Abstract
Spinal cord injuries (SCIs) are associated with tremendous physical, social, and financial costs for millions of individuals and families worldwide. Rapid delivery of specialized medical and surgical care has reduced mortality; however, long‐term functional recovery remains limited. Cell‐based therapies represent an exciting neuroprotective and neuroregenerative strategy for SCI. This article summarizes the most promising preclinical and clinical cell approaches to date including transplantation of mesenchymal stem cells, neural stem cells, oligodendrocyte progenitor cells, Schwann cells, and olfactory ensheathing cells, as well as strategies to activate endogenous multipotent cell pools. Throughout, we emphasize the fundamental biology of cell‐based therapies, critical features in the pathophysiology of spinal cord injury, and the strengths and limitations of each approach. We also highlight salient completed and ongoing clinical trials worldwide and the bidirectional translation of their findings. We then provide an overview of key adjunct strategies such as trophic factor support to optimize graft survival and differentiation, engineered biomaterials to provide a support scaffold, electrical fields to stimulate migration, and novel approaches to degrade the glial scar. We also discuss important considerations when initiating a clinical trial for a cell therapy such as the logistics of clinical‐grade cell line scale‐up, cell storage and transportation, and the delivery of cells into humans. We conclude with an outlook on the future of cell‐based treatments for SCI and opportunities for interdisciplinary collaboration in the field.
Keywords: clinical trials, neuroprotection, neuroregeneration, spinal cord injury, stem cells
![]()
Significance statement.
Traumatic spinal cord injuries (SCIs) result in tremendous lifelong disability and financial burden for millions of patients and caregivers worldwide. Cell‐based therapies have emerged as an exciting neuroprotective and neuroregenerative strategy for SCI. This review highlights key preclinical and clinical data in cell therapy with an emphasis on the pathobiology and mechanisms of recovery. Also discussed are adjunct treatments to maximize the efficacy of the grafts. Finally, important translational considerations such as clinical‐grade scale‐up and delivery techniques are discussed. The article succinctly provides readers with a working knowledge of SCI and cell therapies at the leading edge of research.
1. INTRODUCTION
Spinal cord injuries (SCIs) have tremendous physical, social, and financial consequences for over 1 million North Americans and their families. 1 , 2 Direct lifetime costs of care range from $1.1 to $4.7 million per person not including lost wages and productivity. 2 Rapid delivery of specialized medical and surgical care has significantly reduced mortality; however, long‐term functional recovery remains limited. 3 , 4 , 5 , 6 Cell‐based therapies have emerged as an exciting strategy to neuroprotect and regenerate the injured cord through multiple mechanisms such as immunomodulation, paracrine signaling, extracellular matrix (ECM) modification, and lost cell replacement. 7 , 8 Herein, we summarize the most promising preclinical and clinical cell therapies, adjunct strategies to enhance transplant success, as well as key translational considerations such as sex and age. Throughout, we emphasize the fundamental biology of stem cells, critical features in the pathophysiology of spinal cord injury and provide meaningful discussions on the strengths and limitations of each therapeutic approach.
1.1. Epidemiology
The epidemiology of SCI is an important consideration when designing clinical trials. Traumatic SCI is more common in males (79.8%) than females (20.2%). Most injuries are cervical (~60%) followed by thoracic (32%) and lumbosacral (9%). 9 There is a bimodal age distribution with one peak occurring from 15 to 29 years of age and a second, smaller but growing peak, occurring after age 50. 10 , 11 High‐energy motor vehicle collisions (MVCs) and sports‐related injuries disproportionately affect younger individuals. Low‐energy trauma, such as falls, are more common in those over 60 years old where underlying degenerative spinal conditions, such as degenerative cervical myelopathy, are more prevalent. 11 , 12 Interestingly, MVCs account for a declining majority (38%) of SCIs in North America, 9 whereas falls are increasing and account for 31% of injuries followed by sports‐related impacts at 10% to 17%. 11 , 12
2. PATHOPHYSIOLOGY
2.1. Acute injury and the postinjury milieu
The initial traumatic event causes permeabilization of cell membranes, ion and small molecule dysregulation, and ischemia due to damage to the sensitive microvascular supply. 13 , 14 Together, these events initiate a secondary injury cascade which generates further permanent damage (Figure 1A). Over several hours, progressive edema and hemorrhage cyclically add to the harsh postinjury milieu. The compromised blood‐spinal cord barrier (BSCB) exposes the vulnerable cord to inflammatory cells, vasoactive peptides, and cytokines such as tumor necrosis factor and interleukin‐1β. 16 Ongoing cell death releases DNA, ATP, and K+ into the microenvironment; microglia respond by secreting additional pro‐inflammatory cytokines and promoting the infiltration of large numbers of macrophages, neutrophils, and nearby microglia. This activates astrocytes and endothelial cells which further secrete factors such as BMPs, TGF‐β, and Notch activating ligand, Jagged. Activated phagocytes can clear myelin debris within the injury but also produce oxygen free radicals (eg, O2−, peroxynitrite and hydrogen peroxide) and cytotoxic by‐products which generate additional cell death through lipid peroxidation, protein oxidation, and DNA damage. 17 , 18 Extracellular glutamate accumulates as neurons die and astrocytes' reuptake capacity is lost. 19 , 20 This leads to excitotoxic cell death of the remaining neurons through NMDA, kainate, and AMPA receptor overactivation combined with ATP‐dependent ion pump dysfunction and subsequent sodium dysregulation (Figure 1B). 21 , 22
FIGURE 1.
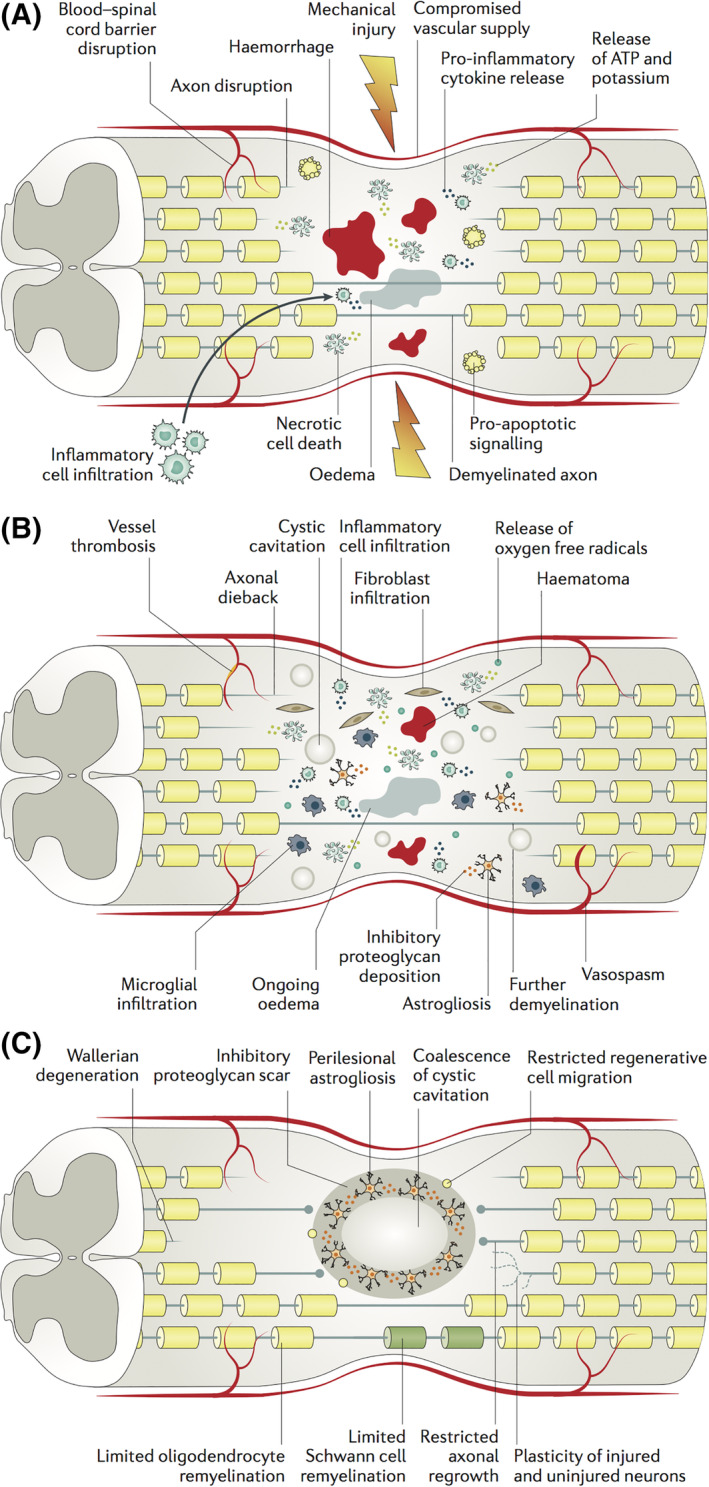
Pathophysiology of traumatic spinal cord injury. “(a) The initial mechanical trauma to the spinal cord initiates a secondary injury cascade that is characterized in the acute phase (that is, 0–48 hours after injury) by oedema, haemorrhage, ischaemia, inflammatory cell infiltration, the release of cytotoxic products and cell death. This secondary injury leads to necrosis and/or apoptosis of neurons and glial cells, such as oligodendrocytes, which can lead to demyelination and the loss of neural circuits. (b) In the subacute phase (2–4 days after injury), further ischaemia occurs owing to ongoing oedema, vessel thrombosis and vasospasm. Persistent inflammatory cell infiltration causes further cell death, and cystic microcavities form, as cells and the extracellular architecture of the cord are damaged. In addition, astrocytes proliferate and deposit extracellular matrix molecules into the perilesional area. (c) In the intermediate and chronic phases (2 weeks to 6 months), axons continue to degenerate and the astroglial scar matures to become a potent inhibitor of regeneration. Cystic cavities coalesce to further restrict axonal regrowth and cell migration.” Republished with permission from Ahuja et al 15
At a systemic level, poor respiratory function can cause hypoxia whereas loss of sympathetic innervation to the vasculature can result in profound hypotension. Combined with the impaired autoregulatory capacity of the cord, this can contribute to ongoing ischemia for days to weeks postinjury. 23 The multiple causes of acute and subacute cell death in this injury cascade represent important targets for cell‐based neuroprotective approaches.
2.2. Barriers to recovery
In the intermediate‐chronic phase, acute inflammation subsides and the cord undergoes alterations in ECM composition, attempts at remyelination, and remodeling of neural networks. 24 Although this can result in limited recovery, multiple barriers to local circuit and long‐tract regeneration persist.
Neuroglial cell death and degeneration in the early phase disrupts the cord's structural framework and leads to ex vacuo formation of microcystic cavitations containing extracellular fluid with thin bands of connective tissue. 25 These cavities coalesce into larger collections which lack substrate for directed axonal regrowth and regenerative cell migration. 26 , 27 Additionally, oligodendrocytes are susceptible to necrotic and apoptotic cell death. The denuded axons they leave behind cannot utilize rapid saltatory conduction and are particularly susceptible to nonfunctional electrogenesis which further contributes to poor recovery. 28
Early after injury, astrocytes also proliferate within the perilesional zone and tightly interweave an irregular mesh of processes to sequester the injured region (Figure 1C). Resident neural stem and progenitor cells surrounding the central canal can also differentiate to astrocytes and contribute to this astrogliosis. The astrocytes, pericytes, and ependymal cells in the region generate dense deposits of chondroitin sulfate proteoglycans (CSPGs), NG2, and tenascin which form the fibrous component of the glial scar. 29 , 30 , 31 , 32 Although literature exists supporting the beneficial aspects of scar, the balance of evidence suggests that chronic scarring potently inhibits axonal regeneration and neurite outgrowth by acting as a physical barrier and tightly binding transmembrane protein tyrosine phosphatase receptors. 33 , 34 , 35
Furthermore, CNS myelin‐ and neuron‐associated ligands, such as myelin associated glycoprotein (MAG), oligodendrocyte myelin glycoprotein (OMgp), neurite outgrowth inhibitor (NOGO), and semaphorin 3A/4D, bind NOGO receptor‐p75 complexes (NgR) and plexins to activate Rho GTPase and its downstream effector, Rho‐associated protein kinase (ROCK). 36 , 37 , 38 , 39 This results in a change in actomyosin contractility and collapse of the axonal growth cone and further inhibition of regeneration. There are other potent inhibitors of axonal regeneration such as Repulsive Guidance Molecule A which are upregulated in the injured cord. 40 , 41 , 42
Multiple cell strategies discussed below aim to preserve and/or regenerate functional, myelinated neural circuits to enhance functional recovery.
3. CELL‐BASED THERAPIES
Cell‐based therapies include cell transplantation and harnessing the potential of endogenous neural precursor cells. Cell treatments can immunomodulate, alter the microenvironment, and replace lost cells depending on the cell type, cell state, delivery route (eg, systemic vs local), and timing of administration. 43 , 44 , 45 , 46 The most commonly studied and promising cell types include mesenchymal stem cells (MSCs), neural stem cells (NSCs), oligodendrocyte progenitor cells (OPCs), Schwann cells (SCs), and olfactory ensheathing cells (OECs). 47 , 48 , 49 , 50 This section outlines the mechanisms of action for each and summarizes progress along the translational research spectrum. Key preclinical studies are highlighted in Table 1, and completed and ongoing clinical trials are summarized in Tables 2 and 3, respectively.
TABLE 1.
Key preclinical studies of cell therapies for spinal cord injury
| Cell type | Species; source | SCI model; injury level; host; transplant interval; route of cell delivery; immunosuppression | Behavioral outcome | Histological outcome |
|---|---|---|---|---|
| BMSC | Human BMSC 51 | T8 contusion (MASCIS Impactor; mild, moderate, severe); Sprague Dawley rats; subacute (7 days); epicenter injections in mild and severe injury, additional rostral and caudal injections in moderate injury group; cyclosporine immunosuppression (10 mg/kg/day s.c.) | Improvement in BBB score in mild SCI group at endpoint; in moderate SCI group BBB score higher at 1, 3, and 7 wk post‐transplantation but not sustained; transient effect in severe SCI group; no improvement in grid walk and no difference in thermal sensitivity | In moderate SCI group more axons found within BMSC grafts relative to control; low graft survival in severe SCI group |
| BMSC | Human BMSC 52 | T8‐9 modified balloon compression; Wistar rats; subacute (7 days); intravenous injection; cyclosporine immunosuppression (10 mg/kg/day s.c.) | Improvement in BBB score at 21 and 28 days post‐SCI | Transplanted BMSC detected in ventrolateral white matter and in segments rostral and caudal to injury epicenter |
| BMSC | Adult rat BMSC 53 | T9 contusion (NYU impactor); Lewis rats; acute and subacute (7 days); epicenter and rostral and caudal injections; no immunosuppression | In acute groups, no difference between BMSC and control groups; in subactute groups, BMSC grafts improved BBB score | Better survival of grafts with subacute transplants; BMSC formed bundles bridging the epicenter of the injury |
| BMSC | Adult rat BMSC 54 | T8‐9 contusion (NYU impactor); Sprague Dawley rats; acute; epicenter injection; no immunosuppression | BMSC treated rats showed higher BBB with weight supported stepping | Less cavitation in BMSC group |
| BMSC | Adult rat BMSC 55 | T8‐9 contusion (NYU impactor; mild and severe SCI); Sprague Dawley rats; acute; intrathecal injection into fourth ventricle; FK506 immunosuppression | Improvement in BBB score for mild injury and at endpoint for severe SCI | Transplanted BMSC were found attached to spinal surface at initial time point and undetectable by 3 wk post‐transplant; smaller lesion cavity in BMSC treated rats |
| BMSC | Adult rat BMSC 56 | T8 contusion (OSU Impactor); Wistar rats; subacute (2 days); epicenter injection; group with additional injection at T11; no immunosupression | No significant differences in BBB and subscore; more rats with BMSC grafts showed hindlimb airstepping | Spared tissue area rostral and caudal to epicenter in BMSC transplanted groups; more axonal fibers at lesion site |
| BM‐MNC | Adult rat BM‐MNC 57 | T8‐9 balloon compression; Wistar rats; subacute (7 days); intravenous injection; Depo‐Medrol immunosuppression (2 mg/rat/wk, i.m.) | Improvement in BBB score from 2 wk post‐SCI | BMSC transplanted groups showed spared white matter rostral and caudal to epicenter, and some spared gray matter |
| Umbilical cord‐derived MSC | Human umbilical cord‐derived MSC 58 | T9 contusion (NYU); Sprague Dawley rats; subacute (7 days); intraspinal injections intralesional | Improvement in BBB score from 2 wk after transplantation | Reduced cavity volume |
| Adipose‐derived MSC | Human adipose‐derived MSC 59 | T8‐9 balloon compression; Sprague Dawley rats; acute; intraspinal injection rostral to lesion | Increased BBB score throughout time course | Tissue preservation, restricting inflammation, stimulation of axonal growth; laminin at lesion site associated with MSC grafts |
| NSPC and BMSC | Adult rat spinal cord derived NSPC alone or co‐grafted with adult rat BMSC 60 | T8 clip compression; Sprague Dawley rats; subacute (9 days) transplants of NSPC, acute transplants of BMSC, alone or in combination; intraspinal rostral and caudal injections; cyclosporine immunosuppression (15 mg/kg/day s.c.) | Improved recovery on BBB and horizontal ladder with subacute NSPC transplants only | Grafted NSPC ensheathed axons at injury site; increased sparing of long tracts |
| NSPC | Adult rat spinal cord derived NSPC 61 | T8 clip compression; Sprague Dawley rats; acute, subacute (9 days) and chronic (6 wk); intraspinal rostral and caudal injections; cyclosporine immunosuppression (15 mg/kg/day s.c.) | Functional recovery only examined in acute transplant groups and no significant differences | NSPC transplants showed primarily glial differentiation; better graft survival with subacute transplants |
| NSPC | Adult rat spinal cord derived NSPC and adult NSPC transduced to express neurogenin‐2 53 | T8‐9 contusion (weight drop); Sprague Dawley rats; subacute (7 days); intraspinal around the lesion site | Increased pain sensation with NSPC grafts but not with neurogenin‐2 transduced NSPC which also showed improved BBB and grid walk scores | NSPC transplants primarily differentiated into astrocytes whereas neurogenin‐2 transduced NSPC grafts showed neuronal phenotypes, enhanced myelination, white matter sparing, and axonal sprouting |
| NSPC | Adult mouse SVZ derived NSPC 62 | T7 clip compression; Wistar rats; subacute (14 days) and chronic (56 days); intraspinal rostral and caudal injections; growth factors (EGF, bFGF, PDGF‐AA) infused intrathecally at time of transplant for 1 wk; minocycline for 10 days (starting 3 days prior to transplantation); daily cyclosporine immunosuppression | Subacutely transplanted NSPC promoted recovery from 3 wk post‐transplant on BBB; fewer footfalls on gridwalk; no improvement in chronic group | NSPC‐derived oligodendrocytes produced MPB when transplanted subacutely; low survival in chronic transplants |
| NSPC | Adult mouse SVZ derived NSPC 63 | T7 clip compression; Wistar rats; chronic (7 wk); intraspinal rostral and caudal injections; ChABC infused intrathecally 1 wk prior to transplant; growth factors (EGF, bFGF, PDGF‐AA) infused intrathecally at time of transplant for 1 wk; minocycline for 10 days; daily cyclosporine immunosuppression | Improved BBB score and fewer footfall errors on grid walk with combination treatment; grafts did not cause allodynia | ChABC infusion reduced CSPG and improved NSPC graft survival; NSPC primarily differentiated into oligodendrocytes; combination enhanced axonal plasticity |
| NSPC | Human fetal NSPC (hCNS‐SC) 64 | T9 contusion (Infinite Horizon); NOD‐SCID mice; subacute (9 days) | Improvement in BBB and horizontal ladder beam task in NSPC group; effects lost when diphtheria toxin was used to kill the grafted cells | Neuronal differentiation of grafted cells; wrapping of spared axons |
| NSPC | Human fetal NSPC 65 | C5 contusion (modified NYU impactor); common marmosets; subacute (9 days); epicenter injection; cyclosporine (10 mg/kg/day) | NSPC transplants improved bar grip power and spontaneous motor activity | Axonal bundles in NSPC grafts filling lesion; MRI shows smaller lesions in NSPC transplanted group |
| iPSC‐derived NSPC | Human iPSC‐derived NSPC 66 | T10 contusion (Infinite Horizon); NOD‐SCID mice; subacute (9 days); epicenter injections | Improvement in BMS score and rotarod test | Grafted cells expressed neurotrophic factors; stimulation of angiogenesis and axonal growth; increased myelination; synapse formation between graft‐derived neurons and host neurons |
| iPSC‐derived NSPC | Human iPSC‐derived NSPC 67 | T9‐10 contusion (Infinite Horizon); NOD‐SCID mice; subacute (7 days); epicenter injections | Improvement in BMS at 2 wk post‐transplantation and motor‐evoked potentials | Sparing of endogenous neurons; synapse formation between graft‐derived neurons and host neurons |
| ESC‐derived OPC | Mouse ESC‐derived NSPCs 68 | T9‐10 contusion (NYU); Long Evans rats; subacute (9 days); intraspinal into lesion site; cyclosporine (10 mg/kg/day s.c.) | Improvement in BBB at 5 wk post‐transplantation | Grafted cells differentiated into neuronal and glial phenotypes |
| ESC‐derived OPC | Human ESC‐derived OPC 69 | T8‐11 contusion (Infinite Horizon); Sprague Dawley rats; subacute (7 days) and chronic (10 mo); intraspinal rostral and caudal injections; cyclosporine (10 mg/kg/day s.c.) | Subacutely transplanted hESC‐derived OPC promoted recovery from 3 wk post‐SCI on BBB and certain gait parameters; no improvement in chronic groups | Subacute transplants increased oligodendrocyte remyelination and decreased the density of demyelinated axons; no change in chronic groups |
| ESC‐derived OPC | Human ESC‐derived OPC 70 | C5 contusion (Infinite Horizon); Sprague Dawley rats; subacute (7 days); intraspinal rostral and caudal injections; cyclosporine (20 mg/kg/day s.c.) | Improved specific gait parameters of forelimb motor function | Tissue sparing; preservation of motor neurons |
| Schwann Cells | Adult human Schwann cells; peripheral nerve 71 | 4‐5 mm segment of cord removed at T8; athymic nude rats; Schwann cells implanted acutely in PAN/PVC channels; in combination with methylprednisolone (30 mg/kg, i.v to all animals at 5 min, 2 and 4 hours) | Rats implanted with bridging Schwann cell grafts in PAN/PVC channels showed higher scores on BBB and inclined plane at 6 wk post‐SCI | Schwann cell grafts without channels showed more myelinated fibers than grafts in channel; 5‐HT+, CGRP+ axons were present within the grafts but did not exit grafts |
| Schwann Cells and OEC | Adult rat Schwann cells and OEC from nerve fiber layer 72 | T9 contusion (NYU impactor); Fischer rats; subacute (7 days); intraspinal injection into lesion of Schwann cells, OEC, or Schwann cell + OEC grafts | Improved BBB score in Schwann cell group only | More myelinated axons in Schwann cell grafts compared to OEC or OEC + Schwann cell; less cavitation and more sparing in all grafted groups |
| Schwann Cells and OEC | Adult rat Schwann cells and OEC from olfactory bulb 73 | T9 contusion (NYU/MASCIS); Fischer rats; chronic (8 wk); intraspinal injections of Schwann cell or OEC grafts | Schwann cell but not OEC grafts improved BBB score and base of support and hindpaw rotation in footprint analysis | Schwann cells survived better than OEC and Schwann cell grafts contained more sensory axons but not CST ingrowth |
| Schwann Cells and OEC | Adult rat Schwann cells and OEC from olfactory bulb 74 | T9 contusion (NYU/MASCIS); Fischer rats; subacute (7 days); intraspinal injections of Schwann cells, OEC, or Schwann cell + OEC grafts | Improved BBB score only with Schwann cell + OEC grafts but no improvement in gait parameters | More myelinated axons found within regions of grafted Schwann cells but not OEC; both grafts increased host Schwann cell infiltration but no sensory or supraspinal axon ingrowth; OEC grafts survived poorly |
| OEC | Adult rat OEC from olfactory bulb 75 | Cervical CST hemisection; acute; intraspinal transplant into lesion site | Rats in which OEC grafts formed continuous bridge across lesion were able to use affected forepaw for directed reaching | OEC grafts promoted growth of lesioned axons |
| OEC | Adult rat OEC 76 | T8/T9 complete transection; acute; intraspinal transplants into cord stumps | Improved locomotor function and sensorimotor reflexes in climbing test | Regeneration of motor axons caudally in OEC grafts |
TABLE 2.
Key completed clinical trials of cell therapies for spinal cord injury
| Cell type | Sponsor; country | Phase; Clinicaltrials.gov identifier | # Participants; age | Injury level; severity; transplant interval after SCI | Route of cell delivery | Completion date |
|---|---|---|---|---|---|---|
| Autologous BMSC | Puerta de Hierro University Hospital, Spain | Phase II; NCT02570932 | 10; 18‐70 yr | ASIA A‐D; more than 6 mo | Intrathecal; 3 injections 3 mo apart | Dec 2017 |
| Autologous BMSC | Indian Spinal Injuries Centre, India | Phase I/II; NCT02260713 | 21; 18‐50 yr | T1‐T12; ASIA A; 10‐14 days | Intrathecal (single injection) or intraspinal | Nov 2017 77 |
| Autologous BMSC | Hospital Sao Rafael, Brazil | Phase I; NCT01325103 | 14; 18‐50 yr | Thoracic and lumbar; ASIA A; more than 6 mo | Intraspinal | Dec 2012 78 |
| Autologous BMSC | International Stemcell Services Limited, India | Phase I; NCT01186679 | 12; 20‐55 yr | C4‐T12; ASIA A‐C; acute within 2 wk, subacute 2‐8 wk, chronic more than 6 mo | Intrathecal for acute and subacute; intraspinal for chronic | Aug 2010 |
| Autologous BMSC | Cairo University, Egypt | Phase I/II; NCT00816803 | 80; 10‐36 yr | C3‐T12; ASIA A‐B; 10 mo to 3 yr | Intrathecal | Dec 2008 79 |
| Autologous MSC | Hospital Sao Rafael, Brazil | Phase I; NCT02152657 | 5; 18‐65 yr | T8 and below; ASIA A; more than 6 mo | Percutaneous injection | Dec 2016 |
| Autologous Adipose‐derived MSC | Biostar, Korea University Anam Hospital, Korea | Phase I/II; NCT01769872 | 15; 19‐70 yr | ASIA A‐C; more than 3 mo | Intravenous, intrathecal, and intraspinal; each single injections | Jan 2016 80 |
| Autologous Adipose‐derived MSC | Biostar, Anyang Sam Hospital, Korea | Phase I; NCT01274975 | 8; 19‐60 yr | ASIA A‐C; more than 2 mo | Intravenous, single injection | Feb 2010 81 |
| Autologous BMSC vs Adipose‐derived MSC | University of Jordan, Jordan | Phase I/II; NCT02981576 | 14; 18‐70 yr | AISA A‐C; more than 2 wk | Intrathecal; total of three injections | Jan 2019 |
| Autologous BM‐MNC | Armed Forces Bone Marrow Transplant Center, Pakistan | Phase I; NCT02482194 | 9; 18‐50 yr | Thoracic; ASIA A; more than 2 wk | Intrathecal | Mar 2016 82 |
| Autologous BM‐MNC | Neurogen Brain and Spine Institute, India | Phase I; NCT02027246 | 166; 8 mo to 63 yr | Any SCI | Intrathecal | Feb 2013 |
| Autologous BM‐MNC | China Spinal Cord Injury Network, China | Phase I/II; NCT01354483 | 20; 18‐60 yr | C5‐T11; ASIA A; more than 1 yr | Intraspinal; dose escalation | Dec 2013 |
| Human Central Nervous System Stem Cells (HuCNS‐SC) | StemCells, Inc, Canada and Switzerland | Phase I/II; NCT01321333 | 12; 18‐60 yr | T2‐T11; ASIA A‐C; 3‐12 mo | Intraspinal | Apr 2015 83 |
| Human Central Nervous System Stem Cells (HuCNS‐SC) | StemCells, Inc, Canada and United States | Phase II; NCT02163876; terminated (based on a business decision unrelated to any safety concerns) | 31; 18‐60 yr | C5‐C7; ASIA B‐C; more than 12 wk | Intraspinal | May 2016 |
| ESC‐derived OPC (GRNOPC1) | Asterias Biotherapeutics, Inc, United States | Phase 1; NCT01217008 | 5; 18‐65 yr | T3‐T11; ASIA A; 1‐2 wk | Intraspinal | July 2013 |
| ESC‐derived OPC (AST‐OPC1) | Asterias Biotherapeutics, Inc, United States | Phase I/IIa; NCT02302157; | 25; 18‐69 yr | C4‐7; ASIA A‐B; 21‐42 days | Intraspinal; dose escalation study | Dec 2018 |
| Autologous Human Schwann Cells (ahSC) | The Miami Project to Cure Paralysis, University of Miami, United States | Phase I; NCT01739023 | 9; 18‐60 yr | T3‐T11; ISNCSCI grade A; 30‐72 days | Intraspinal | Aug 2016 50 |
| Autologous Human Schwann Cells (ahSC) | The Miami Project to Cure Paralysis, University of Miami, United States | Phase I; NCT02354625; recruiting | 8; 18‐65 yr | C5‐T12; ASIA A‐C; more than 12 mo | Intraspinal | Aug 2019 |
Note: Clinical trials that are completed are identified with the NCT number listed on www.ClinicalTrials.gov. Published results of clinical trials, if available, are referenced.
Abbreviations: BM‐MNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow‐derived mesenchymal stem cells; ESC, embryonic stem cell; ISNCSCI, International Standards for Neurological Classification of Spinal Cord Injury; MSC, mesenchymal stem cells; NSPC, neural stem/progenitor cells; OPC, oligodendrocyte precursor cells.
TABLE 3.
Key ongoing clinical trials of cell therapies for spinal cord injury
| Cell type | Sponsor; country | Phase; Clinicaltrials.gov identifier; study status | Estimated enrollment; age | Injury level; severity; transplant interval after SCI | Route of cell delivery | Estimated completion date |
|---|---|---|---|---|---|---|
| Autologous MSC | Hospital Sao Rafael, Brazil | Phase I; NCT02574572; recruiting | 10; 18‐65 yr | C5‐C7; ASIA A; more than 12 mo | Intraspinal | Jun 2020 |
| Autologous MSC | Hospital Sao Rafael, Brazil | Phase II; NCT02574585; not yet recruiting | 40; 18‐65 yr | T1‐L2; ASIA A; more than 12 mo | Percutaneous; 2 injections 3 mo apart | Jan 2022 |
| Autologous MSC | Pharmicell Co., Ltd., Seoul, Korea | Phase II/III; NCT01676441; active, not recruiting | 32; 16‐65 yr | Cervical; ASIA B; more than 12 mo | Intraspinal and intrathecal | Dec 2020 |
| Autologous Adipose‐derived MSC | Allan Dietz, Mayo Clinic, United States | Phase I; NCT03308565; recruiting | 10; 18 yr and older | AISA A‐B; 2 wk to 1 yr | Intrathecal; single injection | Nov 2023 |
| Autologous BM‐MNC | Da Nang Hospital, Vietnam | Phase I/II; NCT02923817; recruiting | 30; 20‐60 yr | ASIA A‐B; 3 wk to 12 mo | Intrathecal | Jun 2019 a |
| Allogeneic UC‐derived MSC | The Third Affiliated Hospital, Sun Yat‐Sen University, Guangdong, China | Phase I/II; NCT03505034; recruiting | 43; 18‐65 yr | ASIA A‐D; more than 12 mo | Intrathecal | Dec 2021 |
| Allogeneic UC‐derived MSC | Limin Rong, Third Affiliated Hospital, Sun Yat‐Sen University, Guangdong, China | Phase I/II; NCT02481440; recruiting | 44; 18‐65 yr | ASIA A‐D; more than 2 wk | Intrathecal; monthly injections for 4 mo | Dec 2018 a |
| Allogeneic UC‐derived MSC | The Third Affiliated Hospital, Sun Yat‐Sen University, Guangdong, China | Phase II; NCT03521323; recruiting | 92; 18‐65 yr | ASIA A‐D; 2‐12 mo | Intrathecal; monthly injections for 4 mo | Dec 2022 |
| Allogeneic UC‐derived MSC | The Third Affiliated Hospital, Sun Yat‐Sen University, Guangdong, China | Phase II; NCT03521336; recruiting | 130; 18‐65 yr | ASIA A‐D; subacute (2 wk to 2 mo), early chronic (2‐12 mo), chronic (more than 12 mo) | Intrathecal; monthly injections for 4 mo | Dec 2022 |
| Allogeneic WJ‐derived MSC | Banc de Sang i Teixits, Barcelona, Spain | Phase I/II; NCT03003364; active, not recruiting | 10; 18‐65 yr | T2‐T11; ASIA A; 1‐5 yr | Intrathecal | Apr 2020 |
| Human Spinal Cord‐derived NSC | Neuralstem Inc, United States | Phase I; NCT01772810; recruiting | 8; 18‐65 yr | T2‐T12 or C5‐C7; ASIA A; 1‐2 yr | Intraspinal | Dec 2022 |
| Autologous OEC | Wroclaw Medical University, Poland | Phase I; NCT01231893; unknown status | 10; 16‐65 yr | C5‐L5; ASIA A; Interval N/A | Intraspinal | N/A a |
Note: Clinical trials currently recruiting or ongoing are identified with the NCT number listed on www.ClinicalTrials.gov.
Abbreviations: BM‐MNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow‐derived mesenchymal stem cells; ESC, embryonic stem cell; MSC, mesenchymal stem cells; NSPC, neural stem/progenitor cells; OEC, olfactory ensheathing cells; OPC, oligodendrocyte precursor cells; UC‐derived MSC, umbilical cord‐derived mesenchymal stem cells; WJ‐derived MSC, Wharton's jelly‐derived mesenchymal stem cells.
Status unknown or not updated on clinicaltrials.gov.
3.1. Cell source
MSCs, SCs, and OECs can all be harvested from an adult allogeneic source to generate standardized stocks depending on the success of proliferation. MSCs, SCs, and OECs can also be derived directly from the patient to avoid post‐transplant immunosuppression. 84 However, autologous primary cells are typically more costly requiring harvest surgery, in vitro expansion and extensive characterization prior to transplant.
CNS cells, such as NSCs, OPCs, astrocytes and microglia, are more challenging to isolate from adult allogeneic donors, and the performance of a line is influenced by donor age, genetics, and harvest conditions. 85 , 86 , 87 Furthermore, autologous CNS tissue is inaccessible. As a result, these cells are often derived from embryonic stem cell (ESC) sources. 88 , 89 ESCs can be propagated indefinitely and can generate cells of any germ layer. However, ESC‐derived grafts have ethical issues surrounding their use and may show karyotypic instability or hold the potential for tumorigenesis due to incomplete or aberrant differentiation. More recently, induced pluripotent stem cells (iPSCs) have allowed derivation of NSCs and OPCs from autologous, accessible cells such as bone marrow and skin fibroblasts. This has been further adapted to allow direct reprogramming of adult somatic cells to multipotent neuroglial cells while bypassing the pluripotent state. 7 In more recent protocols, it has also become possible to convert easily accessible somatic cells directly into neurons, 90 , 91 neuronal subtypes, 92 , 93 and oligodendrocytes progenitors. 94 Some limitations associated with these approaches such as reprogramming efficiency, line variability, lineage‐specific differentiation, and retention of epigenetic memory are being investigated.
3.2. Neural stem cells
NSCs are tripotent, self‐renewing cells which have attracted great interest as they can potentially replace the neurons, oligodendrocytes, and astrocytes lost after injury. 88 , 95 , 96 , 97 During embryological development, NSCs are found throughout the neural tube where they acquire unique identities based on their position and temporal exposure to patterning morphogens. 98 , 99 , 100 , 101 In adults, they are found in a more limited number of regions such as the subventricular zone in the brain 95 , 96 , 97 and around the central canal in the spinal cord. 102 , 103 , 104 , 105 There are two distinct NSC populations that can be isolated from the adult spinal cord: (a) primitive NSCs (pNSCs) and (b) the definitive NSCs (dNSCs) they give rise to (Figure 2). 106 , 107 pNSCs are rare cells expressing pluripotency marker, Oct4, and are responsive to leukemia inhibitory factor in vitro. 108 , 109 , 110 dNSCs are more abundant in adults, express astrocyte marker, GFAP, and respond to epidermal and fibroblast growth factors (EGF and FGF) in vitro. Both populations can proliferate and generate neurons, astrocytes and oligodendrocytes.
FIGURE 2.
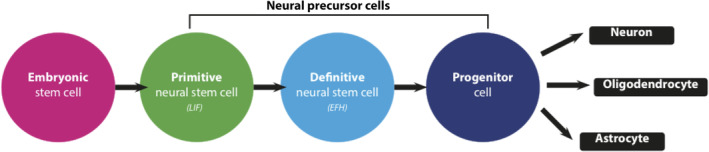
A simplified schematic representation of a proposed endogenous neural stem cell (NCS) lineage. Within the central nervous system, the proposed lineage suggests two types of NSCs are present. Primitive NSCs (pNSCs) are a population of rare, leukemia inhibitory factor (LIF) responsive cells that give rise to more abundant definitive NSCs (dNSCs). dNSCs are responsive to EGF and FGF2 (EFH). NSC progeny (progenitor cells) give rise to neurons, astrocytes, and oligodendrocytes upon differentiation. This pathway is exploited for ESC‐ and iPSC‐based generation of NSCs, neurons and glia. Direct reprogramming allows somatic cells to enter the NSC or later stage without passing through the pluripotent state
Numerous small and large animal preclinical studies have provided evidence supporting the therapeutic efficacy of transplanting NSCs derived from ESCs or allogeneic adult sources. 60 , 62 , 84 More recently, autologous iPSC‐derived and directly reprogrammed NSCs have emerged as translationally relevant options which mitigate immunorejection concerns and generate highly pure populations of cells over several weeks. A 2016 meta‐analysis (N = 74 studies) found all sources of NSCs to provide significant motor recovery in models of SCI (pooled SMD = 1.45; 95% confidence interval [CI]: 1.23‐1.67; P < .001). 111 Although the mechanisms underlying recovery continue to be investigated in preclinical studies, it is likely that trophic signaling (eg, BDNF, GDNF, IGF‐1, etc.), remyelination of denuded axons, partial regeneration and remodeling of neural circuitry, and ECM deposition play a role. 7 , 84 , 112 Recent work demonstrating the self‐assembly of grafted spinal cord NSCs into organotypic, dorsal horn‐like domains highlights their plasticity and the potential for further optimization as a therapy. 113
Currently, a phase I/II study in China (N = 30; NCT02688049) is comparing 1 × 106 MSCs vs NSCs supported by a linearly ordered collagen biomaterial, NeuroRegen scaffold, in individuals with chronic AIS Grade A C5‐T12 injuries. The study is expected to conclude in 2020 and report on AIS grade, somatosensory evoked potentials, motor evoked potentials, Functional Independence Measure, and MRI assessments. Neuralstem is also conducting a trial (N = 8; NCT01772810) of NSI‐566, human fetal spinal cord NSCs, delivered through six intraspinal injections into patients with 1 to 2 year old thoracic injuries. In 2016, a pair of phase II trials led by Stem Cells Inc were terminated prior to completion. The studies were assessing the effects of human CNS stem cell transplants for thoracic (NCT01321333) and cervical (NCT02153876) SCI. 114 A recently published report on the studies found that escalating doses up to 4.0 × 106 were well tolerated with no significant increase in serious adverse events related to the cells or free‐hand manual injection technique. 83 Based on emerging preclinical data, it is likely that further modifying the cells in vitro and/or altering the local environment will be necessary to enhance functional recovery.
3.3. Oligodendrocyte progenitor cells
OPCs are tripotent cells which predominantly differentiate into oligodendrocytes to remyelinate axons. They are suited for regeneration in SCI as they respond rapidly to injuries, can self‐renew, and address an important component of the injury pathophysiology. Animal studies have shown that transplanted OPCs can promote white matter preservation, increase the number of surviving endogenous oligodendrocytes, and reduce cavity volume, resulting in enhanced motor recovery. The underlying mechanism may be a combination of remyelination, local immunomodulation, trophic factor secretion, and provision of a physical scaffold to support growing axons. 69 , 70 , 115 , 116 The cells also possess a favorable secretome, 117 consisting of growth factors, neurotrophins, chemokines and cytokines, and can form glutamatergic synapses with neurons, an area of ongoing discovery. 118 It remains unclear whether improved outcomes are due to these factors or direct remyelination of denuded axons, and to what extent remyelination can itself enhance function after injury. 119 , 120
A large clinical trial by Geron Inc assessing human ESC‐derived OPCs was discontinued for financial reasons. Renewed funding allowed Asterias Biotherapeutics Inc to extend the trial of these cells, termed AST‐OPC1, in a phase I/IIa open‐label dose‐escalation study (N = 25; NCT02302157). 2 × 106, 1 × 107, or 2 × 107 cells were transplanted 21 to 42 days after SCI patients with AIS grade A or B injuries at C4 to C7. The study completed in 2019 and BioTime, which later changed its name to Lineage Cell Therapeutics, acquired the company. Key findings were no increase in significant adverse events and evidence of regeneration on MRI at 12 months. Ninety‐five percent of patients improved by at least 1 AIS grade and 32% of patients improved by at least 2 AIS grades by 12 months after receiving the 1 × 107 and 2 × 107 doses. 114 Longer term follow‐up is continuing and the company plans to further optimize the cell manufacturing process. 121
3.4. Olfactory ensheathing cells
OECs are specialized glial cells that encircle olfactory neurons and clear bacteria and debris at the CNS‐nasal mucosa transition. 122 , 123 , 124 , 125 They also secrete neurotrophic factors 126 and maintain a favorable environment for neuronal function. When the olfactory nerve or epithelium is damaged, OECs support the growth of olfactory epithelium‐derived neurons into the olfactory bulb. They differ from typical glia but share a number of morphological and molecular markers with astrocytes and SCs. After transplantation, OECs form a cellular substrate through which injured axons can regenerate across a spinal cord transection lesion site. 127 OECs can be harvested from the nasal mucosa or directly from the olfactory bulb and transplanted into the cord parenchyma as a bridging, nonrelay approach. 75 OECs have also been shown to enhance neurite outgrowth, promote remyelination, neuroprotect, provide guidance cues, and locally immunomodulate. 49 , 128 , 129
Several clinical trials have transplanted OECs for subacute and chronic SCI. Early work confirmed the safety of a purified population of OECs; however, subsequent studies using mucosal tissue reported conflicting results. 130 , 131 , 132 To clarify the discrepancy, a meta‐analysis of key clinical trials (pooled N = 1193) was conducted which found no statistically significant increase in serious adverse events; however, efficacy has not been definitively established due to technical and methodological concerns with existing studies. 133 Recently, a small phase I trial (N = 6) of autologous mucosal OECs and olfactory fibroblasts demonstrated sensorimotor improvements after transplant into patients with AIS grade A injuries; however, a larger sample size and extended follow‐up will be required to confirm safety and efficacy. 45 , 129 , 131
3.5. Schwann cells
SCs are myelinating cells in the peripheral nervous system (PNS) and are an important component of the robust, spontaneous regeneration observed in the PNS. They provide a structural scaffold acting as a conduit to guide growing axons. They also produce a favorable environment by expressing growth factors and extracellular proteins. In animal models of SCI, they have been shown to promote tissue sparing, reduce cystic cavitation, enhance CNS axon regeneration, remyelinate axons, and enhance endogenous SC myelination resulting in sensorimotor recovery. 134 , 135 , 136 , 137 , 138 The growth‐promoting properties of SCs are also being exploited in combinatorial therapies. For example, neuroprotectant D15A (a modified human NT3 that can activate both TrkB and TrkC receptors) has been combined with phosphodiesterase‐4 inhibitor, rolipram, and SCs to enhance axonal sparing and growth of serotonergic fibers into and beyond the SC graft. 139 SCs have also been combined with NSC, 140 OEC, 74 and BM‐MSC 141 transplants to enhance cell survival and promote additional remyelination.
In humans, The Miami Project to Cure Paralysis conducted a phase I, open‐label study (N = 8; NCT02354625) of autologous SC transplants in individuals with chronic AIS grade A‐C C5‐T12 injuries. The study completed in August 2019 with results pending. The same group conducted an open‐label, nonrandomized, noncontrolled, dose‐escalation phase I study (N = 6) of autologous SCs transplanted into the lesion epicenter for subacute‐intermediate AIS grade A thoracic injuries. SCs were harvested from the sural nerve within 5 to 30 days of injury, expanded in vitro, and transplanted within 4 to 7 weeks of injury. After 1 year, there were no medical, surgical, or neurological complications to indicate that the treatment was unsafe. 50
3.6. Mesenchymal stem cell
MSCs are multipotent, self‐renewing connective tissue progenitor cells found throughout the body, particularly in the perivascular region. They have the capacity to regenerate muscles (myocytes), cartilage (chondrocytes), bone (osteoblasts), and fat (adipocytes). 142 , 143 They also have favored properties for conducting a clinical trial. Multipotent MSCs expand rapidly, 144 remain viable after −80°C or liquid nitrogen cryostorage, 145 demonstrate minimal immunoreactivity after allogenic transplant, 146 and can be harvested from accessible tissue such as fat, bone marrow, and skeletal muscle. 147 This has encouraged their translation in multiple fields such as sepsis, 148 multiple sclerosis, 149 and arthritis. 150 In SCI, MSCs have been shown to promote angiogenesis and significantly enhance tissue sparing through neurotrophic signaling and immunomodulation. 8 , 56 , 151 , 152 There is also evidence that MSCs transplanted directly into the spinal cord can modulate activation of macrophages and promote tissue sparing. 153 , 154 However, MSC transplants have shown considerable variability with some studies showing positive effects whereas other studies have shown no benefit (Table 1).
Multiple adipocyte‐derived MSC clinical trials are ongoing to assess safety, dosing, and efficacy. The Mayo Clinic is conducting a phase I study (N = 10; clinicaltrials.gov identifier NCT03308565) of 1 × 108 autologous, adipose‐derived MSCs delivered intrathecally into patients with American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade A/B/C injuries from 2 weeks to 1 year prior to transplant. The study will be completed by 2023.
Bone marrow‐derived MSCs are also being studied. A phase II/III trial (N = 32; NCT01676441) conducted by Pharmicell Co is assessing 1.6 × 107 intraparenchymal and 3.2 × 107 intrathecal autologous bone marrow‐derived MSCs in chronic cervical AIS grade B patients with 12 month follow‐up of ASIA motor scores, MR Diffusion Tensor Imaging, and electrophysiological parameters. The study is expected to conclude in 2020.
The umbilical cord is an alternate source of MSCs (UC‐MSCs). UC‐MSCs can be isolated from cord tissue, cord blood, or Wharton's jelly, a gelatinous substance within the umbilical cord that insulates the blood vessels. Umbilical cord tissue is readily accessible and frequently discarded, and MSCs from the umbilical cord are less prone to rejection, as evidenced by a lower risk of developing graft vs host disease. 155 Compared with adult sources, the number of MSCs obtained from cord blood or placental tissues is small, although they can be readily expanded and tissue can be frozen and used later for isolation. 156 MSCs derived from the umbilical cord have also been shown to have immunomodulatory properties. 157 Several recently registered phase I/II trials (NCT03505034; NCT02481440; NCT03521323) are recruiting to assess allogeneic UC‐derived MSCs for subacute and chronic SCI. A phase I/II open‐label study (N = 14; NCT02981576) in Jordan directly compared adverse effects and AIS improvement with intrathecal adipose‐ vs bone marrow‐derived MSCs in AIS A/B/C patients 2 or more weeks postinjury. The study completed in January 2019 with results pending.
3.7. Endogenous stem cell therapies
An alternative approach to promote neural repair is to harness the potential of resident stem cells, such as NSCs within the injured CNS. 158 , 159 This approach circumvents immunorejection, cell delivery challenges and logistical hurdles such as good manufacturing practice (GMP) scale‐up, cell storage, and transport. Within the spinal cord, it has been demonstrated that NSCs respond to injury by proliferating 160 and migrating to the lesion, although the effect is likely insufficient to generate recovery. 29 , 159 Recently, small molecules, such as the FDA‐approved drugs cyclosporin A and Metformin, have been found to increase the size of the endogenous pNSC and dNSC pool to augment this intrinsic injury response. 161 , 162 Cyclosporin A is an immunosuppressive medication that has been shown to enhance survival of NSCs and promote recovery within the brain. 161 , 162 Activation of NSCs with cyclosporin A has also been observed in the spinal cord and ongoing investigations aim to elucidate its effect following SCI. 161 , 162 Metformin, a drug commonly used to treat type II diabetes, has also been shown to activate endogenous NSCs and direct their differentiation toward neurons and oligodendrocytes. 110 , 159 Although molecular mechanisms underlying these effects have not been elucidated, metformin administration has been shown to improve functional recovery after insult to the brain. 110 , 159 Studies of metformin in SCI animal models are ongoing. Additional targets being explored to enhance the endogenous NSC response are the C‐Kit and ErbB2 signaling pathways. 163
4. ENHANCING CELL TRANSPLANTATION THERAPIES
Transplanted cell survival has historically been low in animal models of SCI. 164 This is typically overcome by delivering excess numbers of cells to compensate for losses; however, this approach introduces variability into the therapy, contributes cytotoxic by‐products of cell death to the microenvironment, and becomes infeasible when large numbers of surviving cells are required. Ongoing work seeks to enhance cell survival, migration, and axonal outgrowth by overcoming key barriers in the SCI microenvironment.
4.1. Growth factors
To support graft survival, growth factors (eg, PDGF, EGF, and IGF‐1), neurotrophins (eg, BDNF, NT3, NGF), and anti‐inflammatory agents (eg, minocycline) have all been successfully delivered via intrathecal injections and pumps. 63 , 164 , 165 , 166 Unique biomaterials have also been engineered to gradually deliver key factors to support grafts. 167 Growth factors and treatments such as ferritin are also associated with enhanced endogenous OPC proliferation and oligodendrogenesis. However, pumps are prone to failure, require refilling and explant procedures, and expose growth factors to mammalian body temperatures for prolonged periods. As a result, alternate approaches continue to be investigated such as the codelivery of cells with biomaterials capable of slowly releasing growth factors directly into the environment. This unique strategy is discussed further in the Biomaterials section below. Another approach is the in vitro genetic modification of cells or the in vivo transfection of endogenous cells to secrete the necessary factors. For example, MSCs have been successfully engineered to express bFGF, 168 HGF, 169 NT3, 170 BDNF, 171 and GDNF 172 in vivo for various applications. SCs have also been transduced to overexpress BDNF and NT3 simultaneously. 173 Similarly, safe and highly efficient methods of engineering human iPSCs, ESCs, and NSCs are currently being developed.
4.2. Rehabilitation
An often overlooked method of promoting endogenous trophic factor release and long‐term cell survival noninvasively is rehabilitation. Physical rehabilitation, with or without electroceutical augmentation, is an integral component of the care plan for individuals with SCI; however, it is underrepresented in preclinical trials. Whether the rehabilitation entails forced treadmill training, free swimming, or task‐specific tests such as forelimb reaching, the functional benefits can be significant. 174 In addition to enhancing cardiorespiratory and musculoskeletal function, treadmill locomotor training has been found to enhance transplanted NSC survival by more than fivefold through increased IGF‐1 signaling. 175 This finding underscores the value of multimodality, interdisciplinary care in SCI.
4.3. Biomaterials
Biomaterials can enhance cell‐based approaches for SCI in several ways. Scaffolds derived from either natural or synthetic polymers have been implanted within the lesion cavity to bridge the gap to serve as a substrate for axonal growth and cell migration. 176 , 177 , 178 , 179 , 180 InVivo Therapeutics' Neuro‐Spinal Scaffold is a porous bioresorbable polymer scaffold shown to promote appositional healing, white matter sparing, and normalization of intraparenchymal tissue pressure in preclinical models of SCI. 181 A recent case study at 6 month follow‐up from a patient enrolled in the clinical trial (NCT02138110) reported no adverse effects related to acute scaffold implantation. 182
Scaffolds can also provide a physical substrate for seeded cells and provide directional guidance for axons. Moreover, biomaterials can also be used as vehicles to deliver cells and release growth factors to aid in graft cell survival, integration, and differentiation. Injectable in situ polymerizing hydrogels can deliver cells and factors directly into a lesion site with less invasive surgical interventions. For example, a polymer blend of hyaluronan/methylcellulose (HAMC) is injectable, in situ gelling, biodegradable, and noncytotoxic. 183 HAMC modified with PDGF‐AA, to enhance graft survival and oligodendrocyte differentiation of cotransplanted rat brain‐derived NSCs, 184 , 185 promoted host oligodendrocyte sparing and improved fine motor function. 186 Further modification of the HAMC hydrogel with RGD peptide promoted the survival, integration, and differentiation of human iPSC‐derived OPCs. 187
Another approach has been the use of fibrin scaffolds which have been shown to promote the survival of transplanted stem cells after SCI and, when codelivered with growth factors, have been used to direct differentiation and enhance recovery. 188 , 189 QL6 is an exciting peptide biomaterial which self‐assembles to form a lattice‐like structure at physiological temperatures. QL6 injected with NSCs improved graft survival, reduced glial scarring and inflammation, and improved forelimb function in cervical models of SCI. 190 , 191 , 192
4.4. Galvanotaxis
Electrical fields (EFs) are a physical environmental cue present within living tissue. During development, multipotent cells rely on these fields for appropriate migration and differentiation. If the fields are disrupted, severe defects can result. 193 EFs have also been shown to guide cells in adults after injury. 194 , 195 Therapeutic galvanotaxis (the directed migration of cells in an electric field) exploits the electrosensitivity of cells to promote migration using externally applied EFs. 196 This has been shown to be feasible with SCs, 197 NSCs, 198 and many other cell types. 199 Both endogenous and transplanted NSCs, but not their differentiated progeny, have been shown to migrate with transcranial direct current electrical stimulation; however, directed migration will require further optimization to establish the ideal current, voltage, phase, lead placement, and timing of EF application. 200 , 201
4.5. Disrupting the glial scar
The glial/CSPG scar is well established in chronic injury and limits axon regeneration through the lesional/perilesional region (Figure 1C). A meta‐analysis of NSC treatments found motor function recovered to a greater extent with cell delivery in the acute phase (SMD = 1.80; 95% CI: 1.36‐2.24) or subacute phase (SMD = 1.38; 95% CI: 1.08‐1.67) than in the chronic phase (SMD = 1.04; 95% CI: 0.47‐1.60; P = .03) of injury 111 highlighting the difficulty in regenerating the chronically injured spinal cord.
As a result, numerous labs have focused on methods to disrupt the glial/CSPG scar during or prior to cell transplant. Chondroitinase ABC (ChABC) is a bacterial enzyme which rapidly degrades the long glycosaminoglycan (GAG) side chains of CSPGs 202 In SCI, intrathecal or intraparenchymal ChABC treatments have been shown to promote neurite outgrowth and enhance anatomic plasticity, by degrading CSPGs within the perineuronal nets, resulting in sensorimotor behavioral recovery. 203 , 204 , 205 This exciting approach is under further development to address key limitations and better combine with cell transplants. First, thermostabilized variants of ChABC are being developed to retain activity for longer periods at mammalian body temperatures. 206 Second, ChABC is being delivered via novel vehicles such as affinity‐release biomaterials or lentivirus transfections of host cells. 207 , 208 Finally, alternate human enzymes with GAG or CSPG core protein degrading activity are being studied to mitigate immunogenicity risks associated with using a bacterial protein. 209
Another exciting approach is to inhibit the association of the protein tyrosine phosphatase σ receptor with its CSPG ligand. 210 An example is Intracellular Sigma Peptide (ISP) which is administered subcutaneously after injury, crosses the BSCB, and results in significant axonal regrowth within the injured cord. 34 Recently, ISP has also been shown to indirectly immunomodulate when combined with leukocyte antigen‐related receptor blockade. 211
5. TRANSLATING STEM CELL THERAPIES
5.1. Graft survival
A major challenge in translating cell therapies to clinic is assessing and optimizing graft survival. Culture and storage conditions, characterization pipelines, and transplant conditions can be significantly different in the laboratory than in clinical trial or routine use. 212 Furthermore, commonly employed graft assessment techniques such as immunohistochemistry and bioluminescent tagging are typically not possible in humans. Strategies discussed previously such as increased trophic support, timed rehabilitation, and codelivering biomaterials may prove to be important adjuvants in advancing cell therapy for humans. These are in early deployment such as Neuro‐Spinal Scaffold (NCT02138110) by InVivo Therapeutics Inc or the phase I/II study of MSCs and NSCs using the NeuroRegen scaffold (NCT02688049). Assessing grafted cells in vivo may also become viable as novel MRI‐based cell trackers are developed. 213 , 214
5.2. Immunorejection
Another important translational hurdle is understanding and overcoming potential immunorejection within the CNS. The extent and temporality of cell graft rejection within the human CNS is currently unknown. Additionally, the cell source (eg, autologous, allogenic, genetically modified, etc.) may significantly affect downstream graft survival in humans. 215 , 216 Enhancing endogenous cell proliferation is one strategy to avoid immunorejection; however, endogenous cell pools may still be limited and optimal methods to drive their differentiation and migration have not yet been established. 217 Recently, genetic techniques which modify major histocompatibility complexes and CD47 have been shown to generate immune‐evasive iPSC lines. 218 This may become an important strategy in the future to protect grafts from immune cells.
5.3. GMP‐grade scale‐up
Effective translation of cell therapies into clinical trial and beyond requires careful planning of GMP facilities and scale‐up strategies. Many challenges of GMP‐grade production are not typically encountered in a research setting and are associated with significant financial costs. For example, all manual handling must be highly reproducible between facilities and occur in stringent GMP‐grade clean rooms. Standard operating procedures must be established and compliance must be traceable throughout the production chain. 212 Cultures are typically free of animal products (eg, proteins, feeder cells, etc.), unless more suitable options are not available, and all reagents require qualification as meeting the standards for GMP‐grade culture. 212 Common viral transduction and nonviral transfection (eg, electroporation, lipofection) techniques require additional steps to validate that risks inherent to viral particles or foreign DNA have been mitigated. Furthermore, master and working cell banks must be established with extensive testing for bacteria, viruses, fungi, mycoplasma, and endotoxins at each step of preparation. It is also important to note that during this translational process, the research cell line with which preclinical efficacy was validated, may not be the same as the final clinical cell line for trial which may necessitate additional efficacy testing in animal models. 219
The importance of cell line and subline testing is apparent when addressing the potential for tumor formation as graft‐derived tumors can occur in rigorously tested and banked lines due to the multiple potential etiologies of tumorigenicity. 220 , 221 As a living therapy, even small variations in transport, subculture, or transplant technique at individual centers hold the potential to alter the phenotype of the graft. Furthermore, lines are typically characterized by sampling a portion of the larger population; however, tumor formation can occur due to a single aberrant cell. Advanced technologies, such as environment‐controlled cell culture robots and high‐throughput total population screening techniques, seek to address these challenges. 222 , 223
Fortunately, the US FDA and European Medicines Agency have provided regulatory frameworks to begin approaching the complexities of GMP cell manufacturing. 224 , 225 Additionally, networks of GMP‐grade facilities, such as the CellCAN Regenerative Medicine and Cell Therapy Network in Canada, have been established to aid in navigating these challenges. 226
5.4. Storage and transport
Conducting clinical trials or treatments across multiple sites requires a coordinated storage and transportation approach capable of accommodating international shipping delays and unexpected package handling conditions. For example, a study of human MSCs found that cells stored at 2°C to 8°C were sensitive to 25 Hz vibrations, 227 leading to cell death and increases in MSC marker expression such as CD29 and CD44. 228 Similarly, temperature and repeated freeze‐thaw cycles have a significant effect on cell viability. Cryoprotectant toxicity, rapid osmotic shifts, ice crystal formation, and activation of apoptosis are key mechanisms underlying cell death during the process. 229 Early studies achieved human cryopreserved pluripotent cell survival rates of only 30% or less. 230 As a result, other cold storage techniques have been developed such as vitrification, the rapid cooling of cells in high concentrations of cryoprotectants to inhibit ice formation. These were found to provide >75% survival but add technical complexity and may be limiting in the production of large‐scale banks. 229 Furthermore, the storage solution (eg, DMSO, polyethylene glycol, etc.) and additives (eg, ROCK‐inhibitor, trehalose, poly‐L‐lysine, etc.) exert their own effects on the survival and differentiation of stored cells. 229 Therefore, each cell type and cell line requires optimization to establish ideal conditions for a functional and reliable supply chain.
5.5. Patient selection
Establishing the ideal patient population for cell transplant in SCI is challenging. Given the currently high cost and limited availability of GMP‐grade cell therapies, one strategy is to focus on populations with the greatest potential gains. Cervical cord injuries are the most common and can result in devastating impairments in activities of daily living (eg, feeding, grooming, transferring) and are often associated with respiratory and autonomic complications. 47 Even modest improvements in key muscle groups such as grip and elbow flexion can have profound benefits for quality of life making this an important population for inclusion in cell‐based studies. It is, however, important to balance these inclusion benefits with potential risks. The cervical SCI population can be more expensive to study in trial as hospital stay, treatment, and rehabilitation costs are higher. 2 Additionally, limiting studies to highly specific study regions can make recruitment more challenging.
Another key consideration is transplant timing. Due to differences in physiology, injury etiology, cord architecture, patient comorbidities, and a host of microenvironmental cell signals, the optimal timing for transplant in animal models likely differs from humans. 15 Furthermore, even with established infrastructure, there is often a lead time associated with delivering banked cells (eg, allogeneic therapy) or generating a cell line (eg, autologous therapy). 231 Therefore, many trials are now conducted in the intermediate to chronic phases of injury where the patients' condition and neurologic status are better established and study recruitment is less complex. However, overcoming regenerative barriers in chronic SCI such as the glial scar and cystic cavitation may require some of the innovative strategies discussed previously.
5.6. Delivery techniques
Adapting delivery techniques is a key facet of translating cell therapies. Systemic or intrathecal treatments avoid many challenges by using existing, well‐established medical techniques; however, the distribution of cells is poorly controlled. For intraparenchymal treatments (Figure 3), trials are increasingly utilizing standardized, tightly controlled delivery systems which must be scaled for human doses, provide high reliability, allow sterilization, and have undergone regulatory approval as a medical device. 232
FIGURE 3.
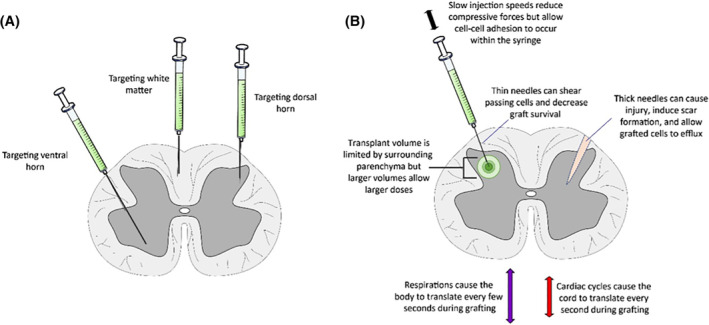
Potential considerations during intraparenchymal transplant of stem cells into the spinal cord. These considerations apply to perilesional parenchymal transplants; however, other considerations apply when transplanting directly into lesion or cavity sites where parenchymal volume and cord architecture are already lost. A, Stem cell grafts can be delivered by fine needles or catheters to the gray matter (ventral horn, dorsal horn, etc.) or white matter (dorsal tracts, lateral tracts, ventral tracts, etc.). The spinal cord is most commonly approached dorsally, however, dorsolateral and ventral techniques are also possible depending on the surgical approach. B, Multiple factors affect graft delivery. Higher syringe injection speeds lead to compressive forces on the graft, however, lower speeds increase operative time and allow cell‐cell adhesion to occur which can clog the needle or cause membrane disruption. Thin needles can causes greater shearing forces on cells as they exit the tip, whereas thick needles cause greater parenchymal damage and potentially allow a wider needle tract for graft efflux. Larger transplant volumes allow larger doses of cells, however, volumes are limited by surrounding tissue. Respiratory and cardiac cycles typically continue during grafting, which can potentially cause microtrauma and cell efflux around the transplant needle
The two main classes of injectors currently in use are table‐mounted and spine‐mounted systems. Table‐mounted injectors 233 , 234 provide a high degree of injection cannula stability along all three axes but do not account for cord movement due to ventilation, cardiovascular pulsations, or other patient movement. Spine‐mounted devices are typically immobilized on pedicle screws or by clamping the posterior vertebral arch to account for respiration but may not account for all cord pulsations. Recently, a floating cannula system has also been developed which compensates for these natural pulsations to further improve targeting. 235 A third class of devices is also under development which utilizes tools mounted on surgical robots, such as the da Vinci Surgical System (Intuitive Surgical Inc), for highly precise localization.
6. OUTLOOK
The multifaceted pathophysiology of SCI and the complexities of neural repair and regeneration necessitate novel approaches to treatment. Cell‐based therapies continue to be very attractive and hopeful strategies for repair of SCI and the rapid pace of innovation continues to increase as our understanding of fundamental cell biology deepens. We predict that as the timing, dose, and delivery of adult‐ and pluripotent stem cell‐derived treatments are optimized, increasing numbers of cell‐based therapies will be translated to humans. It is highly likely that successful approaches will integrate strategies to enhance and support cells, such as genetic engineering, biomaterials, galvanotaxis, and scar degradation to maximize clinical outcomes. Ongoing preclinical and clinical trials highlight the excitement and tremendous progress that has been made in the field and underscore the importance of the collaborative work being conducted by researchers, clinicians, stakeholders, and funding agencies worldwide.
CONFLICT OF INTEREST
Michael G. Fehlings declared advisory role with Fortuna Fix. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
C.S.A., A.M.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.K., J.H.B., E.A.G., D.V.D.K., C.M.M., C.T.: manuscript writing, final approval of manuscript; M.G.F.: conception and design, financial support, manuscript writing, final approval of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Wolfram Tetzlaff from the University of British Columbia, Canada for insightful discussions and providing critical feedback on this article. Michael G. Fehlings would like to acknowledge support from the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration, the Krembil Foundation, and the DeZwirek Family Foundation.
Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: Emerging neuroprotective and neuroregenerative cell‐based therapies for spinal cord injury. STEM CELLS Transl Med. 2020;9:1509–1530. 10.1002/sctm.19-0135
Christopher S. Ahuja and Andrea Mothe contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Christopher & Dana Reeve Foundation . 2010. One degree of separation: paralysis and spinal cord injury in the United States.
- 2. National Spinal Cord Injury Statistical Center . Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2018. [Google Scholar]
- 3. Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury‐repair and regeneration. Neurosurgery. 2017;80:S9‐S22. [DOI] [PubMed] [Google Scholar]
- 5. Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: from point of injury to rehabilitation. J Spinal Cord Med. 2017;40:665‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahuja CS, Schroeder GD, Vaccaro AR, Fehlings MG. Spinal cord injury‐what are the controversies? J Orthop Trauma. 2017;31(Suppl 4):S7‐S13. [DOI] [PubMed] [Google Scholar]
- 7. Khazaei M, Ahuja CS, Fehlings MG. Induced pluripotent stem cells for traumatic spinal cord injury. Front Cell Dev Biol. 2017;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16:1127‐1129. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, He Y, DeVivo MJ. Changing demographics and injury profile of new traumatic spinal cord injuries in the United States, 1972‐2014. Arch Phys Med Rehabil. 2016;97:1610‐1619. [DOI] [PubMed] [Google Scholar]
- 10. van den Berg ME, Castellote JM, Mahillo‐Fernandez I, de Pedro‐Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184‐192. discussion 192. [DOI] [PubMed] [Google Scholar]
- 11. Lenehan B, Street J, Kwon BK, et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine (Phila Pa 1976). 2012;37:321‐329. [DOI] [PubMed] [Google Scholar]
- 12. DeVivo MJ, Chen Y. Trends in new injuries, prevalent cases, and aging with spinal cord injury. Arch Phys Med Rehabil. 2011;92:332‐338. [DOI] [PubMed] [Google Scholar]
- 13. LaPlaca MC, Simon CM, Prado GR, Cullen DK. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13‐26. [DOI] [PubMed] [Google Scholar]
- 14. Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255‐266. [DOI] [PubMed] [Google Scholar]
- 15. Ahuja C, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:1‐21. [DOI] [PubMed] [Google Scholar]
- 16. Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267‐285. [DOI] [PubMed] [Google Scholar]
- 17. Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical‐induced damage to DNA: mechanisms and measurement. This article is part of a series of reviews on “Oxidative DNA Damage and Repair.” The full list of papers may be found on the homepage of the journal. Guest editor: Miral Dizdaroglu. Free Radic Biol Med. 2002;32:1102‐1115. [DOI] [PubMed] [Google Scholar]
- 18. Hausmann ON. Post‐traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369‐378. [DOI] [PubMed] [Google Scholar]
- 19. Liu M, Wu W, Li H, et al. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J Spinal Cord Med. 2015;38:745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Wang H, Tao Y, Zhang S, Wang J, Feng X. Necroptosis inhibitor necrostatin‐1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91‐101. [DOI] [PubMed] [Google Scholar]
- 21. Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+‐dependent glutamate transport. J Neurosci. 1999;19:RC16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li S, Stys PK. Mechanisms of ionotropic glutamate receptor‐mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. [DOI] [PubMed] [Google Scholar]
- 24. Kwon B. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451‐464. [DOI] [PubMed] [Google Scholar]
- 25. Pathania S, Bagler G, Ahuja PS. Differential network analysis reveals evolutionary complexity in secondary metabolism of Rauvolfia serpentina over Catharanthus roseus . Front Plant Sci. 2016;7:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407‐413. [DOI] [PubMed] [Google Scholar]
- 27. Milhorat TH, Capocelli AL, Anzil AP, Kotzen RM, Milhorat RH. Pathological basis of spinal cord cavitation in syringomyelia: analysis of 105 autopsy cases. J Neurosurg. 1995;82:802‐812. [DOI] [PubMed] [Google Scholar]
- 28. Jeffery ND, Blakemore WF. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain. 1997;120(Pt 1):27‐37. [DOI] [PubMed] [Google Scholar]
- 29. Barnabe‐Heider F, Goritz C, Sabelstrom H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470‐482. [DOI] [PubMed] [Google Scholar]
- 30. Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238‐242. [DOI] [PubMed] [Google Scholar]
- 31. Meletis K, Barnabe‐Heider F, Carlen M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soderblom C, Luo X, Blumenthal E, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci. 2013;33:13882‐13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146‐156. [DOI] [PubMed] [Google Scholar]
- 34. Lang BT, Cregg JM, DePaul MA, et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398‐3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forgione N, Fehlings MG. Rho‐ROCK inhibition in the treatment of spinal cord injury. World Neurosurg. 2014;82:e535‐e539. [DOI] [PubMed] [Google Scholar]
- 37. Chen MS, Huber AB, van der Haar ME, et al. Nogo‐A is a myelin‐associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN‐1. Nature. 2000;403:434‐439. [DOI] [PubMed] [Google Scholar]
- 38. Moreau‐Fauvarque C, Kumanogoh A, Camand E, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23:9229‐9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo‐A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825‐6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hata K, Fujitani M, Yasuda Y, et al. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mothe AJ, Tassew NG, Shabanzadeh AP, et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci Rep. 2017;7:10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwab JM, Conrad S, Monnier PP, Julien S, Mueller BK, Schluesener HJ. Spinal cord injury‐induced lesional expression of the repulsive guidance molecule (RGM). Eur J Neurosci. 2005;21:1569‐1576. [DOI] [PubMed] [Google Scholar]
- 43. Rosado IR, Carvalho PH, Alves EG, et al. Immunomodulatory and neuroprotective effect of cryopreserved allogeneic mesenchymal stem cells on spinal cord injury in rats. Genet Mol Res. 2017;16(1). 10.4238/gmr16019555. [DOI] [PubMed] [Google Scholar]
- 44. Khazaei M, Ahuja CS, Nakashima H, et al. GDNF rescues the fate of neural progenitor grafts by attenuating notch signals in the injured spinal cord in rodents. Sci Transl Med. 2020;12:eaau3538. [DOI] [PubMed] [Google Scholar]
- 45. Tabakow P, Jarmundowicz W, Czapiga B, et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22:1591‐1612. [DOI] [PubMed] [Google Scholar]
- 46. Piltti KM, Salazar DL, Uchida N, Cummings BJ, Anderson AJ. Safety of human neural stem cell transplantation in chronic spinal cord injury. Stem Cells Translational Medicine. 2013;2:961‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Badhiwala JH, Ahuja CS, Fehlings MG. Time is spine: a review of translational advances in spinal cord injury. J Neurosurg Spine. 2018;30:1‐18. [DOI] [PubMed] [Google Scholar]
- 48. Torres‐Espin A, Redondo‐Castro E, Hernandez J, Navarro X. Immunosuppression of allogenic mesenchymal stem cells transplantation after spinal cord injury improves graft survival and beneficial outcomes. J Neurotrauma. 2015;32:367‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J, Chen P, Wang Q, et al. Meta analysis of olfactory ensheathing cell transplantation promoting functional recovery of motor nerves in rats with complete spinal cord transection. Neural Regen Res. 2014;9:1850‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson KD, Guest JD, Dietrich WD, et al. Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950‐2963. [DOI] [PubMed] [Google Scholar]
- 51. Himes BT, Neuhuber B, Coleman C, et al. Recovery of function following grafting of human bone marrow‐derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278‐296. [DOI] [PubMed] [Google Scholar]
- 52. Cizkova D, Rosocha J, Vanicky I, Jergova S, Cizek M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol. 2006;26:1167‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu S, Suzuki Y, Ejiri Y, et al. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res. 2003;72:343‐351. [DOI] [PubMed] [Google Scholar]
- 55. Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187:266‐278. [DOI] [PubMed] [Google Scholar]
- 56. Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190:17‐31. [DOI] [PubMed] [Google Scholar]
- 57. Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte‐colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379‐1391. [DOI] [PubMed] [Google Scholar]
- 58. Park SI, Lim JY, Jeong CH, et al. Human umbilical cord blood‐derived mesenchymal stem cell therapy promotes functional recovery of contused rat spinal cord through enhancement of endogenous cell proliferation and oligogenesis. J Biomed Biotechnol. 2012;2012:362473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Menezes K, Nascimento MA, Goncalves JP, et al. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One. 2014;9:e96020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parr AM, Kulbatski I, Zahir T, et al. Transplanted adult spinal cord‐derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155:760‐770. [DOI] [PubMed] [Google Scholar]
- 61. Parr AM, Kulbatski I, Tator CH. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J Neurotrauma. 2007;24:835‐845. [DOI] [PubMed] [Google Scholar]
- 62. Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377‐3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord‐injured mice. Proc Natl Acad Sci USA. 2005;102:14069‐14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iwanami A, Kaneko S, Nakamura M, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005;80:182‐190. [DOI] [PubMed] [Google Scholar]
- 66. Nori S, Okada Y, Yasuda A, et al. Grafted human‐induced pluripotent stem‐cell‐derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci USA. 2011;108:16825‐16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fujimoto Y, Abematsu M, Falk A, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell‐derived long‐term self‐renewing neuroepithelial‐like stem cells. Stem Cells. 2012;30:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 68. McDonald JW. Repairing the damaged spinal cord. Sci Am. 1999;281:64‐73. [DOI] [PubMed] [Google Scholar]
- 69. Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell‐derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694‐4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell‐derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guest JD, Rao A, Olson L, Bunge MB, Bunge RP. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp Neurol. 1997;148:502‐522. [DOI] [PubMed] [Google Scholar]
- 72. Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670‐6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barakat DJ, Gaglani SM, Neravetla SR, et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225‐240. [DOI] [PubMed] [Google Scholar]
- 74. Pearse DD, Sanchez AR, Pereira FC, et al. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association, and functional recovery. Glia. 2007;55:976‐1000. [DOI] [PubMed] [Google Scholar]
- 75. Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000‐2002. [DOI] [PubMed] [Google Scholar]
- 76. Ramon‐Cueto A, Cordero MI, Santos‐Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425‐435. [DOI] [PubMed] [Google Scholar]
- 77. Chhabra HS, Sarda K, Arora M, et al. Autologous bone marrow cell transplantation in acute spinal cord injury—an Indian pilot study. Spinal Cord. 2016;54:57‐64. [DOI] [PubMed] [Google Scholar]
- 78. Mendonca MV, Larocca TF, de Freitas Souza BS, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. El‐Kheir WA, Gabr H, Awad MR, et al. Autologous bone marrow‐derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729‐745. [DOI] [PubMed] [Google Scholar]
- 80. Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose‐derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39:655‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue‐derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297‐1308. [DOI] [PubMed] [Google Scholar]
- 82. Satti HS, Waheed A, Ahmed P, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18:518‐522. [DOI] [PubMed] [Google Scholar]
- 83. Levi AD, Okonkwo DO, Park P, et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Neurosurgery. 2018;82:562‐575. [DOI] [PubMed] [Google Scholar]
- 84. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637‐647. [DOI] [PubMed] [Google Scholar]
- 85. Mothe AJ, Zahir T, Santaguida C, Cook D, Tator CH. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self‐renewing and differentiate after transplantation. PLoS One. 2011;6:e27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73‐85. [DOI] [PubMed] [Google Scholar]
- 87. Rustenhoven J, Park TI, Schweder P, et al. Isolation of highly enriched primary human microglia for functional studies. Sci Rep. 2016;6:19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chaddah R, Arntfield M, Runciman S, Clarke L, van der Kooy D. Clonal neural stem cells from human embryonic stem cell colonies. J Neurosci. 2012;32:7771‐7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Umebayashi D, Coles B, van der Kooy D. Enrichment of oligodendrocyte progenitors from differentiated neural precursors by clonal sphere preparations. Stem Cells Dev. 2016;25:712‐728. [DOI] [PubMed] [Google Scholar]
- 90. Colasante G, Rubio A, Massimino L, Broccoli V. Direct neuronal reprogramming reveals unknown functions for known transcription factors. Front Neurosci. 2019;13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ambasudhan R, Talantova M, Coleman R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Caiazzo M, Dell'Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224‐227. [DOI] [PubMed] [Google Scholar]
- 93. Colasante G, Lignani G, Rubio A, et al. Rapid conversion of fibroblasts into functional forebrain GABAergic interneurons by direct genetic reprogramming. Cell Stem Cell. 2015;17:719‐734. [DOI] [PubMed] [Google Scholar]
- 94. Najm FJ, Lager AM, Zaremba A, et al. Transcription factor‐mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gritti A, Parati EA, Cova L, et al. Multipotential stem cells from the adult mouse brain proliferate and self‐renew in response to basic fibroblast growth factor. J Neurosci. 1996;16:1091‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071‐1082. [DOI] [PubMed] [Google Scholar]
- 97. Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389‐404. [DOI] [PubMed] [Google Scholar]
- 98. Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen‐regulated transcriptional network. Development. 2008;135:2489‐2503. [DOI] [PubMed] [Google Scholar]
- 99. Dessaud E, Ribes V, Balaskas N, et al. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 2010;8:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640‐2649. [DOI] [PubMed] [Google Scholar]
- 101. Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1‐13. [DOI] [PubMed] [Google Scholar]
- 102. Kulbatski I, Mothe AJ, Keating A, Hakamata Y, Kobayashi E, Tator CH. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. J Histochem Cytochem. 2007;55:209‐222. [DOI] [PubMed] [Google Scholar]
- 103. Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16:1045‐1057. [DOI] [PubMed] [Google Scholar]
- 104. Mothe AJ, Zahir T, Santaguida C, Cook D, Tator CH. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self‐renewing and differentiate after transplantation. PLoS One. 2011;6:e27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599‐7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hitoshi S, Seaberg RM, Koscik C, et al. Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of notch signaling. Genes Dev. 2004;18:1806‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xu W, Lakshman N, Morshead CM. Building a central nervous system: the neural stem cell lineage revealed. Neurogenesis (Austin). 2017;4:e1300037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sachewsky N, Morshead CM. Prosurvival factors derived from the embryonic brain promote adult neural stem cell survival. Stem Cells Dev. 2014;23:2469‐2481. [DOI] [PubMed] [Google Scholar]
- 109. Xu W, Lakshman N, Morshead CM. Stem cells in the adult CNS revealed: examining their regulation by myelin basic protein. Neural Regen Res. 2016;11:1916‐1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dadwal P, Mahmud N, Sinai L, et al. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Rep. 2015;5:166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yousefifard M, Rahimi‐Movaghar V, Nasirinezhad F, et al. Neural stem/progenitor cell transplantation for spinal cord injury treatment; a systematic review and meta‐analysis. Neuroscience. 2016;322:377‐397. [DOI] [PubMed] [Google Scholar]
- 112. Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701‐713. [DOI] [PubMed] [Google Scholar]
- 113. Dulin JN, Adler AF, Kumamaru H, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun. 2018;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.ClinicalTrials.gov. National Institutes of Health: USA; 2016.
- 115. All AH, Gharibani P, Gupta S, et al. Early intervention for spinal cord injury with human induced pluripotent stem cells oligodendrocyte progenitors. PLoS One. 2015;10:e0116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kawabata S, Takano M, Numasawa‐Kuroiwa Y, et al. Grafted human iPS cell‐derived oligodendrocyte precursor cells contribute to robust Remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang YW, Denham J, Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 2006;15:943‐952. [DOI] [PubMed] [Google Scholar]
- 118. Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187‐191. [DOI] [PubMed] [Google Scholar]
- 119. Plemel JR, Keough MB, Duncan GJ, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54‐72. [DOI] [PubMed] [Google Scholar]
- 120. Myers SA, Bankston AN, Burke DA, Ohri SS, Whittemore SR. Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Exp Neurol. 2016;283:560‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Asterias Biotherapeutics . Asterias Provides Top Line 12 Month Data Update for its OPC1 Phase 1/2a Clinical Trial in Severe Spinal Cord Injury. Omaha, Nebraska: GlobeNewsWire; 2019. [Google Scholar]
- 122. Windus LC, Lineburg KE, Scott SE, et al. Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions. Cell Mol Life Sci. 2010;67:1735‐1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Silva NA, Cooke MJ, Tam RY, et al. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials. 2012;33:6345‐6354. [DOI] [PubMed] [Google Scholar]
- 124. Zhang J, Chen H, Duan Z, et al. The effects of co‐transplantation of olfactory ensheathing cells and Schwann cells on local inflammation environment in the contused spinal cord of rats. Mol Neurobiol. 2016;54:943‐953. [DOI] [PubMed] [Google Scholar]
- 125. Ekberg JA, St John JA. Olfactory ensheathing cells for spinal cord repair: crucial differences between subpopulations of the glia. Neural Regen Res. 2015;10:1395‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Roet KC, Verhaagen J. Understanding the neural repair‐promoting properties of olfactory ensheathing cells. Exp Neurol. 2014;261:594‐609. [DOI] [PubMed] [Google Scholar]
- 127. Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci. 2004;24:8485‐8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Watzlawick R, Rind J, Sena ES, et al. Olfactory ensheathing cell transplantation in experimental spinal cord injury: effect size and reporting bias of 62 experimental treatments: a systematic review and meta‐analysis. PLoS Biol. 2016;14:e1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473:1‐15. [DOI] [PubMed] [Google Scholar]
- 130. Mackay‐Sim A, Feron F, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3‐year clinical trial. Brain. 2008;131:2376‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dlouhy BJ, Awe O, Rao RC, Kirby PA, Hitchon PW. Autograft‐derived spinal cord mass following olfactory mucosal cell transplantation in a spinal cord injury patient: case report. J Neurosurg Spine. 2014;21:618‐622. [DOI] [PubMed] [Google Scholar]
- 132. Chhabra HS, Lima C, Sachdeva S, et al. Autologous olfactory [corrected] mucosal transplant in chronic spinal cord injury: an Indian Pilot Study. Spinal Cord. 2009;47:887‐895. [DOI] [PubMed] [Google Scholar]
- 133. Li L, Adnan H, Xu B, et al. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: a systematic review and meta‐analysis. Eur Spine J. 2015;24:919‐930. [DOI] [PubMed] [Google Scholar]
- 134. Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB. Schwann cell transplantation for spinal cord injury repair: its significant therapeutic potential and prospectus. Rev Neurosci. 2015;26:121‐128. [DOI] [PubMed] [Google Scholar]
- 135. Wiliams RR, Bunge MB. Schwann cell transplantation: a repair strategy for spinal cord injury? Prog Brain Res. 2012;201:295‐312. [DOI] [PubMed] [Google Scholar]
- 136. Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199‐3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sparling JS, Bretzner F, Biernaskie J, et al. Schwann cells generated from neonatal skin‐derived precursors or neonatal peripheral nerve improve functional recovery after acute transplantation into the partially injured cervical spinal cord of the rat. J Neurosci. 2015;35:6714‐6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Biernaskie J, Sparling JS, Liu J, et al. Skin‐derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545‐9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610‐616. [DOI] [PubMed] [Google Scholar]
- 140. Niapour A, Karamali F, Nemati S, et al. Cotransplantation of human embryonic stem cell‐derived neural progenitors and Schwann cells in a rat spinal cord contusion injury model elicits a distinct neurogenesis and functional recovery. Cell Transplant. 2012;21:827‐843. [DOI] [PubMed] [Google Scholar]
- 141. Pourheydar B, Joghataei MT, Bakhtiari M, Mehdizadeh M, Yekta Z, Najafzadeh N. Co‐transplantation of bone marrow stromal cells with Schwann cells evokes mechanical allodynia in the contusion model of spinal cord injury in rats. Cell J. 2012;13:213‐222. [PMC free article] [PubMed] [Google Scholar]
- 142. Fellows CR, Matta C, Zakany R, Khan IM, Mobasheri A. Adipose, bone marrow and synovial joint‐derived mesenchymal stem cells for cartilage repair. Front Genet. 2016;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. 2013;2:22‐38. [PMC free article] [PubMed] [Google Scholar]
- 144. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530‐541. [DOI] [PubMed] [Google Scholar]
- 145. Kotobuki N, Hirose M, Takakura Y, Ohgushi H. Cultured autologous human cells for hard tissue regeneration: preparation and characterization of mesenchymal stem cells from bone marrow. Artif Organs. 2004;28:33‐39. [DOI] [PubMed] [Google Scholar]
- 146. Carrade DD, Affolter VK, Outerbridge CA, et al. Intradermal injections of equine allogeneic umbilical cord‐derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13:1180‐1192. [DOI] [PubMed] [Google Scholar]
- 147. Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells. 2014;6:120‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lalu MM, Sullivan KJ, Mei SH, et al. Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta‐analyses prior to initiating a first‐in‐human trial. Elife. 2016;5:e17850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Dulamea A. Mesenchymal stem cells in multiple sclerosis ‐ translation to clinical trials. J Med Life. 2015;8:24‐27. [PMC free article] [PubMed] [Google Scholar]
- 150. Yan X, Cen Y, Wang Q. Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA‐regulated IkappaB expression. Sci Rep. 2016;6:28915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623‐6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sasaki M, Radtke C, Tan AM, et al. BDNF‐hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009;29:14932‐14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Boido M, Garbossa D, Fontanella M, Ducati A, Vercelli A. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014;81:183‐190. [DOI] [PubMed] [Google Scholar]
- 155. Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical‐cord blood from unrelated donors. N Engl J Med. 2001;344:1815‐1822. [DOI] [PubMed] [Google Scholar]
- 156. Park DH, Lee JH, Borlongan CV, Sanberg PR, Chung YG, Cho TH. Transplantation of umbilical cord blood stem cells for treating spinal cord injury. Stem Cell Rev. 2011;7:181‐194. [DOI] [PubMed] [Google Scholar]
- 157. Zhou C, Yang B, Tian Y, et al. Immunomodulatory effect of human umbilical cord Wharton's jelly‐derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gregoire CA, Goldenstein BL, Floriddia EM, Barnabe‐Heider F, Fernandes KJ. Endogenous neural stem cell responses to stroke and spinal cord injury. Glia. 2015;63:1469‐1482. [DOI] [PubMed] [Google Scholar]
- 159. Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC‐CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23‐35. [DOI] [PubMed] [Google Scholar]
- 160. Namiki J, Tator CH. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J Neuropathol Exp Neurol. 1999;58:489‐498. [DOI] [PubMed] [Google Scholar]
- 161. Hunt J, Cheng A, Hoyles A, Jervis E, Morshead CM. Cyclosporin A has direct effects on adult neural precursor cells. J Neurosci. 2010;30:2888‐2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sachewsky N, Hunt J, Cooke MJ, et al. Cyclosporin A enhances neural precursor cell survival in mice through a calcineurin‐independent pathway. Dis Model Mech. 2014;7:953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Reeve RL, Yammine SZ, DeVeale B, van der Kooy D. Targeted activation of primitive neural stem cells in the mouse brain. Eur J Neurosci. 2016;43:1474‐1485. [DOI] [PubMed] [Google Scholar]
- 164. Tetzlaff W, Okon EB, Karimi‐Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611‐1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19:223‐238. [DOI] [PubMed] [Google Scholar]
- 166. Kojima A, Tator CH. Epidermal growth factor and fibroblast growth factor 2 cause proliferation of ependymal precursor cells in the adult rat spinal cord in vivo. J Neuropathol Exp Neurol. 2000;59:687‐697. [DOI] [PubMed] [Google Scholar]
- 167. Kim H, Zahir T, Tator CH, Shoichet MS. Effects of dibutyryl cyclic‐AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLoS One. 2011;6:e21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liu WG, Wang ZY, Huang ZS. Bone marrow‐derived mesenchymal stem cells expressing the bFGF transgene promote axon regeneration and functional recovery after spinal cord injury in rats. Neurol Res. 2011;33:686‐693. [DOI] [PubMed] [Google Scholar]
- 169. Jung WS, Han SM, Kim SM, et al. Stimulatory effect of HGF‐overexpressing adipose tissue‐derived mesenchymal stem cells on thymus regeneration in a rat thymus involution model. Cell Biol Int. 2014;38:1106‐1117. [DOI] [PubMed] [Google Scholar]
- 170. Kim YH, Cho SH, Lee SJ, et al. Growth‐inhibitory effect of neurotrophin‐3‐secreting adipose tissue‐derived mesenchymal stem cells on the D283‐MED human medulloblastoma cell line. J Neurooncol. 2012;106:89‐98. [DOI] [PubMed] [Google Scholar]
- 171. Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain‐derived neurotrophic factor‐expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma. 2015;32:185‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rooney GE, McMahon SS, Ritter T, et al. Neurotrophic factor‐expressing mesenchymal stem cells survive transplantation into the contused spinal cord without differentiating into neural cells. Tissue Eng Part A. 2009;15:3049‐3059. [DOI] [PubMed] [Google Scholar]
- 173. Girard C, Bemelmans AP, Dufour N, et al. Grafts of brain‐derived neurotrophic factor and neurotrophin 3‐transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924‐7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137:654‐667. [DOI] [PubMed] [Google Scholar]
- 175. Hwang DH, Shin HY, Kwon MJ, Choi JY, Ryu BY, Kim BG. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin‐like growth factor‐1 signaling. J Neurosci. 2014;34:12788‐12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Bozkurt G, Mothe AJ, Zahir T, Kim H, Shoichet MS, Tator CH. Chitosan channels containing spinal cord‐derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery. 2010;67:1733‐1744. [DOI] [PubMed] [Google Scholar]
- 177. Guo X, Zahir T, Mothe A, et al. The effect of growth factors and soluble Nogo‐66 receptor protein on transplanted neural stem/progenitor survival and axonal regeneration after complete transection of rat spinal cord. Cell Transplant. 2012;21:1177‐1197. [DOI] [PubMed] [Google Scholar]
- 178. Rauch MF, Hynes SR, Bertram J, et al. Engineering angiogenesis following spinal cord injury: a coculture of neural progenitor and endothelial cells in a degradable polymer implant leads to an increase in vessel density and formation of the blood‐spinal cord barrier. Eur J Neurosci. 2009;29:132‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Tsai EC, Dalton PD, Shoichet MS, Tator CH. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004;21:789‐804. [DOI] [PubMed] [Google Scholar]
- 180. Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27:519‐533. [DOI] [PubMed] [Google Scholar]
- 181. Layer RT, Ulich TR, Coric D, et al. New clinical‐pathological classification of intraspinal injury following traumatic acute complete thoracic spinal cord injury: postdurotomy/myelotomy observations from the INSPIRE trial. Neurosurgery. 2017;64:105‐109. [DOI] [PubMed] [Google Scholar]
- 182. Theodore N, Hlubek R, Danielson J, et al. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery. 2016;79:E305‐E312. [DOI] [PubMed] [Google Scholar]
- 183. Gupta D, Tator CH, Shoichet MS. Fast‐gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370‐2379. [DOI] [PubMed] [Google Scholar]
- 184. Caicco MJ, Zahir T, Mothe AJ, et al. Characterization of hyaluronan‐methylcellulose hydrogels for cell delivery to the injured spinal cord. J Biomed Mater Res A. 2013;101:1472‐1477. [DOI] [PubMed] [Google Scholar]
- 185. Fisher SA, Tam RY, Shoichet MS. Tissue mimetics: engineered hydrogel matrices provide biomimetic environments for cell growth. Tissue Eng Part A. 2014;20:895‐898. [DOI] [PubMed] [Google Scholar]
- 186. Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan‐based hydrogel. Biomaterials. 2013;34:3775‐3783. [DOI] [PubMed] [Google Scholar]
- 187. Fuhrmann T, Tam RY, Ballarin B, et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell‐derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23‐36. [DOI] [PubMed] [Google Scholar]
- 188. Johnson PJ, Parker SR, Sakiyama‐Elbert SE. Fibrin‐based tissue engineering scaffolds enhance neural fiber sprouting and delay the accumulation of reactive astrocytes at the lesion in a subacute model of spinal cord injury. J Biomed Mater Res A. 2010;92:152‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lu P, Woodruff G, Wang Y, et al. Long‐distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Iwasaki M, Wilcox JT, Nishimura Y, et al. Synergistic effects of self‐assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617‐2629. [DOI] [PubMed] [Google Scholar]
- 191. Liu Y, Ye H, Satkunendrarajah K, Yao GS, Bayon Y, Fehlings MG. A self‐assembling peptide reduces glial scarring, attenuates post‐traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075‐8088. [DOI] [PubMed] [Google Scholar]
- 192. Zweckberger K, Ahuja CS, Liu Y, Wang J, Fehlings MG. Self‐assembling peptides optimize the post‐traumatic milieu and synergistically enhance the effects of neural stem cell therapy after cervical spinal cord injury. Acta Biomater. 2016;42:77‐89. [DOI] [PubMed] [Google Scholar]
- 193. Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985‐996. [DOI] [PubMed] [Google Scholar]
- 194. Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry. 2003;106:375‐383. [DOI] [PubMed] [Google Scholar]
- 195. Chiang MC, Cragoe EJ Jr, Vanable JW Jr. Intrinsic electric fields promote epithelization of wounds in the newt, Notophthalmus viridescens . Dev Biol. 1991;146:377‐385. [DOI] [PubMed] [Google Scholar]
- 196. Ross CL, Syed I, Smith TL, Harrison BS. The regenerative effects of electromagnetic field on spinal cord injury. Electromagn Biol Med. 2017;36:74‐87. [DOI] [PubMed] [Google Scholar]
- 197. McKasson MJ, Huang L, Robinson KR. Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp Neurol. 2008;211:585‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Babona‐Pilipos R, Droujinine IA, Popovic MR, Morshead CM. Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS One. 2011;6:e23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Iwasa SN, Babona‐Pilipos R, Morshead CM. Environmental factors that influence stem cell migration: an "electric field". Stem Cells Int. 2017;2017:4276927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Keuters MH, Aswendt M, Tennstaedt A, et al. Transcranial direct current stimulation promotes the mobility of engrafted NSCs in the rat brain. NMR Biomed. 2015;28:231‐239. [DOI] [PubMed] [Google Scholar]
- 201. Rueger MA, Keuters MH, Walberer M, et al. Multi‐session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7:e43776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636‐640. [DOI] [PubMed] [Google Scholar]
- 203. Barritt AW, Davies M, Marchand F, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856‐10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bradbury EJ, Moon LDF, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636‐640. [DOI] [PubMed] [Google Scholar]
- 205. Suzuki H, Ahuja CS, Salewski RP, et al. Neural stem cell mediated recovery is enhanced by chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS One. 2017;12:e0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci USA. 2010;107:3340‐3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bartus K, James ND, Didangelos A, et al. Large‐scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci. 2014;34:4822‐4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Pakulska MM, Tator CH, Shoichet MS. Local delivery of chondroitinase ABC with or without stromal cell‐derived factor 1‐alpha promotes functional repair in the injured rat spinal cord. Biomaterials. 2017;134:13‐21. [DOI] [PubMed] [Google Scholar]
- 209. Tauchi R, Imagama S, Natori T, et al. The endogenous proteoglycan‐degrading enzyme ADAMTS‐4 promotes functional recovery after spinal cord injury. J Neuroinflammation. 2012;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Filous AR, Tran A, Howell CJ, et al. Entrapment via synaptic‐like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci. 2014;34:16369‐16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Dyck S, Kataria H, Alizadeh A, et al. Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPsigma receptors promotes a beneficial inflammatory response following spinal cord injury. J Neuroinflammation. 2018;15:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Neofytou E, O'Brien CG, Couture LA, Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551‐2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Moghaddam SE, Hernandez‐Rivera M, Zaibaq NG, et al. A new high‐performance gadonanotube‐polymer hybrid material for stem cell labeling and tracking by MRI. Contrast Media Mol Imaging. 2018;2018:2853736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Ariza de Schellenberger A, Kratz H, Farr TD, et al. Labeling of mesenchymal stem cells for MRI with single‐cell sensitivity. Int J Nanomedicine. 2016;11:1517‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17:R84‐R92. [DOI] [PubMed] [Google Scholar]
- 216. Hoornaert CJ, Le Blon D, Quarta A, et al. Concise review: innate and adaptive immune recognition of allogeneic and xenogeneic cell transplants in the central nervous system. Stem Cells Translational Medicine. 2017;6:1434‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Mao Y, Nguyen T, Sutherland T, Gorrie CA. Endogenous neural progenitor cells in the repair of the injured spinal cord. Neural Regen Res. 2016;11:1075‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Deuse T, Hu X, Gravina A, et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol. 2019;37:252‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Anderson AJ, Piltti KM, Hooshmand MJ, Nishi RA, Cummings BJ. Preclinical efficacy failure of human CNS‐derived stem cells for use in the pathway study of cervical spinal cord injury. Stem Cell Rep. 2017;8:249‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Tan Y, Ooi S, Wang L. Immunogenicity and tumorigenicity of pluripotent stem cells and their derivatives: genetic and epigenetic perspectives. Curr Stem Cell Res Ther. 2014;9:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Deng J, Zhang Y, Xie Y, Zhang L, Tang P. Cell transplantation for spinal cord injury: tumorigenicity of induced pluripotent stem cell‐derived neural stem/progenitor cells. Stem Cells Int. 2018;2018:5653787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kami D, Watakabe K, Yamazaki‐Inoue M, et al. Large‐scale cell production of stem cells for clinical application using the automated cell processing machine. BMC Biotechnol. 2013;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Rafiq QA, Twomey K, Kulik M, et al. Developing an automated robotic factory for novel stem cell therapy production. Regen Med. 2016;11:351‐354. [DOI] [PubMed] [Google Scholar]
- 224. U.S. Food & Drug Administration . Cellular & gene therapy guidances Vaccines, Blood & Biologics. Silver Sprong, Maryland: Author; 2018. [Google Scholar]
- 225. European Medicines Agency . Multidisciplinary: cell therapy and tissue engineering.
- 226. Roy DC, Alarco AM, Isasi R. CellCAN: a unique enabler of regenerative medicine and cell therapy in Canada. Stem Cells Dev. 2014;23(Suppl 1):24‐28. [DOI] [PubMed] [Google Scholar]
- 227. Prażnowski K, Mamala J. Classification of the road surface condition on the basis of vibrations of the sprung mass in a passenger car. Paper presented at: IOP Conference Series: Materials Science and Engineering; 2016:012022.
- 228. Nikolaev NI, Liu Y, Hussein H, Williams DJ. The sensitivity of human mesenchymal stem cells to vibration and cold storage conditions representative of cold transportation. J R Soc Interface. 2012;9:2503‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Hunt CJ. Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother. 2011;38:107‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Heng BC, Kuleshova LL, Bested SM, Liu H, Cao T. The cryopreservation of human embryonic stem cells. Biotechnol Appl Biochem. 2005;41:97‐104. [DOI] [PubMed] [Google Scholar]
- 231. Abraham E, Ahmadian BB, Holderness K, Levinson Y, McAfee E. Platforms for manufacturing allogeneic, autologous and iPSC cell therapy products: an industry perspective. Adv Biochem Eng Biotechnol. 2018;165:323‐350. [DOI] [PubMed] [Google Scholar]
- 232. Donnelly EM, Lamanna J, Boulis NM. Stem cell therapy for the spinal cord. Stem Cell Res Ther. 2012;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Mazzini L, Mareschi K, Ferrero I, et al. Stem cell treatment in amyotrophic lateral sclerosis. J Neurol Sci. 2008;265:78‐83. [DOI] [PubMed] [Google Scholar]
- 234. Feron F, Perry C, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951‐2960. [DOI] [PubMed] [Google Scholar]
- 235. Raore B, Federici T, Taub J, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976). 2011;36:E164‐E171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


