Abstract
Mesenchymal stromal cells (MSCs) offer great potential for the treatment of cardiovascular diseases (CVDs) such as myocardial infarction and heart failure. Studies have revealed that the efficacy of MSCs is mainly attributed to their capacity to secrete numerous trophic factors that promote angiogenesis, inhibit apoptosis, and modulate the immune response. There is growing evidence that MSC‐derived extracellular vesicles (EVs) containing a cargo of lipids, proteins, metabolites, and RNAs play a key role in this paracrine mechanism. In particular, encapsulated microRNAs have been identified as important positive regulators of angiogenesis in pathological settings of insufficient blood supply to the heart, thus opening a new path for the treatment of CVD. In the present review, we discuss the current knowledge related to the proangiogenic potential of MSCs and MSC‐derived EVs as well as methods to enhance their biological activities for improved cardiac tissue repair. Increasing our understanding of mechanisms supporting angiogenesis will help optimize future approaches to CVD intervention.
Keywords: angiogenesis, cardiovascular disease, extracellular vesicles, mesenchymal stromal cells, treatment
Mesenchymal stromal cells (MSCs) offer tremendous potential for the treatment of cardiovascular disease. Their predominant mode‐of action is the paracrine secretion of trophic factors and extracellular vesicles (EVs) that promote angiogenesis, inhibit apoptosis, and modulate immune responses. In this review, we discuss different strategies to enhance the angiogenic potential of MSCs and MSC‐derived EVs for improved cardiac tissue repair.
Significance statement.
Mesenchymal stromal cells (MSCs) are currently being evaluated in clinical trials for the treatment of numerous diseases. Their therapeutic potential is mainly due to the factors they secrete. Studies have demonstrated that MSCs also produce extracellular vesicles that carry proteins, metabolites, lipids, and various RNAs. Based on their multifunctional properties, extracelullar vesicles are of great importance and interest in the development of future medicine. This study provides an overview of the current knowledge on the therapeutic potential of MSCs and MSC‐derived extracelullar vesicles, as well as methods for improving their biological activities to promote angiogenesis and tissue repair.
1. INTRODUCTION
In both developed and developing countries, cardiovascular disease (CVD) is a major cause of morbidity and mortality, 1 and most importantly, ischemic heart disease such as acute myocardial infarction (MI) is a leading cause of heart failure. While obstruction to blood flow can be effectively treated by common surgical and catheter‐based interventions, achieving cures for microvascular disease remains an elusive goal. The concept of promoting the perfusion of ischemic tissue through angiogenesis has been considered as a highly promising treatment strategy for CVD. Previous attempts to induce neocapillarization in ischemic tissue involved the targeted delivery of various proangiogenic growth factors and nucleic acids encoding them, as well as physical interventions to stimulate angiogenic processes. 2 However, as none of them proved to be sufficiently effective to reverse end‐organ ischemia and prevent loss‐of‐function, other strategies had to be pursued. With the advent of cell therapies for nonhematological disorders in the 1990s, the idea of using viable cells to ameliorate or reverse tissue ischemia has rapidly gained traction. 3 Given their ease of isolation, robustness in culture, multilineage differentiation potential in vitro, and partially restricted immunogenicity, 4 mesenchymal stromal cells (MSCs) have been proposed as a promising tool for translational research in cardiology. In recent years, much work has been done to improve the functional properties of MSCs in terms of cell retention and survival of grafted cells, and to elicit their proangiogenic effects. For example, it has been hypothesized that the microenvironment of injured tissue is not conducive for cell engraftment and retention, and that the paracrine effect of transplanted MSCs lasts for only 24 to 48 hours. 5 To overcome these limitations, various scaffolds for cell transplantation were tested and showed promising results for the use in cardiac applications. 6 , 7 MSC transplantation to repair damage caused by MI and restore cardiac function has been demonstrated in both animal experiments and patients. 8 , 9 , 10 , 11 However, recent meta‐analyses failed to show consistent improvement in infarct size or left ventricular function. 12 , 13 Consequently, the initial assumption that transplanted stem or progenitor cells support neovascularization by differentiation into endothelial cells was soon replaced by the notion of their predominantly paracrine function by producing and secreting small molecules responsible for proangiogenic effects, such as cytokines, chemokines, and growth factors. 14 Besides releasing a variety of soluble factors, MSCs have been shown to secrete extracellular vesicles (EVs) that are important mediators of cell‐to‐cell communication. 15 Among the known subtypes of EVs, endosome‐derived exosomes carrying proteins, metabolites, lipids, and various RNAs have emerged as physiologically relevant components of the MSC secretome 16 (Figure 1). Earlier reports demonstrated that the paracrine activity of the MSC secretome has a therapeutic effect on a wide range of diseases and tissue injury in myocardium, kidney, liver, and lung. 17 , 18 , 19 , 20 The elucidation of paracrine effects thus not only improves our understanding of vascular pathologies, but also enhances the ability to facilitate neocapillarization (ie, endothelial sprouting) for regeneration purposes. In this article, we summarize ways to stimulate angiogenesis with the help of MSCs and their derived EVs, thereby enhancing tissue repair in a variety of pathologies associated with insufficient angiogenesis. We also present the latest advances in the identification of regulatory microRNAs (miRNAs) encapsulated in EVs and discuss their role in promoting angiogenesis.
FIGURE 1.
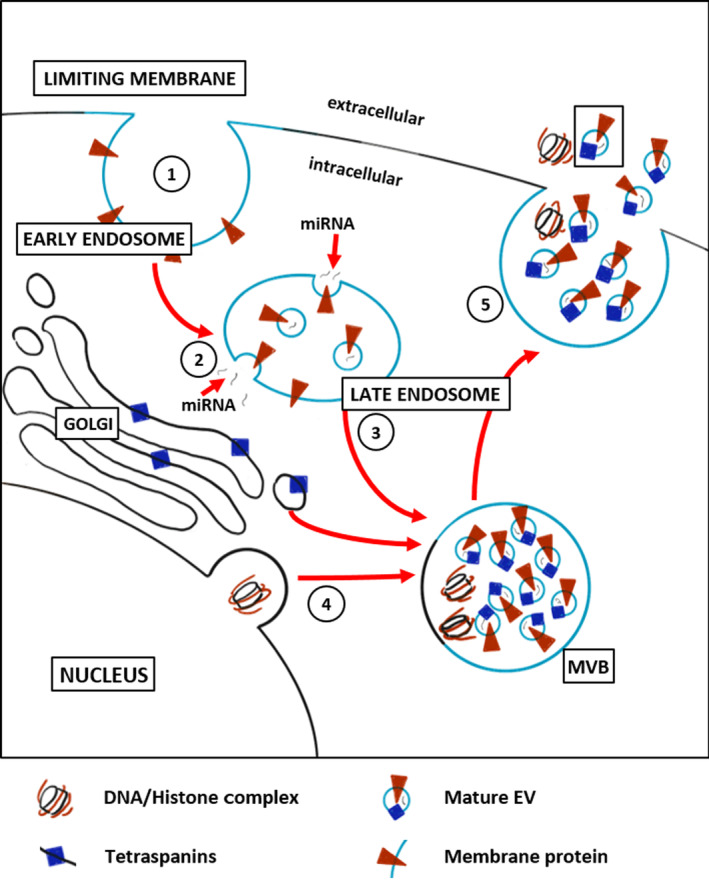
Exosome biosynthesis. (1) Early endosomes are formed by inward budding of the limiting membrane of cells. Surface proteins (orange triangles) may be incorporated into the early endosomal membrane. (2) Early endosomes undergo a maturation process to form late endosomes, in which the biogenesis of exosomes occurs by continuous invagination of the limiting membrane. (3) This particular type of late endosome, which ends up accumulating numerous small intraluminal vesicles with a diameter of 40 to 150 nm is called multivesicular body (MVB). During this process, cytosolic components (eg, miRNAs) are actively packed into the vesicles. In addition, communication with the Golgi apparatus through bidirectional vesicle exchange leads to the incorporation of tetraspanins (blue rectangles) into the membrane of the vesicles. (4) Besides that, cytosolic histone‐bound DNA fragments can be transported to MVBs via the autophagosome pathway. (5) Finally, MVBs either fuse with the plasma membrane causing the release of their content into the extracellular environment, or fuse with lysosomes for degradation of their cargo.
2. ROLE OF MSCs IN ANGIOGENESIS
The human body contains approximately 90 000 km of blood vessels that supply all cells and tissues with vital nutrients and oxygen needed for survival and proliferation. 21 The stimulation of new blood capillary vessel formation through the process of angiogenesis is an integral part of tissue growth and repair. It has been hypothesized that MSCs are part of the perivascular niche in various organs and play an important role in the orchestration of neocapillarization, 22 , 23 which has rapidly attracted considerable interest in the scientific community. In addition, due to their in vitro multipotent differentiation potential into mesenchymal lineages, including osteoblasts, chondrocytes, myocytes, and adipocytes, the idea was raised that they could also replenish lost tissue in vivo. 24 The therapeutic rationale for MSC treatment, for example, for acute MI patients, is to repair damaged heart tissue by cardiomyocyte differentiation and to provide growth factors to induce angiogenesis, to stimulate resident cardiac stem cell migration and commitment to cardiomyocytes. Most evidence suggests that the beneficial effects of MSCs are mainly caused by the secretion of a variety of bioactive paracrine factors. 25 Especially for bone marrow‐derived MSCs, numerous small molecules have been demonstrated to induce angiogenesis both in vitro and in vivo; key factors are summarized in Table 1.
TABLE 1.
Key proangiogenic factors secreted by MSCs
| Short name | Long name | Reference |
|---|---|---|
| ANG | Angiogenin | 26 |
| ANGPT1 | Angiopoietin‐1 | 27 |
| EGF | Epidermal growth factor | 28 |
| FGF‐2 | Fibroblast growth factor‐2 | 29 |
| G‐CSF | Granulocyte‐colony stimulating factor | 30 |
| HGF | Hepatocyte growth factor | 31 |
| IL‐6 | Interleukin‐6 | 32 |
| IL‐8 | Interleukin‐8 | 33 |
| MCP‐1 | Monocyte chemotactic protein‐1 | 34 |
| PDGF | Platelet‐derived growth factor | 35 |
| PlGF | Placental growth factor | 36 |
| SDF‐1 | Stromal cell‐derived factor‐1 | 37 |
| TGF‐alpha | Transforming growth factor alpha | 38 |
| TGF‐beta | Transforming growth factor beta | 39 |
| TNF‐alpha | Tumor necrosis factor alpha | 39 |
| VEGF | Vascular endothelial growth factor | 39 |
Abbreviation: MSCs, mesenchymal stromal cells.
Vascular endothelial growth factor (VEGF) and fibroblast growth factor‐2 (FGF‐2) are two of the most studied factors that regulate angiogenesis. 39 Given that elevated levels can induce cell proliferation and migration of endothelial cells, coordinated regulation of VEGF and FGF‐2 expression is required to elicit the proangiogenic effects of MSCs. Another interesting proangiogenic protein is tumor necrosis factor alpha (TNF‐alpha), as its effect on angiogenesis depends on the concentration and the duration of treatment. Therefore, it might have a dual role in angiogenesis: high doses of TNF‐alpha were found to inhibit angiogenesis in mice in vivo, while low doses promoted it. 40 In addition to classical angiogenic factors, MSCs also secret EVs that carry a variety of biomolecules capable of regulating angiogenesis both in vitro and in vivo. 41 , 42 EVs were proposed as key agents in the modulation of angiogenesis 43 and have been shown to improve angiogenesis in a number of studies, including mouse and rat models of burn injuries, skin wounds, acute kidney injury, acute MI, and limb ischemia. 44 , 45 , 46
3. ENHANCEMENT OF THE ANGIOGENIC POTENTIAL OF MSCs
MSCs can be obtained from a variety of tissues, such as bone marrow, adipose tissue, and umbilical cord tissue, with bone marrow being the most common stem cell source. 47 Efforts to maximize the secretion of proangiogenic factors by MSCs are expected to substantially increase the beneficial role of MSCs in regenerative medicine. Several studies have shown that preconditioning of MSCs by hypoxia enhances the proangiogenic effects of MSCs, 48 , 49 which might be a valuable strategy for boosting their clinical potential and therapeutic efficacy upon transplantation. Exposure of MSCs to reduced oxygen partial pressure induces the expression of genes involved in migration and homing, mainly regulated by hypoxia‐inducible factor‐1 alpha (HIF‐1 alpha). 50 HIF‐1 alpha is constitutively expressed in most cell types, including cardiac cells. Under normoxic conditions, it is inactive due to ubiquitin‐mediated proteasomal degradation and transcriptional inhibition. 51 However, under hypoxic conditions, HIF‐1 alpha becomes rapidly stabilized and its accumulation results in higher gene expression of proangiogenic factors, such as VEGF and transforming growth factor beta, 52 , 53 as well as increased release of EVs from MSCs. 54 Overexpression of HIF‐1 alpha also promotes incorporation of Jagged1, a Notch ligand that increases angiogenesis, into MSC‐derived EVs, suggesting that an active HIF‐1 alpha phenotype can be transmitted to surrounding cells. 55 In addition, hypoxia‐preconditioned MSCs show a higher cell viability, enhanced proliferation potential, decreased production of reactive oxygen species, increased antioxidant glutathione production, and higher superoxide dismutase levels. 56 Other stress conditions that may be of interest for enhancing the angiogenic potential of MSCs include pH variation and calorie restriction. 57 However, given that modification of culture conditions is a rather indirect process for increasing the angiogenic activity of MSCs, as it affects not only one specific molecule but many factors, it may in turn lead to serious side effects. Apart from altering the overall culture environment, several growth factors have been shown to enhance the regenerative capacity of MSCs in vitro. For instance, pretreatment of MSCs with epidermal growth factor or transforming growth factor alpha increased the release of proangiogenic factors such as VEGF and hepatocyte growth factor, which play a central role in inducing angiogenesis and improving oxygen supply to ischemic tissues. 58 , 59 In addition, it has been shown that MSCs, when cultured on collagen‐coated patches, are less fibrogenic and secrete more cardiotrophic factors. 60 Besides modulating culture conditions or using additives, the genetic modification of MSCs was also investigated. 61 Although MSCs naturally possess an enormous inherent therapeutic potential, gene therapy is being used to modify MSCs to further enhance their efficacy and even extend the range of diseases for which MSCs could be applied. MSCs can be easily transduced by clinically available viral vector systems, including retrovirus and lentivirus. 62 This technique leads to efficient production of angiogenic factors and, because viral vectors can be integrated into the host genome, to long‐term gene expression. 63 Numerous animal studies have reported the success of genetically engineered MSCs as a gene delivery vehicle. For example, Xu et al 64 have used a lentiviral vector to generate MSCs that overexpress angiopoietin‐1, a proangiogenic protein that induces endothelial survival and vascular stabilization. Another study by Song et al 65 showed that the introduction of v‐myc into human MSCs using a lentiviral gene delivery system resulted in increased MSC secretion of VEGF and thus increased vessel formation. However, since applications of these vectors elicited adverse side effects including toxicities, immuno‐ and oncogenicity, 66 many clinical studies using viral vectors were terminated. Therefore, nonviral vectors have been continuously studied and have become an attractive alternative for MSC modification. 67 As one example, Bandara et al 68 described a novel nonviral minicircle vector to deliver the endothelial nitric oxide synthase (eNOS) transgene to MSCs. Overexpression of eNOS has been shown to improve the ability of MSCs to treat ischemic heart damage following coronary artery occlusion. In a rat model of acute MI, the authors demonstrated that transplantation of eNOS‐overexpressing MSCs significantly reduced MI size, increased capillary density, and corrected hemodynamic parameters. In addition, in recent years, transfection of MSCs by modified mRNAs has gained considerable traction as a promising strategy to prime MSCs for targeted delivery of therapeutic molecules at a controlled rate. 69 Another approach to multiply the therapeutic potential of systemically applied MSCs is to module their homing and interaction with target cells by surface coating. For example, Chou et al 70 showed that bone marrow‐derived MSCs transfected with 1,3‐fucosyltransferase VI, an enzyme transforming native CD44 on MSCs into a hematopoietic cell E‐/L‐selectin ligand, increased homing to injured endothelial cells. Similarly, Zou et al 71 coated mouse adipose tissue‐derived MSCs (AMSCs) with antibodies to kidney injury molecule‐1, a protein that is upregulated in damaged kidneys, and injected them into mice with renal artery stenosis. These AMSCs showed selective homing compared to untreated AMSCs, leading to improved renal perfusion and capillary density as well as attenuation of oxidative damage and fibrosis. Besides improving the homing efficiency to and retention of MSCs in a target tissue, enhancing MSC survival is a major milestone in improving the effectiveness of MSC‐based therapy. 72 Strategies like preconditioning with hyperoxia or repeated episodes of short‐term exposure to hypoxia have been found to promote the viability and proliferation of MSCs. 73 In addition, studies have provided evidence that stromal cell‐derived factor‐1 alpha (SDF‐1 alpha) can suppress apoptosis in MSCs and promote cardiomyocyte survival. Tang et al 74 found that 1 week after cell implantation, the number of SDF‐1 alpha‐modified MSCs was five times higher than that of wild‐type MSCs in a rat model of MI. Nevertheless, more studies are needed to further improve the survival of MSCs after transplantation in heart tissue.
To date, it has not been conclusively investigated whether MSCs transplantation and systemic application can promote or cause neoplasia and possibly cancer. 75 Studies have shown that due to their perivascular origin, MSCs can differentiate into pericytes or endothelial cells, which ultimately supports tumor vascularization and growth. However, the data on the interaction of MSCs with different tumor types are ambiguous. 76 There is evidence in the literature to support the hypothesis that MSCs can inhibit capillarization in vitro in a dose‐dependent manner. 77 Furthermore, the group of Otsu et al 77 showed tumor recession in vivo after coinoculation of melanoma cells with MSCs. Most clinical trials using MSCs for myocardial regeneration screened their subjects for tumor formation after MSCs injections. In clinical safety studies with unmodified MSCs for myocardial regeneration, no neoplasms associated with MSC application were observed. 78 Nevertheless, since proangiogenic modifications of MSCs carry the risk of promoting existing tumor growth, it is important to carefully examine patients for pre‐existing neoplasms.
4. MSC‐DERIVED EVs AS AN ALTERNATIVE TO MSC TRANSPLANTATION
MSCs could be used in an autologous setting to exclude immune responses of the recipient and thereby preserve their regenerative properties. 79 However, autologous MSC applications have some limitations, including availability and decreased biological activity when isolated from elderly donors and patients with systemic diseases. For example, MSCs isolated from older patients showed a reduction in superoxide dismutase activity and an increase in reactive oxygen species, resulting in oxidative damage in MSCs and, consequently, apoptosis and senescence. 80 In addition, autologous MSC extraction and in vitro expansion prior to implantation is time‐consuming, making it difficult to use them to treat acute diseases such as MI. These shortcomings, coupled with the evidence that MSCs have immunomodulatory properties and are less immunogenic compared to other cell types 14 have stimulated the development of allogeneic MSC products obtained from young and healthy donors. Given their anti‐inflammatory and immune‐evasive mechanisms, off‐the‐shelf allogeneic MSCs that can be administered immediately were considered as a promising option for tissue repair. However, in clinical trials, the overall therapeutic effect was limited, similar to autologous MSCs. 12 Additionally, the use of viable cells still carries inherent risks such as microvasculature obstruction, immune rejection, and proarrhythmic side effects. EVs derived from MSCs can overcome many of these concerns associated with the use of living cells, while having therapeutic effects similar to those achievable by the originating MSCs themselves. 81 In conclusion, rather than transplanting exogenous MSCs, MSC‐derived EVs, even from allogeneic sources, offer a great alternative because they are nonproliferative, less immunogenic, and easier to store and deliver than MSCs. 82 However, as a prerequisite for application, it must be ensured that MSC‐derived EVs can be produced in sufficient quantity and quality and that they are able to effectively mediate pro‐regenerative and immunomodulatory effects of the parental cells.
5. PROANGIOGENIC CHARACTERISTICS OF MSC‐DERIVED EVs
EVs, such as exosomes, are small secretory vesicles carrying a large number of bioactive molecules, including proteins, metabolites, lipids, and RNAs. 83 Besides other and larger types of EVs, they are produced by most cell types under normal and pathophysiological conditions and serve as messengers of the intercellular network, allowing the exchange of cellular components between cells. 42 In detail, classical exosomes are generated in multivesicular bodies and excreted in the extracellular environment when these compartments fuse with the plasma membrane 84 (Figure 1). They can then either be taken up by target cells localized in the microenvironment or transported to distant sites via biological fluids. Upon arrival at the target cells, exosomes can deliver their content directly into the cytoplasm of the target cell or may be surrounded by the plasma membrane and be disintegrated in the cytoplasm, where their content is released. 85 For recognition and internalization by the target cells, exosomes have specific proteins on their surface, such as tetraspanins. 42 In regenerative medicine, EVs secreted by MSCs can stimulate proliferation and inhibit apoptosis of recipient cells. Accordingly, their proangiogenic effects are related to their ability to sustain the viability and proliferation of endothelial cells. 86 However, studies have also reported that MSC‐derived EVs are potent regulators of tumorigenesis. For example, Zhu et al 87 showed an increase in tumor incidence and growth when human gastric and colon cancer cell lines were mixed with MSC‐derived EVs and then injected subcutaneously into mice. Similarly, Ren et al 88 have shown, using a xenograft model, that intravenous injection of hypoxia‐conditioned MSC‐EVs significantly increases tumor development. It is therefore critical to identify which molecules transferred by EVs induce cancer pathways and which tumor types can benefit from MSC‐EV treatment.
Several studies have shown that MSC‐derived EVs contain cytokines and growth factors, and accumulating evidence indicates that angiogenesis can also be specifically regulated by different encapsulated RNAs, including miRNAs. 89 MiRNA is a class of highly conserved, single‐stranded, 19 to 22 nucleotide long, noncoding small RNAs that regulate gene expression at the post‐transcriptional level by targeting 3′‐untranslated regions of specific mRNAs. 90 Upon binding, miRNAs inhibit mRNA translation or cause mRNA degradation, thus suppressing protein synthesis. Although more than 2000 miRNAs are present in humans 91 and nearly 800 miRNAs have been identified in the human heart at this time, 92 the nature of target transcripts is unknown for many of them. Meanwhile, only a few miRNAs have been described to promote angiogenesis; Table 2 shows a selection of known miRNAs with proangiogenic activity.
TABLE 2.
Selection of miRNAs with proangiogenic properties
| miRNA | Regulated targets (selection) | Reference |
|---|---|---|
| miR‐let‐7 | ALK5, FASLG, TSP‐2 | 93, 94, 95 |
| miR‐9 a | ECAD, SOCS5 | 96, 97, 98 |
| miR‐10a a | KLF4, PTEN | 99, 100 |
| miR‐10b a | HOXD10, KLF4, SDC1 | 101, 102 |
| miRNA‐17~92 a | CTGF, TSP‐1 | 103 |
| miR‐21 a | CHIP, PDCD4, PTEN, SMAD7, SPRY1, STAT3 | 104, 105, 106, 107, 108 |
| miR‐23a a | PHD1, PHD2, TSGA10, ZO‐1 | 109, 110 |
| miR‐26b | COX2, CTGF, OCT4, SMAD1 | 111, 112, 113 |
| miR‐27b | DLL4, SPRY2 | 114, 115 |
| miR‐30b | DLL4, JDP2 | 116, 117 |
| miR‐30d a | MYPT1 | 118 |
| miR‐31 | FIH‐1 | 119 |
| miR‐93 a | ITGB8 | 120 |
| miR‐125a | DLL‐4 | 121 |
| miR‐126 | PIK3R2, SPRED1 | 122, 123, 124 |
| miR‐130a a | GAX, HOXA5, RUNX3, TFPI2 | 125, 126, 127 |
| miR‐132 a | p120RasGAP | 128 |
| miR‐135b a | FIH‐1, LATS2 | 129, 130 |
| miR‐145 | TMOD3 | 131 |
| miR‐146a a | BRCA1, NF2, PAK1, RAC1 | 132, 133 |
| miR‐150 a | c‐Myb, SRCIN1, TP53 | 134, 135, 136, 137, 138, 139 |
| miR‐155 a | VHL | 140 |
| miR‐181a a | SRCIN1 | 141 |
| miR‐181b a | GATA6, PDCD10, | 142 |
| miR‐182 a | BRCA1, FOXO3, HMGA2, MITF‐M, MTSS1 | 143 |
| miR‐194 a | TSP‐1 | 144 |
| miR‐210 a | EFNA3 | 145, 146 |
| miR‐214 | ATM | 147 |
| miR‐217 | FOXO3A, KRAS, SIRT1 | 148, 149 |
| miR‐296 a | HGS | 150 |
| miR‐378 a | FUS‐1, SUFU | 151 |
| miR‐382 a | PTEN | 152 |
| miR‐424 | CUL2 | 153 |
| miR‐433 | DKK1 | 154 |
| miR‐467 a | TSP‐1 | 155 |
| miR‐494 a | CASP2 | 156 |
| miR‐1246 a | PML | 157 |
Abbreviations: ALK5, activin receptor‐like kinase 5; ATM, ataxia telangiectasia mutated protein; BRCA1, breast cancer protein 1; CASP2, caspase‐2; CHIP, carboxyl terminus of the heat‐shock cognate 70‐interacting protein; COX2, cyclooxygenase‐2; CTGF, connective tissue growth factor; CUL2, cullin 2; DKK1, dickkopf Wnt signaling pathway inhibitor 1; DLL4, delta‐like ligand 4; ECAD, e‐cadherin; FASLG, Fas ligand; FIH‐1, factor‐inhibiting hypoxia‐inducible factor 1; FOXO3, forkhead‐box‐protein O3; GATA6, GATA‐binding factor 6; GAX, growth arrest‐specific homeobox; HGS, hepatocyte growth factor‐regulated tyrosine kinase substrate; HMGA2, high‐mobility group AT‐hook 2; HOXA5, homeobox A5; HOXD10, homeobox D10; ITGB8, integrin B8; JDP2, jun dimerization protein 2; KLF4, Krüppel‐like factor 4; LATS2, large tumor suppressor kinase 2; MITF‐M, microphthalmia‐associated transcription factor type M; MTSS‐1, metastasis suppressor‐1; MYPT1, myosin phosphatase targeting subunit 1; NF2, neurofibromin 2; PAK1, p21‐activated kinase 1; PDCD4, programmed cell death protein 4; PDCD10, programmed cell death protein 10; PHD1, prolyl hydroxylase 1; PHD2, prolyl hydroxylase 2; PIK3R2, phosphoinositide‐3‐kinase regulatory subunit 2; PML, promyelocytic leukemia protein; PTEN, phosphatase and tensin homolog; RAC1, Ras‐related C3 botulinum toxin substrate 1; p120RasGAP, Ras GTPase‐activating protein 1; RUNX3, Runt‐related transcription factor 3; SDC1, syndecan‐1; SOCS5, suppressor of cytokine signaling 5; SPRED1, sprouty‐related EVH1 domain containing 1; SPRY1, sprouty homologue 1; SPRY2, sprouty homologue 2; SRCIN1, SRC kinase signaling inhibitor 1; STAT3, signal transducer and activator of transcription 3; SUFU, suppressor of fused; TFPI2, tissue factor pathway inhibitor 2; TMOD3, tropomodulin 3; TP53, tumor protein p53; TSP‐1, thrombospondin‐1; TSP‐2, thrombospondin‐2; VHL, von Hippel‐Lindau tumor suppressor; ZO‐1, zonula occludens‐1.
miRNAs that have been shown to also play a role in promoting angiogenesis in tumors.
For instance, with regard to miRNAs incorporated into MSC‐derived EVs, miRNA‐21 activates the protein kinase B/extracellular signal‐regulated kinase signaling pathway leading to the overproduction of VEGF. 104 MiR‐126 exerts its activity by targeting phosphoinositide‐3‐kinase regulatory subunit 2 and sprouty‐related EVH1 domain containing 1, two negative regulators of VEGF signaling. 122 MiR‐130a is a strong positive regulator of angiogenesis because it targets, for example, the antiangiogenic factors growth arrest‐specific homeobox and homeobox A5. 125 MiR‐135b and miR‐31 contribute to angiogenesis by accelerating HIF‐1 alpha transcriptional activity via inhibition of factor‐inhibiting hypoxia‐inducible factor 1, an asparaginyl hydroxylase enzyme that suppresses HIF‐1 alpha. 119 , 129 Likewise, miR‐23a directly targets prolyl hydroxylase 1 and 2, leading to HIF‐1 alpha stabilization. 109 However, despite the benefits of selective miRNAs for regenerative medicine approaches by inducing angiogenesis, there is a close relationship between vascularity and tumor expansion. 158 For example, the miRNA‐17~92 cluster has been shown to increase angiogenesis both in vitro and in vivo, and its predominance was observed in a variety of human cancers. 159 Similarly, plasma miRNA‐21 levels have been described as a marker for various types of tumors, such as breast, colon, prostate, ovarian, pancreatic, and lung cancer. 160 In addition, since miRNAs do not perfectly complement their target mRNAs, they may target multiple genes whose protein products act on different signaling pathways and thus dysregulate several networks in tumor cells. 161 Given their pivotal role in carcinogenesis, off‐target effects of miRNAs should be well characterized before evaluating their use in clinical settings. Taken together, although aberrant angiogenesis may contribute to pathological conditions, including growth and dissemination of tumors, miRNA application is more welcome for the induction of angiogenesis than its repression. 162 , 163
6. EVs AS VEHICLES FOR THE TARGETED DELIVERY OF PROANGIOGENIC MOLECULES
An EV‐based delivery system for proangiogenic factors offers great benefits such as low toxicity, low immunogenicity, high blood circulation stability, biocompatibility, and biological barrier permeability. Apart from the fact that stress situations, such as hypoxia, can alter the composition of EVs, the loading of EVs with proangiogenic factors is a more specific approach to facilitate angiogenesis. To date, various methods have been proposed for loading EVs which can be classified into either cargo loading during formation or after isolation. One promising approach for cargo loading during EV formation is the transfection of MSCs with DNA encoding therapeutically relevant compounds, which are then released into secreted EVs. However, because overexpression of a particular factor does not ensure increased presence in EVs, loading of EVs after release with proangiogenic factors or the vectors encoding them was considered. For instance, by applying an electric field to a suspension of EVs and the therapeutic cargo, pores are created in the membrane, thereby facilitating movement of the cargo into the lumen of EVs. 164 Besides the efficacy of EVs, methods for optimal delivery to the heart are still being investigated. Previous studies have used both intracoronary and intramyocardial injections, with the latter being more effective. For example, Gallet et al 165 showed that EVs from human cardiosphere‐derived cells administered to pigs in both acute and chronic models of cardiac ischemia lead to reduced infarct size and preserved systolic function after intramyocardial but not intracoronary delivery. Whether these improvements will be maintained in the long‐term remains to be investigated. However, although intramyocardial injections are acceptable in animal studies, this method is not clinically appealing because of its invasive nature. Ideally, EVs should be administered intravenously. Nonetheless, a challenge with the use of exogenously administered EVs is that they may be nonspecifically trapped in nontargeted organs, particularly in the lungs and liver, resulting in insufficient targeting of myocardial ischemia. 166 As with cells, attempts to modify EVs as effective tools directly targeting ischemic myocardium have been considered. One method is to restructure transmembrane proteins of EVs to fuse them with ligands or homing peptides, thereby conferring the ability of EVs for targeting tissues bearing the corresponding receptors. Recently, a new peptide sequence, CSTSMLKAC, has been discovered that can preferentially target the ischemic region of the heart, resulting in increased specificity and efficiency of EVs targeting the ischemic myocardium. 166 Another major challenge to the clinical application of EVs is that a high dose is required to improve angiogenesis to a physiologically relevant extent. 167 Although methods for isolating EVs are continuously being developed and optimized, the typical yield of an EV isolation can be less than 1 μg of total EV protein from 1 mL of culture medium, 168 while the therapeutic dose of EVs is normally in the range of 10 to 100 μg of protein in mouse models. 169 In turn, the effective dose in humans could be an order of magnitude or more to compensate for the rapid clearance of EVs from the body. In sum, EVs are promising carriers for proangiogenic molecules, and future efforts should investigate their specific delivery to target organs and the optimal dose. Other important issues to be addressed are the precise mechanism of action of exogenously administered EVs in vivo, the appropriate time window for EV administration, and the route of administration that achieves maximum efficacy without side effects. 170 , 171
7. CONCLUSIONS
MSCs have been explored as a versatile and widely used cell source in regenerative medicine and tissue engineering. Owing to their proangiogenic potential, which is mainly mediated by paracrine factors, they are a promising treatment strategy for diseases caused by insufficient angiogenesis such as MI. But instead of transplanting autologous or allogenic MSCs, a new option is cell‐free therapy, where MSCs are first cultivated and their EVs are then isolated and further manipulated to achieve a more proangiogenic cargo. Evidence supports the superiority of this approach over stromal cell transplantation. Although there are still some drawbacks with respect to the production of sufficiently large amounts of EVs, sample impurities and the inability to produce EVs without the use of cells, EVs constitute a major focus of proangiogenic therapy. Proteins, mRNAs, and miRNAs contained in EVs can be transferred to recipient cells in order to induce their reprogramming to promote angiogenesis. Interestingly, encapsulated miRNAs have recently emerged as key positive regulators of angiogenesis in pathological settings of insufficient blood supply and thus represent promising new tools for CVD treatment. However, to manifest this potential, several challenges for miRNA therapeutics need to be addressed, including the efficiency of the delivery system and the currently incomplete understanding of their biology, as some miRNAs also play a role in promoting angiogenesis in tumors. Future mechanistic studies with EVs should carefully monitor potential off‐target and dose‐dependent effects.
In conclusion, cell‐free MSC‐derived EVs loaded with proangiogenic factors represent a feasible option in situations of insufficient angiogenesis, such as acute MI and ischemia. Consequently, EVs may constitute a promising platform for noncellular regenerative therapies to complement or even replace the use of MSCs in tissue regeneration and repair.
CONFLICT OF INTEREST
V.F. declared consultant/advisory role with Novartis Pharma, Berlin Heart, Biotronik, Edwards, Medtronic, grant funding from Abbott, Medtronic, Boston Scientific, JOTEC, and reimbursements from Edwards, JOTEC, Abbott, Medtronic. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
T.N.S., S.N.: conceptualized and wrote the article and performed the literature search; A.G.D., E.B., Z.X.: supported the literature search; M.S., C.S., V.F.: supported with writing and proof‐reading the article.
ACKNOWLEDGMENTS
The work was funded by the German Centre for Cardiovascular Research, the German Federal Ministry of Education and Research, and the federal states of Berlin and Brandenburg (FKZ 81Z0100302, FKZ 1315848A, FKZ 13GW0099). Dr. Nazari‐Shafti is participant in the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Nazari‐Shafti TZ, Neuber S, Garcia Duran A, et al. Human mesenchymal stromal cells and derived extracellular vesicles: Translational strategies to increase their proangiogenic potential for the treatment of cardiovascular disease. STEM CELLS Transl Med. 2020;9:1558–1569. 10.1002/sctm.19-0432
Timo Z. Nazari‐Shafti and Sebastian Neuber contributed equally to this work.
Funding information German Centre for Cardiovascular Research, the German Federal Ministry of Education and Research, and the federal states of Berlin and Brandenburg, Grant/Award Numbers: FKZ 81Z0100302, FKZ 1315848A, FKZ 13GW0099; Charité − Universitätsmedizin Berlin and the Berlin Institute of Health
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lloyd‐Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948‐954. 10.1161/circulationaha.109.192666. [DOI] [PubMed] [Google Scholar]
- 2. Vale PR, Losordo DW, Symes JF, Isner JM. Growth factors for therapeutic angiogenesis in cardiovascular diseases. Rev Esp Cardiol. 2001;54(10):1210‐1224. [DOI] [PubMed] [Google Scholar]
- 3. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641‐650. 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4. Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3(6):877‐892. 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy O, Zhao W, Mortensen LJ, et al. mRNA‐engineered mesenchymal stem cells for targeted delivery of interleukin‐10 to sites of inflammation. Blood. 2013;122(14):e23‐e32. 10.1182/blood-2013-04-495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker M, Maring J, Schneider M, et al. Towards a novel patch material for cardiac applications: tissue‐specific extracellular matrix introduces essential key features to decellularized amniotic membrane. Int J Mol Sci. 2018;19(4). 10.3390/ijms19041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ciuffreda MC, Malpasso G, Chokoza C, et al. Synthetic extracellular matrix mimic hydrogel improves efficacy of mesenchymal stromal cell therapy for ischemic cardiomyopathy. Acta Biomater. 2018;70:71‐83. 10.1016/j.actbio.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 8. Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30(22):2722‐2732. 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93‐98. 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 10. Perin EC, Sanz‐Ruiz R, Sánchez PL, et al. Adipose‐derived regenerative cells in patients with ischemic cardiomyopathy: the PRECISE trial. Am Heart J. 2014;168(1):88‐95.e2. 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369‐2379. 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nowbar AN, Mielewczik M, Karavassilis M, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta‐analysis. BMJ. 2014;348:g2688 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta‐analysis and update on clinical trials. Circ Cardiovasc Interv. 2014;7(2):156‐167. 10.1161/circinterventions.113.001009. [DOI] [PubMed] [Google Scholar]
- 14. Wang M, Yuan Q. Mesenchymal stem cell‐based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:3057624 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214‐222. 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 16. Bellin G, Gardin C, Ferroni L, Chachques JC, Rogante M. Exosome in cardiovascular diseases: a complex world full of hope. Cells. 2019;8(2). 10.3390/cells8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banas A, Teratani T, Yamamoto Y, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26(10):2705‐2712. 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 18. Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt‐modified mesenchymal stem cell‐mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661‐669. 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 19. Kunter U, Rong S, Djuric Z, et al. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol. 2006;17(8):2202‐2212. 10.1681/asn.2005080815. [DOI] [PubMed] [Google Scholar]
- 20. Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002‐11007. 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev. 2018;98(1):3‐58. 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25‐53. 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Souidi N, Stolk M, Rudeck J, et al. Stromal cells act as guardians for endothelial progenitors by reducing their immunogenicity after co‐transplantation. Stem Cells. 2017;35(5):1233‐1245. 10.1002/stem.2573. [DOI] [PubMed] [Google Scholar]
- 24. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41‐49. 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 25. Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014;9(9):e107001 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tello‐Montoliu A, Patel JV, Lip GY. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006;4(9):1864‐1874. [DOI] [PubMed] [Google Scholar]
- 27. Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328(1):18‐26. [DOI] [PubMed] [Google Scholar]
- 28. van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer. 2005;117(6):883‐888. [DOI] [PubMed] [Google Scholar]
- 29. Esser JS, Rahner S, Deckler M, Bode C, Patterson C, Moser M. Fibroblast growth factor signaling pathway in endothelial cells is activated by BMPER to promote angiogenesis. Arter. Thromb Vasc Biol. 2015;35(2):358‐367. [DOI] [PubMed] [Google Scholar]
- 30. Ohki Y, Heissig B, Sato Y, et al. Granulocyte colony‐stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005;19(14):2005‐2007. [DOI] [PubMed] [Google Scholar]
- 31. Ding S, Merkulova‐Rainon T, Han ZC, Tobelem G. HGF receptor up‐regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101(12):4816‐4822. [DOI] [PubMed] [Google Scholar]
- 32. Nilsson MB, Langley RR, Fidler IJ. Interleukin‐6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65(23):10794‐10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ning Y, Manegold PC, Hong YK, et al. Interleukin‐8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128(9):2038‐2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)‐1 promotes angiogenesis via a novel transcription factor, MCP‐1‐induced protein (MCPIP). J Biol Chem. 2008;283(21):14542‐14551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andrae J, Gallini R, Betsholtz C. Role of platelet‐derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deshane J, Chen S, Caballero S, et al. Stromal cell‐derived factor 1 promotes angiogenesis via a heme oxygenase 1‐dependent mechanism. J Exp Med. 2007;204(3):605‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leker RR, Toth ZE, Shahar T, et al. Transforming growth factor alpha induces angiogenesis and neurogenesis following stroke. Neuroscience. 2009;163(1):233‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ucuzian AA, Gassman AA, East AT, Greisler HP. Molecular mediators of angiogenesis. J Burn Care Res. 2010;31(1):158‐175. 10.1097/BCR.0b013e3181c7ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor‐alpha in angiogenesis. Am J Pathol. 1992;140(3):539‐544. [PMC free article] [PubMed] [Google Scholar]
- 41. Bian X, Ma K, Zhang C, Fu X. Therapeutic angiogenesis using stem cell‐derived extracellular vesicles: an emerging approach for treatment of ischemic diseases. Stem Cell Res Ther. 2019;10(1):158 10.1186/s13287-019-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jeppesen DK, Fenix A, Franklin J, et al. Reassessment of exosome composition. Cell. 2019;177(2):428‐445. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shabbir A, Cox A, Rodriguez‐Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635‐1647. 10.1089/scd.2014.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang B, Wu X, Zhang X, et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/beta‐catenin pathway. Stem Cells Translational Medicine. 2015;4(5):513‐522. 10.5966/sctm.2014-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin KC, Yip HK, Shao PL, et al. Combination of adipose‐derived mesenchymal stem cells (ADMSC) and ADMSC‐derived exosomes for protecting kidney from acute ischemia‐reperfusion injury. Int J Cardiol. 2016;216:173‐185. 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 46. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med. 2014;92(4):387‐397. 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 47. Fitzsimmons REB, Mazurek MS. Mesenchymal stromal/stem cells in regenerative medicine and tissue engineering. Stem Cells Int. 2018;2018:8031718 10.1155/2018/8031718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26(8):2173‐2182. 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beegle J, Lakatos K, Kalomoiris S, et al. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33(6):1818‐1828. 10.1002/stem.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nat Med. 2004;10(8):858‐864. 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 51. Salceda S, Caro J. Hypoxia‐inducible factor 1alpha (HIF‐1alpha) protein is rapidly degraded by the ubiquitin‐proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox‐induced changes. J Biol Chem. 1997;272(36):22642‐22647. 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 52. Zimna A, Kurpisz M. Hypoxia‐inducible factor‐1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou G, Dada LA, Wu M, et al. Hypoxia‐induced alveolar epithelial‐mesenchymal transition requires mitochondrial ROS and hypoxia‐inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297(6):L1120‐L1130. 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang HC, Liu XB, Huang S, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21(18):3289‐3297. 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gonzalez‐King H, Garcia NA, Ontoria‐Oviedo I, Ciria M, Montero JA, Sepulveda P. Hypoxia inducible factor‐1α potentiates jagged 1‐mediated angiogenesis by mesenchymal stem cell‐derived exosomes. Stem Cells. 2017;35(7):1747‐1759. 10.1002/stem.2618. [DOI] [PubMed] [Google Scholar]
- 56. Stubbs SL, Hsiao ST, Peshavariya HM, Lim SY, Dusting GJ, Dilley RJ. Hypoxic preconditioning enhances survival of human adipose‐derived stem cells and conditions endothelial cells in vitro. Stem Cells Dev. 2012;21(11):1887‐1896. 10.1089/scd.2011.0289. [DOI] [PubMed] [Google Scholar]
- 57. Alcayaga‐Miranda F, Varas‐Godoy M, Khoury M. Harnessing the angiogenic potential of stem cell‐derived exosomes for vascular regeneration. Stem Cells Int. 2016;2016:3409169 10.1155/2016/3409169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tamama K, Kawasaki H, Wells A. Epidermal growth factor (EGF) treatment on multipotential stromal cells (MSCs). Possible enhancement of therapeutic potential of MSC. J Biomed Biotechnol. 2010;2010:10 10.1155/2010/795385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kwon YW, Heo SC, Jeong GO, et al. Tumor necrosis factor‐alpha‐activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832(12):2136‐2144. 10.1016/j.bbadis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 60. Rashedi I, Talele N, Wang XH, Hinz B, Radisic M, Keating A. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS One. 2017;12(10):e0187348 10.1371/journal.pone.0187348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wei W, Huang Y, Li D, Gou HF, Wang W. Improved therapeutic potential of MSCs by genetic modification. Gene Ther. 2018;25(8):538‐547. 10.1038/s41434-018-0041-8. [DOI] [PubMed] [Google Scholar]
- 62. Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181‐196. 10.1016/j.pharmthera.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 63. Porada CD, Almeida‐Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156‐1166. 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu J, Qu J, Cao L, et al. Mesenchymal stem cell‐based angiopoietin‐1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214(4):472‐481. 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 65. Song SH, Lee MO, Lee JS, et al. Genetic modification of human adipose‐derived stem cells for promoting wound healing. J Dermatol Sci. 2012;66(2):98‐107. 10.1016/j.jdermsci.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 66. Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60(2):249‐271. 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 67. Li SD, Huang L. Non‐viral is superior to viral gene delivery. J Control Release. 2007;123(3):181‐183. 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 68. Bandara N, Gurusinghe S, Chen H, et al. Minicircle DNA‐mediated endothelial nitric oxide synthase gene transfer enhances angiogenic responses of bone marrow‐derived mesenchymal stem cells. Stem Cell Res Ther. 2016;7:48 10.1186/s13287-016-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Badieyan ZS, Evans T. Concise review: application of chemically modified mRNA in cell fate conversion and tissue engineering. Stem Cells Translational Medicine. 2019;8(8):833‐843. 10.1002/sctm.18-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chou K‐J, Lee PT, Chen CL, et al. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp Cell Res. 2017;350(1):91‐102. 10.1016/j.yexcr.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 71. Zou X, Jiang K, Puranik AS, et al. Targeting murine mesenchymal stem cells to kidney injury molecule‐1 improves their therapeutic efficacy in chronic ischemic kidney injury. Stem Cells Translational Medicine. 2018;7(5):394‐403. 10.1002/sctm.17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Matsuura K, Honda A, Nagai T, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119(8):2204‐2217. 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li L, Chen X, Wang WE, Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:9682757 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang J, Wang J, Guo L, et al. Mesenchymal stem cells modified with stromal cell‐derived factor 1 alpha improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29(1):9‐19. 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 75. Lee H‐Y, Hong I‐S. Double‐edged sword of mesenchymal stem cells: cancer‐promoting versus therapeutic potential. Cancer Sci. 2017;108(10):1939‐1946. 10.1111/cas.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29(1):11‐19. 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. Concentration‐dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197‐4205. 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta‐analysis of clinical trials. PLoS One. 2012;7(10):e47559 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87‐117. 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stolzing A, Jones E, McGonagle D, Scutt A. Age‐related changes in human bone marrow‐derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163‐173. 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 81. Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell‐derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301‐312. 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 82. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287‐296. 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15 10.3410/b3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell‐to‐cell communication. Kidney Int. 2010;78(9):838‐848. 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 86. Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes function in pro‐ and anti‐angiogenesis. Curr Angiogenes. 2013;2(1):54‐59. 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28‐37. 10.1016/j.canlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 88. Ren W, Hou J, Yang C, et al. Extracellular vesicles secreted by hypoxia pre‐challenged mesenchymal stem cells promote non‐small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR‐21‐5p delivery. J Exp Clin Cancer Res. 2019;38(1):62 10.1186/s13046-019-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Phinney DG, Pittenger MF. Concise review: MSC‐derived exosomes for cell‐free therapy. Stem Cells. 2017;35(4):851‐858. 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 90. Felekkis K, Touvana E, Stefanou C, Deltas C. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14(4):236‐240. [PMC free article] [PubMed] [Google Scholar]
- 91. Friedlander MR, Lizano E, Houben AJS, et al. Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biol. 2014;15(4):R57 10.1186/gb-2014-15-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Leptidis S, el Azzouzi H, Lok SI, et al. A deep sequencing approach to uncover the miRNOME in the human heart. PLoS One. 2013;8(2):e57800 10.1371/journal.pone.0057800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bae ON, Wang JM, Baek SH, Wang Q, Yuan H, Chen AF. Oxidative stress‐mediated thrombospondin‐2 upregulation impairs bone marrow‐derived angiogenic cell function in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2013;33(8):1920‐1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dhahri W, Dussault S, Haddad P, et al. Reduced expression of let‐7f activates TGF‐beta/ALK5 pathway and leads to impaired ischaemia‐induced neovascularization after cigarette smoke exposure. J Cell Mol Med. 2017;21(9):2211‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kong L, Du X, Hu N, et al. Downregulation of let‐7e‐5p contributes to endothelial progenitor cell dysfunction in deep vein thrombosis via targeting FASLG. Thromb Res. 2016;138:30‐36. [DOI] [PubMed] [Google Scholar]
- 96. Ma L, Young J, Prabhala H, et al. miR‐9, a MYC/MYCN‐activated microRNA, regulates E‐cadherin and cancer metastasis. Nat Cell Biol. 2010;12(3):247‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Seashols‐Williams SJ, Budd W, Clark GC, et al. miR‐9 acts as an OncomiR in prostate cancer through multiple pathways that drive tumour progression and metastasis. PLoS One. 2016;11(7):e0159601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhuang G, Wu X, Jiang Z, et al. Tumour‐secreted miR‐9 promotes endothelial cell migration and angiogenesis by activating the JAK‐STAT pathway. EMBO J. 2012;31(17):3513‐3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dong J, Zhang Z, Huang H, et al. miR‐10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res Ther. 2018;9(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tu J, Cheung H‐H, Lu G, Chen Z, Chan W‐Y. MicroRNA‐10a promotes granulosa cells tumor development via PTEN‐AKT/Wnt regulatory axis. Cell Death Dis. 2018;9(11):1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tian Y, Luo A, Cai Y, et al. MicroRNA‐10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010;285(11):7986‐7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sheedy P, Medarova Z. The fundamental role of miR‐10b in metastatic cancer. Am J Cancer Res. 2018;8(9):1674‐1688. [PMC free article] [PubMed] [Google Scholar]
- 103. Dews M, Homayouni A, Yu D, et al. Augmentation of tumor angiogenesis by a Myc‐activated microRNA cluster. Nat Genet. 2006;38(9):1060‐1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu LZ, Li C, Chen Q, et al. MiR‐21 induced angiogenesis through AKT and ERK activation and HIF‐1alpha expression. PLoS One. 2011;6(4):e19139 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu Y, Luo F, Wang B, et al. STAT3‐regulated exosomal miR‐21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125‐135. [DOI] [PubMed] [Google Scholar]
- 106. Haque R, Iuvone PM, He L, et al. The MicroRNA‐21 signaling pathway is involved in prorenin receptor (PRR) ‐induced VEGF expression in ARPE‐19 cells under a hyperglycemic condition. Mol Vis. 2017;23:251‐262. [PMC free article] [PubMed] [Google Scholar]
- 107. Zhou Y, Zhu Y, Zhang L, et al. Human stem cells overexpressing miR‐21 promote angiogenesis in critical limb ischemia by targeting CHIP to enhance HIF‐1alpha activity. Stem Cells. 2016;34(4):924‐934. [DOI] [PubMed] [Google Scholar]
- 108. Cappellesso R, Tinazzi A, Giurici T, et al. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122(9):685‐693. [DOI] [PubMed] [Google Scholar]
- 109. Hsu YL, Hung JY, Chang WA, et al. Hypoxic lung cancer‐secreted exosomal miR‐23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO‐1. Oncogene. 2017;36(34):4929‐4942. 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 110. Bao L, You B, Shi S, et al. Metastasis‐associated miR‐23a from nasopharyngeal carcinoma‐derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene. 2018;37(21):2873‐2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lin J, Zhang L, Huang H, et al. MiR‐26b/KPNA2 axis inhibits epithelial ovarian carcinoma proliferation and metastasis through downregulating OCT4. Oncotarget. 2015;6(27):23793‐23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Duan G, Ren C, Zhang Y, Feng S. MicroRNA‐26b inhibits metastasis of osteosarcoma via targeting CTGF and Smad1. Tumour Biol. 2015;36(8):6201‐6209. [DOI] [PubMed] [Google Scholar]
- 113. Xia M, Duan ML, Tong JH, Xu JG. MiR‐26b suppresses tumor cell proliferation, migration and invasion by directly targeting COX‐2 in lung cancer. Eur Rev Med Pharmacol Sci. 2015;19(24):4728‐4737. [PubMed] [Google Scholar]
- 114. Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581‐588. [DOI] [PubMed] [Google Scholar]
- 115. Biyashev D, Veliceasa D, Topczewski J, et al. miR‐27b controls venous specification and tip cell fate. Blood. 2012;119(11):2679‐2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gong M, Yu B, Wang J, et al. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget. 2017;8(28):45200‐45212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Howe GA, Kazda K, Addison CL. MicroRNA‐30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2. PLoS One. 2017;12(10):e0185619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin ZY, Chen G, Zhang YQ, et al. MicroRNA‐30d promotes angiogenesis and tumor growth via MYPT1/c‐JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol Cancer. 2017;16(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119. Kang T, Jones TM, Naddell C, et al. Adipose‐derived stem cells induce angiogenesis via microvesicle transport of miRNA‐31. Stem Cells Translational Medicine. 2016;5(4):440‐450. 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fang L, Deng Z, Shatseva T, et al. MicroRNA miR‐93 promotes tumor growth and angiogenesis by targeting integrin‐beta8. Oncogene. 2011;30(7):806‐821. [DOI] [PubMed] [Google Scholar]
- 121. Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR‐125a. J Cell Sci. 2016;129(11):2182‐2189. [DOI] [PubMed] [Google Scholar]
- 122. Fish JE, Santoro MM, Morton SU, et al. miR‐126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272‐284. 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Huang F, Fang ZF, Hu XQ, Tang L, Zhou SH, Huang JP. Overexpression of miR‐126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors. Biol Chem. 2013;394(9):1223‐1233. [DOI] [PubMed] [Google Scholar]
- 124. Wang S, Aurora AB, Johnson BA, et al. The endothelial‐specific microRNA miR‐126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR‐130a) that down‐regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111(3):1217‐1226. 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lee SH, Jung YD, Choi YS, Lee YM. Targeting of RUNX3 by miR‐130a and miR‐495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget. 2015;6(32):33269‐33278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gao F, Wang F‐G, Liu R‐R, et al. Epigenetic silencing of miR‐130a ameliorates hemangioma by targeting tissue factor pathway inhibitor 2 through FAK/PI3K/Rac1/mdm2 signaling. Int J Oncol. 2017;50(5):1821‐1831. [DOI] [PubMed] [Google Scholar]
- 128. Anand S, Majeti BK, Acevedo LM, et al. MicroRNA‐132‐mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16(8):909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR‐135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor‐inhibiting HIF‐1. Blood. 2014;124(25):3748‐3757. 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hua K, Jin J, Zhao J, et al. miR‐135b, upregulated in breast cancer, promotes cell growth and disrupts the cell cycle by regulating LATS2. Int J Oncol. 2016;48(5):1997‐2006. [DOI] [PubMed] [Google Scholar]
- 131. Liu CH, Wang Z, Huang S, Sun Y, Chen J. MicroRNA‐145 regulates pathological retinal angiogenesis by suppression of TMOD3. Mol Ther Nucleic Acids. 2019;16:335‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Seo HH, Lee S‐Y, Lee CY, et al. Exogenous miRNA‐146a enhances the therapeutic efficacy of human mesenchymal stem cells by increasing vascular endothelial growth factor secretion in the ischemia/reperfusion‐injured heart. J Vasc Res. 2017;54(2):100‐108. [DOI] [PubMed] [Google Scholar]
- 133. Zhu K, Pan Q, Zhang X, et al. MiR‐146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis. 2013;34(9):2071‐2079. [DOI] [PubMed] [Google Scholar]
- 134. Desjarlais M, Dussault S, Dhahri W, Mathieu R, Rivard A. MicroRNA‐150 modulates ischemia‐induced neovascularization in atherosclerotic conditions. Arterioscler Thromb Vasc Biol. 2017;37(5):900‐908. [DOI] [PubMed] [Google Scholar]
- 135. Wang W, Li C, Li W, et al. MiR‐150 enhances the motility of EPCs in vitro and promotes EPCs homing and thrombus resolving in vivo. Thromb Res. 2014;133(4):590‐598. [DOI] [PubMed] [Google Scholar]
- 136. Li J, Zhang Y, Liu Y, et al. Microvesicle‐mediated transfer of microRNA‐150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem. 2013;288(32):23586‐23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vang S, Wu H‐T, Fischer A, et al. Identification of ovarian cancer metastatic miRNAs. PLoS One. 2013;8(3):e58226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lu Q, Guo Z, Qian H. Role of microRNA‐150‐5p/SRCIN1 axis in the progression of breast cancer. Exp Ther Med. 2019;17(3):2221‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Liu F, Di Wang X. miR‐150‐5p represses TP53 tumor suppressor gene to promote proliferation of colon adenocarcinoma. Sci Rep. 2019;9(1):6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kong W, He L, Richards EJ, et al. Upregulation of miRNA‐155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple‐negative breast cancer. Oncogene. 2014;33(6):679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sun W, Wang X, Li J, et al. MicroRNA‐181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 2018;9(4):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xu X, Ge S, Jia R, et al. Hypoxia‐induced miR‐181b enhances angiogenesis of retinoblastoma cells by targeting PDCD10 and GATA6. Oncol Rep. 2015;33(6):2789‐2796. [DOI] [PubMed] [Google Scholar]
- 143. Liu Z, Liu J, Segura MF, et al. MiR‐182 overexpression in tumourigenesis of high‐grade serous ovarian carcinoma. J Pathol. 2012;228(2):204‐215. [DOI] [PubMed] [Google Scholar]
- 144. Sundaram P, Hultine S, Smith LM, et al. p53‐responsive miR‐194 inhibits thrombospondin‐1 and promotes angiogenesis in colon cancers. Cancer Res. 2011;71(24):7490‐7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Fasanaro P, D'Alessandra Y, Di Stefano V, et al. MicroRNA‐210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin‐A3. J Biol Chem. 2008;283(23):15878‐15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang Z, Yin B, Wang B, Ma Z, Liu W, Lv G. MicroRNA‐210 promotes proliferation and invasion of peripheral nerve sheath tumor cells targeting EFNA3. Oncol Res. 2013;21(3):145‐154. [DOI] [PubMed] [Google Scholar]
- 147. van Balkom BW, de Jong OG, Smits M, et al. Endothelial cells require miR‐214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121(19):3997‐4006. [DOI] [PubMed] [Google Scholar]
- 148. Zhang S, Liu L, Wang R, et al. MicroRNA‐217 promotes angiogenesis of human cytomegalovirus‐infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS One. 2013;8(12):e83620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR‐217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis. 2010;31(10):1726‐1733. [DOI] [PubMed] [Google Scholar]
- 150. Wurdinger T, Tannous BA, Saydam O, et al. miR‐296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14(5):382‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee DY, Deng Z, Wang CH, Yang BB. MicroRNA‐378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus‐1 expression. Proc Natl Acad Sci USA. 2007;104(51):20350‐20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Seok JK, Lee SH, Kim MJ, Lee YM. MicroRNA‐382 induced by HIF‐1alpha is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014;42(12):8062‐8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ghosh G, Subramanian IV, Adhikari N, et al. Hypoxia‐induced microRNA‐424 expression in human endothelial cells regulates HIF‐alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120(11):4141‐4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sun J, Chen J, Cao J, Li T, Zhuang S, Jiang X. IL‐1beta‐stimulated beta‐catenin up‐regulation promotes angiogenesis in human lung‐derived mesenchymal stromal cells through a NF‐kappaB‐dependent microRNA‐433 induction. Oncotarget. 2016;7(37):59429‐59440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bhattacharyya S, Sul K, Krukovets I, Nestor C, Li J, Adognravi OS. Novel tissue‐specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J Am Heart Assoc. 2012;1(6):e005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhang Q, Li Y, Zhao M, et al. MiR‐494 acts as a tumor promoter by targeting CASP2 in non‐small cell lung cancer. Sci Rep. 2019;9(1):3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell‐derived microvesicles containing microRNA‐1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down‐regulation in endothelial cells. Biochim Biophys Acta. 2014;1839(11):1256‐1272. [DOI] [PubMed] [Google Scholar]
- 158. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fuziwara CS, Kimura ET. Insights into regulation of the miR‐17‐92 cluster of miRNAs in cancer. Front Med. 2015;2:64 10.3389/fmed.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Feng YH, Tsao CJ. Emerging role of microRNA‐21 in cancer. Biomed Rep. 2016;5(4):395‐402. 10.3892/br.2016.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18(12):1121‐1126. 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shang J, Liu H, Zhou Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage‐related diseases. J Cell Mol Med. 2013;17(12):1515‐1524. 10.1111/jcmm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110(3):496‐507. 10.1161/circresaha.111.247916. [DOI] [PubMed] [Google Scholar]
- 164. Bunggulawa EJ, Wang W, Yin T, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16(1):81 10.1186/s12951-018-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gallet R, Dawkins J, Valle J, et al. Exosomes secreted by cardiosphere‐derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38(3):201‐211. 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang X, Chen Y, Zhao Z, et al. Engineered exosomes with ischemic myocardium‐targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. 2018;7(15):e008737 10.1161/jaha.118.008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Nooshabadi VT, Verdi J, Ebrahimi‐Barough S, et al. Endometrial mesenchymal stem cell‐derived exosome promote endothelial cell angiogenesis in a dose dependent manner: a new perspective on regenerative medicine and cell‐free therapy. Arch Neurosci. 2019;6(4):e94041 10.5812/ans.94041. [DOI] [Google Scholar]
- 168. Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y. Cell type‐specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316‐322. 10.1016/j.ejps.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 169. Willis GR, Kourembanas S, Mitsialis SA. Toward exosome‐based therapeutics: isolation, heterogeneity, and fit‐for‐purpose potency. Front Cardiovasc Med. 2017;4:63 10.3389/fcvm.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cheng L, Zhang K, Wu S. Focus on mesenchymal stem cell‐derived exosomes: opportunities and challenges in cell‐free therapy. Stem Cells Int. 2017;2017:6305295 10.1155/2017/6305295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Nazari‐Shafti TZ, Stamm C, Falk V, Emmert MY. Exosomes for cardioprotection: are we ready for clinical translation? Eur Heart J. 2019;40(12):953‐956. 10.1093/eurheartj/ehz106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


