Abstract
Background
Replacement therapy is the most common treatment for reduction of bleeding and control of episodic bleeding in individuals with hemophilia. Despite the proven effectiveness of factor replacement therapy, repeated intravenous administration is a heavy burden to individuals with hemophilia.
Objectives
To reduce the burden, therapeutic agents that can be subcutaneously administered need to be developed, and an anti–tissue factor pathway inhibitor (TFPI) antibody may be a suitable candidate for this purpose.
Methods
MG1113 is an IgG4 monoclonal antibody that binds to Kunitz‐2 domain (KD2) of TFPI. To confirm the coagulation potential of MG1113, several tests were conducted using factor VIII (FVIII)‐ or IX (FIX)‐deficient plasma. For the ex vivo spiking test, platelet‐poor plasma samples from 14 individuals with hemophilia were spiked with MG1113. The in vivo efficacy was determined using blood loss tests, modified prothrombin time (mPT), and free TFPI quantification after intravenous or subcutaneous administration of MG1113 into hemophilia A (HA)‐induced rabbits.
Results
Radiographic crystallography demonstrated the specific binding site between MG1113 and KD2. In FVIII‐deficient plasma and the plasma of individuals with hemophilia, peak thrombin and endogenous thrombin levels were increased by MG1113 in a concentration‐dependent manner. Rotational thromboelastometry assay revealed that clotting time, clot formation time, and maximum clot firmness were normalized in MG1113‐treated blood of patients. Intravenous or subcutaneous injection of MG1113 into HA‐induced rabbits resulted in rebalancing of blood loss, mPT, and free TFPI levels.
Conclusions
These results indicate that subcutaneous administration of MG1113 neutralizes the function of TFPI and regulates bleeding in individuals with hemophilia.
Keywords: antibody, coagulation, hemophilia A, hemophilia B, tissue factor pathway inhibitor
Essentials.
Hemophilia therapy has a heavy burden due to repeated intravenous administration.
MG1113 binds to the Kunitz‐2 domain of tissue factor pathway inhibitor (TFPI) and neutralizes the function of TFPI‐α and ‐β.
MG1113 promoted hemostasis in plasma and whole blood of individuals with hemophilia.
Subcutaneous administration of MG1113 rebalanced blood loss in hemophilia A–induced rabbits.
1. INTRODUCTION
Hemophilia A (HA) and B (HB) are rare hemorrhagic diseases caused by the deficiency of the coagulation factors VIII (FVIII) or IX (FIX). 1 Replacement therapy of the deficient factor is the most widely used treatment for individuals with hemophilia. Prophylaxis is established as a standard of care to reduce episodic bleeding and enable residual factor activity at 1% or higher. However, intravenous administration repeated two to three times per week poses a heavy burden to patients and their families. Moreover, the development of inhibitors against therapeutics can decrease drug efficacy and cause difficulties in bleeding management. Hemophilia therapeutics should be improved regarding the route of administration and to reduce the risk of inhibitor formation.
Tissue factor pathway inhibitor (TFPI) exists in two forms, α and β. TFPI‐α is composed of three Kunitz‐type serine protease inhibitor domains (KD1, KD2, and KD3). 2 , 3 KD1 combines with the extrinsic factor Xase (FXase), which is a complex consisting of activated factor VII (FVIIa) and tissue factor (TF). Such combination is mediated by KD2, which suppresses the activity of activated factor X (FXa). KD3 does not exist in the β form. Further, positively charged amino acids present at the carboxyl terminus of TFPI‐α suppresses the activity of specific prothrombinase by combining with activated factor V. 3 As TFPI regulates the extrinsic pathway by inhibiting activities of FXa and extrinsic FXase, the activity of the extrinsic pathway can be increased by neutralizing TFPI. 4 , 5 Preceding studies have reported that inhibition of TFPI upregulated the extrinsic coagulation pathway and induced thrombin generation. 4 , 5 , 6 The purpose of the present study is to demonstrate the possibility of subcutaneous administration using an anti‐TFPI antibody as an alternative therapeutic for hemophilia.
2. MATERIALS AND METHODS
2.1. TFPI‐binding domain confirmation test
The sequence and domain information regarding human TFPI‐α (accession number: P10646‐1) were obtained from the UniProt database. cDNA encoding a total of five fragments (KD1KD2: a.a.29‐209; KD2KD3: a.a.108‐304; KD1KD3: a.a.29‐124 & a.a.176‐304; ΔKD2 epitope: a.a.29‐127 & a.a.155‐304), including one at full length, were synthesized and cloned into pcDNA4‐HisMaxC. The expression vector was transfected into human embryonic kidney (HEK) 293 cells using lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Immunoprecipitation was then performed by adding MG1113 and protein A sepharose beads (Cytiva) to the cell lysates. Bound proteins were eluted, resolved using SDS‐PAGE, and immunoblotted with the anti‐Xpress antibody (Thermo Fisher Scientific).
2.2. Crystallization, structure determination, and refinement
KD2 of human TFPI was produced in Escherichia coli. The Fab of MG1113 was secured through papain treatment. Details are provided in Appendix S1. The Fab of MG1113 and the KD2 complex (8.8 mg/mL) was crystallized in a solution containing 200 mM of sodium citrate tribasic and 18% (v/v) polyethylene glycol 3350 using the hanging‐drop vapor diffusion method at 20°C. For cryoprotection, the crystals were soaked in the crystallization solution containing 15% (v/v) glycerol. A radiographic diffraction data set was collected on the beamline 5C at the Pohang Accelerator Laboratory at POSTECH. All diffraction data were processed with the HKL2000 suit. 7 The structure of the complex was determined by molecular replacement using the structure of the reference (PDB entry 4DTG) as a search model with the program PHASER. 8 The asymmetric unit of the crystal contained four complexes. Model building and structure refinement were carried out using the programs COOT 9 and CNS. 10 Crystallographic data statistics are summarized in Table S1.
2.3. Measurement of TFPI‐binding affinity
An analysis on the binding of TFPI‐α and MG1113 was carried out using the surface plasmon resonance (SPR) method using Biacore T200 (Cytiva, Emeryville, CA, USA). Binding affinity was measured by reacting TFPI‐α with MG1113 fixed to a Series S sensor Chip Protein A. Kinetics and binding constants were calculated using Biacore T200 evaluation software (Cytiva), assuming that TFPI and MG1113 would interact in a 1:1 stoichiometric ratio.
We analyzed the binding of TFPI‐β and MG1113 by observing the binding of human umbilical vein endothelial cells (HUVECs) and MG1113. HUVECs were seeded on a 96‐well plate 1 day before the experiment. HUVECs were exposed to MG1113 and incubated for 1 hour at 2‐8°C. After washing, anti‐human IgG‐HRP (Sigma‐Aldrich, St Louis, MO) was added and incubated for 1 hour at 2‐8°C. Thereafter, MG1113 bound to HUVECs was detected through a change in absorbance using TMB (3,3',5,5'‐tetramethylbenzidine) treatment. We then calculated the half maximal effective concentration (EC50) based on the experimental results with four‐parameter regression.
2.4. FXa activity assay
MG1113 and recombinant human (rh) TFPI (Creative Biomart, Shirley, NY, USA) were incubated at 37°C for 10 minutes. rhFXa (Enzyme Research Laboratories, South Bend, IN, USA) was then added and incubated at 37°C for 30 minutes, followed by the addition of FXa substrate, S‐2765 (Instrumentation Laboratory, Bedford, MA, USA). The absorbance was measured using a VersaMax plate reader (Molecular Devices, San Jose, CA, USA) at 405 nm.
2.5. Extrinsic FXase activity assay
Relipidated rhTF (Sekisui Diagnostics) and rhFVIIa (Novo Nordisk) were placed onto a 96‐well plate and then reacted at 37°C for 10 minutes. Thereafter, the mixture was treated with MG1113 and rhTFPI and reacted at 37℃. Next, the mixture was treated with rhFX (HYPHEN BioMed, Neuville‐sur‐Oise, France) and reacted at 37℃ for 15 minutes shaking at 500 rpm with ThermoMixer® C (Eppendorf, Hamburg, Germany). The mixture was then treated with EDTA and reacted at 37°C for 5 minutes with shaking at 500 rpm with ThermoMixer® C (Eppendorf, Hamburg, Germany) to stop the reaction. The mixture was then treated with S‐2765. The absorbance was measured at 405 nm after the reaction.
2.6. Neutralization of TFPI‐β on HUVECs
HUVECs were cultured to confluence in EBM‐2 medium (Lonza Group, Basel, Switzerland) and stimulated with 20 ng/mL of tumor necrosis factor‐α (TNF)‐α and 20 ng/mL IL‐1β for 1 hour before testing. 5 Stimulated HUVECs were treated with or without MG1113. Thereafter, rhFX and rhFVIIa were added. EDTA was used to halt the reaction. rhFXa generation were measured using S‐2765. The absorbance was measured at 405 nm on a VersaMax plate reader at 90 minutes.
2.7. Modified Prothrombin Time Assay
The modified prothrombin time (mPT) assay was performed by mixing the plasma sample (50 μL) with relipidated rhTF solution (50 μL). MG1113 was diluted with FVIII‐ or FIX‐deficient plasma. rhTF was diluted to 0.2 ng/mL with phosphate buffered saline before mixing with plasma. Clotting time (CT) was recorded on a Start 4 Hemostasis Analyzer (Stago, Asnières sur Seine, France).
2.8. Thrombin generation assay
Thrombin generation assay (TGA) was performed using a Fluoroskan Ascent (Thermo Fisher Scientific) fluorescence plate reader. Plasma samples used were normal control plasma (Instrumentation Laboratory), FVIII‐deficient plasma (Siemens Healthcare, Erlangen, Germany), and FVIII‐deficient plasma with diluted MG1113. Plasma samples were mixed with PPP‐reagent LOW containing TF and phospholipids. Triplicate samples were incubated at 37°C in an Immulon microtiter 2HB‐High Binding 96‐well plate (Thermo Fisher Scientific). In control wells, plasma was mixed with a Thrombin Calibrator reagent. Before the reaction, fluorogenic thrombin substrate was mixed with prewarmed Flu‐Ca reagent and vortexed. The reaction began upon addition of the Flu‐Ca reagent mixture. TGA‐related reagents were obtained from Thrombinoscope. Analysis was performed using Thrombinoscope Analysis Version 3.0. All three reagents were manufactured by Thrombinoscope BV, Maastricht, the Nethelands.
2.9. Rotational thromboelastometry
We employed rotational thromboelastometry (ROTEM) for an in vitro spike test using whole blood samples from healthy volunteers and individuals with hemophilia to determine the coagulation effect of MG1113 (Table S2). For all samples used for the assay, approval was obtained from an institutional review board (IRB No. GCRL2014‐03), and consent was obtained from each subject before the assay was performed. For blood samples secured from healthy volunteers, the assay was carried out after incubating anti‐FVIII antibody (Haematologic Technologies, Essex Junction, VT, USA) at 37℃ for 30 minutes. The blood samples with neutralized FVIII activity from healthy volunteers or the blood samples from individuals with hemophilia were treated with MG1113 and incubated at 37℃ for 90 minutes. These samples were tested by NATEM (trigger: Ca++) using ROTEM delta (Tem International GmbH, Basel, Switzerland) according to the manufacturer’s instructions.
2.10. Animals
New Zealand White male rabbits were purchased from Samtako and individually raised in a well‐controlled facility. The present experiment was approved by the Animal Ethics Committee of GC Pharma (Approval No. GC‐17‐010A). An induced hemophilia model was produced by administering 10 mg/kg of FVIII neutralizing antibodies (NAbs). The level of FVIII was analyzed using a COAMATIC FACTOR VIII kit (Instrumentation Laboratory).
2.11. Rabbit cuticle bleeding model
After NAbs were administered for 30 minutes, the rabbit was anesthetized with 25 mg/kg pentobarbital sodium. After 2 mm of the apex of the cuticle was cut, the leg was placed into a 50‐mL conical tube containing sterile normal saline prewarmed to 37℃. The amount of hemoglobin was measured after collecting the blood for 60 minutes. After 60 minutes, blood was collected from the jugular vein, and then the rabbit was euthanized. A hemoglobin assay was conducted using a hemoglobin assay kit (MAK115‐1KT, Sigma‐Aldrich). The concentration of TFPI in the blood was measured as described previously. 11
3. RESULTS
3.1. The MG1113‐binding interface of KD2 overlaps with the FXa‐binding surface on KD2
To confirm the domain where MG1113 and TFPI could bind to each other, DNA constructs lacking KD1, KD2, and KD3, including full length, were designed. For each construct inserted into an expression vector, immunoprecipitation (IP) was conducted using MG1113 after transfecting the construct into HEK 293 cells. Western blot results showed the correct bands only in lanes 2, 3, and 4 because these constructs contained KD2 (Figure 1A). These results showed that MG1113 could bind only to KD2. To determine the epitope and paratope of KD2 of TFPI and MG1113, radiographic crystallography was performed on KD2 and the Fab fragment of papain digested MG1113. The crystal structure of the Fab MG1113‐KD2 complex at 2.8 Å resolution was determined (Table S1; Figure 1B). Epitopes of KD2 and paratopes of MG1113 were determined by identifying residues within an intermolecular distance of 4.5 Å (Table 1). More heavy‐chain residues than light‐chain residues of MG1113 interacted with KD2. Both electrostatic and hydrophobic interactions contributed to the antigen‐antibody interaction, burying a surface area of 1862.24 Å2 in one subunit. The MG1113‐binding interface of KD2 overlapped with the FXa‐binding surface on KD2 (Figure 1B), consistent with the potent anti‐hemophilia activity of MG1113. For additional verification, a mutation was induced by removing amino acids from the KD2 epitope section (amino acid 128‐154). As a result, in samples without the KD2 epitope area, no immunoprecipitated band was observed (Figure 1C). Binding affinity of MG1113 and TFPI‐α was measured to be 4.665 ± 0.371 × 10‐11 M (kon 2.825 ± 0.669 × 106 M‐1·s‐1; koff 1.308 ± 0.274 × 10‐4 s‐1) by SPR. This was the highest binding affinity among several candidate clones.
Figure 1.
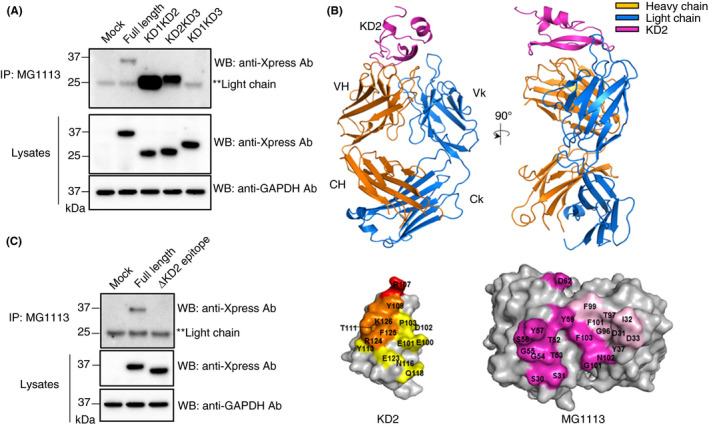
MG1113 binds to KD2 of tissue factor pathway inhibitor (TFPI). (A) TFPI expression vector was transfected into HEK 293 cells. Binding of Kunitz‐2 domain (KD2) of TFPI with MG1113 was then confirmed using immunoprecipitation (IP). A band was observed by western blot (WB) only in cases of TFPI constructs possessing KD2. Mock: human embryonic kidney (HEK) 293 cells were transfected without TFPI expression vector. (B) Two perpendicular views are shown with three polypeptides in different colors. Immunoglobulin (Ig) heavy chain is denoted by VH and CH. Ig kappa light chain is indicated by Vk and Ck. Mapping of epitopes and paratopes, defined as residues within the intersubunit distance of 4.5 Å, on surface representations of KD2 and Fab of MG1113. Left, epitopes on KD2 are shown in three different colors. Orange, yellow, and red indicate putative activated factor X (FXa)‐binding residues, which overlap with Fab of MG1113‐binding residues of KD2. Arg107 (in red) is a key residue in the inhibition of FXa. Right, paratopes on MG1113 are shown in magenta for VH residues and in pink for Vk residues. (C) MG1113 no longer binds to the construct from which the epitope of TFPI is removed. Ab, antibody; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase
Table 1.
Antigen‐antibody interactions (intermolecular distances ≤ 4.5 Å)
| KD2 | MG1113 | Type of interaction | |
|---|---|---|---|
| VH | Vk | ||
| Glu100 | Thr52 | … | H bonding |
| Glu100 | Gly54 | … | H bonding |
| Glu100 | Gly55 | … | H bonding |
| Glu100 | Ser56 | … | H bonding |
| Glu100 | Tyr57 | … | Van der Waals |
| Glu101 | Thr53 | … | H bonding |
| Glu101 | Tyr57 | … | OH‐π electrons |
| Asp102 | Tyr57 | … | Van der Waals |
| Pro103 | Thr52 | … | Van der Waals |
| Pro103 | Tyr57 | … | Van der Waals |
| Pro103 | Tyr59 | … | Van der Waals |
| Arg107 | Asp62 | … | Ionic interaction |
| Tyr109 | Tyr59 | … | Van der Waals |
| Tyr109 | … | Phe99 | Van der Waals |
| Thr111 | … | Asp31 | H bonding |
| Thr111 | … | Ile32 | Van der Waals |
| Tyr113 | … | Asp33 | H bonding |
| Tyr113 | … | Tyr37 | H bonding |
| Asn116 | Ser31 | … | H bonding |
| Gln118 | Ser30 | … | H bonding |
| Gln118 | Ser31 | … | Van der Waals |
| Glu123 | Gly101 | … | Van der Waals |
| Arg124 | Gly101 | … | H bonding |
| Arg124 | Asn102 | … | H bonding |
| Arg124 | Phe103 | … | Van der Waals |
| Arg124 | … | Asp31 | H bonding |
| Arg124 | … | Tyr37 | NH‐π electrons |
| Arg124 | … | Gly96 | H bonding |
| Arg124 | … | Thr97 | H bonding |
| Phe125 | Tyr59 | … | OH‐π electrons |
| Phe125 | Phe103 | … | Van der Waals |
| Lys126 | Tyr59 | … | H bonding |
| Lys126 | Phe103 | … | Van der Waals |
| Lys126 | … | Thr97 | H bonding |
| Lys126 | … | Phe99 | Van der Waals |
| Lys126 | … | Phe101 | Van der Waals |
Abbreviations: VH, heavy chain; Vk, kappa light chain.
3.2. MG1113 restores hemostatic rebalance by neutralizing the function of TFPI
In vitro assays were performed to observe whether MG1113 could neutralize the suppression of FXa and extrinsic FXase complex activities by TFPI. When FXa was treated with rhTFPI‐α, the absorbance decreased by approximately 94.2% due to suppressed FXa activity (Figure 2A). Thereafter, when FXa was treated with MG1113 under relevant conditions, the absorbance increased as concentration increased, and the residual activity of FXa was recovered up to 89.8% with the same concentration as TFPI treatment (Figure 2A). The EC50 of MG1113 for TFPI 10 nM measured 4.54 ± 0.07 nM. Regarding the effect of MG1113 on the extrinsic FXase complex, we observed that the activity increased in a concentration‐dependent manner (Figure 2B). The EC50 of MG1113 for TFPI 9 nM measured 5.69 ± 0.38 nM.
Figure 2.
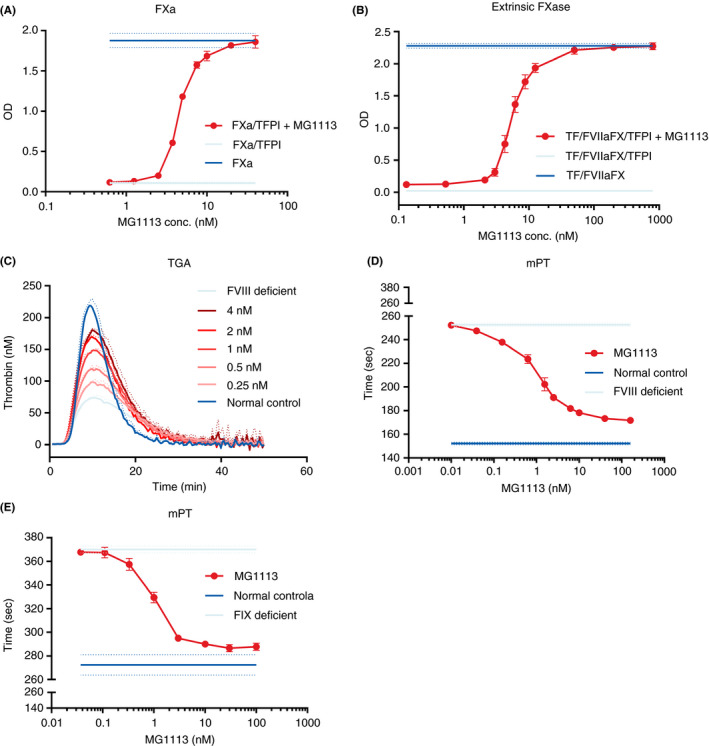
Neutralizing effect of MG1113 on the function of tissue factor pathway inhibitor‐α (TFPI‐α). (A) The activity of 1 nM activated factor X (FXa) is reduced by 10 nM TFPI. The activity of FXa is recovered (n = 3) after treatment with MG1113 (0.625‐40 nM). (B) Activation of 10 nM factor X (FX) by extrinsic FXase, comprised of 10.5 pM tissue factor (TF) and 0.5 nM activated factor VII (FVIIa), is inhibited by 9 nM TFPI. Generation and activity of FXa are recovered (n = 4) after treatment with MG1113 (0.13‐800 nM). (C) After factor VIII (FVIII) deficient plasma is treated with MG1113 through thrombin generation assay, with increasing concentration, thrombin generation is also increased (n = 3). (D, E) In a modified prothrombin time (mPT) assay, when FVIII or factor IX (FIX) deficient plasma is treated with MG1113, with increasing concentration, clotting time is further shortened (n = 3). The graph represents mean and standard deviation
Since the in vitro experiment revealed that MG1113 effectively neutralized the function of TFPI, we next determined whether thrombin generation is changed when FVIII‐deficient plasma was treated with MG1113. TGA showed that when the concentration of MG1113 was increased, endogenous thrombin potential (ETP) and thrombin peak level were also increased (Figure 2C). The ETP was measured to be about 128% that of normal plasma, and the thrombin peak was recovered up to about 81%. The recovery of ETP and thrombin peaks was similar to that of concizumab. 12
mPT was measured to determine the extent by which CT could be reduced via activation of the extrinsic pathway by MG1113 in FVIII‐deficient conditions. When the concentration of MG1113 used for treatment was increased, CT was further decreased for FVIII‐ or FIX‐deficient plasma (Figure 2D, E). The CT shortened from 252.6 ± 1.4 seconds to 171.7 ± 0.8 seconds in the case of FVIII‐deficient plasma, while it shortened from 370.1 ± 2.9 seconds to 287.9 ± 3.1 seconds in the case of FIX‐deficient plasma.
The TFPI‐β form possesses only KD1 and KD2. The β form exists on the cell surface where it binds to glycosylphosphatidylinositol anchors. 2 , 3 Accordingly, we determined whether MG1113 could bind to TFPI‐β possessing KD2 and further neutralize its function. First, binding of MG1113 to HUVECs known to express TFPI‐β 13 was observed. MG1113 exhibited increased binding to HUVECs than human IgG, a negative control (Figure 3A). The EC50 of MG1113 with HUVECs was 0.605 ± 0.075 nM. Next, a test was conducted to determine whether MG1113 could neutralize the function of TFPI‐β. We induced the development of TF by exposing HUVECs to TNF‐α and interleukin‐1β (IL‐1β). We then added FVIIa and FX to induce the generation of extrinsic FXase on the surface of cells with various concentrations of MG1113. When the concentration of MG1113 was increased, the amount of FXa generated from the TF/FVIIa/FX complex was also increased (Figure 3B).
Figure 3.
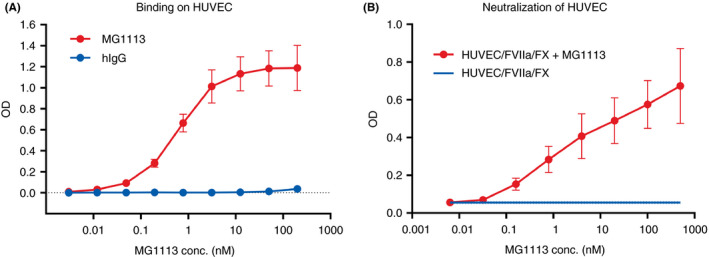
MG1113 not only binds to tissue factor pathway inhibitor‐β (TFPI‐β) but also neutralizes it. (A) Binding of human umbilical vein endothelial cells (HUVECs) and MG1113 is confirmed. Differently from human IgG (hIgG), which is a negative control, with increasing concentration of MG1113 (0.003 ‐ 200 nM), increased binding of MG1113 to HUVECs (n = 3) is observed. (B) The factor X (FX) is activated by activated factor VII (FVIIa) and tissue factor, which is expressed on stimulated HUVECs by tumor necrosis factor‐α. With increasing concentration of MG1113 (0.006‐500 nM), generation of activated factor X (FXa) is increased (n = 2). The graph represents mean and standard deviation
3.3. MG1113 rebalances hemostasis under hemophilia conditions
An acquired HA condition was created by treating blood secured from healthy volunteers with FVIII NAb. The effect of MG1113 was then observed through ROTEM. The CT of FVIII neutralized blood increased approximately 5.7 times (from 874 ± 121 seconds to 4961 ± 610 seconds; Figure 4A). After treatment with MG1113, all four parameters reverted to patterns shown in normal blood. Such changes were dependent on the concentration of MG1113 for all parameters (Figure 4).
Figure 4.
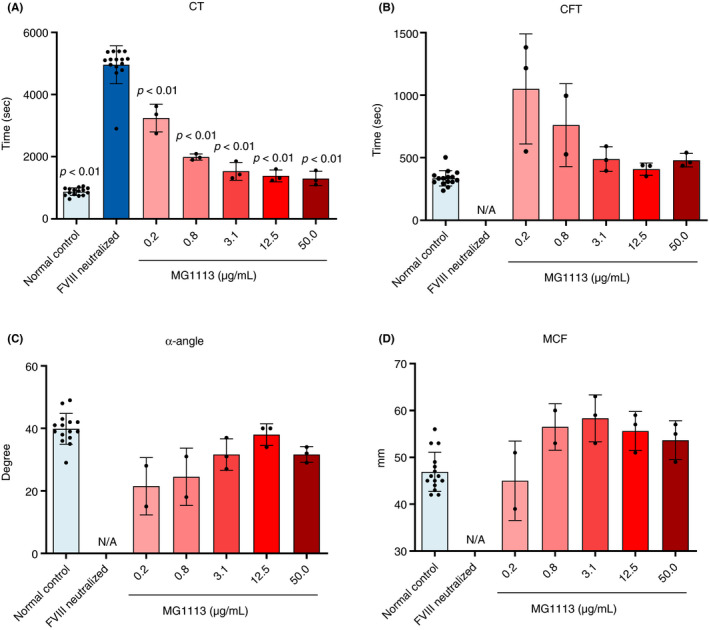
Confirmation for the coagulation inducing ability of MG1113 in factor VIII (FVIII) neutralized blood through rotational thromboelastometry. All values of (A) clotting time (CT), (B) clot formation time (CFT), (C) α‐angle, and (D) maximum clot firmness (MCF) changed to normal blood levels as concentration of MG1113 increased. The graph represents mean and standard deviation. Normal control (n = 15) and FVIII neutralized sample (n = 15), MG1113 treated sample (n = 3). A one‐way analysis of variance was conducted, assuming a normal distribution. The significance level is set at P < .05. The difference is analyzed based on FVIII neutralized sample results using a Dunnett post hoc analysis
Next, the effect of MG1113 was confirmed using whole blood secured from individuals with hemophilia. Blood was secured from 12 patients with HA and 2 with hemophilia B (HB). The patient’s whole blood was treated with 150 μg/mL MG1113 and then ROTEM was conducted. All four parameters reverted to patterns shown in healthy volunteers (Figure 5A‐D), similar to results observed earlier with the acquired HA condition. After treatment with 150 μg/mL MG1113, CT decreased from 2874 ± 721 seconds to 1455 ± 475 seconds, clot formation time decreased from 777 ± 271 seconds to 507 ± 215 seconds, α‐angle increased from 22 ± 6° to 33 ± 8°, and maximum clot firmness increased from 29 ± 17 mm to 51 ± 10 mm. Additionally, TGA and mPT assays were conducted. The average thrombin peak level in the patient’s plasma was 10.1 ± 6.2 nM, which increased to 117 ± 21 nM and 124 ± 20 nM when the patient’s plasma was treated with 3.13 μg/mL and 150 μg/mL MG1113, respectively. The average mPT of the patient’s plasma was 394.5 ± 119.6 sec. It shortened to 192.3 ± 35.5 seconds and 186.6 ± 34.5 seconds after the patient’s plasma was treated with 3.13 μg/mL and 150 μg/mL MG1113, respectively (Figure 5E, F).
Figure 5.
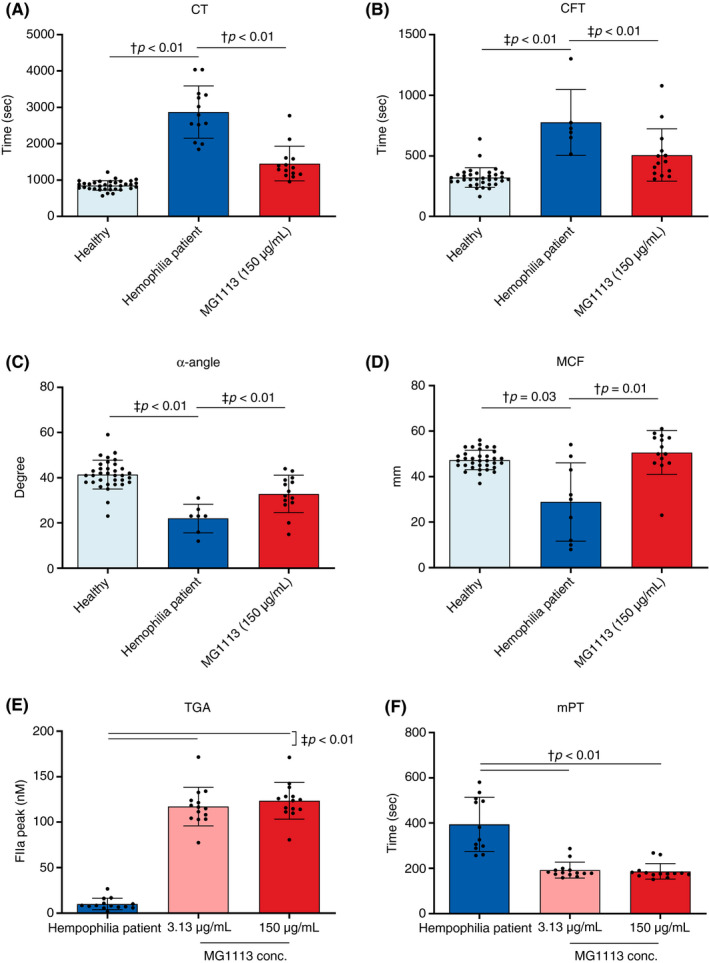
Hemostasis of blood and plasma of individuals with hemophilia was promoted by MG1113 treatment. All values of (A) clotting time (CT), (B) clot formation time (CFT), (C) α‐angle, (D) maximum clot firmness (MCF), (E) thrombin generation assay (TGA), and (F) modified prothrombin time (mPT) changed to the normal state after MG1113 treatment. The graph represents mean and standard deviation. Each dot represents the value of one person (healthy [n = 34]/individual with hemophilia [n = 6‐13]/MG1113 [n = 14]). In the case of the blood of individuals with hemophilia , certain parameters were not measured, in which case values of the relevant patients’ blood samples were excluded for relevant parameters. A one‐way analysis of variance was conducted, assuming a normal distribution. The significance level was set at P < .05. Each group was analyzed using †Games‐Howell pairwise comparison. Or the difference was analyzed based on treatment result of hemophilia sample or 0 μg/mL MG1113 using ‡Dunnett post hoc analysis
3.4. Confirmation on the effect of MG1113 in a cuticle bleeding model using an HA‐induced rabbit
The cross‐reactivity test showed that MG1113 binds to rabbit, monkey, and human TFPI (data not shown). Several NAbs against rabbit FVIII were produced to establish an HA‐induced model. MG1113 was administered through the marginal ear vein 10 minutes after administering NAbs (Figure 6). Regarding blood loss resulting from administration of MG1113 in a cuticle bleeding rabbit model, hemoglobin level in the blood flowing from a front cuticle to the saline of an anesthetized rabbit was measured. There was a statistically significant difference in the result of blood loss compared to normal rabbits. When blood losses for different administration doses were compared, hemoglobin values showed a decreasing trend as the administration dose of MG1113 was increased (Figure 6A). A statistically significant decrease of hemoglobin was observed in groups in which ≥0.25 mg/kg MG1113 was administered (Figure 6A). In addition to the measurement of blood loss in the cuticle bleeding experiment, activated partial thromboplastin time (aPTT) and mPT were measured as indicators of blood coagulation. As a result of analyzing aPTT to measure the intrinsic coagulation pathway, although there was a statistically significant delay compared to the control group, there was no difference in aPTT between groups administered MG1113 (Figure 6B). Conversely, as a result of mPT analysis to measure the extrinsic coagulation pathway, statistically significant shortening of mPT was shown when MG1113 was administered into the HA‐induced rabbit, although there was no difference between normal rabbits and the HA‐induced rabbit (Figure 6C). At 0.1 mg/kg and 0.25 mg/kg MG1113 dosing conditions, mPT was dose‐dependently shortened, but no dose‐dependent results were observed at 1.0 mg/kg and 5.0 mg/kg dosing conditions. If MG1113 is administered at 0.25 mg/kg or more, the saturation point of the effect is reached, and mPT is considered shortened to its maximum value (Figure 6C). This result showed that MG1113 functions in the extrinsic pathway rather than the intrinsic pathway. The level of free TFPI not bound to MG1113 was selected as a biomarker for the effect of MG1113. As a result of measuring the free TFPI level, although there was no statistically significant difference between induced HA groups and vehicle administered WT (wild type), a statistically significant decrease in the free TFPI level was found when MG1113 was administered depending on its concentration (Figure 6D). When cuticle bleeding results and free TFPI levels were compared, blood loss decreased when the rabbit’s free TFPI level was maintained below 20%‐22% (Figure 6A, D).
Figure 6.
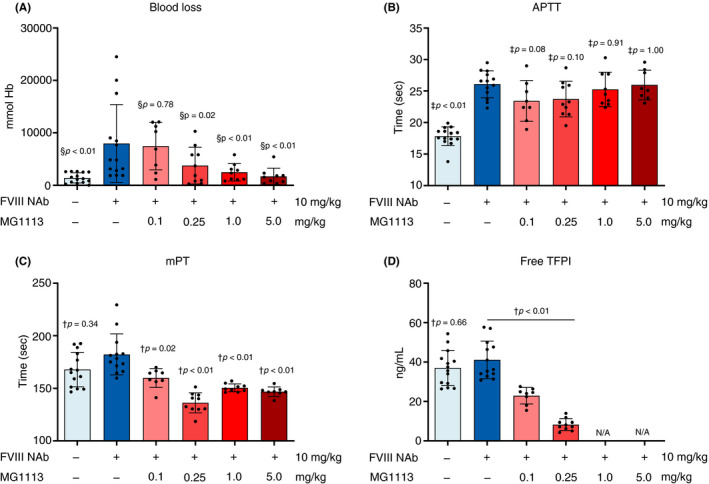
MG1113 restores blood loss and clotting time of a hemophilia A (HA)‐induced rabbit. (A) Rabbits received saline or factor VIII (FVIII) neutralizing antibody (Nab) (10 mg/kg) 45 minutes before bleeding induction. Rabbits received saline or MG1113 (0.1, 0.25, 1, 5 mg/kg) 35 min before bleeding induction. Bleeding was observed for 1 hour. Hemoglobin level was reduced by MG1113 treatment in a concentration‐dependent manner. (B) Activated partial thromboplastin time (aPTT) was delayed after treatment with FVIII NAb, but not changed after MG1113 treatment. (C) Modified prothrombin time (mPT) was shortened by MG1113 treatment. (D) Free tissue factor pathway inhibitor (TFPI) level was not changed by FVIII neutralizing antibody (NAb) treatment. However, it was reduced by MG1113 treatment. The graph represents mean and standard deviation. In the test group of 0.25 mg/kg, at which MG1113 started to affect, free TFPI level remained at approximately 20%‐22% of that of the group not treated with MG1113. Each dot represents an entity (n = 8‐15). Outliers were excluded from the data set (Grubb’s test, P < .01). The group not treated with MG1113 and other groups were analyzed using one‐way analysis of variance, assuming a normal distribution. The significance level was set at P < .05. § Two‐stage linear step‐up procedure; † Games‐Howell pairwise comparison; ‡ Dunnett post hoc analysis
To determine the possibility of subcutaneous administration, 5 mg/kg MG1113 was administered through intravenous and subcutaneous routes. Assays carried out previously were conducted similarly. Regardless of the route of administration, blood loss was reduced to WT control levels (Figure 7A). Although aPTT was not affected by the administration of MG1113, a statistically significant shortening of mPT was found after intravenous or subcutaneous administration of MG1113 (Figure 7B, C). The free TFPI level was also low in both cases (intravenous and subcutaneous administration) (Figure 7D). When blood loss and biomarkers resulting from the administration route of MG1113 were compared, there was no difference between the intravenous and subcutaneous administration groups of 5 mg/kg MG1113.
Figure 7.
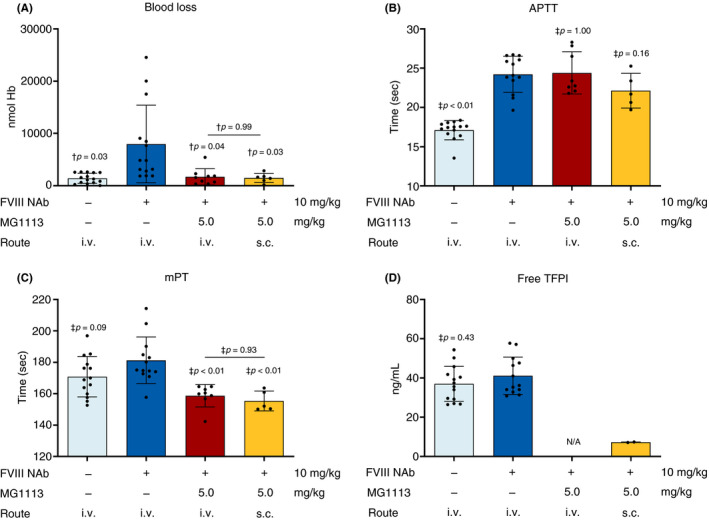
Confirmation for the ability of MG1113 in an HA‐induced rabbit through subcutaneous administration. The same concentration of MG1113 was administered intravenously or subcutaneously. Blood loss, activated partial thromboplastin time (aPTT), modified prothrombin time (mPT), and free tissue factor pathway inhibitor (TFPI) levels were then measured. (A) Hemoglobin level, (C) mPT, and (D) the level of free TFPI are reduced by MG1113 treatment through subcutaneous administration. (B) aPTT is delayed by FVIII neutralizing antibody treatment, but not changed by MG1113 treatment. The graph represents mean and standard deviation. Each dot represents an entity (n = 6‐15). The group not treated with MG1113 and other groups were analyzed using one‐way analysis of variance assuming a normal distribution. The significance level was set at P < .05. † Games‐Howell pairwise comparison; ‡ Dunnett post hoc analysis
4. DISCUSSION
To determine the binding site of MG1113 and TFPI, DNA constructs deficient of each domain were produced and transfected. Specific binding sites were observed using IP–western blotting. Thereafter, the exact epitope sequence was confirmed using radiographic crystallography. This epitope sequence is known to differ from that of concizumab or marstacimab. When the amino acid sequence of the binding epitope was deleted, binding could no longer occur (Figure 1C).
MG1113 exhibits high binding affinity to TFPI. In addition, its binding epitope overlaps with the binding site sequence of the FXa‐TFPI complex. Thus, it could effectively neutralize the function of TFPI that suppresses activities of FXa and extrinsic FXase. In vitro assays revealed that MG1113 recovered activities of FXa and extrinsic FXase. When FVIII‐ or FIX‐deficient plasma and the plasma secured from individuals with HA and HB were treated with MG1113, thrombin generation increased and CT shortened. It is thought that MG1113 bound to TFPI to suppress its function and normalize coagulation by increasing the activity of the extrinsic pathway. When the blood secured from individuals with HA and HB was treated with MG1113, various parameters of ROTEM were normalized. The HUVEC binding assay result indicates that MG1113 bound to TFPI‐β existing on the cell membrane and neutralized the function.
Finally, MG1113 was evaluated using an HA‐induced rabbit model produced by treating with anti‐rabbit FVIII NAbs. Blood loss decreased after intravenous administration of MG1113 into the cuticle bleeding model in a concentration‐dependent manner. Also, mPT and the free TFPI level decreased.
The generation of thrombin is very quickly amplified by the intrinsic pathway, which enables sufficient blood coagulation to be induced. However, in individuals with HA and HB who display difficulties in the intrinsic pathways, effective blood coagulation cannot be induced due to the lack of thrombin amplification, leading to bleeding. Based on such facts, we can hypothesize that, if sufficient thrombin generation can be secured for individuals with HA and HB to induce coagulation, blood coagulation can be regulated independently from FVIII and FIX. 14 , 15 , 16 Generation of FXa in the extrinsic blood coagulation pathway is brought about by TF/FVIIa, 1 , 17 , 18 and is suppressed by TFPI. 2 , 3 Accordingly, if TFPI in the extrinsic pathway can be suppressed, a higher amount of FXa generation can be secured. TFPI suppresses the activity of FXa itself and extrinsic FXase by combining with FXa. 2 , 3 As KD2 is known to directly bind to FXa among the three KDs that compose TFPI, it is thought that binding of MG1113 to KD2 of TFPI can maintain the function of FXa and extrinsic FXase, which enables bleeding to be regulated by inducing continuous thrombin generation.
The most widely used method for treating individuals with HA and HB is replacement therapy. Although replacement therapy has an advantage in that bleeding can be regulated and prevented, there are difficulties regarding repeated intravenous administration and inhibitor generation. 19 , 20 The best way to solve such problems may be through the development of humanized or fully human antibody‐based procoagulants, of which the immunogenicity is known to be low. 21 , 22 Such procoagulation also displays high bioavailability in subcutaneous injection. A total of four anti‐TFPI antibody therapeutics (MG113, concizumab, BAY 1 093 884, and marstacimab) are being developed to meet the needs described above. 11 , 23 , 24 Concizumab 23 and marstacimab (ClinicalTrials.gov: NCT02974855) reduced the annual bleeding rate when administered subcutaneously to hemophilia patients with or without inhibitors. However, BAY 1 093 884 terminated clinical trials due to thrombosis (ClinicalTrials.gov: NCT03597022), and clinical recruitment for concizumab was suspended due to the occurrence of nonfatal thrombotic events (ClinicalTrials.gov: NCT04083781, NCT04082429). MG1113 functions using the same mechanism of action; therefore, it is carefully monitored for thrombosis concerns during clinical phase 1.
Throughout this study, we determined that MG1113 had a novel epitope of TFPI that is different from other anti‐TFPI antibody therapeutics. MG1113 specifically bound to the KD2 of TFPI and covered the residues that interacted with FXa. MG1113 showed potential for use as a therapeutic agent for hemophilia in the future because it can induce thrombin generation at approximately 80% as that of normal control plasma. Additionally, MG1113 can recover blood loss to normal levels when 5 mg/kg is injected into an HA‐induced rabbit model through the subcutaneous route of administration.
RELATIONSHIP DISCLOSURE
Heechun Kwak, Sumin Lee, Seunghyun Jo, Young Eun Kwon, Hyunju Kang, Gahee Choi, Myung Eun Jung, and Sung Ho Hwang are employees of GC Pharma. Dong‐Sik Kim is an employee of MOGAM Institute for Biomedical Research. MOGAM was granted research funds from GC Pharma. Mi‐Jeong Kwak, Seonghoon Kim, and Byung‐Ha Oh report nothing to disclose.
AUTHOR CONTRIBUTIONS
HK, SL, YEK, HK, GC, and DSK performed the in vitro research and data analysis. MJK, SK, and BHO performed radiographic crystallography and data analysis. SJ and MEJ performed in vivo study and data analysis. SHH designed the research study.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study made use of Beamline 5C at the Pohang Accelerator Laboratory.
Kwak H, Lee S, Jo S, et al. MG1113, a specific anti–tissue factor pathway inhibitor antibody, rebalances the coagulation system and promotes hemostasis in hemophilia. Res Pract Thromb Haemost 2020;4:1301–1312. 10.1002/rth2.12438
Handling Editor: Dr Alisa Wolberg.
REFERENCES
- 1. Peters R, Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17(7):493–508. [DOI] [PubMed] [Google Scholar]
- 2. Crawley JT, Lane DA. The haemostatic role of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2008;28(2):233–42. [DOI] [PubMed] [Google Scholar]
- 3. Wood JP, Ellery PE, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. 2014;123(19):2934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang JY, Chantrathammachart P, Monroe DM, Key NS. Studies on the mechanism of action of the aptamer BAX499, an inhibitor of tissue factor pathway inhibitor. Thromb Res. 2012;130(3):e151–e157. [DOI] [PubMed] [Google Scholar]
- 5. Hilden I, Lauritzen B, Sorensen BB, Clausen JT, Jespersgaard C, Krogh BO, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119(24):5871–8. [DOI] [PubMed] [Google Scholar]
- 6. Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, et al. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117(20):5514–22. [DOI] [PubMed] [Google Scholar]
- 7. Otwinowski Z, Minor W. Processing of X‐ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–26. [DOI] [PubMed] [Google Scholar]
- 8. McCoy AJ, Grosse‐Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40(Pt 4):658–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse‐Kunstleve RW, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 5):905–21. [DOI] [PubMed] [Google Scholar]
- 11. Gu JM, Zhao XY, Schwarz T, Schuhmacher J, Baumann A, Ho E, et al. Mechanistic Modeling of the Pharmacodynamic and Pharmacokinetic Relationship of Tissue Factor Pathway Inhibitor‐Neutralizing Antibody (BAY 1093884) in Cynomolgus Monkeys. AAPS J. 2017;19(4):1186–95. [DOI] [PubMed] [Google Scholar]
- 12. Waters EK, Sigh J, Friedrich U, Hilden I, Sorensen BB. Concizumab, an anti‐tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia. 2017;23(5):769–76. [DOI] [PubMed] [Google Scholar]
- 13. Girard TJ, Tuley E, Broze GJ Jr. TFPIbeta is the GPI‐anchored TFPI isoform on human endothelial cells and placental microsomes. Blood. 2012;119(5):1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brummel‐Ziedins KE, Whelihan MF, Rivard GE, Butenas S. Activated protein C inhibitor for correction of thrombin generation in hemophilia A blood and plasma1. J Thromb Haemost. 2011;9(11):2262–7. [DOI] [PubMed] [Google Scholar]
- 15. Sehgal A, Barros S, Ivanciu L, Cooley B, Qin J, Racie T, et al. An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat Med. 2015;21(5):492–7. [DOI] [PubMed] [Google Scholar]
- 16. Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of hemophilia plasma. Thromb Haemost. 1991;66(4):464–7. [PubMed] [Google Scholar]
- 17. Hoffman M, Monroe DM 3rd. A cell‐based model of hemostasis. Thromb Haemost. 2001;85(6):958–65. [PubMed] [Google Scholar]
- 18. Smith SA. The cell‐based model of coagulation. J Vet Emerg Crit Care (San Antonio). 2009;19(1):3–10. [DOI] [PubMed] [Google Scholar]
- 19. Dargaud Y, Pavlova A, Lacroix‐Desmazes S, Fischer K, Soucie M, Claeyssens S, et al. Achievements, challenges and unmet needs for haemophilia patients with inhibitors: Report from a symposium in Paris, France on 20 November 2014. Haemophilia. 2016;22(suppl 1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iorio A, Kearon C, Filippucci E, Marcucci M, Macura A, Pengo V, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710–6. [DOI] [PubMed] [Google Scholar]
- 21. Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36(1):3–10. [DOI] [PubMed] [Google Scholar]
- 22. Kitazawa T, Shima M. Emicizumab, a humanized bispecific antibody to coagulation factors IXa and X with a factor VIIIa‐cofactor activity. Int J Hematol. 2020;111(1):20–30. [DOI] [PubMed] [Google Scholar]
- 23. Shapiro AD, Angchaisuksiri P, Astermark J, Benson G, Castaman G, Chowdary P, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. 2019;134(22):1973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel‐Hett S, Martin EJ, Mohammed BM, Rakhe S, Sun P, Barrett JC, et al. Marstacimab, a tissue factor pathway inhibitor neutralizing antibody, improves coagulation parameters of ex vivo dosed haemophilic blood and plasmas. Haemophilia. 2019;25(5):797–806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


