Abstract
Since the identification of the first human coronavirus in the 1960s, a total of six coronaviruses that are known to affect humans have been identified: 229E, OC43, severe acute respiratory syndrome coronavirus (SARS-CoV), NL63, HKU1, and Middle East respiratory syndrome coronavirus (MERS-CoV). Presently, the human world is affected by a novel version of the coronavirus family known as SARS-CoV-2, which has an extremely high contagion rate. Although the infection fatality rate (IFR) of this rapidly spreading virus is not high (ranging from 0.00% to 1.54% across 51 different locations), the increasing number of infections and deaths has created a worldwide pandemic situation. To provide therapy to severely infected patients, instant therapeutic support is urgently needed and the repurposing of already approved drugs is presently in progress. In this regard, the development of nanoparticles as effective transporters for therapeutic drugs or as alternative medicines is highly encouraged and currently needed. The size range of the viruses is within 60–140 nm, which is slightly larger than the diameters of nanoparticles, making nanomaterials efficacious tools with antiviral properties. Silver-based nanomaterials (AgNMs) demonstrate antimicrobial and disinfectant effects mostly by generating reactive oxygen species (ROS) and are presently considered as a versatile tool for the treatment of COVID-19 patients. Other metal-based nanoparticles have been primarily reported as delivery agents or surface modifying agents, vaccine adjuvant against coronavirus. The present review summarizes and discusses the possible effectiveness of various surface-modified AgNMs against animal coronaviruses and presents a concept for AgNM-based therapeutic treatment of SARS-CoV-2 in the near future.
Keywords: silver nanomaterials, coronavirus, silver nanocomposites, antiviral, SARS-CoV
Introduction
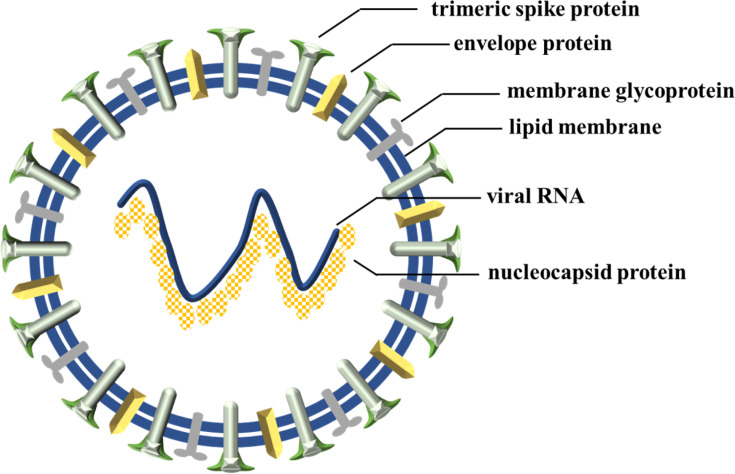
Coronaviridae is an emerging family of coronaviruses and comprises two subfamilies, coronavirinae and torovirinae.1 Coronavirinae is sub-categorized into four genera, namely alpha, beta, gamma, and delta coronaviruses.2 Until now, humans have been mostly affected by alpha (229E, NL63) and beta genera (OC43, SARS-CoV, HKU1, MERS-CoV and SARS-CoV-2).3 As a point of fact, the alpha and beta genera infect mammals while the delta and gamma genera mainly infect birds.4 In 1930, the first bird coronavirus was discovered when domestic chickens were infected by an unknown pathogen named an infectious bronchitis virus (IBV).5 Later, in 1965, the first human coronavirus was reported6 with common cold symptoms. In 1968, eight scientists proposed the name “corona” (which means “crown” or “wreath” in Latin) for the newly discovered viruses based on detailed findings of their structures.7 This structural exploration showed that four types of proteins are present in all coronavirus structures: spike (S), envelope (E), membrane (M), and nucleocapsid (N).8 A positive sense single-strand ribonucleic acid (RNA) (+ssRNA), which is 26–32 kilobases in length, is packed inside an N-protein shell with S-, E-, and M- proteins surrounding it (Figure 1).9
Figure 1.
Schematic diagram of coronavirus.
The S-protein is believed to be the principle and only receptor-attachable part of each viral body.10 The S-protein of the SARS virus binds to the angiotensin-converting enzyme-2 (ACE2) receptor while, for the MERS virus, the S-protein binds to host dipeptidyl peptidase-4 (DPP4) receptors.11
The recently discovered SARS-CoV-2 genome sequence matches about 79% of previous versions of SARS-CoV and around 50% of MERS-CoV.12 The newly discovered SARS-CoV-2 was first reported to affect humans in late 2019 with common pneumonia-like symptoms.13 Based on the degree of infection, other symptoms were also observed including respiratory problems, fever, cough, diarrhea, shortness of breath, dyspnea, kidney failure, and even death.14 Scientists termed this new virus SARS-CoV-215,16 because of its similarity to SARS-CoV and refer to it as a “novel coronavirus” because of its dissimilarity with previously reported coronaviruses.17 This virus has already infected more than 39 million people of which nearly 1.1 million people have died as of October 18, 2020, a number which continues to increase daily.18 The World Health Organization (WHO) has proposed that widespread testing is the best way to reduce these numbers.
Repurposing antiviral drugs or drugs used for other types of therapy to provide therapeutic support to SARS-CoV-2 patients is a temporary solution. Targeted therapeutic treatments will take some time to develop and test. Vaccination against the virus is the only treatment that can boost our immune system and grow antibodies that work against SARS-CoV-2. However, a development time of about 12–18 months is needed to prepare a completely new vaccine, although researchers are trying to speed up the development.19,20 Hence, in this situation, there is an urgent need for better and instant drug support for SARS-CoV-2 patients.
Nanoparticles (NPs) have emerged as one of the most important therapeutic materials used in the medical field.21 Recently, metal,22–24 metal oxide,25,26 metal sulfide,27,28 and inorganic NPs have been developed with proper surface modifications which show promise for important therapeutic applications in various fields.29 Apart from inorganic NPs, there are also several kinds of organic NPs and among them, some of the most extensively studied are nanospheres,30 dendrimers,31 liposomes,32 micelles,33 and solid lipid nanoparticles,34,35 Presently, four organic nanoparticle-based vaccines are in clinical phase trials against COVID-19 and have shown promising results in terms of immunogenicity and safety.36
Among inorganic materials, AgNMs have been extensively studied and shown to possess different biological activities, i.e., antibacterial, antifungal, and anticancer. As a result, they are gaining importance in the medical field.37–41 AgNMs are also being investigated due to their catalytic properties,42,43 non-toxic nature, high quantum efficiency,44,45 enhanced conductivity,46,47 antibacterial properties,48,49 anticancer properties,50,51 disinfectant capacity,52,53 stability,54 biochemical capacity as sensors,55,56 water purification properties,57–60 and straight forward synthesis.61–65 While AgNMs are still not a well-explored topic in the field of virology, some anti-viral actions against a few known viruses, i.e., human immunodeficiency virus (HIV),66 H1N1 influenza A virus,67 hepatitis B virus,68 herpes simplex virus type1,69 and monkeypox virus70 have been reported. Although such nanoparticles are almost undefined and yet to be considered for SARS-CoV-2 therapy, previous results from virologic studies give some hope about their therapeutic use against SARS-CoV-2. Researchers are currently trying to utilize different metals, metal oxides, metal sulfides, and other inorganic nanomaterials for possible therapeutic support against SARS-CoV-2.71–73 Few AgNMs have been reported as therapeutic agents towards some animal coronaviruses and other metal-based nanomaterials are reported as analytical sensors, vaccine adjuvant, and nanocarriers. This review is primarily focused on the formulation of AgNMs to provide therapeutic support against coronaviruses.
Mechanism of Virus Entry into Host Cells
SARS-CoV-2 transmission occurs mainly through active and passive pathways. In the active pathway, mucus droplets released by an infected person through coughing, sneezing, or talking directly attack the healthy person whereas, in the passive pathway, the virus attacks through secondary sources such as mucus droplets released by an infected person which are evaporated and end up as dried nuclei, which later attach to objects such as tables, clothes, and door handles and infect a healthy person.74
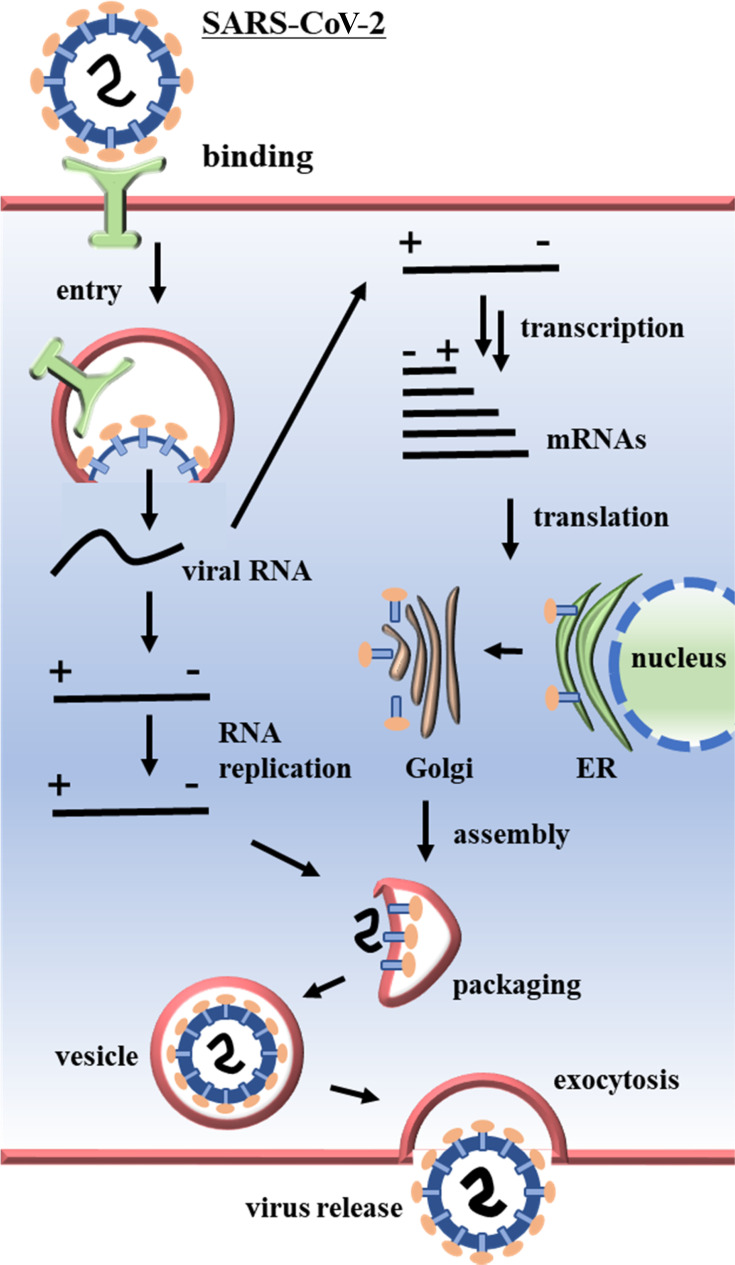
The primary site of SARS-CoV-2 attack is the human respiratory mucosa and the replication of the virus in the organism involves several events such as attachment of the virus to the host cell using ACE2 receptors present on the cell membrane and attachment S-proteins present in the viral capsids, diffusion, uncoating, replication of the virus within the host cell, assembly, and excretion (Figure 2).75
Figure 2.
Coronavirus replication mechanism within host cells.
Therapeutic Strategies for AgNMs Against Animal Coronaviruses
There are three representative strategies used to develop drugs as therapies against coronaviruses.
Repurposing of “broad spectrum” antiviral drugs which are already on the market for therapeutic purposes. Repurposing therapy has the advantage of a known drug mechanism of action, common dosages, and easy production, but unknown side effects in a new disease and drug efficacy for severe conditions are the disadvantages of this approach.
High throughput screening of drugs already approved for a different therapeutic purpose that may have therapeutic effects against SARS-CoV-2.
Development of new drugs based on genomic information, the pathological features of various coronaviruses, and investigation of the mechanism of their actions against coronaviruses. Several therapeutic pathways, their efficacies, side effects, and efficient drug delivery systems, preferably by nanoparticles, can be investigated.
So far, four types of silver nanoparticles have been reported as possible candidates for antiviral therapy as follows.
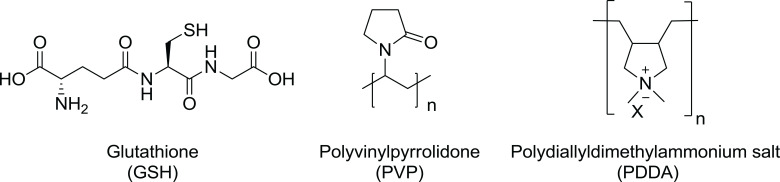
Glutathione-capped silver sulfide nanoclusters (GSH-Ag2S NCs) (Figure 3)
PVP-coated silver nanomaterials (PVP-AgNMs), which include silver nanowires (AgNWs) and silver nanoparticles (AgNPs) (Figure 3)
Silver nanoparticle-anchored graphene oxide nanoparticles (GO-AgNPs)
PDDA-coated PVP functionalized graphene oxide-silver nanocomposites (PDDA-PVP-GO-AgNCs) (Figure 3)
Figure 3.
Chemical structures of organic capping agents.
Synthesis and Characterization of AgNMs
AgNMs have already been successfully tested for antiviral activity in many studies.76 AgNMs are synthesized by various physical and chemical methods that apply common and simplified techniques.
Physical Methods
The basic and key feature of the physical methods employed for the synthesis of AgNMs are processes that include evaporation, condensation, laser ablation, electric irradiation, gamma irradiation, and lithography, which do not use chemicals such as redox reagents, polymers, and electrolytes of colloid stabilizers. Methods such as matrix isolation, gas flow cold trap, gas flow solution trap, and the pulse photo acoustic (PA) technique are used to study the synthesis of AgNPs.77
Chemical Methods
There are several chemical methods used for the synthesis of AgNMs of different shapes and sizes based on the preparation method. Herein, we are interested in the synthesis of four different kinds of AgNMs used against coronavirus.
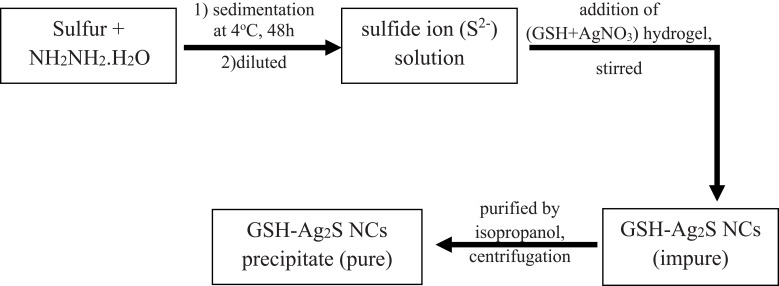
Preparation of GSH-Ag2S NCs
A suitable capping agent is the key factor used to control the size, stability, and morphology of Ag2S NCs. Therefore, glutathione (a small peptide that contains three amino acids) was used as a capping agent to prevent the growth of large NCs. The water solubility of GSH-Ag2S NCs is due to the presence of a multiplicative functional group in glutathione. Du et al reported the synthesis of GSH-Ag2S NCs, following the steps mentioned in Figure 4.78
Figure 4.
Flowchart for stepwise synthesis of GSH-Ag2S NCs.
Preparation of PVP-AgNMs
Commercially available AgNMs supplied from the Institute for Health and Consumer Protection (IHCP) were used in a study by Lv et al. They used four AgNMs:AgNPs (NM-300) (average size of <20 nm), two kinds of AgNWs (60 nm and 400 nm in diameter), and silver colloids (approximately 10 nm).79 The stabilizing agent for the AgNPs (NM-300) was a mixture of 7% ammonium nitrate, 4% polyoxyethylene glycerol trioleate, and 4% Tween20. The stabilizing agents for the two AgNWs were <0.5 wt% PVP and 2 wt% PVP for the silver colloids.
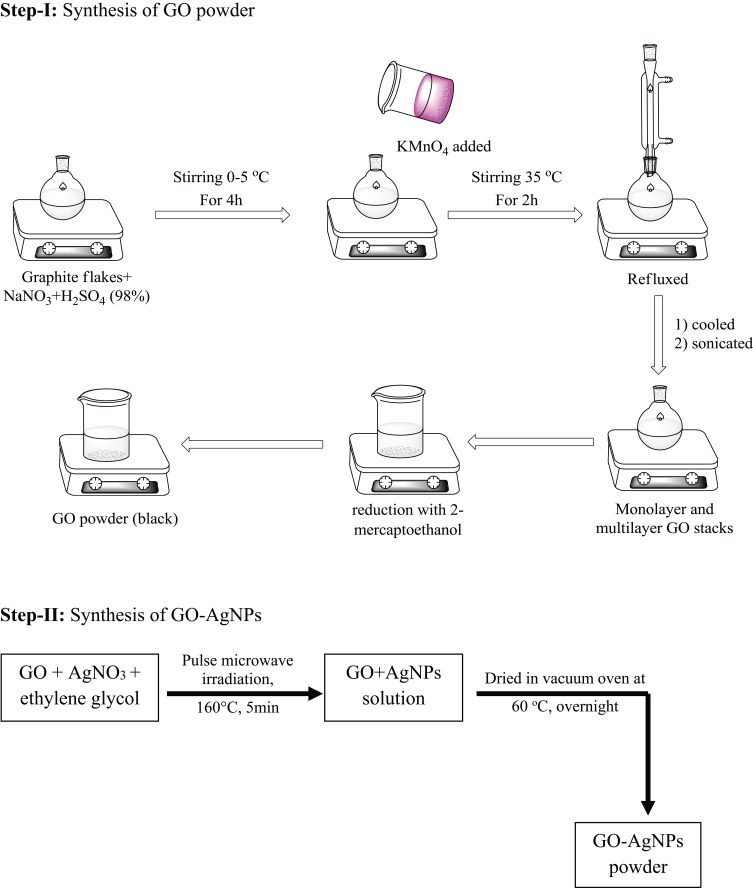
Preparation of GO-AgNPs
Chen et al synthesized GO-AgNPs to investigate their antiviral activity against Feline coronavirus (FCoV) coronaviruses. The GO-AgNPs were synthesized through a number of steps starting with commercially available graphite powder following Hummer’s method (Figure 5).80
Figure 5.
Flowchart for stepwise synthesis of GO-AgNPs.
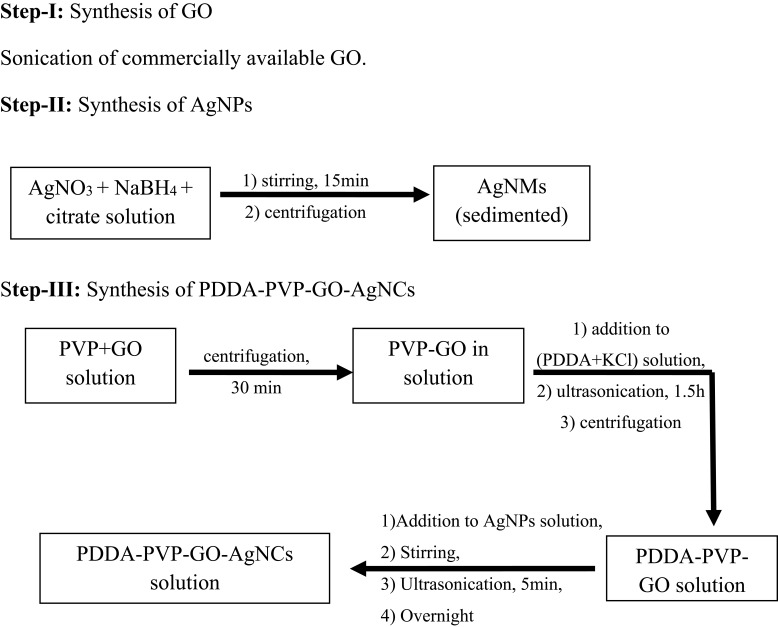
Preparation of PDDA-PVP-GO-AgNCs
Du et al synthesized GO-AgNCs using commercially available GO and silver nitrate (AgNO3) following the three steps mentioned below.
Preparation of PDDA-coated PVP functionalized graphene oxide: PVP-functionalized GO was obtained simply by adding PVP to a GO solution followed by centrifugation. To obtain PDDA-PVP-GO, PDDA and KCl solutions were mixed followed by PVP-capped GO (Figure 6).
Figure 6.
Flowchart for stepwise synthesis of PDDA-PVP-GO-AgNCs.
Synthesis of PDDA-PVP-GO-AgNCs: The AgNPs were synthesized by reducing AgNO3 with NaBH4, followed by the addition of a citrate solution under vigorous stirring. The PDDA-PVP-GO suspension was then injected into the silver nanoparticle suspension and was kept overnight to obtain the PDDA-PVP-GO-AgNCs (Figure 6).81
Characterization of the AgNMs
Characterization is an essential component of synthesized AgNMs. The size, shape, surface charge, crystal structure, and surface chemistry have been studied by different techniques. The UV-visible absorption (UV-Vis) and dynamic light scattering (DLS) data support the formation of AgNMs at room temperature. Topographical imaging, including the size, shape, impurities, and surface stability of respective nanomaterials, was evaluated by scanning electron microscopy (SEM), atomic force microscope (AFM) surface enhanced raman spectroscopy (SERS), and transmission electron microscopy (TEM). Further, the presence of surface modifiers was confirmed using Fourier transform infrared spectroscopy (FTIR) data or UV-Vis spectral analysis. The structures for the crystalline nanomaterials were confirmed by powder X-ray diffractometry (XRD). The thickness of the GO sheets and the layer number were evaluated by AFM analysis. The deposition of AgNMs on GO sheets was confirmed by thermogravimetric analysis (TGA).
In summary, the characterization techniques used for the four AgNMs are as follows.
GSH-Ag2S NCs: UV-Vis, FTIR, HRTEM, XRD, DLS.
PVP-AgNMs: Environmental scanning electron microscopy (ESEM), TEM, and nanoparticle tracking analysis (NTA).
GO-AgNPs: HRTEM, FESEM, XRD, X-ray photoelectron spectroscopy (XPS), AFM, and TGA.
PDDA-PVP-GO-AgNCs: UV-Vis, FTIR, DLS, XPS, SERS, and TEM.
Biological Screening for Antiviral Assays
In this regard, prior studies have been carried out to investigate the antiviral activities of AgNMs against coronaviruses including porcine epidemic diarrhea virus (PEDV), transmissible gastroenteritis virus (TGEV), and FCoV. The antiviral assays for the nanomaterials are generally screened according to the following procedures.76,82
Cell Viability Assays
The cell viability in virology is the percentage of cells which survived after applying an antiviral agent. The cell viability assay is carried out using techniques including Resazurin reduction, tetrazolium reduction, caspase or mitochondrial activity, flow cytometry, and ATP assays. In this study, the optimum concentration of NMs was used to demonstrate a viricidal effect in virus-infected cells.
Plaque Assays
The plaque assay determines the infectivity of a virus and estimates the antiviral ability of functionalized NMs. Herein, the zone of infected cells (plaque) is formed after the virus progeny is released by the cell at room temperature. The plaque-forming titer for the virus stock is represented as plaque-forming units per milliliter (PFU/mL) and the PFU value denotes the antiviral ability of the functionalized NMs.
Indirect Immunofluorescence Assay (IFA)
IFA helps to determine the inhibitory impact of NMs on the expression of viral antigens via antigen–antibody interactions. The virus inhibitory effect can be determined by a comparative analysis of the fluorescence intensity of the NM-treated cells and the control experiment (NM non-treated cells).
Western Blot
After treating the virus with functionalized NMs, the protein degradation is recorded by qualitative detection. The first step is denaturation, followed by gel electrophoresis characterization, and finally generation of an antibody and binding to a proper target. Indirect detection of binding to a proper target protein can be performed by approaches such as staining, immunofluorescence, and radioactivity.
Real-Time Quantitative Polymerase Chain Reaction (qRTPCR)
RT-PCR or the quantitative real-time polymerase chain reaction (qRTPCR) involves the polymerase chain reaction (PCR), which is basically the amplification of targeted DNA/RNA. This procedure is the most useful approach to explore viral DNA/RNA and can detect the gene sequence of host cells to test viral infectivity. Hence, after applying functionalized NMs, PCR provides an indirect investigation of the ability of being antiviral in nature.
The biological screening for the four AgNMs is summarized as follows.
GSH-Ag2S NCs: Cell viability, plaque assay, IFA, western blot, attachment assay, penetration assay, release analysis
PVP-AgNMs: Cell viability, antiviral activity, qRTPCR, western blot, IFA, flow cytometry analysis
GO-AgNPs: Virus inhibitory assay, cytotoxicity
PDDA-PVP-GO-AgNCs: Plaque assay, virus entry assay
Antiviral Activity of AgNMs
Various inorganic nanomaterials have been studied related to coronavirus and among them, the most effective nanomaterials were silver-related nanomaterials. AgNMs have a broad spectrum as potential virucidal agents and drug carriers. They have been successfully applied against HIV, influenza virus, and hepatitis virus.64 The key steps of action of AgNMs involve the inhibition of virus entry into the cells and the generation of radicals (reactive oxygen species) by interacting with biomolecules, causing disruption of the cell membrane and reacting within the cell prompting DNA and RNA damage. Few AgNMs have been reported to exhibit antiviral properties against coronaviruses (only animal coronaviruses from the beta genus). The antiviral evaluation of these materials is discussed in the following section.
Effect of Ag2S NCs on PEDV-Infected Vero Cells
Du et al showed that Ag2S NCs with a glutathione coating at a concentration of 46 µg/mL were successful in preventing viral infection and resulted in retention of more than 90% cell viability of Vero cells, even after 48 h of infection by PEDV.81 A plaque reduction assay showed that, at a definite concentration (46 µg/mL), the Ag2S NCs had very good antiviral effects against viral replication compared to a negative control. They also showed that smaller (2.5±0.6 nm) Ag2S NCs had better antiviral efficacy than larger NCs (4.1±1.5 nm) since their small size enables them to penetrate deeper than larger nanoparticles.79 Ag2S NCs were observed to have a 3.0 log-fold reduction in viral titers at 12 hpi, suggesting a high efficacy against PEDV infections. Ag2S NC treatment was observed to block viral negative-strand RNA synthesis and prevent viral budding. Ag2S NCs were also observed to inhibit PEDV infections by producing IFN-stimulating genes (ISGs) and proinflammation cytokines in Vero cells (Table 1).
Table 1.
AgNMs Used as Antiviral Agents Toward Coronaviruses and Their Mechanisms
| Type of CoV | Host | NMs Used as Antiviral Agent | Size (nm) | Cultured Cell Used | Applied Conc. of NMs | Antiviral Mechanism |
|---|---|---|---|---|---|---|
| PEDV | Pig | GSH-Ag2S NCs | 2.5 ±0.6, 4.1 ±1.5 | Vero cells | 23–184 μg/ml | Prevent -ssRNA synthesis, inhibit viral binding |
| PDDA-PVP-GO-Ag nanocomposites | 17 ±3.4 | MARC-145 cells | 0.5–8.0 μg/ml | Prevent viral entry | ||
| TGEV | Pig | PVP-AgNWs | 60–400 | ST cells | 3.125–50 μg/ml | Disable cell apoptosis |
| PVP-Ag colloids | ~10 | |||||
| PVP-AgNPs | <20 | |||||
| FCoVs | Cat | GO-Ag NPs | 5–25 | fcwf-4 | 0.390625–50 mg/ml | Inhibition of viral entry |
Effect of AgNMs on TGEV-Infected ST Cells
Lv et al observed that PVP-coated AgNMs at concentrations of 25 and 50 μg/mL were highly toxic to ST cells and showed 80% cell viability at 12.5 μg/mL.79 qRTPCR showed that TGEV 3CLpro and S-X were the preferential targets of AgNMs and AgNWs. The inhibitory effects of AgNPs and AgNWs were examined by IFA to observe the amount of TEGV in ST cells after treatment with both and it was observed that both types of AgNMs can significantly reduce the amount of TGEV in ST cells, whereas only TGEV infection of host cells resulted in cell apoptosis. The results of the analysis by annexin V-FITC and a PI dual staining kit suggested that the rate of apoptosis of the virus control (without AgNMs) was 10.07%, but pretreatment with AgNMs decreased the cell apoptosis to 5.33% (AgNPs), 4.97% (AgNW60), and 4.93% (AgNW400) (Table 1).
Effect of GO-AgNPs Towards FCoV-Infected Fcwf-4 Cells
Chen et al studied the antiviral activity of graphene oxide (GO) sheets and GO sheets coupled with silver nanoparticles (GO-AgNPs) against FCoV. They reported that when the concentrations of GO and GO-Ag nanomaterials were less than 1.5625 mg/mL, the cell viability was 90% and the cytotoxicity concentrations 50% (CC50) values were 17.4 mg/mL for GO and 19.7 mg/mL for GO-Ag nanoparticles.83 They also observed that GO-Ag had a greater antiviral effect on FCoV-infected cells compared to GO. The effective inhibitory concentration of GO-Ag nanoparticles was 0.1 mg/mL. They hypothesized that the reactive oxygen species produced by the GO-Ag nanoparticles damaged the viral RNA or blocked the host cell’s receptors. In addition, pretreatment with GO-Ag nanoparticles led to physical or chemical interactions between GO sheets and the coronavirus envelope, resulting in decreased infectivity (Table 1).
Effect of PDDA-PVP-GO-AgNCs on PEDV-Infected MARC-145 Cells
MARC-145 cells were cultured with PDDA-PVP-GO-AgNCs (0.5–4.0 µg/mL) for 24 and 48 h. At the optimum concentration of 4.0 µg/mL, the viability was reported to be over 85% but at 8.0 µg/mL, the viability decreased to below 80%. Additionally, an inhibitory effect of PVP-GO-AgNCs was observed on PEDV-infected MARC-145 cells and the inhibitory rate increased with increasing nanocomposite concentration.81 According to Du et al, the possible mechanism was the inhibition of viral entry (Table 1).
Pre-Clinical Efficacy Studies of AgNPs
Although AgNMs are widely known for their in vitro viricidal activity, only a few in vivo studies have been reported. PVP-coated AgNPs have been reported as a potential vaginal microbicide preventing HIV-1 infection transmission.84 Morris et al evaluated the antiviral and immunomodulatory effects of PVP-coated AgNPs in respiratory syncytial virus (RSV) infections. Bagg and Albino common strain of laboratory (BALB)/c mice were inoculated with RSV pre-incubated with AgNPs and they observed significant reductions in viral titer in the lung tissues as compared to untreated mice infected with RSV.85 With regards to influenza infections, Xiang et al showed that AgNPs were successful in preventing A/Human/Hubei/3/2005 (H3N2) influenza virus infection in an in vivo mice model by destroying their morphologic structures in a time-dependent manner.86 In addition, AgNPs, when administered intranasally, resulted in the inhibition of virus growth in lungs and the development of lung lesions, which led to significantly enhanced survival benefits in mice. Zhang et al reported that BALB/c mice inoculated with Rhesus rotavirus resulted in Biliary atresia. They observed that mice treated with AgNPs showed a significant increase in survival rates that led to a reduction in jaundice, restoration of liver enzymes and bilirubin metabolism to normal levels, and improved body weight. Additionally, the viral load decreased and upregulation of TGF-β mRNA transcripts was observed upon treatment with AgNPs.87
However, Stebounova et al showed that silver nanoparticles when inoculated in mice at subacute concentrations resulted in minimal pulmonary inflammation or cytotoxicity.88 Further, the effect of longer-term exposures in mice have yet to be analyzed based on higher lung burdens of AgNPs, resulting in the underscoring of eventual chronic effects.
From the above pre-clinical data, it can be concluded that AgNMs showed positive changes in mice health status. Also, in an in vivo study, AgNMs showed almost no toxicity. Therefore, the application of AgNMs in an advanced clinical study can be safely performed in the future.37,89
Mechanism of Action of AgNMs
Nanomaterial-based therapeutics offer a versatile tool for antiviral therapy researchers. AgNMs exhibit unique therapeutic efficacy, pharmacokinetics, and superior biological functions as antimicrobial agents. Recently, the antiviral properties of AgNMs have been reported in many studies which inhibit the replication of HIV-1, a receptor of the Tacaribe virus (TCRV), and monkeypox virus (MPV). The mode of action of AgNMs (against coronavirus infection) remains unclear but it has been reported that Ag+ ions participate in the generation of oxidative stress, induction of antibody responses, cytokine production, and inhibition of viral RNA synthesis by blocking the interactions between virus and ACE-2 cell receptors or glycoprotein120 (gp120). AgNMs interact with the gp120 subunit of the viral envelope glycoprotein to inactivate the virus before host cell binding.74,76,90,91
AgNMs work as an adjuvant to improve the immune response of the vaccine. It has been reported that the addition of AgNMs with the influenza vaccine efficiently induced an immune response in infected mice. However, the AuNP performance was not as good compared to AgNMs.92
Moreover, AgNPs and chitosan conjugates were also tested against H1N1 influenza A virus and it was observed that synergistically, the conjugates showed remarkable antiviral effect against the virus compared to AgNMs or chitosan alone.75
AgNMs as Vaccines
Vaccines are biological particles which facilitate building of acquired immunity against infectious diseases. To fight against infectious diseases, vaccination is one of the most cost-efficient and simple methods. Typically, a vaccine consists of virus-like particles (VLP), attenuated viruses, or protein-subunit antigens, which stimulate an immune response against infectious diseases. With the advent of nanotechnology, efforts were made to determine the immunoactivity of natural and engineered NMs.93
Several researchers reported the impact of AgNMs on the inflammatory response.94 Silver was reported to react with immune cells and affect the suppression or stimulation of various pathological conditions. It was reported that an increase in the size of AgNMs resulted in an increase in inflammatory cytokine secretion in rat alveolar macrophages,95 toxicological effects on macrophage U937,96 and also elicited an immune response in macrophages.97 Park et al reported that the repeated oral administration of AgNPs leads to an increased level of cytokine production, inflammatory cell infiltration, and B cell distribution in mice.98 AgNPs were also used as adjuvants and showed effects in vitro and in vivo using model antigens of oval albumin (OVA) and bovine serum albumin (BSA).99
Therapeutic Effect of Oral Inhalation of Silver Nanoparticles
The antibacterial100–102 and antiviral103 properties of silver nanoparticles provide a promising regimen in the fight against COVID-19. Colloidal silver solution104 inhalation is reported to be a promising therapy to tackle the aggravation of respiratory system infections. For antiviral applications, the nanoparticle size should be in the range of 3–7 nm. It was reported that the antibacterial effect of an ionic silver solution in water105 has a much higher minimum inhibitory concentration (MIC) than a colloidal silver solution. However, in the case of an antiviral effect, as was observed in the case of HIV, a colloidal silver solution was 10 times more potent than an ionic silver solution.66
For the oral inhalation of colloidal silver nanoparticles, it is important to determine the MIC level. A study showed that the MIC is very sensitive to the nanoparticle size.106 Smaller particles contain a greater number of particles at a specific weight fraction, producing a higher particle density that can interact with the pathogen best at low concentrations (MIC value).
It has already been shown that silver nanoparticles 10 nm or smaller are much more effective than particle sizes of 25–50 nm against HIV.107 Smaller AgNPs are easily permeable and hence highly incorporated into the cells, thus exert more toxicity in cells.108–110 It was observed that nanoparticles that are bound to the virus were exclusively within the range of 1–10 nm. While the HIV particle size is ~120 nm, the SARS virus size varies between ~90–100 nm. The MIC of AgNPs for HIV was observed to be 10 µg/ml. Since SARS has a similar size as HIV, it is expected that it can also be susceptible to AgNPs at a similar MIC value.
Application of Other Metal, Metal Sulfide, and Metal Oxide-Based Nanoparticles Against Coronavirus
Apart from AgNMs, there are also other metal, metallic oxide and metallic sulfide nanomaterials which are effective in the treatment of coronaviruses. These nanomaterials are utilized as various agents other than therapeutic treatments like nano-carriers, vaccine adjuvants, and analytical sensors. Gold nanoparticles (AuNPs) stand out because of their antimicrobial, photonic, electrical, and catalytic properties. AuNPs were used against coronavirus as a vaccine carrier towards TGEV-infected mice111 and as a vaccine adjuvant towards SARS-CoV-infected BALB/c mice.112 AuNPs are also used as an electrochemiluminescence towards MERS-CoV infected cells,113 as a chiroimmunosensor towards IBV-infected cells,114 and as an immunochromatographic strip towards IBV.115 Wang et al synthesized AuNPs as an electrochemiluminescence for studying HCoV and towards PEDV as a non-nest PCR material.116 Other nanomaterials like MoS2 nanosheets,117 zirconium quantum dots,118 ferritin-based nanoparticles,119 and magnetoplasmonic nanoparticles were also used as chemosensors and vaccine carriers. Khaiboullina et al used TiO2 nanoparticles as a surface modifier.120 In their in vitro study, they showed effectivity against alpha coronavirus HCoV-NL63, which is highly similar to the SARS-CoV-2 (Table 2).
Table 2.
Some Representative Nanomaterials Other Than AgNMs Used as Antiviral Agents Toward Coronaviruses and Their Mechanisms
| Nanoparticle | Virus/Antigen | Targeted Living System | Host | Purpose of Use/Acts as |
|---|---|---|---|---|
| Gold nanoparticle | Swine TGEV | Mice/Rabbit | Pig | Nano carrier of vaccine111 |
| SARS-CoV | BALB/c mice | Human | Vaccine adjuvant112 | |
| MERS-CoV | - | Camel | Electrochemiluminescence (analytical sensor)113 |
|
| HCoV | - | Human | Electrochemiluminescence (analytical sensor)113 |
|
| IBV | - | Chicken | Chiroimmunosensing (analytical sensor)114 | |
| IBV | - | Chicken | Immunochromatographic strip (analytical sensor)115 | |
| PEDV | - | Pig | Nano-nest PCR (analytical sensor)116 | |
| MoS2 nanosheet | IBV | - | Chicken | Immunosensing (nanosensor for diagnosis)117 |
| Zirconium QDs and magnetoplasmonic nanoparticles | IBV | - | Chicken | Photoluminescence (nanosensor)118 |
| Ferritin-based nanoparticle | MERS-CoV | Female BALB/C mice | Camel | Vaccine119 |
| TiO2 nanoparticle | HCoV | - | Human | Surface modifier agent120 |
Toxicity of Silver Nanoparticles
It can be hypothesized that silver nanoparticle toxicity is due to the attachment of the AgNMs directly to the viral protein surface.76 Hence, proper surface modification can be carried out by investigating the exact interacting site. It has been shown that silver nanomaterials are broad-spectrum antiviral agents and can efficiently reduce viral infectivity when applied to cultured cells.76 It has been reported that these nanomaterials are highly cytotoxic to mammalian cells because of their interaction with biomolecules that generate reactive oxygen species by interfering with defensive antioxidant mechanisms, thus posing harmful effects that damage lipids, proteins, and DNA through oxidation.121 An in vitro study revealed that the toxicity level of such nanomaterials varies depending on the dose. Although the use of nanomaterials is still debatable due to their toxicity or side effects on normal human cells, silver nanomaterials provide a promising means to carry antiviral or other drugs throughout the body. In order to reduce toxicity, surface modifications of the nanoparticles need to be made so that the metal surfaces do not directly attach to cells. Also, their concentration in the interior of a cell should not be high within a particular cell compartment. Although the progression of nanoparticle research is presently ongoing, the detailed mechanisms of nanomaterial actions are not clear yet, which requires improvements with respect to their safety in order to optimize clinical advancements.
Conclusion
Experimental findings suggested that small nanoparticles show greater effectiveness than larger nanoparticles. Hence, AgNPs smaller than 20 nm are more efficient than AgNWs (60 nm and 400 nm) in PEDV-infected cells. Smaller (2.5±0.6 nm) nanomaterials were also observed to be more efficacious compared to larger ones (4.1±1.5 nm), such as Ag2S NCs in the case of TGEV-infected cells. In the case of GO-Ag NPs, they are effective against FCoV-infected cells at a diameter of 5–25 nm but no size dependent experiments have been carried out with these NPs. A GO-Ag nanocomposite with a diameter of 17±3.4 nm was also very efficient against the PEDV coronavirus. Although Ag and Ag-related nanomaterials are well-explored antiviral and antibacterial agents, there are no such clinically approved antivirals known currently. The recent pandemic demands continuous therapeutic advancements and hence, such nanomaterials or nanoclusters should be under consideration as an alternative research topic for therapies aimed against SARS-CoV-2, as they are still less studied than other types of antiviral therapy.
Future Perspectives
It has been around ten months since SARS-CoV-2 was declared a pandemic by the WHO and the search for targeted therapies is far from providing therapies that can be used in the clinical setting. The repurposing of some antiviral drugs is in clinical trial throughout the world and has provided some prospective results that have been published recently. In this scenario, the use of nanoparticles is considered to be a suitable alternative therapy. AgNMs are good and effective antimicrobials, although their mechanisms of action are not clear yet. Their antiviral activity against some viruses has been extensively studied and has been found to be good enough to expand this research area to include nanotherapies effective against coronaviruses. Although some research has been carried out for nanoparticle use against a few animal coronaviruses, the respective nanomaterials have still not been studied in order to identify their effects against human coronaviruses. With respect to the SARS-related coronaviruses which are raging across the world currently, the use of nanomaterials in personal protection equipment (PPE) including face masks by coating PPEs with these various nanomaterials is becoming popular and safe.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) with a grant funded by the Korea government (MSIT; No. NRF-2019R1H1A2039759). This work was also supported by the Soonchunhyang University Research Fund.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Payne S. Family Coronaviridae In: Viruses. Elsevier; Acquisitions Editor: Linda Versteeg-Buschman 2017:149–158. doi: 10.1016/B978-0-12-803109-4.00017-9 [DOI] [Google Scholar]
- 2.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, eds. Family - Coronaviridae. In: Virus Taxonomy. Elsevier; 2012:806-828. doi: 10.1016/B978-0-12-384684-6.00068-9. [DOI] [Google Scholar]
- 3.Yang P, Wang X. COVID-19: a new challenge for human beings. Cell Mol Immunol. 2020;17(5):555–557. doi: 10.1038/s41423-020-0407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Y, Zhao K, Shi Z-L, Zhou P. Bat Coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bande F, Arshad SS, Omar AR, Bejo MH, Abubakar MS, Abba Y. Pathogenesis and diagnostic approaches of avian infectious bronchitis. Adv Virol. 2016;2016:1–11. doi: 10.1155/2016/4621659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(Supplement):S223–S227. doi: 10.1097/01.inf.0000188166.17324.60 [DOI] [PubMed] [Google Scholar]
- 7.Virology: coronaviruses. Nature. 1968;220(5168):650. doi: 10.1038/220650b0 [DOI] [Google Scholar]
- 8.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsin W-C, Chang C-H, Chang C-Y, et al. Nucleocapsid protein-dependent assembly of the RNA packaging signal of Middle East respiratory syndrome coronavirus. J Biomed Sci. 2018;25(1):47. doi: 10.1186/s12929-018-0449-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–620. doi: 10.1038/s41423-020-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi Y, Lagniton PNP, Ye S, Li E, Xu R-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15..Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Ho W, Huang Y, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. The Lancet. 2020;395(10228):949–950. doi: 10.1016/S0140-6736(20)30557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y-Z, Holmes EC. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181(2):223–227. doi: 10.1016/j.cell.2020.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int Accessed October18, 2020.
- 19.Mullard A. COVID-19 vaccine development pipeline gears up. The Lancet. 2020;395(10239):1751–1752. doi: 10.1016/S0140-6736(20)31252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Thanh T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5 [DOI] [PubMed] [Google Scholar]
- 21.Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khandel P, Yadaw RK, Soni DK, Kanwar L, Shahi SK. Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J Nanostructure Chem. 2018;8(3):217–254. doi: 10.1007/s40097-018-0267-4 [DOI] [Google Scholar]
- 23.Elahi N, Kamali M, Baghersad MH. Recent biomedical applications of gold nanoparticles: a review. Talanta. 2018;184:537–556. doi: 10.1016/j.talanta.2018.02.088 [DOI] [PubMed] [Google Scholar]
- 24.Azharuddin M, Zhu GH, Das D, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun. 2019;55(49):6964–6996. doi: 10.1039/C9CC01741K [DOI] [PubMed] [Google Scholar]
- 25.Dukhinova MS, Prilepskii A, Shtil AA, Vinogradov VV. Metal oxide nanoparticles in therapeutic regulation of macrophage functions. Nanomaterials. 2019;9(11):1631. doi: 10.3390/nano9111631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das C, Sen S, Singh T, et al. Green synthesis, characterization and application of natural product coated magnetite nanoparticles for wastewater treatment. Nanomaterials. 2020;10(8):1615. doi: 10.3390/nano10081615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim -Y-Y, Walsh D. Metal sulfide nanoparticles synthesized via enzyme treatment of biopolymer stabilized nanosuspensions. Nanoscale. 2010;2(2):240–247. doi: 10.1039/B9NR00194H [DOI] [PubMed] [Google Scholar]
- 28.Wang L. Synthetic methods of CuS nanoparticles and their applications for imaging and cancer therapy. RSC Adv. 2016;6(86):82596–82615. doi: 10.1039/C6RA18355G [DOI] [Google Scholar]
- 29.Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011 [DOI] [Google Scholar]
- 30.Chen J, Huang Y, Wei X, et al. Covalent organic nanospheres: facile preparation and application in high-resolution gas chromatographic separation. Chem Commun. 2019;55(73):10908–10911. doi: 10.1039/C9CC05307G [DOI] [PubMed] [Google Scholar]
- 31.Abbasi E, Aval S, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi: 10.1186/1556-276X-9-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Çağdaş M, Sezer AD, Bucak S. Liposomes as potential drug carrier systems for drug delivery In: Sezer AD editor. Application of Nanotechnology in Drug Delivery. InTech;2014. doi: 10.5772/58459 [DOI] [Google Scholar]
- 33.Ahmad Z, Shah A, Siddiq M, Kraatz H-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–17038. doi: 10.1039/C3RA47370H [DOI] [Google Scholar]
- 34.Pandey A. Solid lipid nanoparticles: a multidimensional drug delivery system In: Daima HK, Pn N, Ranjan S, Dasgupta N, Lichtfouse E editors. Nanoscience in Medicine Vol. 1. Vol 39. Environmental Chemistry for a Sustainable World. Springer International Publishing; 2020:249–295. doi: 10.1007/978-3-030-29207-2_8. [DOI] [Google Scholar]
- 35.Khalid K, Tan X, Mohd Zaid HF, et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11(1):328–355. doi: 10.1080/21655979.2020.1736240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz‐Hitzky E, Darder M, Wicklein B, et al. Nanotechnology responses to COVID‐19. Adv Healthc Mater. 2020;September:2000979. doi: 10.1002/adhm.202000979 [DOI] [PubMed] [Google Scholar]
- 37.Burdușel A-C, Gherasim O, Grumezescu AM, Mogoantă L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials. 2018;8(9):681. doi: 10.3390/nano8090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova N, Gugleva V, Dobreva M, Pehlivanov I, Stefanov S, Andonova V. Silver nanoparticles as multi-functional drug delivery systems In: Akhyar Farrukh M editor. Nanomedicines. IntechOpen;2019. doi: 10.5772/intechopen.80238 [DOI] [Google Scholar]
- 39.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(1):32. doi: 10.1186/2228-5326-2-32 [DOI] [Google Scholar]
- 40.Roy CN, Ghosh D, Mondal S, Kundu S, Maiti S, Saha A. SERS enhancement on the basis of temperature-dependent chemisorption: microcalorimetric evidence. ChemPhysChem. 2016;17(24):4144–4148. doi: 10.1002/cphc.201600941 [DOI] [PubMed] [Google Scholar]
- 41.Roy CN, Ghosh D, Mondal S, Saha A. Reductant control on particle size, size distribution and morphology in the process of surface enhanced raman spectroscopy active silver colloid synthesis. J Nanosci Nanotechnol. 2015;15(2):1771–1779. doi: 10.1166/jnn.2015.9511 [DOI] [PubMed] [Google Scholar]
- 42.Bhosale A, Bhanage M. Silver nanoparticles: synthesis, characterization and their application as a sustainable catalyst for organic transformations. Curr Org Chem. 2015;19(8):708–727. doi: 10.2174/1385272819666150207001154 [DOI] [Google Scholar]
- 43.Jiang Z-J, Liu C-Y, Sun L-W. Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B. 2005;109(5):1730–1735. doi: 10.1021/jp046032g [DOI] [PubMed] [Google Scholar]
- 44.Das TK, Karmakar S, Maiti S, Kundu S, Saha A. Room temperature synthesis of NIR emitting Ag2S nanoparticles through aqueous route and its influence on structural modulation of DNA. Spectrochim Acta A Mol Biomol Spectrosc. 2020;227:117536. doi: 10.1016/j.saa.2019.117536 [DOI] [PubMed] [Google Scholar]
- 45.Basheer NS, Kumar BR, Kurian A, George SD. Silver nanoparticle size–dependent measurement of quantum efficiency of Rhodamine 6G. Appl Phys B. 2013;113(4):581–587. doi: 10.1007/s00340-013-5513-3 [DOI] [Google Scholar]
- 46.Cornelis G, Pang L, Doolette C, Kirby JK, McLaughlin MJ. Transport of silver nanoparticles in saturated columns of natural soils. Sci Total Environ. 2013;463–464:120–130. doi: 10.1016/j.scitotenv.2013.05.089 [DOI] [PubMed] [Google Scholar]
- 47.Mahdi KNM, Peters R, van der Ploeg M, Ritsema C, Geissen V. Tracking the transport of silver nanoparticles in soil: a saturated column experiment. Water Air Soil Pollut. 2018;229(10):334. doi: 10.1007/s11270-018-3985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das G, Patra JK, Shin H-S. Biosynthesis, and potential effect of fern mediated biocompatible silver nanoparticles by cytotoxicity, antidiabetic, antioxidant and antibacterial, studies. Mater Sci Eng C. 2020;114:111011. doi: 10.1016/j.msec.2020.111011 [DOI] [PubMed] [Google Scholar]
- 49.Loo YY, Rukayadi Y, Nor-Khaizura M-A-R, et al. In Vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front Microbiol. 2018;9:1555. doi: 10.3389/fmicb.2018.01555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn E-Y, Park Y. Anticancer prospects of silver nanoparticles green-synthesized by plant extracts. Mater Sci Eng C. 2020;116:111253. doi: 10.1016/j.msec.2020.111253 [DOI] [PubMed] [Google Scholar]
- 51.Gomathi AC, Xavier Rajarathinam SR, Mohammed Sadiq A, Rajeshkumar S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol. 2020;55:101376. doi: 10.1016/j.jddst.2019.101376 [DOI] [Google Scholar]
- 52.Deshmukh SP, Patil SM, Mullani SB, Delekar SD. Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu S, Gao W, Gu HY. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 2008;34(5):623–628. doi: 10.1016/j.burns.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 54.Rao YN, Das SK, Saha A. Room temperature aqueous synthesis of bipyramidal silver nanostructures. J Nanosci Nanotechnol. 2012;12(3):2014–2021. doi: 10.1166/jnn.2012.5165 [DOI] [PubMed] [Google Scholar]
- 55.Shrivas K, Nirmalkar N, Deb MK, Dewangan K, Nirmalkar J, Kumar S. Application of functionalized silver nanoparticles as a biochemical sensor for selective detection of lysozyme protein in milk sample. Spectrochim Acta A Mol Biomol Spectrosc. 2019;213:127–133. doi: 10.1016/j.saa.2019.01.039 [DOI] [PubMed] [Google Scholar]
- 56.Loiseau A, Asila V, Boitel-Aullen G, Lam M, Salmain M, Boujday S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors. 2019;9(2):78. doi: 10.3390/bios9020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasool K, Lee DS. Inhibitory effects of silver nanoparticles on removal of organic pollutants and sulfate in an anaerobic biological wastewater treatment process. J Nanosci Nanotechnol. 2016;16(5):4456–4463. doi: 10.1166/jnn.2016.10984 [DOI] [PubMed] [Google Scholar]
- 58.Singh J, Kumar V, Singh Jolly S, et al. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J Ind Eng Chem. 2019;80:247–257. doi: 10.1039/B9NR00194H [DOI] [Google Scholar]
- 59.Sumesh E, Bootharaju MS, Anshup PT. A practical silver nanoparticle-based adsorbent for the removal of Hg2+ from water. J Hazard Mater. 2011;189(1–2):450–457. doi: 10.1016/j.jhazmat.2011.02.061 [DOI] [PubMed] [Google Scholar]
- 60.Al-Qahtani KM. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt J Aquat Res. 2017;43(4):269–274. doi: 10.1007/s11270-018-3985-9 [DOI] [Google Scholar]
- 61.Pandiarajan J, Krishnan M. Properties, synthesis and toxicity of silver nanoparticles. Environ Chem Lett. 2017;15(3):387–397. doi: 10.1007/s10311-017-0624-4 [DOI] [Google Scholar]
- 62.Loiseau A, Asila V, Boitel-Aullen G, Lam M, Salmain M, Boujday S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors. 2019;9(2):78. doi: 10.3390/bios9020078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siddiqi KS, Husen A, Rao RAK. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnology. 2018;16(1):14. doi: 10.1186/s12951-018-0334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X-F, Liu Z-G, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chouhan N. Silver nanoparticles: synthesis, characterization and applications In: Maaz K editor. Silver Nanoparticles - Fabrication, Characterization and Applications. InTech;2018. doi: 10.5772/intechopen.75611 [DOI] [Google Scholar]
- 66.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology. 2010;8(1):1. doi: 10.1186/1477-3155-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Z, Li Y, Guo M, et al. The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 2017;7(2):742–750. doi: 10.1039/C6RA25010F [DOI] [Google Scholar]
- 68.Chai H, Zhao Y, Zhao C, Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem. 2006;14(4):911–917. doi: 10.1016/j.bmc.2005.08.041 [DOI] [PubMed] [Google Scholar]
- 69.Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R. Inhibition of Herpes Simplex Virus Type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem. 2009;20(8):1497–1502. doi: 10.1021/bc900215b [DOI] [PubMed] [Google Scholar]
- 70.Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett. 2008;3(4):129–133. doi: 10.1007/s11671-008-9128-2 [DOI] [Google Scholar]
- 71.Weiss C, Carriere M, Fusco L, et al. Toward nanotechnology-enabled approaches against the COVID-19 Pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697 [DOI] [PubMed] [Google Scholar]
- 72.Chan WCW. Nano research for COVID-19. ACS Nano. 2020;14(4):3719–3720. doi: 10.1021/acsnano.0c02540 [DOI] [PubMed] [Google Scholar]
- 73.Łoczechin A, Séron K, Barras A, et al. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl Mater Interfaces. 2019;11(46):42964–42974. doi: 10.1021/acsami.9b15032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nikaeen G, Abbaszadeh S, Yousefinejad S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomed. 2020;15(15):1501–1512. doi: 10.2217/nnm-2020-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurunathan S, Qasim M, Choi Y, et al. Antiviral potential of nanoparticles—can nanoparticles fight against coronaviruses?. Nanomaterials. 2020;10(9):1645. doi: 10.3390/nano10091645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16(10):8894–8918. doi: 10.3390/molecules16108894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Shen W, Xue J, et al. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res Lett. 2018;13(1):54. doi: 10.1186/s11671-018-2450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du T, Liang J, Dong N, et al. Glutathione-Capped Ag 2 S nanoclusters inhibit coronavirus proliferation through blockage of Viral RNA synthesis and budding. ACS Appl Mater Interfaces. 2018;10(5):4369–4378. doi: 10.1021/acsami.7b13811 [DOI] [PubMed] [Google Scholar]
- 79.Lv X, Wang P, Bai R, et al. Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials. 2014;35(13):4195–4203. doi: 10.1016/j.biomaterials.2014.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80(6):1339. doi: 10.1021/ja01539a017 [DOI] [Google Scholar]
- 81.Du T, Lu J, Liu L, et al. Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl Bio Mater. 2018;1(5):1286–1293. doi: 10.1021/acsabm.8b00154 [DOI] [PubMed] [Google Scholar]
- 82.Huy TQ, Hien Thanh NT, Thuy NT, et al. Cytotoxicity and antiviral activity of electrochemical – synthesized silver nanoparticles against poliovirus. J Virol Methods. 2017;241:52–57. doi: 10.1016/j.jviromet.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 83.Chen Y-N, Hsueh Y-H, Hsieh C-T, Tzou D-Y, Chang P-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int J Environ Res Public Health. 2016;13(4):430. doi: 10.3390/ijerph13040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. J Nanobiotechnology. 2010;8(1):15. doi: 10.1186/1477-3155-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morris D, Ansar M, Speshock J, et al. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019;11(8):732. doi: 10.3390/v11080732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiang D, Zheng C, Zheng Y, et al.. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int J Nanomedicine. 2013:4103. doi: 10.2147/IJN.S53622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang R, Lin Z, Lui VCH, et al. Silver nanoparticle treatment ameliorates biliary atresia syndrome in rhesus rotavirus inoculated mice. Nanomedicine Nanotechnol Biol Med. 2017;13(3):1041–1050. doi: 10.1016/j.nano.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 88.Stebounova LV, Adamcakova-Dodd A, Kim J, et al. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Part Fibre Toxicol. 2011;8(1):5. doi: 10.1186/1743-8977-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura S, Sato M, Sato Y, et al. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int J Mol Sci. 2019;20(15):3620. doi: 10.3390/ijms20153620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang E-J, Kim S, Kim JS, Choi I-H. Inflammasome formation and IL-1β release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012;33(28):6858–6867. doi: 10.1016/j.biomaterials.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 91.Alphandéry E. The potential of various nanotechnologies for coronavirus diagnosis/treatment highlighted through a literature analysis. Bioconjug Chem. 2020;31(8):1873–1882. doi: 10.1021/acs.bioconjchem.0c00287 [DOI] [PubMed] [Google Scholar]
- 92.Sanchez-Guzman D, Le Guen P, Villeret B, et al. Silver nanoparticle-adjuvanted vaccine protects against lethal influenza infection through inducing BALT and IgA-mediated mucosal immunity. Biomaterials. 2019;217:119308. doi: 10.1016/j.biomaterials.2019.119308 [DOI] [PubMed] [Google Scholar]
- 93.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–495. doi: 10.1021/mp800032f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ninan N, Goswami N, Vasilev K. The impact of engineered silver nanomaterials on the immune system. Nanomaterials. 2020;10(5):967. doi: 10.3390/nano10050967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–13619. doi: 10.1021/jp712087m [DOI] [PubMed] [Google Scholar]
- 96.Park J, Lim D-H, Lim H-J, et al. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem Commun. 2011;47(15):4382. doi: 10.1039/c1cc10357a [DOI] [PubMed] [Google Scholar]
- 97.Martínez-Gutierrez F, Thi EP, Silverman JM, et al. Antibacterial activity, inflammatory response, coagulation and cytotoxicity effects of silver nanoparticles. Nanomedicine Nanotechnol Biol Med. 2012;8(3):328–336. doi: 10.1016/j.nano.2011.06.014 [DOI] [PubMed] [Google Scholar]
- 98.Park E-J, Bae E, Yi J, et al. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30(2):162–168. doi: 10.1016/j.etap.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 99.Xu Y, Tang H, Liu J, Wang H, Liu Y. Evaluation of the adjuvant effect of silver nanoparticles both in vitro and in vivo. Toxicol Lett. 2013;219(1):42–48. doi: 10.1016/j.toxlet.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 100.Aderibigbe B. Metal-based nanoparticles for the treatment of infectious diseases. Molecules. 2017;22(8):1370. doi: 10.3390/molecules22081370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Ouay B, Stellacci F. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10(3):339–354. doi: 10.1016/j.nantod.2015.04.002 [DOI] [Google Scholar]
- 102.Liao S, Zhang Y, Pan X, et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int J Nanomedicine. 2019;14:1469–1487. doi: 10.2147/IJN.S191340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haggag E, Elshamy A, Rabeh M, et al. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int J Nanomedicine. 2019;14:6217–6229. doi: 10.2147/IJN.S214171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zachar O. Formulations for COVID-19 Early Stage Treatment via Silver Nanoparticles Inhalation Delivery at Home and Hospital; 2020. doi: 10.14293/S2199-1006.1.SOR-.PPHBJEO.v1 [DOI] [Google Scholar]
- 105.Tien D-C, Tseng K-H, Liao C-Y, Tsung -T-T. Identification and quantification of ionic silver from colloidal silver prepared by electric spark discharge system and its antimicrobial potency study. J Alloys Compd. 2009;473(1–2):298–302. doi: 10.1016/j.jallcom.2008.05.063 [DOI] [Google Scholar]
- 106.Dong Y, Zhu H, Shen Y, Zhang W, Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. Mukherjee A, ed. PLOS ONE. 2019;14(9):e0222322. doi: 10.1371/journal.pone.0222322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elechiguerra J, Burt JL, Morones JR, et al. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnology. 2005;3(1):6. doi: 10.1186/1477-3155-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pazos-Ortiz E, Roque-Ruiz JH, Hinojos-Márquez EA, et al. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J Nanomater. 2017;2017:1–9. doi: 10.1155/2017/4752314 [DOI] [Google Scholar]
- 109.Cho Y-M, Mizuta Y, Akagi J, Toyoda T, Sone M, Ogawa K. Size-dependent acute toxicity of silver nanoparticles in mice. J Toxicol Pathol. 2018;31(1):73–80. doi: 10.1293/tox.2017-0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams KM, Gokulan K, Cerniglia CE, Khare S. Size and dose dependent effects of silver nanoparticle exposure on intestinal permeability in an in vitro model of the human gut epithelium. J Nanobiotechnology. 2016;14(1):62. doi: 10.1186/s12951-016-0214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Staroverov SA, Volkov AA, Mezhenny PV, et al. Prospects for the use of spherical gold nanoparticles in immunization. Appl Microbiol Biotechnol. 2019;103(1):437–447. doi: 10.1007/s00253-018-9476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sekimukai H, Iwata‐Yoshikawa N, Fukushi S, et al. Gold nanoparticle‐adjuvanted S protein induces a strong antigen‐specific IgG response against severe acute respiratory syndrome‐related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol Immunol. 2020;64(1):33–51. doi: 10.1111/1348-0421.12754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Layqah LA, Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim Acta. 2019;186(4):224. doi: 10.1007/s00604-019-3345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahmed SR, É N, Neethirajan S. Self-assembled star-shaped chiroplasmonic gold nanoparticles for an ultrasensitive chiro-immunosensor for viruses. RSC Adv. 2017;7(65):40849–40857. doi: 10.1039/C7RA07175B [DOI] [Google Scholar]
- 115.Liu I-L, Lin Y-C, Lin Y-C, et al.. Strip for antigen detection of avian infectious bronchitis virus. Int J Mol Sci. 2019;20(9):2216. doi: 10.3390/ijms20092216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang K, Zhu J, Dong H, Pei Z, Zhou T, Hu G. Rapid detection of variant and classical porcine epidemic diarrhea virus by nano-nest PCR. Pak Vet J Published Online. 2017;5. [Google Scholar]
- 117.Weng X, Neethirajan S. Immunosensor based on antibody-functionalized MoS 2 for rapid detection of avian coronavirus on cotton thread. IEEE Sens J. 2018;18(11):4358–4363. doi: 10.1109/JSEN.2018.2829084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahmed SR, Kang SW, Oh S, Lee J, Neethirajan S. Chiral zirconium quantum dots: a new class of nanocrystals for optical detection of coronavirus. Heliyon. 2018;4(8):e00766. doi: 10.1016/j.heliyon.2018.e00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim Y-S, Son A, Kim J, et al. Chaperna-mediated assembly of ferritin-based middle east respiratory syndrome-coronavirus nanoparticles. Front Immunol. 2018;9:1093. doi: 10.3389/fimmu.2018.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khaiboullina S, Uppal T, Dhabarde N, Subramanian VR, Verma SC. Vitro Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: throwing Light on SARS-CoV-2. Microbiology. 2020. doi: 10.1101/2020.08.25.265223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Villanueva-Flores F, Castro-Lugo A, Ramírez OT, Palomares LA. Understanding cellular interactions with nanomaterials: towards a rational design of medical nanodevices. Nanotechnology. 2020;31(13):132002. doi: 10.1088/1361-6528/ab5bc8 [DOI] [PMC free article] [PubMed] [Google Scholar]