Abstract
Massive and irreparable rotator cuff tears remain a difficult condition to treat. Fatty infiltration of the muscles and excessive retraction of the tendons predispose to high failure rates of arthroscopic repair techniques. In recent years, studies on the superior capsule have shown that it plays a key role in reducing superior humeral head translation and restoring balance to the force couples required for dynamic shoulder function. Superior capsule reconstruction has become common in clinical practice. Several techniques with different types of grafts have been described, such as fascia lata autograft, dermal allograft patch, and long head of the biceps tendon autograft. More recently, an open technique with semitendinosus tendon autograft has been proposed. Our aim is to describe an all-arthroscopic technique for superior capsule reconstruction using a doubled semitendinosus tendon autograft in a box-shaped configuration. We believe that the technique can combine the advantages of other techniques, such as graft availability, low harvest-site morbidity, limited cost, and mechanical strength.
The treatment of massive and irreparable rotator cuff tears remains a challenge. By “irreparable,” we mean the inability to repair the tendon to its native footprint. The failure rate of these massive tears is very high.1 Excessive tension at the repair site, poor tissue quality, and a possible mismatch in the tension of the articular and bursa layers of the cuff in large delaminated tears are surely possible mechanisms of failure.
Over the years, several joint-preserving surgical options have been adopted, such as debridement with or without biceps tenotomy, partial repair, tendon transfer, subacromial spacer placement, and interposition graft placement.2,3 In the past decade, a deeper knowledge of the anatomy of the rotator cuff tendons has given importance to the role of the superior capsule.4 Mihata et al.5 first highlighted the importance of the superior capsule of the shoulder as a stabilizing structure and its crucial role in improving shoulder function by re-centering the humeral head into the glenoid socket, thus restoring the force couples to efficiently elevate the arm in case of an irreparable superior cuff tear. Subsequently, Mihata et al.6 reported the preliminary results of superior capsule reconstruction (SCR) and described a surgical technique using an autologous tendon graft of fascia lata for the treatment of irreparable superior cuff tears. Since then, several variations of the original technique have been proposed, introducing different graft sources, thicknesses, and fixation configurations.7, 8, 9
Recently, Rosales-Varo et al.10 proposed an open technique for SCR with autologous semitendinosus tendon graft in a reverse V–shaped configuration (single fixation point on the glenoid and double fixation on the greater tuberosity). The technique is safe and reliable and has some potential advantages related to cost and lesser morbidity than fascia lata graft. Moreover, the tendon length allows for different configurations (double strand, V shaped, reverse V shaped, or box shaped). This Technical Note aims to present an all-arthroscopic technique for SCR using an autologous semitendinosus tendon graft.
Surgical Technique
Indications
The diagnosis of a rotator cuff tear is always made through patient history, physical examination, and imaging. The routine imaging study consists of a standard radiographic evaluation to assess arthritic changes, as well as to rule out calcific tendinitis, and magnetic resonance imaging to assess tear characteristics, fatty infiltration, and muscle atrophy.
Our current indications for SCR are massive contracted tears of the superior cuff (supraspinatus and upper part of infraspinatus tendon) with grade III to IV fatty infiltration, upper migration of the humeral head, intact or at least repairable subscapularis tendon, intact teres minor, and mild cuff tear arthropathy (stage I-III according to the Hamada classification). However, the definitive indication for SCR is always confirmed at the time of surgery when actual tear reparability can be tested. Contraindications are severe cuff tear arthropathy (Hamada stage IV or higher), shoulder stiffness, and damage to the axillary nerve.
Patient Position and Surgical Fields
The procedure can be performed with the patient under general anesthesia or an interscalene block (or a combination thereof). The beach-chair position is preferable because it allows easy preparation of the surgical fields both at the operative shoulder and at the ipsilateral lower limb from which the semitendinosus tendon graft will be taken.
Diagnostic Arthroscopy and Portal Placement
After preparation of both surgical fields (shoulder and knee), the following bony landmarks at the shoulder are identified with a marking pen: spine of the scapula, acromion, clavicle, and coracoid process. The following standard portals are used:
-
•
Posterior portal, used as a viewing portal or as a working portal for suture management when the scope is in the lateral portal
-
•
Anterosuperior portal, used for suture management
-
•
Lateral portal, used as a viewing portal or as a working portal for suture management when the scope is in the posterior portal
-
•
Posterolateral portal, used as a viewing portal when the graft is passed through the lateral portal
The following accessory portals are used:
-
•
One or two superolateral portals, just beneath the lateral edge of the acromion, for optimal positioning of the anchors on the humeral head
-
•
Accessory posterior portal, about 2 cm medial and 1 cm superior to the standard posterior portal, for optimal positioning of the posterior anchor on the glenoid
-
•
Accessory anterosuperior portal, just anterior to the acromioclavicular joint, for optimal positioning of the anterior anchor on the glenoid
Intra-articular Procedures
Arthroscopic procedures always begin with an intra-articular diagnostic evaluation in air through the posterior portal. This is necessary to exclude advanced degenerative joint changes and possible lesions of the subscapularis tendon. The anterosuperior portal is then established, and an outflow cannula is inserted into the joint through the rotator cuff tear.
Biceps tenotomy or tenodesis is performed according the status of the tendon, as well as the patient’s age and demand. If a tear is present in the subscapularis tendon, it is repaired at this time, using the fixation technique more appropriate for the specific injury, which we will not discuss in this article.
Bursoscopy
When this first intra-articular phase is completed, the scope is passed into the subacromial space through the posterior portal, and by use of a shaver and an electrocautery device, bursectomy is performed through the lateral portal. The scope is then switched to the lateral portal so that bursectomy and release of eventual scar adhesions can be completed through the posterior or anterosuperior portal. At this point, it is possible to clearly assess the characteristics of the tear: Size, shape, tissue quality and delamination, retraction, and reducibility of the tear are estimated, thus confirming the indication for SCR. An arthroscopic graduated probe is used to estimate the possible length of the graft by summing the distance between the fixation points on the glenoid and greater tuberosity (posteriorly and anteriorly) and the distance between the 2 fixation points on the humerus (Fig 1).
Fig 1.
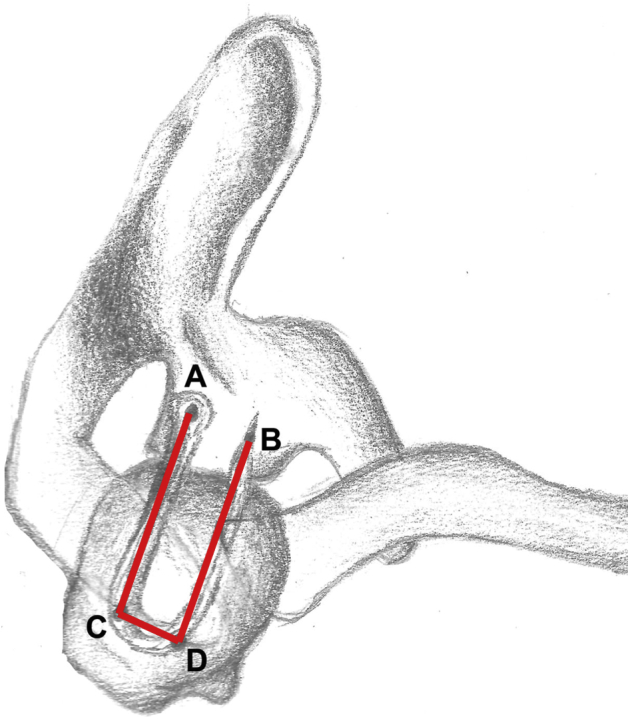
Superior view of shoulder. The graft length is estimated with a graduated arthroscopic probe by summing the distance between the fixation points on the glenoid and greater tuberosity, posteriorly (AC) and anteriorly (BD), and the distance between the 2 fixation points on the humerus (CD): Graft length = AC + BD + CD.
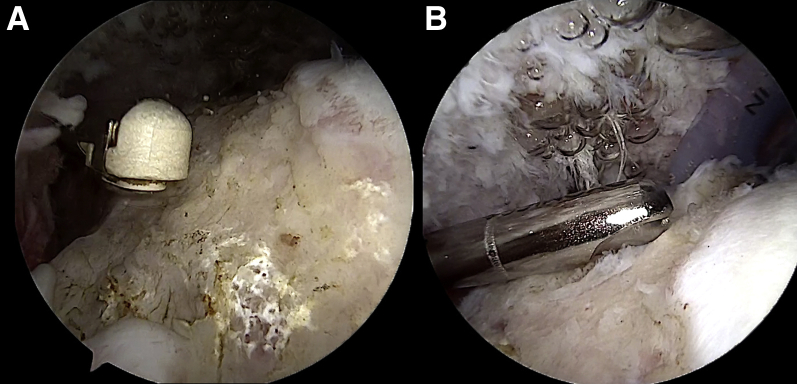
Tendon Harvesting and Preparation
Once the indication for SCR is confirmed, the tendon of the semitendinosus muscle is identified through a small incision (2-3 cm) at the medial aspect of the proximal tibia, centered on the insertion of the pes anserinus, and is harvested using a tendon stripper. On an appropriate workstation, the graft is debrided of any residual muscular tissue; it is then doubled and whipstitched at both ends with No. 2 high-strength nonabsorbable sutures (FiberWire; Arthrex, Naples, FL) (Fig 2). Once prepared, the graft is tensioned over the workstation in manual maximum tension and covered with wet gauze. It is useful to maintain the sutures as long as possible so that they can be easily used as a transport for the graft.
Fig 2.
Graft preparation on workstation. The semitendinosus tendon is debrided of any residual muscular tissue and is doubled and whipstitched at both ends with No. 2 high-strength nonabsorbable sutures (FiberWire).
Glenoid Preparation
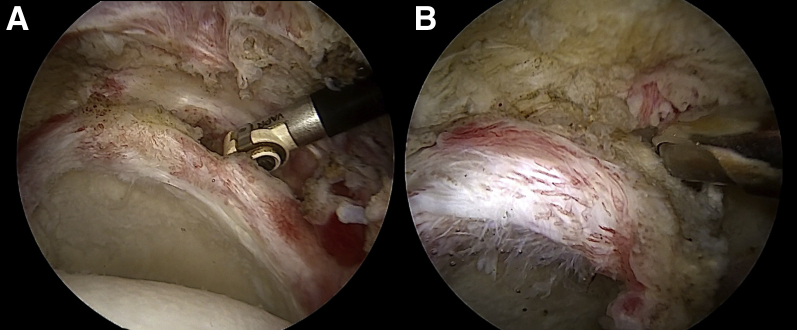
With the scope in the lateral portal, a radiofrequency device is used from the anterosuperior portal and from the posterior portal—or from accessory portals as described earlier—to clean off all soft tissue from the superior part of the glenoid neck (from the 10- to 2-o’clock position). A shaver is also used through the same portals to abrade some cortical bone (Fig 3). Care must be taken to avoid removal of the superior labrum, which may contribute as a restraint against superior translation of the humeral head.
Fig 3.
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through lateral portal. (A) A radiofrequency device is used to clean off all soft tissue from the anterosuperior and posterosuperior parts of the glenoid neck. (B) A second step is performed with a shaver blade to decorticate the superior aspect of the glenoid neck.
Greater Tuberosity Preparation
The radiofrequency device and shaver are used through the lateral and/or anterosuperior portal to clean off the footprint of the posterosuperior rotator cuff while the scope is maintained in the posterior portal. The viewing portal can be shifted to lateral to complete the procedure from the posterior portal (Fig 4).
Fig 4.
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through posterior portal. Preparation of the greater tuberosity is performed using a radiofrequency device for the soft tissues (A) and a shaver blade for the cortical bone (B) from the lateral and anterosuperior portals. The viewing portal can be shifted to the lateral portal to complete the procedure with instruments from the posterior and/or anterosuperior portal.
Graft Fixation on Glenoid
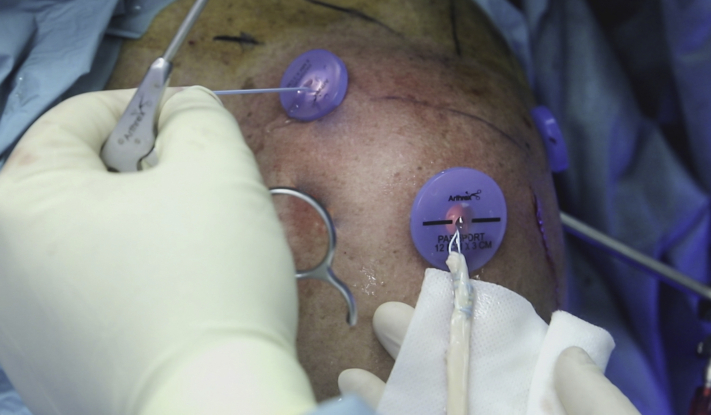
With the scope in the posterolateral portal, the graft is introduced through the lateral portal into the joint for anterior fixation first. The traction sutures of the loop end of the graft are retrieved from the anterosuperior portal (or through the accessory anterosuperior portal), and the graft is pulled into the joint through a lateral 12-mm flexible plastic cannula (PassPort; Arthrex) (Fig 5). Before graft passage, a free No. 2 FiberWire is looped around the tendon at about the midpoint of its length and used to apply tension from the lateral side when the second end of the graft will be passed and fixed on the posterior aspect of the glenoid.
Fig 5.
Lateral view of left shoulder with patient in beach-chair position. The graft is pulled into the joint from the lateral portal and through a 12-mm flexible plastic cannula (PassPort). Traction sutures are retrieved from the anterosuperior portal.
A pilot hole for anchor fixation is made from the same portal in which the graft was retrieved on the anterosuperior aspect of the glenoid neck, at the 2-o’clock position for a right shoulder. Then, traction sutures are loaded on a knotless PEEK (polyether ether ketone) anchor (2.9-mm PushLock; Arthrex), and onlay fixation of the loop end of the graft is achieved (Fig 6).
Fig 6.
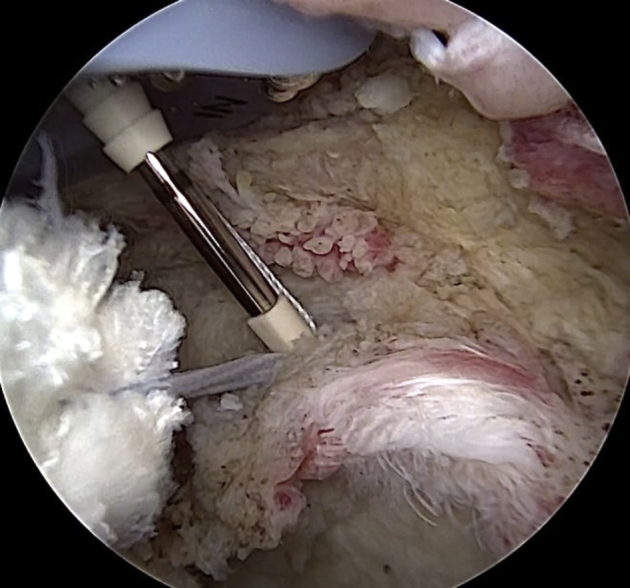
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through lateral portal. After the creation of a pilot hole for the anterior glenoid anchor through the accessory anterosuperior portal, one graft end is shifted into the joint. Onlay fixation on the anterosuperior aspect of the glenoid neck (at the 10-o’clock position for a left shoulder) is achieved by means of a knotless PEEK anchor (2.9-mm PushLock), appropriately loaded with the traction sutures of the graft.
Thereafter, while the scope is maintained in the posterolateral portal, the traction sutures of the free ends (sutured together) of the graft are retrieved from the posterior portal (or accessory posterior portal). The steps described to achieve anterior fixation are repeated to achieve posterior glenoid fixation while the graft is maintained in tension from the lateral portal using the free suture applied at its midsubstance (Fig 7).
Fig 7.
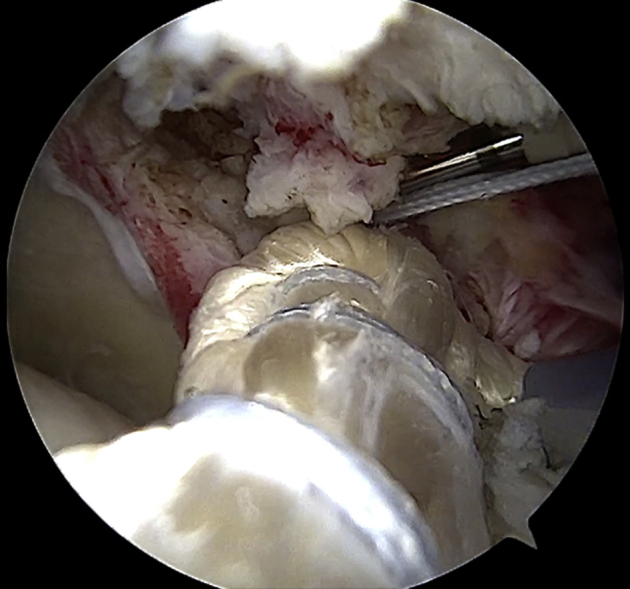
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through posterolateral portal. The graft is fixed on the posterosuperior aspect of the glenoid neck (at the 2-o’clock position for a left shoulder) by means of a knotless PEEK anchor (2.9-mm PushLock), properly loaded with the traction sutures of the graft. The anchor is introduced through the accessory posterior portal.
Graft Fixation on Greater Tuberosity
Lateral fixation is achieved with 2 knotless PEEK anchors (4.75-mm SwiveLock; Arthrex) placed close to the edge of the articular surface of the humeral head by creating 2 parallel bands (anterior and posterior) with a box-shaped configuration of the graft (Fig 8). The anterior anchor is placed through an accessory superolateral percutaneous portal while viewing from the posterolateral portal. A lasso loop is created around the tendon and adjusted to correspond to the insertion site of the anchor. The loop is loaded on the anchor and fixed in a knotless configuration. The same procedure is performed for the posterior anchor. During this step, the scope is shifted to the lateral portal and the anchor is introduced through the posterolateral portal. The previously applied midsubstance traction suture can be used to fix the graft with the posterior anchor. Alternatively, a new lasso loop can be loaded around the graft and the midsubstance traction suture can still be maintained in tension from the lateral portal during fixation. If the graft remains lax between the 2 fixation points on the humerus, 1 or 2 supplementary anchors can be used to fix the midsubstance of the graft on the lateral aspect of the footprint in a double-row configuration.
Fig 8.
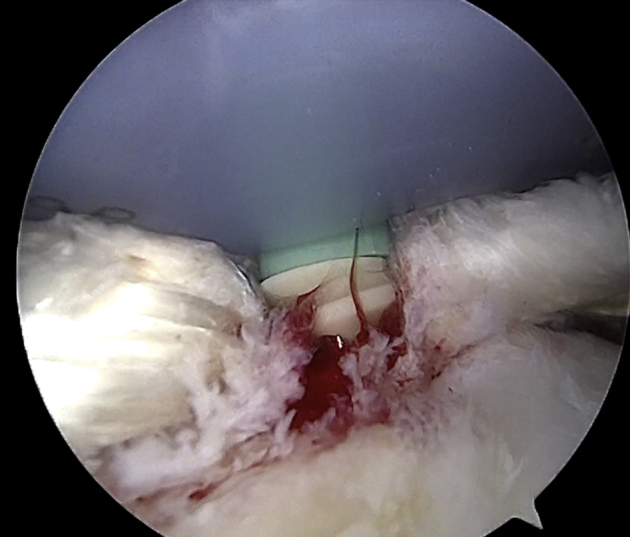
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through posterior portal. Fixation of the graft on the greater tuberosity is performed through an accessory superolateral portal with a knotless PEEK anchor (4.75-mm SwiveLock).
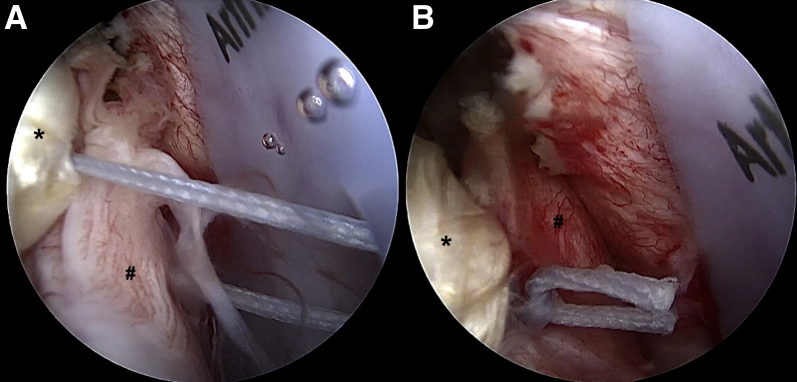
At the end of the procedure, the posterior cuff can be partially repaired to the greater tuberosity and/or approximated to the posterior band of the graft with some side-to-side No. 2 FiberWire sutures (Fig 9). The final construct appears like a rectangular box that acts as a barrier against superior migration of the humeral head, thus simulating the effect of a patch graft (e.g., fascia lata or extracellular dermal matrix) (Fig 10). The surgical technique is shown in detail in Video 1.
Fig 9.
Arthroscopic view of left shoulder with patient in beach-chair position: intra-articular view through posterolateral portal. (A, B) The posterior cuff (pound signs) is partially repaired to the posterior band of the graft (asterisks) with some side-to-side No. 2 FiberWire sutures.
Fig 10.
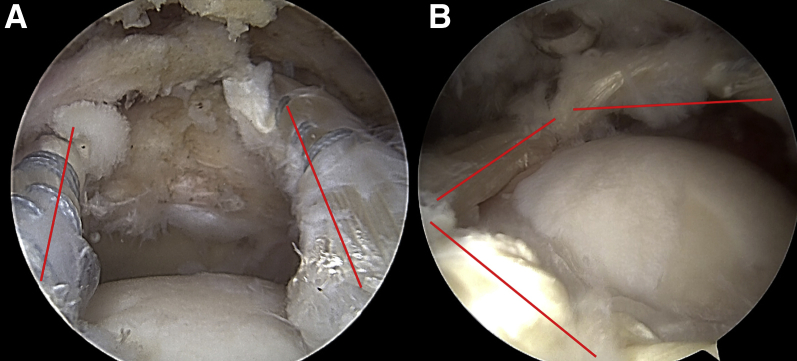
Arthroscopic view of left shoulder with patient in beach-chair position. (A) Intra-articular view through lateral portal. The 2 parallel bands (anterior and posterior) of the graft tensioned from the humeral head to the superior aspect of the glenoid neck are marked (red lines). (B) Intra-articular view through posterior portal. The box-shaped configuration of the graft is marked (red lines).
Postoperative Care
The arm is immobilized in an abduction sling in neutral rotation for 6 weeks. The rehabilitation protocol starts 6 weeks after surgery according to the following phases:
-
•
Phase 1 (6-8 weeks after surgery): massotherapy and physical modalities for the management of pain, inflammation, and muscle contractures and passive range-of-motion (ROM) exercises
-
•
Phase 2 (9-12 weeks after surgery): active-assisted ROM exercises and closed–kinetic chain exercises to strengthen the residual rotator cuff, subscapularis, biceps, deltoid, pectoralis major, and scapular stabilizers
-
•
Phase 3 (13-16 weeks after surgery): active ROM exercises and open–kinetic chain exercises, proprioceptive and plyometric exercises, and postural rehabilitation of the kinetic chain (lumbo-pelvic, thoracolumbar, and scapulothoracic muscles)
Return to heavy manual work or sports activities is allowed 6 months after surgery. Magnetic resonance imaging is routinely performed at 6 months’ follow-up. Pearls and pitfalls of the described technique are presented in Table 1, and advantages and disadvantages are listed in Table 2.
Table 1.
Pearls and Pitfalls of Technique
| Pearls |
| Preparation of the glenoid and greater tuberosity footprint should be performed before graft introduction. |
| Medial and lateral accessory portals should be used for optimal positioning of the anchors. |
| Graft tensioning from the lateral portal must be maintained during fixation on the greater tuberosity. |
| Pitfalls |
| Anchor placement on the HH can affect tensioning (more distance, more tension). |
| The graft length should be carefully estimated because it can affect tension of the construct. |
| The arthroscopic view may be compromised by the size of the graft (too thick). |
HH, humeral head.
Table 2.
Advantages and Disadvantages of Technique
| Advantages |
| Low cost |
| Reduced donor-site morbidity compared with other autografts (i.e., fascia lata) |
| Good option in revision surgery |
| Less cumbersome to manage during arthroscopy (vs fascia lata or dermal patch) |
| Disadvantages |
| High surgical skills required |
| Additional surgical time needed for tendon harvest |
| Need for biomechanical studies |
| No clinical evidence |
Discussion
In the past several years, arthroscopic SCR has gained considerable popularity as one of the best options available for the treatment of massive and irreparable rotator cuff tears. This hypothesis has been progressively confirmed, starting by the studies of Mihata et al.5,6 and subsequently by clinical reports published in recent years.7, 8, 9 In support of this, some recent literature reviews have confirmed the effectiveness of the technique, in terms of both objective functional and subjective outcomes.11,12 The original SCR technique described by Mihata et al.5 with the use of an autologous fascia lata graft has some disadvantages related to harvest-site morbidity, even through a minimally invasive technique has been described by Ângelo and de Campos Azevedo.13
The main advantages of extracellular matrix (ECM) consist of graft uniformity and avoidance of donor-site morbidity. However, no definitive information has been provided about local adverse reactions and the inflammatory response, integration with the surrounding tissues, and maintenance of integrity and mechanical behavior over time. Particularly, ECM patch grafts have created some concerns in terms of cost, availability (when allograft is used), mechanical strength, and structural integrity (especially with xenograft).14
The long head of the biceps tendon (LHBT) as an autologous graft for SCR was proposed in some articles using different techniques.15 Recent biomechanical studies have shown that SCR with LHBT graft restores shoulder stability in irreparable rotator cuff tears by re-centering the humeral head on the glenoid.16,17 Particularly, single-strand LHBT graft was shown to be biomechanically equivalent to—and potentially even stronger than—fascia lata autograft in the prevention of superior humeral migration.16,17
SCR with LHBT graft is an attractive and reliable option because of limited donor-site morbidity, very limited costs (1 or 2 anchors on the greater tuberosity), and ease and speed of execution. However, a major drawback of this technique is related to the status of the biceps tendon. Severe degeneration with delamination and partial or complete rupture of the LHBT limit the use of the procedure. Similarly, in revision surgery, the LHBT is almost always unavailable because of previous tenotomy or tenodesis.
We believe that the described technique represents a possible alternative for SCR, which combines the advantages of other techniques. Indeed, graft availability is almost guaranteed (except in rare cases of patients who previously underwent ligament reconstruction procedures for which both semitendinosus tendons were used); donor-site morbidity is rather limited; costs are reduced in comparison with other techniques, such as ECM graft; and mechanical strength is potentially greater than that of single-strand LHBT graft and, therefore, that of fascia lata.
Certainly, the technique is challenging, and optimization of surgical steps will improve its reproducibility. There is no doubt that future studies, both biomechanical and clinical, are needed to prove the effectiveness of the technique and to compare it with the most established techniques. Nonetheless, semitendinosus tendon graft can be considered a valid graft option, especially for surgeons who use the LHBT for SCR. Use of the semitendinosus could bridge the gap between patch graft and the LHBT, especially when biceps tendon is unavailable because of a previous partial or complete tear or, in case of revision cuff surgery, previous tenotomy or tenodesis.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: G. M receives personal fees from Arthrex and Smith & Nephew and nonfinancial support from Arthrex, Wright, and FGP, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Superior capsule reconstruction with doubled autologous semitendinosus tendon graft: step-by-step technique.
References
- 1.Chona D.V., Lakomkin N., Lott A. The timing of retears after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2054–2059. doi: 10.1016/j.jse.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Burnier M., Elhassan B.T., Sanchez-Sotelo J. Surgical management of irreparable rotator cuff tears: What works, what does not, and what is coming. J Bone Joint Surg Am. 2019;101:1603–1612. doi: 10.2106/JBJS.18.01392. [DOI] [PubMed] [Google Scholar]
- 3.Carver T.J., Kraeutler M.J., Smith J.R., Bravman J.T., McCarty E.C. Nonarthroplasty surgical treatment options for massive, irreparable rotator cuff tears. Orthop J Sport Med. 2018;6:1–13. doi: 10.1177/2325967118805385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams C.R., DeMartino A.M., Rego G., Denard P.J., Burkhart S.S. The rotator cuff and the superior capsule: Why we need both. Arthroscopy. 2016;32:2628–2637. doi: 10.1016/j.arthro.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Mihata T., McGarry M.H., Pirolo J.M., Kinoshita M., Lee T.Q. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: A biomechanical cadaveric study. Am J Sports Med. 2012;40:2248–2255. doi: 10.1177/0363546512456195. [DOI] [PubMed] [Google Scholar]
- 6.Mihata T., Bui C.N.H., Akeda M. A biomechanical cadaveric study comparing superior capsule reconstruction using fascia lata allograft with human dermal allograft for irreparable rotator cuff tear. J Shoulder Elbow Surg. 2017;26:2158–2166. doi: 10.1016/j.jse.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Tokish J.M., Beicker C. Superior capsule reconstruction technique using an acellular dermal allograft. Arthrosc Tech. 2015;4:e833–e839. doi: 10.1016/j.eats.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petri M., Greenspoon J.A., Millett P.J. Arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthrosc Tech. 2015;4:e751–e755. doi: 10.1016/j.eats.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutter E.G., Godin J.A., Garrigues G.E. All-arthroscopic superior shoulder capsule reconstruction with partial rotator cuff repair. Orthopedics. 2017;40:e735–e738. doi: 10.3928/01477447-20170615-01. [DOI] [PubMed] [Google Scholar]
- 10.Rosales-Varo A.P., Zafra M., García-Espona M.A., Flores-Ruiz M.A., Roda O. Reconstrucción de la cápsula superior en las roturas irreparables del manguito mediante injerto autógeno de isquiotibiales. Rev Esp Cir Ortop Traumatol. 2019;63:1–6. doi: 10.1016/j.recot.2018.08.004. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 11.Catapano M., de Sa D., Ekhtiari S., Lin A., Bedi A., Lesniak B.P. Arthroscopic superior capsular reconstruction for massive, irreparable rotator cuff tears: A systematic review of modern literature. Arthroscopy. 2019;35:1243–1253. doi: 10.1016/j.arthro.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Makovicka J.L., Chung A.S., Patel K.A., Deckey D.G., Hassebrock J.D., Tokish J.M. Superior capsule reconstruction for irreparable rotator cuff tears: A systematic review of biomechanical and clinical outcomes by graft type. J Shoulder Elbow Surg. 2020;29:392–401. doi: 10.1016/j.jse.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Ângelo A.C.L.P.G., de Campos Azevedo C.I. Minimally invasive fascia lata harvesting in ASCR does not produce significant donor site morbidity. Knee Surg Sports Traumatol Arthrosc. 2018;27:245–250. doi: 10.1007/s00167-018-5085-1. [DOI] [PubMed] [Google Scholar]
- 14.Pennington W.T., Bartz B.A., Pauli J.M., Walker C.E., Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: Short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy. 2018;34:1764–1773. doi: 10.1016/j.arthro.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y.S., Lee H.J., Park I., Sung G.Y., Kim D.J., Kim J.H. Arthroscopic in situ superior capsular reconstruction using the long head of the biceps tendon. Arthrosc Tech. 2018;7:e97–e103. doi: 10.1016/j.eats.2017.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park M.C., Itami Y., Lin C.C. Anterior cable reconstruction using the proximal biceps tendon for large rotator cuff defects limits superior migration and subacromial contact without inhibiting range of motion: A biomechanical analysis. Arthroscopy. 2018;34:2590–2600. doi: 10.1016/j.arthro.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Han F., Kong C.H., Hasan M.Y., Ramruttun A.K., Kumar V.P. Superior capsular reconstruction for irreparable supraspinatus tendon tears using the long head of biceps: A biomechanical study on cadavers. Orthop Traumatol Surg Res. 2019;105:257–263. doi: 10.1016/j.otsr.2018.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Superior capsule reconstruction with doubled autologous semitendinosus tendon graft: step-by-step technique.