Abstract
Needle arthroscopy may provide several potential advantages over standard arthroscopy. The smaller camera size and weight allows for a minimally invasive and percutaneous approach with decreased fluid use. As resolution and image quality improve, the potential to expand clinical use for therapeutic applications becomes possible. One promising use is in elbow arthroscopy. Difference in the technology, such as a zero-degree optic and less-rigid instrumentation, necessitate a modified technique to accommodate thorough diagnostic arthroscopy and therapeutic procedures. This manuscript introduces the authors' approach to diagnostic needle arthroscopy of the anterior and posterior elbow compartments and placement of therapeutic instrumentation. This technique could theoretically decrease the risk of iatrogenic neurovascular injuries, reduce postoperative swelling and pain due to decreased fluid use, and potentially lead to faster recovery.
Introduction (With Video Illustration)
Needle arthroscopy (NA) has gained traction as an in-office diagnostic tool. It provides a cost-effective alternative to magnetic resonance imaging1, 2, 3, 4 and may provide better reliability and diagnostic utility in the setting of certain pathologies, previous surgery, or previous hardware.2,4,5 As an in-office procedure, it allows direct visualization without the cost and anesthetic risk associated with formal diagnostic arthroscopy.4,6 However, as resolution and image quality improve, several potential advantages make NA appealing for broader therapeutic applications in the operating room.
Elbow arthroscopy is a technically challenging procedure with some notable risks and limitations. The proximity of important neurovascular structures, including the median, median antebrachial cutaneous, ulnar, and radial nerves as well the brachial artery, leads to a potentially greater risk of complications due to iatrogenic injury.7, 8, 9 The smaller camera size (2 mm) and weight of NA tools allows for a minimally invasive and percutaneous approach, potentially reducing risk to these structures. The inflow sheath results in decreased arthroscopic fluid use, which may reduce postoperative swelling and pain and lead to improved short-term recovery and patient satisfaction. Less fluid use also results in less overall soft-tissue extravasation. Excessive soft-tissue swelling due to arthroscopy fluid is a common limiting factor to the time many surgeons will allot for arthroscopy and the decreased fluid use in NA, theoretically, increases the maximum time allowance for these technically difficult cases.
These differences in instrumentation and technology necessitate a modified technique to accommodate thorough diagnostic arthroscopy and therapeutic application. Specifically, gross movements may need to be modified to avoid bending the inherently more malleable instruments. The viewing angle of zero-degrees may also be unfamiliar to many surgeons who are comfortable with the 30° viewing angle commonly used in standard arthroscopy. This manuscript and accompanying demonstrative video (Video 1) will introduce our preferred approach to diagnostic arthroscopy of the anterior and posterior compartments of the elbow with NA, as well as demonstrate an approach for therapeutic instrumentation.
Surgical Technique
Patient Positioning, Equipment, and Setup
The patient may be positioned in the lateral decubitus or prone position. The authors recommend the lateral decubitus position, with the patient leaning slightly forward to allow intraoperative elbow flexion. A sterile arm holder supports the proximal humerus, allowing the elbow to rest at approximately 90°. A tourniquet is applied but not always inflated. General anesthesia is recommended, as regional anesthesia does not allow postoperative assessment of nerve function and patients may not tolerate the positioning while awake. Protective measures against compressive neuropathies and sterile preparation is the same as with standard arthroscopy. An examination under anesthesia is recommended to evaluate range of motion, crepitus and joint stability.
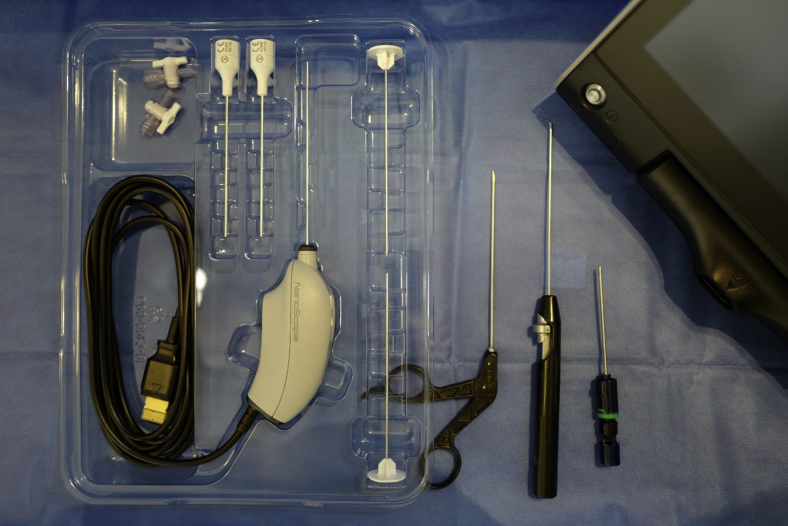
The NA set (NanoScope, Arthrex, Naples, FL) includes a zero-degree arthroscope with power cord, monitor, sharp and blunt trochars with corresponding sheaths including inflow portals. Assorted instruments, including a retractable probe and a 2.0-mm shaver, are also available (Fig 1). With the use of a sterile technique, the cords are attached and the monitor can be relayed to overhead monitors in the operating room via a standard HDMI cable.
Fig 1.
The needle arthroscopy set (NanoScope, Arthrex, Naples, FL) includes a zero-degree arthroscope with power cord, monitor, sharp, and blunt trochars with corresponding sheaths including inflow portals and assorted instruments.
Anatomical Landmarks, Portal Locations, and Insufflation
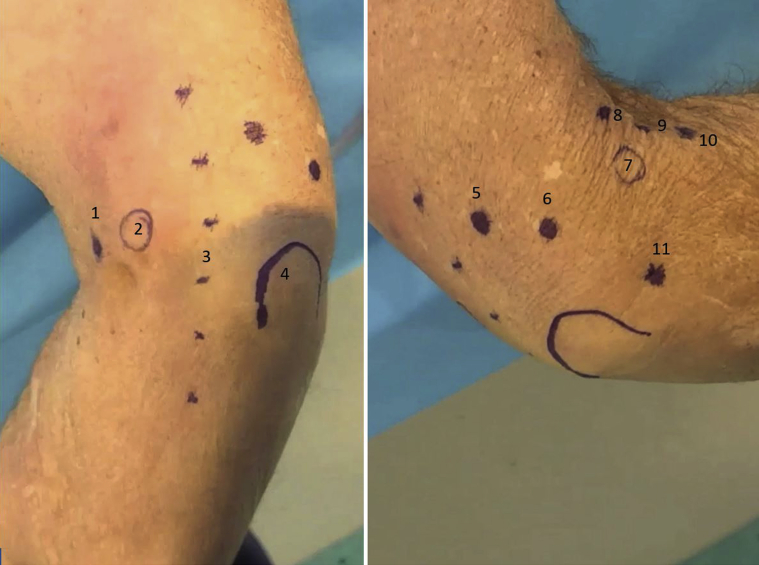
The olecranon, medial epicondyle, lateral epicondyle, and ulnar nerve are identified and marked, as well as the following described portals (Fig 2). For access to the anterior elbow, a modified proximal anteromedial portal (mPAMP), proximal anterolateral portal (PALP), mid-anterolateral portal (MALP), and distal anterolateral portal (DALP) are used. The mPAMP is used as the primary viewing portal and is located 0.5 to 1 cm proximal to the medial epicondyle and immediately anterior to the intermuscular septum. This is a modification of the classic PAMP position, which is located 2 cm proximal to the medial epicondyle and anterior the intermuscular septum. This modification allows for a better inline view of the entire joint and is necessary due to the zero-degree viewing angle of the NA camera. Despite moving the portal slightly more distal, the decreased size of the NA camera sheath (2 mm vs 4-5 mm) makes injury to the median antebrachial cutaneous nerve unlikely. The PALP functions as the inflow portal and is located 2 cm proximal to the lateral epicondyle and 1 cm anterior to the humerus. The MALP is the primary working portal and provides direct inline access to the joint. It is located 1 cm proximal and 1 cm anterior to the lateral epicondyle. The DALP is used as an accessory working portal and is located 1 to 2 cm distal and 1 cm anterior to the lateral epicondyle, just anterior to the radial head.
Fig 2.
Left, Medial external view of a right elbow in the lateral decubitus position. 1 – modified proximal anteromedial portal, 2 – medial epicondyle, 3 – ulnar nerve, 4 – olecronon. Right, Lateral external view of a right elbow in the lateral decubitus position. 5 – transtriceps portal, 6 – posterolateral portal, 7 – lateral epicondyle, 8 – proximal anterolateral portal, 9 – mid-anterolateral portal, 10 – distal anterolateral portal, 11 – soft-spot portal.
Access to the posterior elbow is achieved with a transtriceps portal (TTP), posterolateral portal (PLP), and accessory posterolateral portal (APLP). The TTP is the primary viewing portal and is located 3 cm proximal to the tip of the olecranon through the triceps tendon. The PLP is used as an inflow portal and is located 1.5 cm proximal to the olecranon and just lateral to the triceps tendon. The APLP is a working portal and is located 0.5 to 1 cm proximal to the PLP depending on angle of approach needed for instrumentation.
Insufflation is achieved through the “soft-spot” portal located at the center of a triangle connecting the olecranon tip, lateral epicondyle, and radial head. Before arthroscopy begins, approximately 15 to 20 mL of normal saline is injected with an 18-gauge spinal needle into the soft spot portal to achieve joint insufflation.
Anterior Compartment Arthroscopy
Diagnostic NA begins with the introduction of the needle arthroscope into the mPAMP. By using a NA sheath with sharp trochar, the sheath is introduced through the skin and into the joint space. The trochar is removed and the NA camera is inserted into the sheath. Upon insertion of the camera, visualization should be possible due to previous insufflation. If necessary, inflow tubing can be attached to the camera sheath and the camera can be temporarily removed to assist in fluid delivery and visualization of the joint. Under direct visualization, a second sheath with sharp trochar is introduced into the joint through the PALP. The trochar is removed and inflow tubing is then transferred to this sheath, creating a dedicated inflow port. This is helpful in obtaining excellent visualization of the joint and can help mitigate flow mismatch. It is important to realize visualization in NA relies more upon controlling flow through a dedicated inflow cannula than upon pump pressure and fluid volume.
Once the NA system is established, additional working portals can be made by using the NA sharp trochar (without a sheath). The diameter of the trochar allows for a wide enough percutaneous hole for dedicated NA instrumentation to pass through. The authors recommend the MALP as the primary working portal. The DALP can be created as an accessory working portal as required. Instrumentation can then be inserted, and arthroscopic treatment performed as indicated.
With this approach to the anterior compartment, the radial head and neck, radio-capitellar joint, coronoid fossa, coronoid, and anterior capsule can be easily visualized and accessed with working tools (Fig 3).
Fig 3.
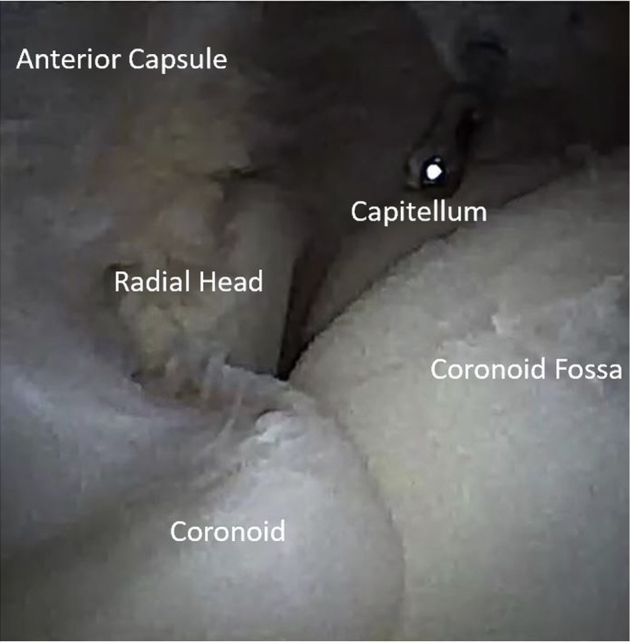
Arthroscopic view, right elbow, lateral decubitus position, from the modified proximal anteromedial portal, which allows direct in-line visualization of key structures. Similarly, the instrument placed through the mid-anterolateral portal enables direct in-line access to key structures.
Posterior Compartment Arthroscopy
Similar to the anterior compartment, NA of the posterior compartment should use a camera sheath and a dedicated inflow sheath. A sheath with sharp trochar is introduced into the TTP and the NA camera is inserted after trochar removal. The dedicated inflow sheath is placed with similar technique in the PLP under direct visualization. A sharp trochar is then introduced into the APLP to form the working portal. A shaver can then be introduced through this portal to clear away the posterior bursa and fat pad, giving a clear intra-articular view of the posterior elbow. Through the viewing TTP, visualization of the olecranon tip, medial gutter, lateral gutter can be easily obtained (Fig 4). Camera viewing and fluid inflow may be switched between the TTP and PLP sheaths as needed.
Fig 4.

Arthroscopic view, right elbow, lateral decubitus position, from the transtriceps portal visualizing the posterior compartment.
This described technique for visualization and placement of working instrumentation allows for thorough diagnostic arthroscopy of all elbow compartments and describes an approach for potential therapeutic applications including loose body removal, osteo-capsular arthroplasty, lateral epicondyle debridement, and fracture fixation.
Closure and Postoperative Protocol
NA results in minimal soft-tissue swelling and disruption. The created percutaneous portals do not require sutures (Fig 5). A simple, compressive soft dressing is placed over the elbow at the conclusion of the procedure and may be removed after 48 to 72 hours. Patients are encouraged to engage in immediate elbow range of motion and, typically, non-narcotic medications are sufficient for postoperative pain control.
Fig 5.
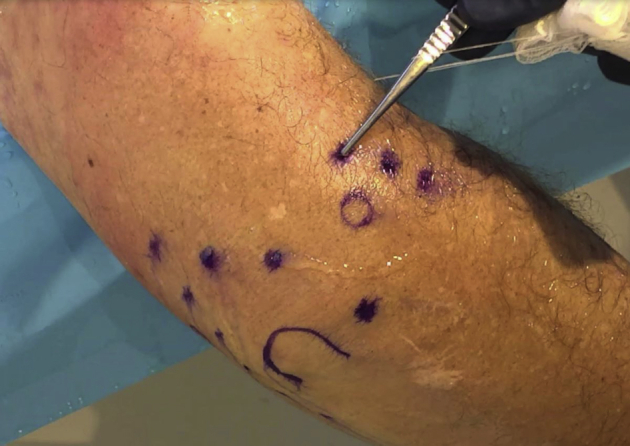
External view of a right elbow in the lateral decubitus position post-arthroscopy, demonstrating minimal soft-tissue disruption. Portals do not require sutures. A simple compressive soft dressing over the elbow for 48-72 hours is usually sufficient for postoperative wound care.
Discussion
The cost effectiveness and diagnostic accuracy of in-office NA have been previously reported.1, 2, 3, 4 However, a recent systematic review highlighted the need to establish defined protocols and indications to expand application and widespread clinical use, particularly in the operating room.10 Despite the exciting prospect of reduced complications due to iatrogenic injury and improved patient outcomes, to our knowledge, there is no English literature on NA use in the elbow to date.
Differences in the technology, namely a zero-degree optic and less-rigid instrumentation, means that standard arthroscopy techniques need to be modified to accommodate thorough arthroscopy with NA. Thus, this manuscript and video (Video 1) presents our technique for diagnostic arthroscopy of the anterior and posterior compartment of the elbow with NA.
Positioning and preparation of the patient are shared features of standard arthroscopy and NA. In standard arthroscopy, patients can be positioned in the lateral decubitus, prone, or supine position. For NA, the authors prefer the lateral decubitus position, as it allows similar access to the elbow as the prone position but with easier positioning and airway access.11 The ability to freely manipulate the elbow intraoperatively and a traction device not being required make it preferable over the supine position.11 Preparation including marking of key anatomical landmarks and portal sites are also synonymous between both approaches to elbow arthroscopy.9 The preferred portals for NA differ slightly from standard arthroscopy to allow direct lines of approach to necessary structures, particularly with the primary viewing anterior mPAMP. The direct lines of approach minimize gross movements, which may otherwise damage the smaller and more malleable NA instrumentation. They also allow for an improved field of view with a zero-degree optic camera.
An important consideration in the described technique is the role of flow in establishing optimal visualization. Visualization in NA relies more upon controlling flow than upon pump pressure and fluid volume. Poiseuille's law stipulates that decreasing the radius of a tube inversely effects resistance and directly reduces flow. Therefore, the narrower cannula of the NA will necessitate adjusting the working instruments, particularly arthroscopic shavers and ablators. A standard large diameter shaver with full suction applied would rapidly remove all the fluid from the joint and create a vacuum. This should, therefore, be managed with smaller shaver diameters and judicious application of suction. The implementation of a dedicated inflow cannula which does not contain the camera also helps mitigate flow mismatch.
Given the smaller diameter of the inflow cannula in NA compared with standard elbow arthroscopy, there is a significantly reduced fluid requirement. This may lead to less tissue distention and less soft-tissue swelling. Decreased postoperative swelling may translate to less postoperative pain, earlier return of function, and improved patient satisfaction. Less fluid use and, by virtue, less soft-tissue extravasation may also theoretically increase the allowable duration of an elbow arthroscopy, which is of concern to even experienced standard arthroscopists.
In addition to primary diagnosis, NA offers widespread potential for therapeutic use, such as we describe in the sections to follow.
Osteocapsular Arthroplasty
Elbow arthritis in the young, active population is not uncommon and presents a therapeutic challenge. Given the issues with longevity of total elbow arthroplasty and the significant soft-tissue disruption required for open osteocapsular arthroplasty, arthroscopic osteocapsular arthroplasty (AOCA) has become more popular. AOCA has been noted by several studies to improve outcomes as well as provide improved postoperative pain and quicker rehabilitation when compared with open osteocapsular arthroplasty.12 AOCA, however, has been associated with a risk of neurovascular injury.13 Using NA for AOCA would provide similar benefits as the standard arthroscopic approach. The decreased camera and instrumentation size along with decreased fluid and soft-tissue disruption could possibly mitigate the neurovascular risks. While this advantage would need to be evaluated with long-term studies, our technique shows that NA can obtain excellent visualization of all commonly affected arthritic elbow structures and instrumentation can be used to reach these anatomic areas.
Fracture Fixation
Open surgical fixation of elbow fractures is technically demanding, associated with a high degree of soft-tissue disruption and carry risks including the formation of heterotopic ossification.14 Arthroscopic fracture fixation has become increasingly used to minimize this soft-tissue disruption and has been shown to be a reliable method for elbow fracture treatment.15 NA provides a similar opportunity as standard arthroscopy, and as demonstrated with the described technique, can produce excellent intra-articular visualization of the joint. This provides an exciting opportunity to capitalize on these emerging arthroscopic skills to improve elbow fracture care with even less invasive technologies.
Second-Look Surgery
Often, diagnostic elbow imaging is compromised by previous surgical intervention ranging from inability to position the patient to hardware artifact. Traditional second-look surgeries incur the cost and risk similar to the index procedure. NA offers the opportunity to minimize these factors and obtaining a potentially more accurate understanding of the elbow following a previous operation. It also offers a minimally invasive and cost-effective research tool to understand the effect certain elbow procedures may have on the joint.
Pearls and Pitfalls
The potential advantages of this modified procedure also carry unique risks. The increased malleability of the instruments makes them inherently less tolerant of manipulation and are prone to bending and even breaking. The ends of the sheaths are sharp and there is a risk of iatrogenic chondral damage, which is increased if a surgeon is not familiar with the instrumentation. Additional pearls and pitfalls of the procedure are discussed in Table 1.
Table 1.
Pearls and Pitfalls
| Pearls |
|
| Pitfalls |
|
3D, 3-dimensional; NA, needle arthroscopy.
Conclusions
We describe an approach for diagnostic and therapeutic procedures in the elbow that uses needle arthroscopy. The technique details portal locations that allow direct in-line visualization and access with working tools; as well as the importance of a dedicated inflow cannula to control flow, decrease overall fluid utilization and maintain excellent visualization.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: B.G. reports personal fees and other from Arthrex, other from Pacific Medical, other from ROM3, other from Stryker, nonfinancial support and other from Smart Medical Devices, other from Synthes, and other from Doximity, outside the submitted work. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Video demonstrating diagnostic small-bore needle arthroscopy and the approach for therapeutic instrumentation, in a right elbow in the lateral decubitus position performed by the senior author.
References
- 1.Amin N., McIntyre L., Carter T., Xerogeanes J., Voigt J. Cost-effectiveness analysis of needle arthroscopy versus magnetic resonance imaging in the diagnosis and treatment of meniscal tears of the knee. Arthroscopy. 2019;35:554–562.e513. doi: 10.1016/j.arthro.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Chapman G.L., Amin N.H. The benefits of an in-office arthroscopy in the diagnosis of unresolved knee pain. Case Rep Orthop. 2018;2018:6125676. doi: 10.1155/2018/6125676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deirmengian C.A., Dines J.S., Vernace J.V., Schwartz M.S., Creighton R.A., Gladstone J.N. Use of a small-bore needle arthroscope to diagnose intra-articular knee pathology: Comparison with magnetic resonance imaging. Am J Orthop (Belle Mead NJ) 2018;47 doi: 10.12788/ajo.2018.0007. [DOI] [PubMed] [Google Scholar]
- 4.Gill T.J., Safran M., Mandelbaum B., Huber B., Gambardella R., Xerogeanes J. A prospective, blinded, multicenter clinical trial to compare the efficacy, accuracy, and safety of in-office diagnostic arthroscopy with magnetic resonance imaging and surgical diagnostic arthroscopy. Arthroscopy. 2018;34:2429–2435. doi: 10.1016/j.arthro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Hong A., Lui J.N., Gowd A.K., Dhawan A., Amin N.H. Reliability and accuracy of MRI in orthopedics: A survey of its use and perceived limitations. Clin Med Insights Arthritis Musculoskelet Disord. 2019;12 doi: 10.1177/1179544119872972. 117954411987972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan S., Saini S., Alyea E., Ford E. Office-based needle arthroscopy: A standardized diagnostic approach to the knee. Arthrosc Tech. 2017;6:e1119–e1124. doi: 10.1016/j.eats.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claessen F.M.A.P., Kachooei A.R., Kolovich G.P. Portal placement in elbow arthroscopy by novice surgeons: Cadaver study. Knee Surg Sports Traumatol Arthrosc. 2017;25:2247–2254. doi: 10.1007/s00167-016-4186-y. [DOI] [PubMed] [Google Scholar]
- 8.Hilgersom N.F.J., Oh L.S., Flipsen M., Eygendaal D., van den Bekeron MPJ Tips to avoid nerve injury in elbow arthroscopy. World J Orthop. 2019;8:99–106. doi: 10.5312/wjo.v8.i2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camp C.L., Degen R.M., Sanchez-Sotelo J., Altchek D.W., Dines J.S. Basics of elbow arthroscopy part I: Surface anatomy, portals, and structures at risk. Arthrosc Tech. 2016;5:e1339–e1343. doi: 10.1016/j.eats.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K., Crum R.J., Samuelsson K., Cadet E., Ayeni O.R., de Sa D. In-office needle arthroscopy: A systematic review of indications and clinical utility. Arthroscopy. 2019;35:2709–2721. doi: 10.1016/j.arthro.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 11.Camp C.L., Degen R.M., Dines J.S., Altchek D.W., Sanchez-Sotelo J. Basics of elbow arthroscopy part III: Positioning and diagnostic arthroscopy in the lateral decubitus position. Arthrosc Tech. 2016;5:e1351–e1355. doi: 10.1016/j.eats.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson G.N., Wu T., Galatz L.M., Yamaguchi K., Keener J.D. Elbow arthroscopy: Early complications and associated risk factors. J Shoulder Elbow Surg. 2014;23:273–278. doi: 10.1016/j.jse.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll S.W., Morrey B.F. Arthroscopy of the elbow. Diagnostic and therapeutic benefits and hazards. J Bone Joint Surg Am. 1992;74:84–94. [PubMed] [Google Scholar]
- 14.Douglas K., Cannada L.K., Archer K.R., Dean D.B., Lee S., Obremskey W. Incidence and risk factors of heterotopic ossification following major elbow trauma. Orthopedics. 2012;35:e815–e822. doi: 10.3928/01477447-20120525-18. [DOI] [PubMed] [Google Scholar]
- 15.Fink Barnes L.A., Parsons B.O., Hausman M. Arthroscopic management of elbow fractures. Hand Clin. 2015;31:651–661. doi: 10.1016/j.hcl.2015.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video demonstrating diagnostic small-bore needle arthroscopy and the approach for therapeutic instrumentation, in a right elbow in the lateral decubitus position performed by the senior author.