Delphi analysis provided uniform diagnostic criteria to diagnose subsegmental pulmonary embolism.
Anticoagulant treatment was favored for prior venous thromboembolism, antiphospholipid syndrome, pregnancy, cancer, and proximal deep vein thrombosis.
1. INTRODUCTION
Multidetector computed tomographic pulmonary angiography (CTPA) has evolved as the imaging test of choice in the diagnostic workup of clinically suspected pulmonary embolism (PE). 1 As CTPA allows better visualization of peripheral pulmonary arteries compared to previously used imaging techniques, small emboli isolated to subsegmental branches of the pulmonary artery tree are increasingly being detected. 2 , 3 , 4 The rate of these so‐called subsegmental PE (ssPE) diagnoses has been reported to have almost doubled to 9%, when earlier studies were compared to more recent studies using computed tomography (CT) scans with more detector rows. 5 Considering that a proportion of ssPE diagnoses would have gone undetected and thus left untreated with former imaging techniques, the clinical significance of these findings is subject to debate. Some observational case series have indeed provided some ground for leaving ssPE untreated in the setting of low risk of (recurrent) venous thromboembolism (VTE) and in the absence of deep vein thrombosis (DVT) of the leg. 6 , 7 On the other hand, unselected patients with ssPE have been described to have a risk of recurrent VTE comparable to that of patients with more proximal PE. 8
Establishing a radiological diagnosis of ssPE is challenging, as demonstrated by a retrospective cohort study in which 11% of routinely established ssPE diagnoses were reinterpreted to be without any evidence of PE, while on the other hand, 37% of patients initially diagnosed with ssPE were reinterpreted to have more proximal PE. 9 As a false‐positive ssPE diagnosis may come with the consequence of unnecessary anticoagulant treatment and its associated potential harms, establishing a correct diagnosis is of utmost importance. 10 Moreover, clinical trials aimed at assessing the optimal management of ssPE would be invalid should the diagnosis not be reproducible.
One of the possible explanations of the poor interobserver reliability for the diagnosis of ssPE is the lack of widely accepted and reproducible diagnostic criteria as well as the existence of related terms such as incidental and isolated. We therefore sought to establish uniform diagnostic criteria for ssPE, guided by systematic review of the literature and consensus of expert thoracic radiologists. Second, we aimed to propose these criteria to clinical VTE specialists and to establish consensus of current best practice with regard to the therapeutic management of ssPE.
2. METHODS
2.1. Study design
The Delphi method was used to assess expert consensus among a panel of thoracic radiologists and VTE specialists. This is a well‐accepted and widely used structured process to build consensus through opinions and feedback from a group of informed experts. 11 , 12 , 13 It is an anonymous process where a predefined research problem, usually lacking empirical evidence, is expressed to an expert panel in the form of a questionnaire. Responses are collated and analyzed, and the process usually continues for several rounds until the best possible level of consensus is achieved. 14 The experts are allowed to adjust their answers in subsequent rounds, based on how they interpret the results of the expert panel as whole, which are provided to them.
The Conducting and Reporting Delphi Studies guidelines were followed for the conducting and reporting of this Delphi study. 15
2.2. Selection of experts
This Delphi analysis consisted of two parts. The first part was designed to establish radiological criteria for a CTPA diagnosis of ssPE, and the second to propose these criteria to clinical VTE specialists and to establish consensus of current best practice about the treatment of ssPE.
For the first part of the Delphi analysis, expert radiologists were selected based on the following criteria: (i) leaders in the field of clinical thoracic radiology as demonstrated by a strong publication track record, (ii) preferably being member of the Fleischner society, and (iii) currently practicing and/or involved in the training of resident radiologists. For the second part, clinical VTE specialists were selected based on the following criteria: (i) leaders in the field of diagnosis and management of VTE as demonstrated by a strong publication track record, (ii) preferably being member of ISTH or the American Society of Hematology, and (iii) currently practicing and/or involved in the training of resident clinicians.
Both expert panels were selected to represent a wide geographic area and to include both females and males. The experts completed the questionnaire anonymously and were unaware of the identity of the other involved experts.
2.3. Delphi process and steering committee
A multinational Delphi steering committee of eight members was established to oversee the process: six clinical experts on venous thromboembolism (PE, MC, CG, FK, MH, GG) and two thoracic radiological experts (LK and CSP). In cooperation with a trained medical librarian, a comprehensive literature review (Appendix 1) was performed by two members (PE and FK) to search for current recommendations in diagnosing PE and, in particular, ssPE. Based on the findings of this review and input from the steering committee, a first version of the Delphi questionnaire was drafted by two members (PE and FK). All members of the steering committee, both the clinical VTE experts and the expert radiologists, provided feedback on the questionnaire and approved its final version. For Part I of our Delphi study, this resulted in a total of 16 statements with primarily multiple‐choice questions (Appendix 2). Each question included a free text box for comments. A final open question was included, which allowed the expert panel to provide input on the best systematic approach for reporting the results of CTPA in the setting of suspected pulmonary embolism with regard to the subsegmental arteries. Subsequent rounds were planned until consensus would have been reached. Following completion of Part I, a second survey (Part II) was constructed with an aim to survey clinical VTE experts. Again, a first version of the Delphi questionnaire was drafted by two members (PE and FK). All members of the steering committee provided feedback on the questionnaire and approved its final version. This resulted in seven questions that were constructed to survey their clinical management approach. In addition, the final results of Part I of the Delphi analysis were provided to the clinical expert panel, to assess whether they would agree with the proposed diagnostic criteria. Subsequent rounds were planned until consensus would have been reached. Consensus in both Parts I and II of the Delphi analysis was defined as a minimum level of agreement of 70%, in line with previous Delphi reports. 16 , 17 , 18 , 19 We planned any number of consecutive Delphi rounds to achieve this consensus. The Delphi process was undertaken with the use of an online survey tool (SurveyMonkey). Responses were filed at the experts’ discretion until a given deadline. Two reminders were sent before reaching the deadline.
2.4. Hypothesis
The normal anatomy of the pulmonary artery follows that of the bronchial anatomy. The main pulmonary artery arises at the base of the right ventricle and extends superiorly for approximately 5 cm before dividing in into the right and left pulmonary artery (Figure 1). The right main pulmonary artery usually divides into an ascending upper lobe artery and a descending interlobar artery that gives rise to the middle and right lower lobe arteries. The left main pulmonary artery, after giving rise to the left upper lobe artery, usually descends and gives rise to the (segmental) lingual arteries and left lower lobe artery. Pulmonary arteries divide by dichotomous branching. Overall, approximately 17 of these divisions are usually present. The further branching of lobar, segmental, and subsegmental pulmonary arteries show considerable variation. To identify a specific lobar or segmental artery branch, it is usually necessary to identify its associated bronchus.
Figure 1.
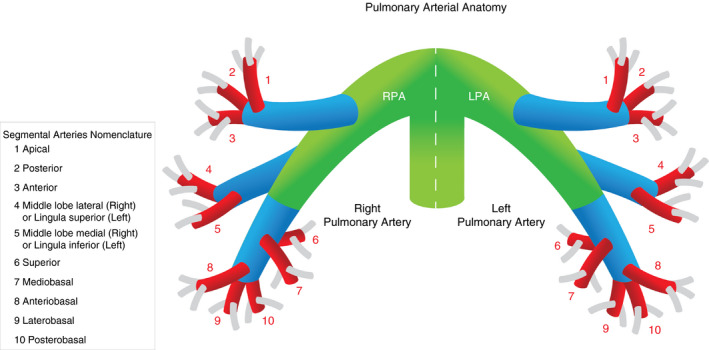
Schematic overview of anatomy of the pulmonary artery; green branches represent main and interlobar arteries, blue branches lobar arteries, red branches segmental arteries, and gray branches subsegmental arteries
The subsegmental pulmonary arteries may have a diameter of up to 6‐7 mm in diameter just after the initial branch, decreasing to <1.5 mm in the peripheral lung. 20 , 21 Vessels smaller than this range are labeled arterioles and are not visible on standard CTPA images, which normally allows detection of arteries as small as 2 mm in diameter. A prerequisite to identify subsegmental PE is the clear and doubtless identification of the subsegmental vessels with an intraluminal contrast defect. The contrast defect can be local and surrounded by intravascular contrast or lead to a complete obstruction and thus cutoff of the vessel. It has been demonstrated that 98% of subsegmental arteries could be identified using 64‐row multidetector computed tomography scanners with 1.2 mm collimation and 0.5 mm ultrathin reconstructed effective slice thickness. 22 Anatomic regions that are not adequately depicted correspond mostly to paracardiac anatomic locations that are exposed to the motion of the beating heart muscle with resulting artefacts.
Consequently, we hypothesized that radiological criteria for ssPE should involve the following aspects: (i) technical quality of the scan (eg, contrast timing, artefacts, identification of all subsegmental arteries), (ii) anatomic location (ie, correlation to bronchus and number of side branches from the lobar artery), and (c) artery diameter. This hypothesis was the basis of the first part of the Delphi analysis.
2.5. Analysis
Descriptive statistics were calculated using SPSS version 25.0.0 (IBM, Armonk, NY, USA).
3. RESULTS
3.1. Literature search
The systematic literature review was performed on October 30, 2017, and resulted in a total of 280 eligible articles. Based on the titles and abstracts, 32 articles were selected for complete review. Diagnostic criteria for a CTPA diagnosis of ssPE could not be extracted from any of the articles.
3.2. Delphi Part I: Radiological definition
In October 2018, the first round of the Delphi questionnaire was distributed among 40 expert radiologists. Of those, 29 consented to participate and completed the questionnaire (73%). The expert panel included radiologists practicing in 12 different countries; 27 of 29 participants completed the full Delphi procedure.
Following compilation of the results of the online survey, consensus was reached on 8 of 16 statements (Figure 2). A vast majority of experts (89%) agreed that the diagnostic criteria for ssPE should be based on the anatomic location within the pulmonary arterial system alone and not on the vessel diameter of the pulmonary artery affected. This was reinforced by another statement in which the expert panel agreed (78%) that the diameter of a certain affected pulmonary artery branch does not contribute to the definition of ssPE. Technical specifications of the CT scanner, a minimum Hounsfield Unit, or maximal collimator width were not considered to be relevant either. Also, 82% of the experts agreed that the term isolated ssPE refers to a solitary embolus in a single subsegmental artery without the presence of other ssPEs or additional emboli more centrally.
Figure 2.
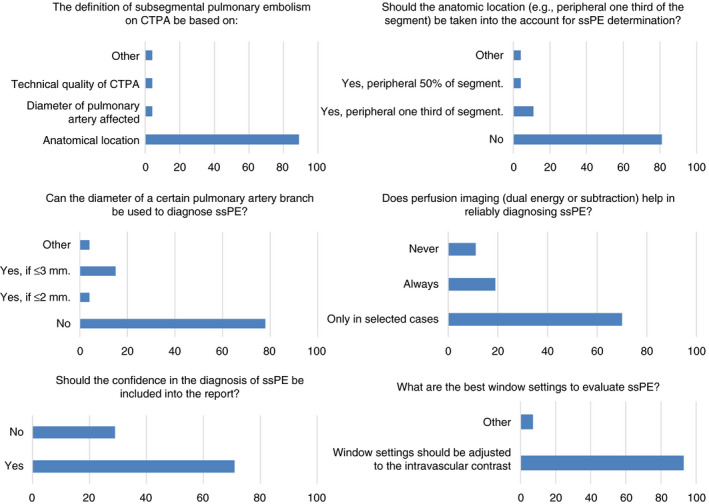
Statements reaching consensus in the first round of the first part of the Delphi analysis. CTPA, computed tomographic pulmonary angiography; ssPE, subsegmental pulmonary embolism
The statements for which consensus was not achieved were discussed within the Delphi steering committee, modified, and sent for a secondary survey round. In this second round, respondents were able to see which of the multiple‐choice options achieved the highest level of consensus. The second survey consisted of nine statements with multiple‐choice answers and were answered by all responders of the first round. Eight of the nine statements achieved consensus (Figure 3). Based on the results of the Delphi, we constructed the following diagnostic criteria for ssPE on CTPA: “A contrast defect in a subsegmental artery, ie, the first arterial branch division of any segmental artery independent of artery diameter, visible in at least two subsequent axial slices, using a CT scanner with a desired maximum collimator width of ≤1 mm.” The agreement on specific recommendations for confirming and reporting of ssPE is summarized in Table 1. A total of 96% of the participants approved these diagnostic criteria and recommendations.
Figure 3.
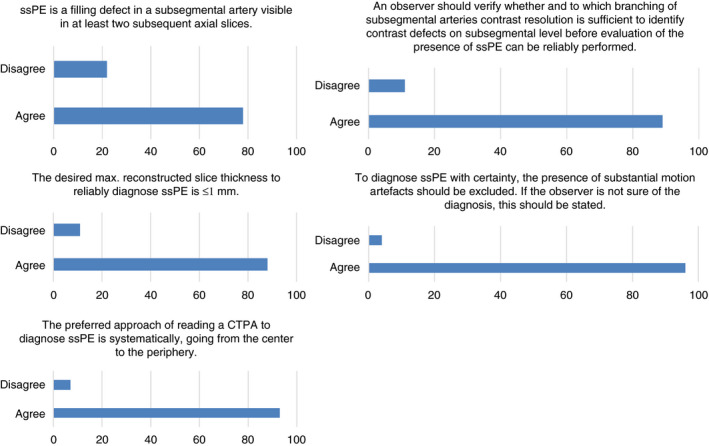
Statements reaching consensus in the second round of the first part of the Delphi analysis. CTPA, computed tomographic pulmonary angiography; ssPE, subsegmental pulmonary embolism
Table 1.
Specific recommendations for confirming and reporting subsegmental pulmonary embolism
| The observer should verify to which branching of the subsegmental arteries (eg, proximal, distal) contrast enhancement is sufficient to identify contrast defects on subsegmental level before evaluation of the presence of subsegmental pulmonary embolism can be reliably performed. |
| To diagnose subsegmental pulmonary embolism with certainty, the presence of substantial motion artefacts should be excluded, preferably in the “lung window,” in addition to affirmation of sufficient contrast enhancement. If the observer is not sure of the diagnosis, this should be stated in the report. |
| The term isolated subsegmental pulmonary embolism is reserved for a subsegmental pulmonary embolism in one subsegmental artery, and the term multiple subsegmental emboli refers to PE in two or more subsegmental arteries (or distal branches).” |
3.3. Delphi Part II: Therapeutic management
In February 2019, the first round of the Delphi questionnaire was distributed among 51 clinical VTE specialists. Of those, 40 consented to participate and completed the questionnaire (78%). The expert panel included clinicians practicing in 11 different countries; 39 of 40 participants completed the full Delphi procedure.
The results of Part II of the Delphi analysis are depicted in Figure 4. A majority of 83% of the experts agreed with the proposed diagnostic criteria derived from the first part of the Delphi. They also agreed on the statement that the diameter of the affected vessel does not contribute to the diagnosis or treatment of ssPE (97% consensus). Of the respondents who did not agree with the diagnostic criteria, three commented that the definition isolated ssPE should include the absence of (proximal) DVT. Two respondents commented that “sufficient contrast” should be specified. One suggested that the number and location of subsegmental emboli should always be included in the report. For one respondent, it was unclear why the phrase “first arterial branch” was needed in the definition. One final respondent suggested to add: “A contrast defect in a subsegmental or lower/smaller/further out artery.”
Figure 4.
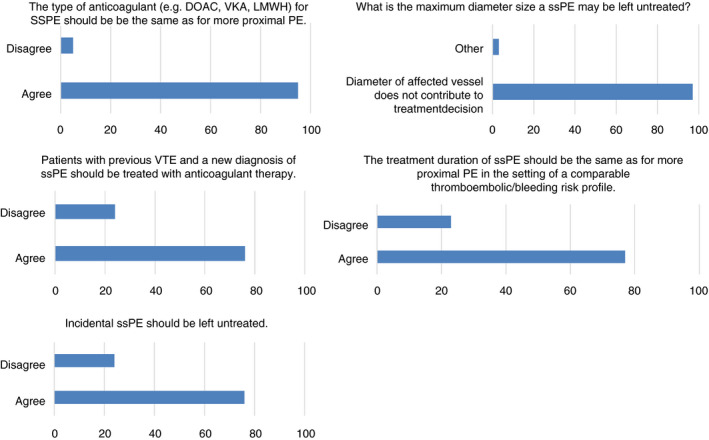
Summary of best clinical practice with regard to management of ssPE resulting from the two rounds of the second part of the Delphi analysis. CTPA, computed tomographic pulmonary angiography; DOAC, direct oral anticoagulant; LMWH, low‐molecular‐weight heparin; PE, pulmonary embolism; ssPE, subsegmental pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism
Over two Delphi rounds, the second round answered by all the responders of the first round, agreement of >70% was achieved that incidental and symptomatic ssPE may be left untreated in the absence of the following five conditions: previous VTE, antiphospholipid syndrome, pregnancy, active malignancy, and asymptomatic or symptomatic proximal DVT. The number of subsegmental emboli, that is, one or multiple, was not found to be a discriminative factor for the decision whether to start treatment. Therefore, the identified conditions necessitating anticoagulant treatment apply to both single or multiple subsegmental pulmonary emboli. In case treatment was indicated, the expert panel agreed (77%) that the treatment duration of ssPE should be the same as for more proximal PE in the setting of a comparable thromboembolic risk profile. It was agreed that the preferred type of anticoagulant (eg, direct oral anticoagulants, vitamin K antagonists, or low‐molecular‐weight heparins) for ssPE should be the same as for more proximal PE.
Based on input from the expert panel during the first Delphi round, we additionally assessed the implications of the bleeding risk for the decision of whether to treat ssPE. It was agreed (90%) that the risk of bleeding complications should carry a greater weight in the decision of whether to treat a patient with ssPE with anticoagulants, and for how long, than for comparable patients with more proximal PE.
4. DISCUSSION
The results of this Delphi analysis have established a clear radiological definition of ssPE with a high level of consensus among both thoracic radiological and clinical VTE experts. These criteria provide guidance in diagnostic and therapeutic decision making. Based on the second part of the Delphi, we also report best practice for the management of ssPE in specific settings as agreed upon by the expert clinicians.
Reported difficulties in diagnosing ssPE stress the need for uniform diagnostic criteria. Although clinical trials have reported the sensitivity of diagnosing ssPE on CTPA to be as high as 82%–100%, this may decrease to 74% when considering single segmental or subsegmental pulmonary emboli. 23 Interobserver agreement between radiologists in diagnosing ssPE has been shown to be low (κ = 0.38; 95% CI, 0.0‐0.89). 24 In another study, reinterpretation by five radiologists showed that at least one radiologist disagreed with the initial interpretation of ssPE in 60% of the cases. 25 This might in part be explained by the high level of expertise that is necessary to diagnose ssPE. Indeed, it has been demonstrated that the sensitivity of ssPE diagnosis is higher among thoracic radiologists than among nonthoracic radiologists and that the diagnosis is more often correctly made among radiologists than among radiology residents. 26 , 27 , 28
In contrast to our hypothesis, the expert radiologists were resolute in their opinion that only the anatomic location of the contrast filling defect accounted for the definition of ssPE. The pulmonary artery diameter, which is known to be variable based on the anatomic location and dependent on anatomic variants and hemodynamic status, was not considered to be relevant. The reason why we explicitly included the arterial diameter in the first Delphi round was that the anatomic definition of subsegmental refers to quite a wide range of arterial diameters from the fourth generation (main‐lobar‐segmental‐subsegmental) of more centrally located arteries to very small peripheral arteries. This indeed allows for a more straightforward definition of ssPE when excluding artery diameter as input criteria, which represents a variable and difficult to reliably measure parameter. Of note, whether this definition leads to higher interobserver agreement and a decline in false‐positive ssPE diagnoses remains to be demonstrated.
Image quality was also rejected as being relevant for the definition of ssPE. The expert radiologists did reach consensus on the statement that “the observer should verify whether and to which branching of the subsegmental arteries contrast enhancement is sufficient to identify contrast defects at the subsegmental level before evaluation of the presence of subsegmental pulmonary embolism can be reliably performed.” This implies that the diagnosis cannot be reliably assessed in a certain segment if “sufficient contrast enhancement” is not reached. In such cases, assuming either an abnormal D‐dimer test or a high or likely clinical pretest probability, 29 a repeat CTPA scan can be considered if it is likely that technical aspects and not limitations of the clinical status of the patient caused the limited quality of the examination. Alternatively, a scintigraphic examination can be considered. Importantly, consensus among radiologists was reached to include the level of confidence into the diagnosis of isolated ssPE into the report, allowing for outweighing the risk of treatment versus a potentially false‐positive diagnosis.
A remarkable observation in this study is that expert radiologists uniformly refer to “isolated ssPE” when it involves a single subsegmental embolus. This definition is more distinct than the one used in clinical studies, where isolated usually refers to one or multiple ssPEs without radiological evidence for more proximal PE or DVT. 5 , 30 This difference in interpretation of ssPE terminology further strengthens the need for criteria that are universally applicable. Importantly, this new definition was approved by a majority of the clinical VTE experts. The fact that isolated refers to a single embolus means that irrespective of its location on a central or peripheral subsegmental artery, its resulting perfusion defect will be limited to a subsegmental area. This can be also seen as a further justification of the fact that the generation of a subsegmental artery and thus the diameter are not considered in the definition.
Until recently, anticoagulant treatment was the management approach of choice for any patient diagnosed with PE, regardless of its anatomic location. Whether patients with ssPEs benefit from anticoagulant treatment is increasingly challenged by reports of patients with ssPEs with an uncomplicated course in the absence of treatment. 31 In addition, although this should be regarded as indirect evidence, population studies have demonstrated that the introduction of modern CT scanners has led to an increased incidence of PE diagnoses without influencing the mortality rate or the 3‐month risk of VTE among patients left untreated following a negative CTPA. 32 Conversely, diagnostic strategies raising the threshold for performing CTPA in the setting of suspected PE are associated with a lower prevalence of ssPE. 33 , 34 Based on these findings, the latest guidelines of the American College of Chest Physicians have suggested that in patients with ssPEs and no proximal DVT in the legs who have a low risk for recurrent VTE, clinical surveillance is preferred over anticoagulation. 35 Definite evidence for the safety of such an approach has yet to arrive from a prospective single‐arm trial (NCT01455818) and a planned randomized controlled trial (NCT04263038). While awaiting the results of this trial, our study provides more clarity on which conditions, as established by clinical VTE specialists, could determine the decision on whether to treat ssPEs. The following conditions were considered to necessitate anticoagulant treatment in case of either single or multiple subsegmental pulmonary emboli: previous VTE, antiphospholipid syndrome, pregnancy, active malignancy, and asymptomatic or symptomatic proximal DVT.
Strengths of this study include the fact that broad and worldwide expert panels were surveyed. Second, by selecting a second expert panel of VTE specialists, we were able to validate consensus for the provided diagnostic criteria in an independent sample of expert clinicians. Third, the results are strengthened by a high level of consensus among the different statements (16/17). In both expert panels, participation initial rates were <80%, which forms a limitation of this study. Still, consensus was reached easily within both panels, with need of few consecutive survey rounds.
In conclusion, this study has provided radiological criteria for ssPEs that may be applied in both clinical trials and practice. Moreover, we also report best practice for the therapeutic management of ssPEs in specific settings as agreed on by the expert clinicians. Future studies should assess to what extent our diagnostic criteria improve the sensitivity and interobserver reliability of diagnosing ssPEs.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interests.
AUTHOR CONTRIBUTIONS
PLE and FAK performed the literature review, designed the questionnaires, performed the analyses, drafted the manuscript and designed the study. LJMK, C G, CLG, CMS, MC, and MV designed the questionnaires and critically reviewed the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
Thoracic radiological experts: L. Meijboom (VU Medical Center, the Netherlands); LF Beenen (Amsterdam Medical Center, the Netherlands); A. de Roos (Leiden University Medical Center, the Netherlands); IJC Hartmann (Maasstad Hospital, the Netherlands); C. Dennie (University of Ottawa, Canada); MP Revel (Hôpitaux Universitaires Paris Centre, France); L. Haramati (Montefiore Medical Center, USA); E. van Beek (University of Edingburh, UK); N. Screaton (Papworth Hospital, UK); G. Ferretti (Universitaire de Grenoble, France); B. Ghaye (Cliniques universitaires Saint‐Luc, Belgium); M. Das (Helios Kliniken, Germany); C. White (University of Maryland, USA); E. Pena Fernandez (University of Ottawa, Canada); N. Paul (University of Toronto, Canada); I. Vlahos (St George’s University Hospital, UK); RD Renapurkar (Cleveland Clinic, USA); J. Ravenel (Medical University of South Carolina College of Medicine, USA); J. Kanne (University of Wisconsin School of Medicine, USA); S. Abbara (UT Southwestern Medical Center, USA); M. Rémy‐Jardin (University Centre of Lille, France); B. Geurts (Radboud University Medical Center; the Netherlands); T. Frauenfelder (University of Zurich, Switzerland); N. Sverzellati (University Hospital of Parma, Italy); H. Prosch (Medical University of Vienna, Austria); JM Goo (Seoul National University College of Medicine, South Korea); J. Vogel‐Claussen (MH Hannover, Germany); PJ MacMahon (University College Dublin, Ireland); S. Bhalla (Washington University Medical Center, USA)
Clinical VTE experts: S. Kahn (McGill University, Canada); S. Shivakumar (Dalhousie University, Canada); P. Wells (University of Ottawa, Canada); M. Rodger (University of Ottawa, Canada); L. Castellucci (University of Ottawa, Canada); L. Duffett (University of Ottawa, Canada); A. Delluc (University of Ottawa, Canada); D. Siegal (McMaster University, Canada); A. Lazo‐Langner (University of Western Ontario, Canada); C. Wu (University of Alberta, Canada); A. Lee (University of British Columbia, Canada); D. Garcia (University of Washington, USA); J. Zwicker (Harvard University, USA); D. Aujesky (University of Lausanne, Aujesky); D. Jimenez (Hospital Ramón y Cajal and Alcalá de Henares University, Spain); M. Righini (Hôpitaux Universitaires de Genève, Switzerland); M. Blondon (Geneva University Hospitals and Faculty of Medicine, Switzerland); C. Ay (Medical University of Vienna, Austria); S. Barco (Mainz University, Germany); PW Kamphuisen (TerGooi Hospital, the Netherlands); M. Ferreira (Department of Internal Medicine, Hospital Garcia de Orta, Almada, Portugal); O. Sanchez (Hôpital Européen Georges‐Pompidou, France); LK Moores (F. Edward Hebert School of Medicine, USA); C. Tromeur (Hôpital La Cavale blanche, France) ; W. Ageno (University of Insubria, Italy); B. Hunt (Guy's and St Thomas’ NHS Foundation Trust, UK); P. Prandoni (Padua University, Italy); M. Monreal (Hospital Universitari Germans Trias i Pujol, Spain); M. Crowther (McMaster University, Canada); PM Roy (Université d’Angers, France); I. Pabinger (Medical University of Vienna, Austria); MP. Donadini (Department of Clinical Medicine, Azienda Socio Sanitaria Territoriale Sette Laghi, Ospedale di Circolo, Italy); F. Moustafa (Clermont‐Ferrand University Hospital, France); L. Jara‐Palomares (Hospital Universitario Virgen del Rocio, Spain); R. Pedroc (Instituto de Investigación Sanitaria del Hospital Clínico San Carlos, Spain); L. Bertoletti (Université Jean‐Monnet, France); P. Verhamme (UZ Leuven, Belgium); HCJ Eikenboom (Leiden University Medical Center, the Netherlands); FJM van der Meer (Leiden University Medical Center, the Netherlands); HR Büller (Amsterdam Medical Center, the Netherlands); N. van Es (Amsterdam Medical Center, the Netherlands).
Appendix 1.
ELECTRONIC DATABASE SEARCH
PubMed
(“subsegmental pulmonary emboli”[tw] OR “subsegmental pulmonary embolism”[tw] OR “subsegmental pulmonary embolisms”[tw] OR “subsegmental pulmonary embolus”[tw] OR “subsegmental pe”[tw] OR “subsegmental pes”[tw] OR “subsegmental pulmonary thromboembolism”[tw] OR “subsegmental sized pulmonary emboli”[tw] OR “sub segmental acute pe”[tw] OR “sub segmental embolisms”[tw] OR “sub segmental pe”[tw] OR “sub segmental pulmonary embolism”[tw] OR ((“subsegmental”[tw] OR subsegment*[tw] OR “sub segmental”[tw] OR sub segment*[tw]) AND (“pulmonary emboli”[tw] OR “pulmonary embolism”[tw] OR “pulmonary embolisms”[tw] OR “pulmonary embolus”[tw] OR “pe”[ti] OR “pes”[ti] OR “pulmonary thromboembolism”[tw] OR “Pulmonary Embolism”[Mesh])) OR (((small branch*[tw] OR smaller branch*[tw] OR smallest branch*[tw]) AND (“Pulmonary Artery”[Mesh] OR “Pulmonary Artery”[tw] OR “Pulmonary Arteries”[tw])) AND (“pulmonary emboli”[tw] OR “pulmonary embolism”[tw] OR “pulmonary embolisms”[tw] OR “pulmonary embolus”[tw] OR “pe”[ti] OR “pes”[ti] OR “pulmonary thromboembolism”[tw] OR “Pulmonary Embolism”[Mesh])) NOT (“Animals”[mesh] NOT “Humans”[mesh]) NOT (“Case Reports”[ptyp] OR “case report”[ti]) AND (english[la] OR “dutch”[la])) NOT (“subsegmental pulmonary emboli”[ti] OR “subsegmental pulmonary embolism”[ti] OR “subsegmental pulmonary embolisms”[ti] OR “subsegmental pulmonary embolus”[ti] OR “subsegmental pe”[ti] OR “subsegmental pes”[ti] OR “subsegmental pulmonary thromboembolism”[ti] OR “subsegmental sized pulmonary emboli”[ti] OR “sub segmental acute pe”[ti] OR “sub segmental embolisms”[ti] OR “sub segmental pe”[ti] OR “sub segmental pulmonary embolism”[ti] OR ((“subsegmental”[ti] OR subsegment*[ti] OR “sub segmental”[ti] OR sub segment*[ti]) AND (“pulmonary emboli”[ti] OR “pulmonary embolism”[ti] OR “pulmonary embolisms”[ti] OR “pulmonary embolus”[ti] OR “pe”[ti] OR “pes”[ti] OR “pulmonary thromboembolism”[ti] OR “Pulmonary Embolism”[majr])) OR (((small branch*[ti] OR smaller branch*[ti] OR smallest branch*[ti]) AND (“Pulmonary Artery”[majr] OR “Pulmonary Artery”[ti] OR “Pulmonary Arteries”[ti])) AND (“pulmonary emboli”[ti] OR “pulmonary embolism”[ti] OR “pulmonary embolisms”[ti] OR “pulmonary embolus”[ti] OR “pe”[ti] OR “pes”[ti] OR “pulmonary thromboembolism”[ti] OR “Pulmonary Embolism”[majr])) NOT (“Animals”[mesh] NOT “Humans”[mesh]) NOT
Appendix 2.
DEPLHI QUESTIONNAIRE SSPE, EXPERT RADIOLOGISTS, FIRST ROUND
1. In your opinion, should the definition of subsegmental pulmonary embolism on CTPA consist of the following parameters (multiple answers possible, please check all that apply)?
The anatomic location within the pulmonary arterial system.
The diameter of the pulmonary artery affected.
The technical quality of the CTPA.
Other, namely: [free text]
2. What is in your opinion correct?
A pulmonary artery is called subsegmental
After the first arterial branch has divided off the segmental artery irrespective of its size (following standard anatomy; Figure 1).
As soon as any arterial branch has divided off the segmental artery irrespective of its size.
Other, namely: [free text]
3. If a pulmonary artery originates from a division of a clearly identifiable segmental artery (following standard anatomy; Figure 1), should the vessel diameter be taken into account for the determination whether it is subsegmental or not? If yes, what is the maximum diameter size a pulmonary branch may be called subsegmental?
≤2 mm.
≤3 mm.
≤4 mm.
≤5 mm.
≤6 mm.
Other, namely: [free text]
The diameter of the affected vessel does not contribute to the definition of subsegmental pulmonary embolism.
4. If a pulmonary artery originates from a division of a clearly identifiable segmental artery (following standard anatomy; Figure 1), should be the anatomic location (eg, peripheral one third of the segment) taken into the account for the determination whether it is subsegmental or not? If yes, what would be your preference?
Peripheral one third of the segment.
Peripheral 50% of the segment.
The anatomic location does not contribute to the definition of subsegmental pulmonary embolism.
Other, namely [free text]:
5. As a general rule, can the diameter of a certain pulmonary artery branch be used to diagnose subsegmental pulmonary embolism? If yes, at what diameter size should a pulmonary branch always be called subsegmental?
≤1 mm.
≤2 mm.
≤3 mm.
≤4 mm.
Other, namely: [free text]
The diameter of the affected vessel does not contribute to the definition of subsegmental pulmonary embolism.
6. What is the desired maximum collimator width to reliably diagnose subsegmental pulmonary embolism?
< 1 mm.
1 mm.
2 mm.
3 mm.
Other, namely: [free text]
The collimator width does not contribute to the diagnosis of subsegmental pulmonary embolism.
7. What is the desired Hounsfield Unit in the main pulmonary artery to reliably diagnose subsegmental pulmonary embolism?
>100 HU.
>150 HU.
>200 HU.
Other, namely: [free text]
The Hounsfield Unit in the main pulmonary artery does not contribute to the definition of subsegmental pulmonary embolism.
8. Should CT images be routinely reviewed in multiplanar reconstructions to reliably diagnose subsegmental pulmonary embolism?
Always.
Never.
On demand, namely: [free text]
9. Should CT images be routinely reviewed using maximum intensity projections to reliably diagnose subsegmental pulmonary embolism?
Always.
Never.
On demand, namely: [free text]
10. Does perfusion imaging (dual energy or subtraction) help in reliably diagnosing subsegmental pulmonary embolism?
Always.
Never.
Only in selected cases, namely: [free text]
11. What are the imaging criteria for the presence of subsegmental pulmonary embolism (several answers are possible)?
Contrast defect in a subsegmental artery visible in a single axial slice.
Contrast defect in a subsegmental artery visible in at least two subsequent axial slices.
Contrast defect in a subsegmental artery visible in at least two directions (axial and coronal or sagittal).
Sudden contrast drop in a subsegmental artery while arteries of comparable diameter in the surrounding show a higher contrast.
Other, namely: [free text]
12. What are the best window settings to evaluate subsegmental pulmonary embolism?
A constant CT angiography window.
The window/ settings should be adjusted to the intravascular contrast.
The window settings should be modified going from the central arteries to the peripheral arteries.
Other, namely: [free text]
Window settings do not contribute to the diagnosis of subsegmental pulmonary embolism.
13. Should technical specifications of the CT scanner be considered for the diagnosis of subsegmental pulmonary embolism?
At least 4‐detector‐row CT is sufficient.
At least 16‐detector‐row CT is sufficient.
At least 64‐detector‐row CT is sufficient.
Yes, but other, namely: [free text]
Technical specifications of the CT scanner are not relevant for the diagnosis of subsegmental pulmonary embolism.
14. What is the best (systematic) approach of reading a CTPA scan that should be used to diagnose subsegmental pulmonary embolism?
Going from the center to the periphery per lobe.
Going caudo‐cranially or cranio‐caudally.
Other, namely: [free text]
No systematic approach is necessary for reading CTPA for subsegmental pulmonary embolism. 14.9%)
15. The term isolated subsegmental pulmonary embolism refers to:
A solitary embolus in a single subsegmental artery without the presence of additional emboli more centrally.
The presence of one or multiple subsegmental emboli without the presence of additional emboli more centrally.
16. Should the confidence into the diagnosis of subsegmental pulmonary embolism be included into the report?
Yes, in this way: [free text]
No.
17. What is the best (systematic) standard of reporting the results of CTPA in the setting of suspected pulmonary embolism with regard to the subsegmental arteries?
[free text]
den Exter PL, Kroft LJM, Gonsalves C, et al. Establishing diagnostic criteria and treatment of subsegmental pulmonary embolism: A Delphi analysis of experts. Res Pract Thromb Haemost. 2020;4:1251–1261. 10.1002/rth2.12422
Handling Editor: Dr Susan Kahn.
Funding information
G. Le Gal is the recipient of an Early Researcher Award from the Province of Ontario, a Heart and Stroke Foundation of Canada Ontario Mid‐Career Investigator Award, and holds the Chair on Diagnosis of Venous Thromboembolism from the Department of Medicine, University of Ottawa.
Contributor Information
Gregoire Le Gal, @grelegal.
Marc Carrier, @marccarrier1.
Frederikus A. Klok, Email: f.a.klok@lumc.nl, @Erik_Klok_MD.
REFERENCES
- 1. Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018;4:18028. [DOI] [PubMed] [Google Scholar]
- 2. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–27. [DOI] [PubMed] [Google Scholar]
- 3. Anderson DR, Kahn SR, Rodger MA, Kovacs MJ, Morris T, Hirsch A, et al. Computed tomographic pulmonary angiography vs ventilation‐perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298:2743–53. [DOI] [PubMed] [Google Scholar]
- 4. Carrier M, Klok FA. Symptomatic subsegmental pulmonary embolism: to treat or not to treat? Hematology American Society of Hematology Education Program. 2017;2017:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta‐analysis of the management outcome studies. J Thromb Haemost. 2010;8(8):1716–22. [DOI] [PubMed] [Google Scholar]
- 6. Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010;126:e266–e270. [DOI] [PubMed] [Google Scholar]
- 7. Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub‐segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 2015;13:214–8. [DOI] [PubMed] [Google Scholar]
- 8. den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJHA, Kamphuisen PW, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122(7):1144–9. [DOI] [PubMed] [Google Scholar]
- 9. Pena E, Kimpton M, Dennie C, Peterson R, Gal GEL, Carrier M. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost. 2012;10:496–8. [DOI] [PubMed] [Google Scholar]
- 10. Swan D, Hitchen S, Klok FA, Thachil J. The problem of under‐diagnosis and over‐diagnosis of pulmonary embolism. Thromb Res. 2019;177:122–9. [DOI] [PubMed] [Google Scholar]
- 11. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphrey‐Murto S, Varpio L, Wood TJ, Gonsalves C, Ufholz LA, Mascioli K, et al. The use of the Delphi and other consensus group methods in medical education research: a review. Acad Med. 2017;92:1491–8. [DOI] [PubMed] [Google Scholar]
- 13. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med. 2016;91:663–8. [DOI] [PubMed] [Google Scholar]
- 14. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 15. Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31:684–706. [DOI] [PubMed] [Google Scholar]
- 16. Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri‐Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137:674–91. [DOI] [PubMed] [Google Scholar]
- 17. Blasi F, Concia E, Del Prato B, Giusti M, Mazzei T, Polistena B, et al. The most appropriate therapeutic strategy for acute lower respiratory tract infections: a Delphi‐based approach. J Chemother (Florence, Italy). 2017;29:274–86. [DOI] [PubMed] [Google Scholar]
- 18. Zafar SY, Currow DC, Cherny N, Strasser F, Fowler R, Abernethy AP. Consensus‐based standards for best supportive care in clinical trials in advanced cancer. Lancet Oncol. 2012;13:e77–e82. [DOI] [PubMed] [Google Scholar]
- 19. Brown AK, O'Connor PJ, Roberts TE, Wakefield RJ, Karim Z, Emery P. Recommendations for musculoskeletal ultrasonography by rheumatologists: setting global standards for best practice by expert consensus. Arthritis Rheum. 2005;53:83–92. [DOI] [PubMed] [Google Scholar]
- 20. Chen GP, Dong HN, Wang XQ, Zheng YF, Xu M, Zhao ZW. Measurement of the diameter of segmental and sub‐segmental pulmonary arteries of the right inferior lobe by multislice spiral CT angiography. Acta Anatomica Sinica. 2014;45:248–52. [Google Scholar]
- 21. Sakuma M, Demachi J, Nawata J, Suzuki J, Takahashi T, Matsubara H, et al. Epoprostenol infusion therapy changes angiographic findings of pulmonary arteries in patients with idiopathic pulmonary arterial hypertension. Circ J. 2008;72:1147–51. [DOI] [PubMed] [Google Scholar]
- 22. Niemann T, Bongartz G. Detectability of subsegmental pulmonary vessels in 64 MDCT‐pulmonary angiography. Internet J Radiol. 2009;12:1–7. [Google Scholar]
- 23. Kligerman SJ, Mitchell JW, Sechrist JW, Meeks AK, Galvin JR, White CS. Radiologist performance in the detection of pulmonary embolism: features that favor correct interpretation and risk factors for errors. J Thorac Imaging. 2018;33:350–7. [DOI] [PubMed] [Google Scholar]
- 24. Ghanima W, Nielssen BE, Holmen LO, Witwit A, Al‐Ashtari A, Sandset PM. Multidetector computed tomography (MDCT) in the diagnosis of pulmonary embolism: interobserver agreement among radiologists with varied levels of experience. Acta Radiol. 2007;48:165–70. [DOI] [PubMed] [Google Scholar]
- 25. Miller WT Jr, Marinari LA, Barbosa E Jr, Litt HI, Schmitt JE, Mahne A, et al. Small pulmonary artery defects are not reliable indicators of pulmonary embolism. Ann Am Thorac Soc. 2015;12:1022–9. [DOI] [PubMed] [Google Scholar]
- 26. Shaham D, Heffez R, Bogot NR, Libson E, Brezis M. CT pulmonary angiography for the detection of pulmonary embolism: interobserver agreement between on‐call radiology residents and specialists (CTPA interobserver agreement). Clin Imaging. 2006;30:266–70. [DOI] [PubMed] [Google Scholar]
- 27. Joshi R, Wu K, Kaicker J, Choudur H. Reliability of on‐call radiology residents' interpretation of 64‐slice CT pulmonary angiography for the detection of pulmonary embolism. Acta Radiol. 2014;55:682–90. [DOI] [PubMed] [Google Scholar]
- 28. Verweij JW, Hofstee HM, Golding RP, van Waesberghe JH, Smulders YM. Interobserver agreement between on‐call radiology residents and radiology specialists in the diagnosis of pulmonary embolism using computed tomography pulmonary angiography. J Comp Assist Tomography. 2009;33:952–5. [DOI] [PubMed] [Google Scholar]
- 29. Huisman MV, Klok FA. How I diagnose acute pulmonary embolism. Blood. 2013;121:4443–8. [DOI] [PubMed] [Google Scholar]
- 30. Carrier M, Righini M, Le GG. Symptomatic sub‐segmental pulmonary embolism: what is the next step? J Thromb Haemost. 2012;10(8):1486–90. [DOI] [PubMed] [Google Scholar]
- 31. Bariteau A, Stewart LK, Emmett TW, Kline JA. Systematic review and meta‐analysis of outcomes of patients with subsegmental pulmonary embolism with and without anticoagulation treatment. Acad Emerg Med. 2018;25:828–35. [DOI] [PubMed] [Google Scholar]
- 32. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Pol LM, Bistervels IM, van Mens TE, van der Hulle T, Beenen LFM, den Exter PL, et al. Lower prevalence of subsegmental pulmonary embolism after application of the YEARS diagnostic algorithm. Br J Haematol. 2018;183:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Hulle T, Cheung WY, Kooij S, Beenen LFM, van Bemmel T, van Es J, e al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390(10091):289–97. [DOI] [PubMed] [Google Scholar]
- 35. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]


