Abstract
This study was designed to compare usnic acid with anti-breast cancer drug molecules (A-BCDM) routinely used in the treatment of breast cancer. The miRNA information of 17 anti-breast cancer drug used in breast cancer treatment was obtained from the Small Molecule-miRNA Network-Based Inferance (SMIR-NBI) tool. We had been determined common and different expressed miRNAs between 17 A-BCDM & usnic acid and were classified according to the common miRNAs to reveal molecular similarity. As a result of the bioinformatic analyzes, 20 common miRNAs were determined between 17 A-BCDM and usnic acid. The common miRNAs were analyzed with bioinformatic tolls for determining pathways and targets. The most common miRNAs for 6 of 17 A-BCDM and usnic acid were determined as miR-374a-5p and miR-26a-5p. We compared the anti-proliferative effect of usnic acid and one of the 17 A-BCDM that tamoxifen on MDA-MB-231 triple negative breast cancer cell with real-time cell analysis system. The real time PCR assay was carried out with miR-26a-5p for evaluate to expression level of MDA-MB-231 breast cancer cell and MCF-12A non-cancerous epithelial breast cell. As a result of study, usnic acid as novel candidate drug molecule showed high similarity ratio with 5-Fluorouracil, Sulindac Sulfide, Curcumin and Cisplatin A-BCDM used in treatment of breast cancer. miR-26a-5p as common response miRNA of usnic acid and tamoxifen was showed a decreased level of expression by validated qRT-PCR assay. The obtained from study, in addition to 17 A-BCDM, usnic acid has also the potential to be used as a candidate molecule in the treatment of breast cancer. Moreover, miR-26a-5p might be used as a biomarker in the treatment of breast cancer but further analysis is required.
Keywords: Usnic acid, Anti-breast cancer drugs, miRNA, Breast cancer
Introduction
Breast cancer is one of the most common types of cancer in the world due to the presence of more than 1.3 million people and causing 450,000 deaths each year (The Cancer Genome Atlas Network 2012). Breast cancer is heterogeneous disease involving complex histopathological patterns and clinical behavior (Simpson et al. 2005; Weigelt and Reis-Filho 2009). Breast cancer is divided into different subtypes based on the presence of estrogen receptor (ER), progesterone receptor (PR) and HER2. The increasing number of studies based on transcriptomic data, the most of ER and PR positive tumors have been classified into common luminal A and luminal B subtypes (Sorlie et al. 2001; Perou et al. 2000). Tumors with HER2 amplification and/or over-expression are evaluated into the HER2-positive subtype. Triple-negative breast cancer (TNBC) were characterized tumors without expression of ER, PR, and HER2. Although TNBC represents only 15–20% of breast cancer, the recurrence and mortality stage in TNBC is significantly higher than in other subtypes of breast cancer (Dent et al. 2007). Because of the high mortality rate of malignancies and metastatic breast cancer, the further studies focusing on TNBC are needed. The surgery is a common treatment option for early stage of breast cancer. In last stage of disease (stage IV), chemotherapy and radiotherapy are the main therapies along with hormone therapy (Fyles et al. 2004). The surgery, radiation, chemotherapy, targeted treatments and immunotherapy separate or in combination are generally used to treatment of breast cancer. Although TNBC is a subtype that responds to chemotherapy (22%), the recurrence and metastasis rates of TNBC patients are higher than as a non-TNBC tumors (Liedtke et al. 2008). Due to the absence of three receptors in TNBC, chemotherapy is still the main treatment option for patients. However, this treatment option may cause different side effects on patients. There is an urgent need to develop new therapeutic approaches for TNBC patients and researchers have focused on determining the effectiveness of new drug candidate molecules in recent years.
Recent studies have revealed the potential use of novel candidate drug molecules obtained from plant, fungi etc. species for the treatment of various cancerous diseases (Kinghorn et al. 2016; Rayan 2017; Lichota and Gwozdzinski 2018; Kılıç et al. 2018; Dinçsoy and Cansaran-Duman 2017). The natural compounds isolated from biologic organisms have long been a used as anti-cancer drug (Hsiao and Liu 2010). Plant-based drugs are generally low cost, abundant, low toxicity and side effects. Usnic acid, a fungi dibenzofuran derivate, is secondary metabolite mainly found in lichen species. Usnic acid has several properties such as antimicrobial, antiviral, antiprotozoal activity. Recently, numerous studies have showed antiproliferative effect of usnic acid in different cancer cells (Dinçsoy and Cansaran-Duman 2017; Geng et al. 2018; Yurdacan et al. 2019; Kumar et al. 2019; Nguyen et al. 2019 Interestingly, usnic acid has low ratio at effect on non-cancerous human cells and therefore usnic acid may be a novel an candidate molecule for the treatment of cancer.
As a result of understood the effective role of miRNAs in cancer, microRNA-based therapies have become a potential research topic and the use of miRNAs as a preclinical model has begun to be investigated in recent years. miRNAs are non-coding small RNAs that regulate gene expression at the post-transcriptional (Bartel 2004; Hsieh et al. 2015). Studies have determined miRNAs are involved in cell development, differentiation, proliferation and apoptosis processes (Esquela-Kerscher and Slack 2006; Yu et al. 2018). In addition to miRNAs used as biomarkers in clinical diagnosis, the idea of being used as a regulator in the treatment of various diseases is very exciting. The ability to function as key regulators in the genome has made miRNAs a drug target and has made these molecules a promising method for future drug based product development. Moreover, the miRNAs' ability to stabilize in the blood and to withstand repeated freeze–thaw periods have made them a promising treatment option. There have been patent applications in the USA and Europe regarding miRNA-based treatment approaches and many patents have been approved (Van Rooij et al. 2012). miRagen Therapeutics, Regulus Therapeutics and Mirna Therapeutics companies have identified various miRNAs to develop drug candidates that play a role and regulate the process in different disease states (Rupaimoole and Slack et al. 2017). The datas obtained from high-throughput biology tools such as miRNA expression assays enabled more detailed evaluation of cellular networks in carcerogenesis. Several network-based methods are needed to predict the new indications of drugs to speed up cancer pharmacogenomics studies (Yu et al. 2016; Yu et al. 2017; Kılıç et al. 2019; Yangın et al. 2019).
Many drugs that are widely used in cancer treatment have a toxic effect on non-cancerous cells so new treatment options need to be developed for breast cancer treatment (Nakamura et al. 2010). miRNAs response to chemotherapeutic drugs are effective target for cancer therapies. In a previous studies in our lab was determined the effect of usnic acid treatment on the miRNA expression profile in breast cancer cells (Kılıç et al. 2019). The results showed the important roles of miRNAs that respond to usnic acid through regulation of different signal pathways. In the light of these data, we compared miRNAs that respond routinely to A-BCDM used in the treatment of breast cancer and usnic acid to identify similarities and differences. miRNA data that respond to 17 A-BCDM used in breast cancer treatment were obtained from the Small Molecule-miRNA Network-Based Inferance (SMIR-NBI) tool (Li et al 2016). In this study was determined the effect of routinely used A-BCDM and usnic acid molecule on breast cancer treatment at miRNA level. The results demonstrated that miR-26a-5p which was the common miRNA of 6 anti-breast cancer drugs and usnic acid response miRNAs was significantly upregulated in MDA-MB-231 breast cancer cells. This study may emphasize a common role of usnic acid and cancer-targeted drugs on the rugulation of miRNAs in breast cancer.
Material and methods
The determination of similarities and differences of 17 anti-breast cancer drugs (A-BCDM) and usnic acid response miRNAs
Li et al. developed SMİR-NBI model to identify miRNAs as potential pharmacogenomic biomarkers in precision cancer medicine (Li et al. 2016). The SMİR-NBI network data have collected from the miREnvironment, sM2miR, MeSH, miRBase, miRTarBase, TarBase, miRecords databases that have been experimentally confirmed to be directly regulated by small molecules. The miRNAs response to 17 A-BCDM were obtained from the SMIR-NBI tool and the names of miRNAs were standardised using Unified Medical Subject Headings (MeSH). The A-BCDM consist of Tamoxifen, Fulvestrant, Letrozole, Cisplatin, Paclitaxel, 5-Fluorouracil Doxorubicin, Docetaxel, Mitoxantrone, Gemcitabine, Capecitabine, Vincristine, Topotecan, Metformin, Sulindac Sulfide, Curcumin and Bicalutamide in SMIR-NBI tool.
In our previous study, usnic acid showed significant cytotoxic activity to MCF-7, BT-474 and MDA-MB-231 breast cancer cells and determined effective dose and time for usnic acid. Usnic acid responsive miRNAs obtained from MCF-7, BT-474 and MDA-MB-231 breast cancer cell lines were identified by microarray analyses (Kılıç et al. 2019).
The number and percentage of miRNAs responsive to 17 A-BCDM and usnic acid obtained from SMIR-NBI tool determined by Microsoft Access Program. The number of common miRNAs to 17 A-BCDM and usnic acid were also performed to Microsoft Access Program.
The percent of similarity of the drug-miRNA were calculated based on the miRNA numbers determined to be common to the respective drugs. Then, the values obtained have shown as a drug-miRNA similarity graph.
The analysis of pathways and target genes of common expressed miRNAs between usnic acid and 17 anti-breast cancer drugs
TargetScanHuman program and KEGG analysis have been used for determining target gene and pathway analysis of 17 A-BCDM and usnic acid responsive miRNAs.
The determination of experimental validation of miR-26a-5p as common miRNA responsive to tamoxifen and usnic acid on MDA-MB-231 triple negative breast cancer cell
Cell culture
MDA-MB-231 breast cancer cell was obtained from American Type Culture Collection (ATCC). Cells were cultured in 10% fetal bovine serum (FBS; Biological Industries) and 1% penicilin/streptomiycin supplemented media in Dulbecco's Modified Eagle's medium (DMEM; Biological Industries). MDA-MB-231 cell cultured at 37 °C in a humidified atmosphere with 5% CO2.
The preparation of usnic acid and tamoxifen
The stock solutions of usnic acid were appropriately diluted to obtain a final concentration of 0.1% DMSO (v/v). A stock solution of 100 μM usnic acid was prepared and diluted with DMEM to obtain final concentrations of 50, 25 and 10 μM. The stock solution of tamoxifen was prepared to 1 M concentration.
Determination of cell proliferation by real time cell analyzer (RTCA) system
Cell proliferation was also continuously monitored using the xCELLigence RTCA S16 Instrument (xCELLigence RTCA, Roche, Germany). MDA-MB-231 breast cancer cells seeded at a density of 1 × 104 cells per well of e-plate. After 24 h, usnic acid was added at different concentrations (100, 50, 25 and 10 μM) on MDA-MB-231 cells. The assay conditions of cell proliferation were also performed by the same method to determine the effect of tamoxifen on MDA-MB-231 cells. Proliferation was monitored every 30 min and time dependent cell index (CI) graph was obtained by the device using the RTCA software program of the manufacturer (xCELLigence RTCA, Roche, Germany).
The verification of miR-26a-5p expression by qRT-PCR
RNA isolation and cDNA synthesis
For RNA isolation, MDA-MB-231 breast cancer cells (5 × 10 5 cells per well) were plated in each well. Cells were incubated in a standard cell culture for 24 h. Then, the IC50 of usnic acid and tamoxifen were applied to MDA-MB-231 cancer cell. Total RNA was isolated with TriPure Isolation Reagent (Roche Life Science, Mannheim, Germany) according to the manufacturer’s instructions. One microgram RNA was reverse transcribed by oligo(dT) primers with Transcriptor High Fidelity cDNA Synthesis Kit (miScript® II RT Kit, Qiagen) at 95ºC for 5 min, and at 48 °C for 60 min, and at 85 °C for 5 min according to the manufacturer’s instructions.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR analysis was performed using a LightCycler 480 PCR (Roche, Germany). miScript SYBR® Green PCR Kit used with miScript Primer Assays or miScript Precursor Assays Kit (Qiagen) was used for real time PCR. The reaction mix was prepared to a total volume of 25 ul (2× QuantiTect SYBR Green PCR Master Mix 12.5 μl, 10× miScript Universal Primer 2.5 μl, 10× miScript Primer Assay 2.5 μl, RNase-free water, Template cDNA ≤ 2.5 μl). The RNU-6 housekeeping gene was used for normalization. PCR condition was performed with initial denaturation at 95 °C for 15 min, followed by amplification for 40 cycles, each cycle consisting of denaturation at 94 °C for 10 s, annealing at 55 °C for 30 s, polymerization at 70 °C for 30 s. The miR-26a-5p primer was 5′UUCAAGUAAUCCAGGAUAGGCU3′. The 2−ΔΔCT method was used to evaluate of expression level of miR-26a-5p. The qRT-PCR experiments were repeated two times and evaluated stu-t test.
Transfection with miR-26a-5p and cell proliferation analysis
3.5 × 105cells were seeded in six-well plates and transfected with 25 nM miR-26a-5p mimic, scrambled control with using HiPerFect Transfection Reagent (Qiagen, Germany). After an transfection period of 24 h, cells were seeded into plate of xCELLigence real-time cell analyser. The antiproliferative effect of miR-26a-5p was evaluated by using real time cell analyzer system.
Results
Comparision of usnic acid and 17 anti-breast cancer drugs by the SMİR-NBI tool
The effect of usnic acid on breast cancer cells was investigated in our previous study (Kılıç et al. 2019). Usnic acid was applied to three different histological subtype breast cancer cells (MDA-MB-231, BT-474, MCF-7). The most highest miRNA response (66 miRNAs) of usnic acid among breast cancer cells were determined on MDA-MB-231 triple negative breast cancer cell. In the current study, the results of our previous study and 17 A-BCDM in the SMIR-NBI tool were compared for determining the common and different of the miRNAs responding to breast cancer treatment. The 42 of miRNAs was specific to usnic acid and 24 miRNA is common with 66 miRNA that respond to MDA-MB-231 and 17 A-BCDM (Table 1). Therefore, while the similarity rate of usnic acid with other A-BCDM in terms of miRNA profile is 63.6%, the uniqueness rate is 36.3% (Table 1). The miRNAs that respond to 17 A-BCDM in the SMIR-NBI tool have shown us that drugs have a different number of miRNA responses. The drug with the highest number of miRNA’s is 5-Fluorouracil with 208 miRNA (Table 1). The 16 A-BCDM other than 5-Fluorouracil in the SMIR-NBI tool showed from 1 to 131 number of miRNA differentiation (Table 1). In addition, the drug-specific miRNAs that is not similar to other drugs, is important for alternative drug treatment option. This uniqueness rate for usnic acid is 87% in MCF-7 cell, 63% in MDA-MB-231 cell, and 60% for 5-Fluorouracil. miRNAs that respond to sulindac sulfur are 40% different from miRNAs that respond to other A-BCDM (Table 1). The results of the study have correlated with the number of miRNAs responsive to 17 A-BCDM loaded into the SMIR-NBI database. However, the bioinformatic data obtained from SMIR-NBI tool indicate that the highest miRNA differentiation was detected in usnic acid compared with 17 A-BCDM, and these miRNAs function via different pathways from other drugs.
Table 1.
The similarities and uniqueness of 17 anti-breast cancer drugs and usnic acid according to miRNAs
| Drug name | Responsible miRNA numbers | Only their unique number miRNA | Number of miRNAs found in any other drugs | Uniqueness ratio (%) | Similarity ratio (%) |
|---|---|---|---|---|---|
| Usnic acid MDA-MB-231 | 66 | 42 | 24 | 63.63 | 36.36 |
| Usnic acid BT-474 | 16 | 8 | 8 | 50 | 50 |
| Usnic acid MCF-7 | 8 | 7 | 1 | 87.50 | 12.50 |
| Tamoxifen | 10 | 1 | 9 | 10 | 90 |
| Gemcitabine | 56 | 13 | 43 | 23.20 | 76.70 |
| Metformin | 10 | – | 10 | 0 | 100 |
| Curcumin | 62 | 13 | 49 | 20.96 | 79.03 |
| Sulindac Sulfide | 131 | 53 | 78 | 40.45 | 59.54 |
| Doxorubicin | 39 | 6 | 33 | 15.38 | 84.61 |
| Vincristine | 1 | 1 | – | 100 | 0 |
| Cisplatin | 56 | 19 | 37 | 33.92 | 66.07 |
| Fulvestrant | 4 | – | 4 | 0 | 100 |
| Bicalutamide | 37 | 12 | 25 | 32.43 | 67.56 |
| 5-Fluorouracil | 208 | 125 | 83 | 60.09 | 39.90 |
| Paclitaxel | 16 | 1 | 15 | 6.25 | 93.75 |
| Letrozole | 1 | – | 1 | 0 | 100 |
| Docetaxel | 20 | 2 | 18 | 10 | 90 |
| Topotecan | 12 | 5 | 7 | 41.66 | 58.33 |
| Mitoxantrone | 2 | – | 2 | 0 | 100 |
| Capecitabine | 2 | – | 2 | 0 | 100 |
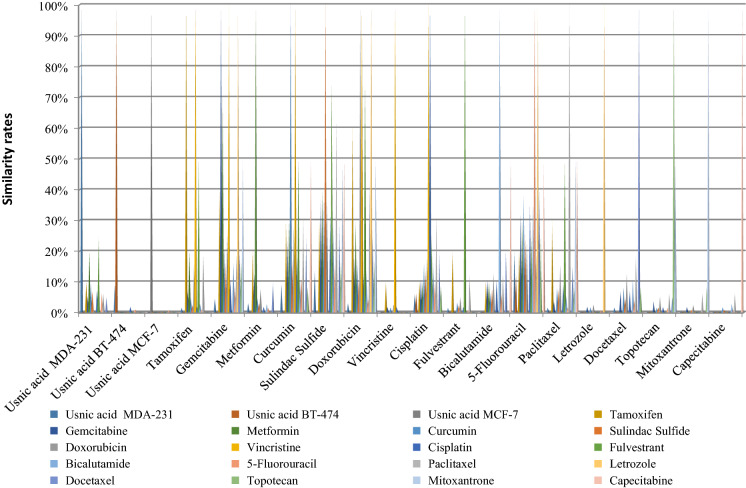
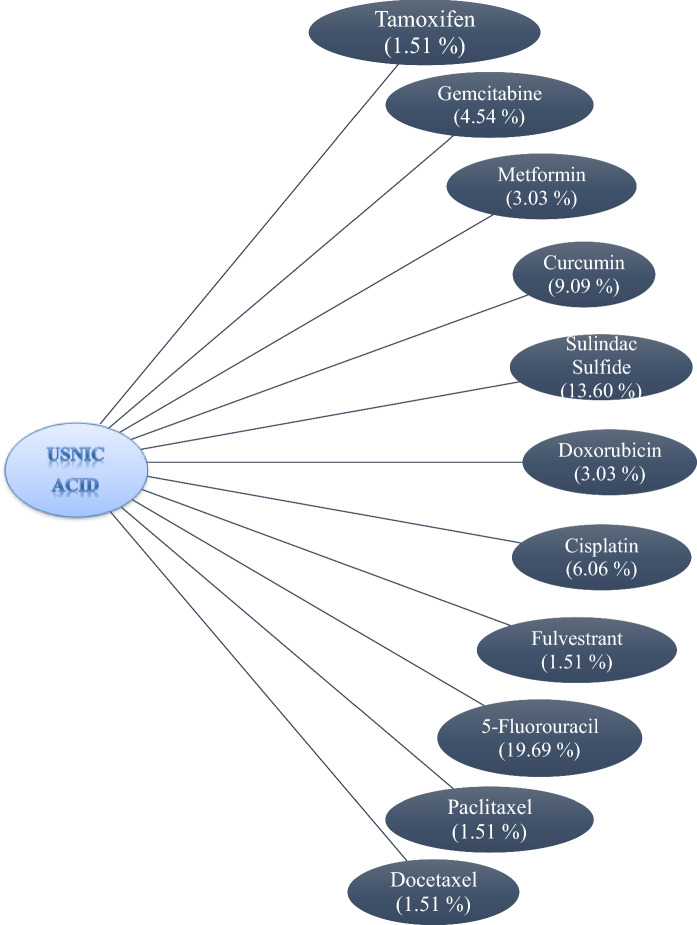
The number of miRNAs, which are common between 17 A-BCDM and usnic acid, were evaluated separately on the basis of drug (Table 2). According to these results, 33 miRNA Sulindac sulfide/5-Fluorouracil; 24 miRNA 5-Fluorouracil / Curcimin; 21 miRNA Sulindac sulfide/Gemcitabine; 18 miRNA Cisplatin / Sulindac sulfide was determined by common miRNA for all combination of 17 A-BCDM and usnic acid. Capecitabine demonstrated the lowest similarity profile which is showed common similarity with only 1 miRNA with 5 A-BCDM (Curcimin, Sulindac sulfide, Bicalutamide, 5-Fluorouracil, Paclitaxel) (Table 2). The similarity percentages of 17 A-BCDM and usnic acid based on miRNA expression profiles in breast cancer treatment are given in Table 3 and Fig. 1. The highest miRNA percentage rates are Metformin and Usnic acid, and therefore miRNAs that respond to two drugs have been compared. Metformin showed 70% similarity to Gemcitabine in terms of miRNA expression profile in breast cancer treatment, 40% similarity with Sulindac Sulfide, 30% similarity with Curcumin, 30% similarity with Doxorobucin, 30% similarity to 5-Fluorouracil, 20% similarity with tamoxifen and usnic acid, 10% similarity to Cisplatin and 10% similarity with Bicalutamide (Table 3). Usnic acid and 5-Fluorouracil have 13 common miRNA expression profiles for the treatment of breast cancer (19.69% similarity) which is the highest result (Fig. 2). Secondly, usnic acid and sulindac sulfide have showed 9 common miRNA profiles (13,60% similarity). Usnic acid was demonstrated the lowest similarity with 1 common miRNA profiles with Tamoxifen, Fulvestrant, Paclitaxel and Docataxel (1,51% similarity) (Fig. 2). When we compared the chemical molecular structure of usnic acid and 17 A-BCDM, we identified 5 different groups. 1. Group: Tertiary, secondary and primary amine group containing molecules: Tamoxifen, Metformin, Topotecan, Vincristine, Paclitaxel, Docetaxel. 2. Group: Fluorouracil-based molecules: Fluorouracil, Capecitabine and Gemcitabine. 3. Group: Sulfo or sulfone group containing molecules: Fulvestrant, Bicalutamide, Sulindac. 4. Group: Molecular structure is completely similar to molecules: Cisplatin and Letrozole. 5. Group: Phenolic molecules containing Acly group: Curcumin, Doxoruicin, Mitoxantrone. The Curcumin molecular structure has a functional part that is common to Usnic acid molecules, which are likely groups that can make similar hydrogen bonds and molecular interactions. Usnic acid and Tamoxifen are similar in that they have radical absorber and planar structure.
Table 2.
Common expressed miRNAs numbers between usnic acid and 17 anti-breast cancers
| Usnic acid MDA-MB-231 | Usnic acid BT-474 | Usnic acid MCF-7 | Tamoxifen | Gemcitabine | Metformin | Curcumin | Sulindac Sulfide | Doxorubicin | Vincristine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Usnic acid MDA-MB-231 | 66 | 6 | – | 1 | 3 | 2 | 6 | 9 | 2 | – |
| Usnic acid BT-474 | – | 16 | – | – | – | – | – | – | – | – |
| Usnic acid MCF-7 | – | – | 8 | – | – | – | – | – | – | – |
| Tamoxifen | 1 | – | – | 10 | 4 | 2 | 3 | 3 | 6 | 1 |
| Gemcitabine | 3 | – | – | 4 | 56 | 7 | 13 | 21 | 10 | 1 |
| Metformin | 2 | – | – | 2 | 7 | 10 | 3 | 4 | 3 | – |
| Curcumin | 6 | – | – | 3 | 13 | 3 | 62 | 23 | 11 | 1 |
| Sulindac sulfide | 9 | – | – | 3 | 21 | 4 | 23 | 131 | 14 | – |
| Doxorubicin | 2 | – | – | 6 | 10 | 3 | 11 | 14 | 39 | 1 |
| Vincristine | – | – | – | 1 | 1 | – | 1 | – | 1 | 1 |
| Cisplatin | 4 | 1 | – | 1 | 6 | 1 | 10 | 18 | 7 | 1 |
| Fulvestrant | 1 | – | – | 2 | – | – | 2 | 3 | 3 | – |
| Bicalutamide | – | – | – | 1 | 6 | 1 | 4 | 11 | 5 | – |
| 5-Fluorouracil | 13 | 2 | 1 | 2 | 18 | 3 | 24 | 33 | 9 | – |
| Paclitaxel | 1 | – | – | 3 | 3 | – | 5 | 10 | 7 | – |
| Letrozole | – | – | – | – | 1 | – | – | – | 1 | – |
| Docetaxel | 1 | – | – | – | 4 | 2 | 5 | 6 | 5 | – |
| Topotecan | – | – | – | – | 2 | – | 1 | 2 | 2 | – |
| Mitoxantrone | – | – | – | – | 1 | – | – | 1 | 1 | – |
| Capecitabine | – | – | – | – | – | – | 1 | 1 | – | – |
| Cisplatin | Fulvestrant | Bicalutamide | 5-Fluorouracil | Paclitaxel | Letrozole | Docetaxel | Topotecan | Mitoxantrone | Capecitabine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Usnic acid MDA-MB-231 | 4 | 1 | – | 13 | 1 | – | 1 | – | – | – |
| Usnic acid BT-474 | 1 | – | – | 2 | – | – | - | – | – | – |
| Usnic acid MCF-7 | – | – | – | 1 | – | – | – | – | – | – |
| Tamoxifen | 1 | 2 | 1 | 2 | 3 | – | – | – | – | – |
| Gemcitabine | 6 | – | 6 | 18 | 2 | 1 | 4 | 2 | 1 | – |
| Metformin | 1 | – | 1 | 3 | – | – | 2 | – | – | – |
| Curcumin | 10 | 2 | 4 | 24 | 5 | 1 | 5 | 1 | – | 1 |
| Sulindac Sulfide | 18 | 3 | 10 | 33 | 10 | – | 6 | 2 | 1 | 1 |
| Doxorubicin | 7 | 2 | 5 | 9 | 6 | 1 | 5 | 2 | 1 | – |
| Vincristine | 1 | – | – | – | – | – | – | – | – | – |
| Cisplatin | 56 | 1 | 6 | 20 | 5 | – | 4 | 1 | – | – |
| Fulvestrant | 1 | 4 | – | 1 | 2 | – | – | – | – | – |
| Bicalutamide | 6 | – | 37 | 13 | 2 | – | 4 | – | – | 1 |
| 5-Fluorouracil | 20 | 1 | 13 | 208 | 3 | 1 | 8 | 3 | – | 1 |
| Paclitaxel | 5 | 2 | 2 | 3 | 16 | – | 3 | 1 | 1 | 1 |
| Letrozole | – | – | – | 1 | – | 1 | – | – | – | – |
| Docetaxel | 4 | – | 4 | 8 | 3 | – | 20 | 1 | – | – |
| Topotecan | 1 | – | – | 3 | 1 | – | 1 | 12 | 1 | – |
| Mitoxantrone | – | – | – | – | 1 | – | – | 1 | 2 | – |
| Capecitabine | – | – | 1 | 1 | 1 | – | – | – | – | 2 |
Table 3.
Similarity rates (%) determined by common expressed miRNA numbers between usnic acid and 17 anti-breast cancer
| Usnic acid MDA-MB-231 | Usnic acid BT-474 | Usnic acid MCF-7 | Tamoxifen | Gemcitabine | Metformin | Curcumin | Sulindac sulfide | Doxorubicin | Vincristine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Usnic acid MDA-MB-231 | 100 | 9.1 | 0 | 1.5 | 4.5 | 3.0 | 9.1 | 13.6 | 3.0 | 0 |
| Usnic acid BT-474 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Usnic acid MCF-7 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tamoxifen | 10 | 0 | 0 | 100 | 40 | 20 | 30 | 30 | 60 | 10 |
| Gemcitabine | 5.4 | 0 | 0 | 7.1 | 100 | 12.5 | 23.2 | 37.5 | 17.9 | 1.8 |
| Metformin | 20 | 0 | 0 | 20 | 70 | 100 | 30 | 40 | 30 | 0 |
| Curcumin | 9.7 | 0 | 0 | 4.8 | 21 | 4.8 | 100 | 37.1 | 17.1 | 1.6 |
| Sulindac sulfide | 6.9 | 0 | 0 | 2.3 | 16.0 | 3.1 | 17.6 | 100 | 10.7 | 0 |
| Doxorubicin | 0.8 | 0 | 0 | 15.4 | 25.6 | 7.7 | 28.2 | 35.9 | 100 | 2.6 |
| Vincristine | 0 | 0 | 0 | 100 | 100 | 0 | 100 | 0 | 100 | 100 |
| Cisplatin | 7.1 | 1.8 | 0 | 1.8 | 10.7 | 1.8 | 17.9 | 32.1 | 12.5 | 1.8 |
| Fulvestrant | 25 | 0 | 0 | 50 | 0 | 0 | 50 | 75 | 75 | 0 |
| Bicalutamide | 0 | 0 | 0 | 2.7 | 16.2 | 2.7 | 10.8 | 29.7 | 13.5 | 0 |
| 5-Fluorouracil | 6.3 | 1 | 0.5 | 1 | 8.7 | 1.4 | 11.5 | 15.9 | 4.3 | 0 |
| Paclitaxel | 6.3 | 0 | 0 | 18.8 | 18.8 | 0 | 31.3 | 62.5 | 43.8 | 0 |
| Letrozole | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 100 | 0 |
| Docetaxel | 5 | 0 | 0 | 0 | 20 | 10 | 25 | 30 | 25 | 0 |
| Topotecan | 0 | 0 | 0 | 0 | 16.7 | 0 | 8.3 | 16.7 | 16.7 | 0 |
| Mitoxantrone | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 50 | 50 | 0 |
| Capecitabine | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | 0 |
| Cisplatin | Fulvestrant | Bicalutamide | 5-Fluorouracil | Paclitaxel | Letrozole | Docetaxel | Topotecan | Mitoxantrone | Capecitabine | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Usnic acid MDA-MB-231 | 6.1 | 1.5 | 0 | 19.7 | 1.5 | 0 | 1.5 | 0 | 0 | 0 | |
| Usnic acid BT-474 | 6.3 | 0 | 0 | 12.5 | - | 0 | 0 | 0 | 0 | 0 | |
| Usnic acid MCF-7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tamoxifen | 10 | 20 | 10 | 20 | 30 | 0 | 0 | 0 | 0 | 0 | |
| Gemcitabine | 10.7 | 0 | 10.7 | 32.1 | 3.6 | 1.8 | 7.1 | 3.6 | 1.8 | 0 | |
| Metformin | 10 | 0 | 10 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Curcumin | 16.1 | 3.2 | 6.5 | 38.7 | 8.1 | 1.6 | 8.1 | 1.6 | 0 | 1.6 | |
| Sulindac Sulfide | 13.7 | 2.3 | 7.6 | 25.2 | 7.6 | 0 | 4.6 | 1.5 | 0.8 | 0.8 | |
| Doxorubicin | 17.9 | 5.1 | 12.8 | 23.1 | 15.4 | 2.6 | 12.8 | 5.1 | 2.6 | 0 | |
| Vincristine | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cisplatin | 100 | 1.8 | 10.7 | 35.7 | 8.9 | 0 | 7.1 | 1.8 | 0 | 0 | |
| Fulvestrant | 25 | 100 | 0 | 25 | 50 | 0 | 0 | 0 | 0 | 0 | |
| Bicalutamide | 16.2 | 0 | 100 | 35.1 | 5.4 | 0 | 10.8 | 0 | 0 | 2.7 | |
| 5-Fluorouracil | 9.6 | 0.5 | 6.3 | 100 | 1.4 | 0.5 | 3.8 | 1.4 | 0 | 0.5 | |
| Paclitaxel | 31.3 | 12.5 | 12.5 | 18.8 | 100 | 0 | 18.8 | 6.3 | 6.3 | 6.3 | |
| Letrozole | 0 | 0 | 0 | 100 | 0 | 100 | 0 | 0 | 0 | 0 | |
| Docetaxel | 20 | 0 | 20 | 40 | 15 | 0 | 100 | 5 | 0 | 0 | |
| Topotecan | 8.3 | 0 | 0 | 25 | 8.3 | 0 | 8.3 | 100 | 8.3 | 0 | |
| Mitoxantrone | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 50 | 100 | 0 | |
| Capecitabine | 0 | 0 | 50 | 50 | 50 | 0 | 0 | 0 | 0 | 100 | |
Fig. 1.
miRNA numbers and similarity rates commonly expressed between usnic acid and 17 anti-breast cancer drugs
Fig. 2.
The similarity relationship between miRNAs responding to the usnic acid and the miRNAs responding to the anti-breast cancer drugs
The expression profiles of miRNAs, which were common in the treatment of breast cancer with 17 A-BCDM and usnic acid, were evaluated. After the treatment of usnic acid, 5 miRNA expression was down-regulated and 16 miRNA expression was upregulated (Fig. 3). The expression of mir-326 which is down-regulated has shown similar expression profile with other drugs. However, the expression of miR-4668-5p, miR-574-3p, miR-574-5p and miR-3149 has shown expression in different profile with other drugs as down-regulated in the treatment of usnic acid (Fig. 3). In the apply with usnic acid, only miR-1275 from 16 miRNAs that were up-regulated showed different expression profiles with other drugs. However, the other upregulated 15 miRNA showed similar expression profiles with 17 A-BCDM (Fig. 3).
Fig. 3.
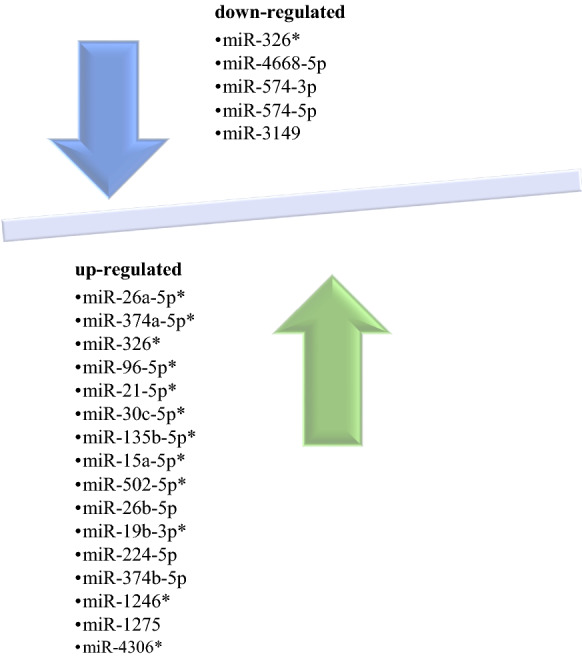
miRNAs of 17 anti-cancer drugs and usnic acid with similar expression levels (*: The common miRNA profiles of 17 anti-cancer drugs and usnic acid
We evaluated the study from another perspective to identify drugs specific to miRNA. In this case, miRNA-drug classification was achieved by identifying miRNAs, which are common expression between 17 A-BCDM and usnic acid in the treatment of breast cancer (Table 4). As a result, usnic acid correlated with 20 miRNA’s of 12 A-BCDM according to the data obtained from SMIR-NBI tool. In this way, 20 classes have created based on the same expression profile compared with the usnic acid and 17 anti-breast cancer drugs (Table 4). As shown in Table 4, mir-26a-5p as one of the 20 classes was determined as common miRNA for Tamoxifen, Gemcitabine, Metformin, Curcumin, Sulindac sulfur and Usnic acid.
Table 4.
Classification of drugs according to miRNAs, which are common expressions between 17 anti breast cancer drugs and usnic acid
| miR-26a-5p | miR-374a-5p | miR-326 | miR-96-5p | miR-21-5p | miR-30c-5p |
|---|---|---|---|---|---|
| Tamoxifen | 5-Fluorouracil | Gemcitabine | Metformin | Curcumin | Curcumin |
| Gemcitabine | Gemcitabine | Usnic acid | Sulindac sulfide | Sulindac Sulfide | Sulindac Sulfide |
| Metformin | Cisplatin | Usnic acid | Doxorubicin | Cisplatin | |
| Curcumin | Curcumin | Cisplatin | Paclitaxel | ||
| Sulindac sulfide | Sulindac sulfide | Fulvestrant | Usnic acid | ||
| Usnic acid | Usnic acid | Usnic acid |
| miR-135b-5p | miR-15a-5p | miR-502-5p | miR-26b-5p | miR-19b-3p | miR-4668-5p |
|---|---|---|---|---|---|
| Curcumin | Curcumin | Sulindac Sulfide | Sulindac Sulfide | Sulindac Sulfide | Cisplatin |
| 5-Fluorouracil | Sulindac sulfide | Docetaxel | 5-Fluorouracil | 5-Fluorouracil | Usnic acid |
| Doxorubicin | 5-Fluorouracil | Usnic acid | Usnic acid | Usnic acid | |
| Usnic acid | Usnic acid |
The target genes and pathways analysis of common miRNAs between usnic acid and 17 anti-breast cancer drugs
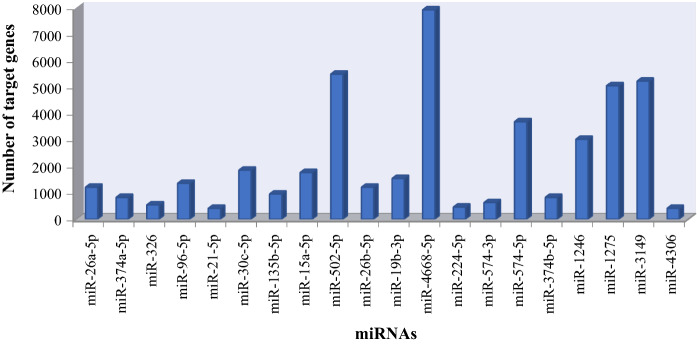
In the treatment of breast cancer, the target gene analysis was performed for miRNAs that were common expression between 17 A-BCDM and usnic acid. When we considered the miRNA target gene numbers, miRNAs, such as miR-4668-5p, miR-3149, seem to target more than 5000 genes. The miR-4306, which was the lowest target gene among the miRNAs, have 415 target genes. The target gene graph of the common expression miRNAs was given in Fig. 4.
Fig. 4.
The number of target gene of common miRNAs between usnic acid and 17 anti-breast cancer drugs
The pathway analysis of common miRNAs of 17 A-BCDM with usnic acid was determined by TargetScanHuman and KEGG analysis tools. Some of the common pathways such as apoptosis, ErbB signal pathway, p53 signal pathway, VEGF signal pathway belong to important pathways related to proliferation, cell cycle and apoptosis (Table 5).
Table 5.
Pathway analysis results of miRNAs common expressed between 17 anti-breast cancer drugs and usnic acid
| KEGG pathways of common miRNA's |
|---|
| hsa04012_ErbB signaling pathway |
| hsa04110_Cell cycle |
| hsa04115_p53 signaling pathway |
| hsa04210_Apoptosis |
| hsa04310_Wnt signaling pathway |
| hsa04510_Focal adhesion |
| hsa04530_Tight junction |
| hsa04722_Neurotrophin signaling pathway |
| hsa04810_Regulation of actin cytoskeleton |
| hsa05200_Pathways in cancer |
| hsa05210_Colorectal cancer |
| hsa05212_Pancreatic cancer |
| hsa05213_Endometrial cancer |
| hsa05214_Glioma |
| hsa05215_Prostate cancer |
| hsa05216_Thyroid cancer |
| hsa05211_Renal cell carcinoma |
| hsa05218_Melanoma |
| hsa05219_Bladder cancer |
| hsa05220_Chronic myeloid leukemia |
| hsa05222_Small cell lung cancer |
| hsa05223_Non small cell lung cancer |
| hsa04010_MAPK signaling pathway |
| hsa00982_Drug metabolism cytochrome P450 |
| hsa03430_Mismatch repair |
| hsa04370_VEGF signaling pathway |
| hsa05221_Acute myeloid leukemia |
Experimental validation of tamoxifen and usnic acid by real-time xCELLigence analysis
Tamoxifen has a low side effect, so it is widely used for treatment of ER-positive breast cancer tumours (White 1999). Many clinical studies determined that because of the lack of hormone receptors in the most aggressive subtype of breast cancer, Triple Negative Breast Cancer (TNBC), it cannot be treated with drugs such as Tamoxifen (Fornander et al. 1989). However, in recent studies have revealed tamoxifen can target not only ERα- but also estrogen receptor subtype beta (ERβ) in TNBC and determine of several ER-independent mechanisms in ER-negative breast cancer cells treated with tamoxifen (Manna and Holz 2015; Huang et al. 2014). All these reasons suggest that tamoxifen may have effect on triple negative breast cancer. We also aimed to determine if usnic acid is as effective as tamoxifen. Since treatment success and survival rates for TNBC are very low, researchers have been focusing on the development of new anti-TNBC agents that can specifically target TNBC with minimal impact on non-cancerous tissue in recent years. The highest level of miRNA response after usnic acid apply in MDA-MB-231 was necessitated the determination of the effect of usnic acid and tamoxifen on the triple negative cell line in this study.
The aim of this experiment is to confirm the similarity between Tamoxifen one of the 17 A-BCDM used in routine treatment and usnic acid at the cellular level. TNBC cell was used in our validation study because the highest number of miRNAs responding after usnic acid administration was MDA-MB-231. MCF-12A cell was used to determine the condition in non-cancerous breast cell. The anti-proliferative effect of usnic acid on MDA-MB-231 and MCF-12A cells was demonstrated by XCELLigence system. Thus, the antiproliferative effect of usnic acid, the drug candidate molecule, was determined by the most sensitive and effective method.
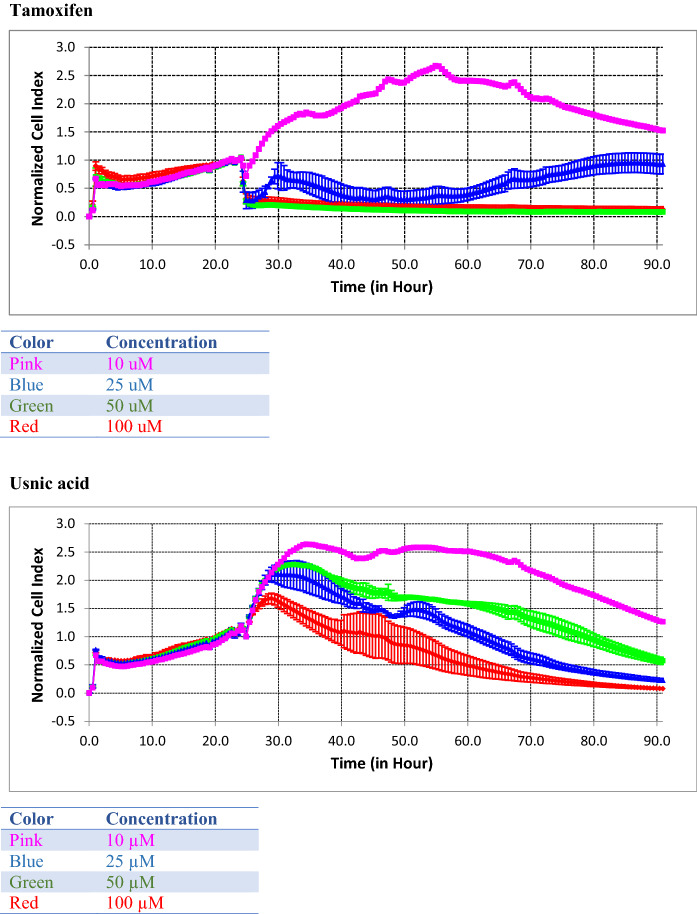
As a result, IC50 concentrations in the MDA-MB-231 cell line were determined for usnic acid and tamoxifen. According to the real-time cell analysis system performed in the triple negative cell line, the IC50 concentration of Tamoxifen is 1.26E−05 M and the IC50 concentration of usnic acid is 1.21E−05 M (Fig. 5). Usnic acid showed effect on TNBC cell at similar concentrations as tamoxifen. This result shows us that usnic acid is a candidate molecule for the treatment of breast cancer, in particular on TNBC cells.
Fig. 5.
The determination of antiproliferative effect for tamoxifen and usnic acid by XCELLigence assay
The validation of miR-26a-5p expression by qRT-PCR
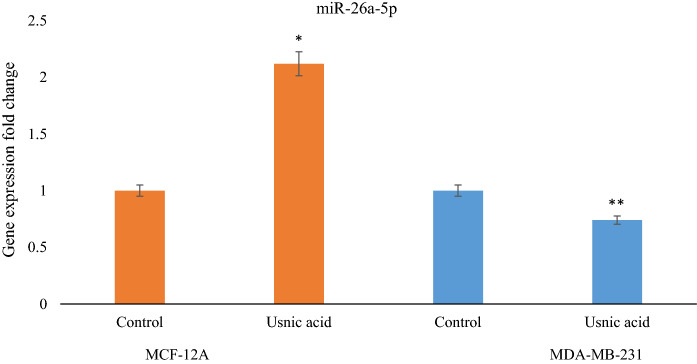
Among the usnic acid and 17 A-BCDM, miRNAs that respond to the largest number of 5 drugs are miR-26a-5p and miR-37a-5p. miR-26a-5p was used in our study because it showed a similar expression profile with tamoxifen. When the miRNA profile differentiation of 5 A-BCDM in breast cancer cell was examined, it was determined that the expression level of miR-26a-5p decreased. The expression level of miR-26a-5p was determined for validation in MDA-MB-231 breast cancer and MCF-12A normal cells after apply to usnic acid. According to the results of qRT-PCR analysis in MDA-MB-231 and MCF-12A cell lines treated with usnic acid, the expression of mir-26a-5p was increased by 2.12 times in normal cell when usnic acid was applied. In the MDA-MB-231 cell line, the expression of mir-26a-5p decreased by 1.34 times when usnic acid was applied (Fig. 6). According to these results, the decrease in the expression level of miR-26a-5p was similar to that of 5 anti-cancer drugs and usnic acid.
Fig. 6.
The expression analyses for miR-26a-5p on MDA-MB-231 and MCF-12A cell lines treated with usnic acid by usnig qRT-PCR assay. (*p < 0.01) (**p < 0.001)
The cell proliferation analysis of miR-26a-5p
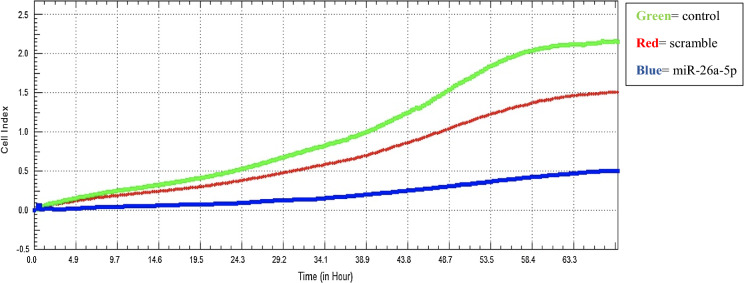
Real time cell analyzer results determined significant antiproliferative effect by miR-26a-5p mimic compared to the scramble control in MDA-MB-231 cell culture (Fig. 7). These findings refer that miR-26a-5p is a potential tumor suppressor and may be used as a potential marker in breast cancer.
Fig. 7.
Antiproliferative effect of miR-26a-5p on MDA-MB-231 (green = control, red = scramble, blue:miR-26a-5p). Each transfection is quantified in duplicate (p < 0.001). (Color figure online)
Discussion
Triple negative breast cancer (TNBC) mainly demonstrate aggressive behaviour among breast cancer cells and have very limited option of therapy. Due to the complexity of TNBC, there are currently no drugs can effectively treatment option. Many anticancer drug are toxic and side effects in cancer patients. Therefore, there is a need to identify new approaches that do not damage normal cells and make it possible to determine the activity of cancer cell-targeted drug candidate molecules. Natural products isolated from biological materials have provided a potential source of therapeutic approach for cancer treatment. A number of in vitro studies have shown an effect of usnic acid on inhibiting the growth of cancer cell (Dinçsoy and Cansaran-Duman 2017; Yurdacan et al. 2019; Tanman et al. 2019; Pyrczak-Felczykowska et al. 2019; Venkata Mallavadhani et al. 2019). Another approach is also that miRNAs that respond to drugs used in cancer treatment can be used in cancer diagnosis and treatment (Rupaimoole and Slack et al. 2017).
Preclinical studies revealed the non-toxic profile of usnic acid and its low cost for large-volume production. Therefore, the effect of usnic acid on breast cancer points out that more research is needed. The further studies and clinical trials will be merged with the development and effective use of usnic acid as a potential anticancer therapy. In a previous studies determined a new role of usnic acid on the regulation of miRNAs in breast cancer (Kılıç et al. 2019). This study, which reveals the relationship between usnic acid and miRNA, is the first assessment in the literature for breast cancer treatment. Our previous studies results demonstrated that usnic acid treatment led to mainly altered expression of miR-4456, miR-1908-3p, miR-6833-3p and miR-7152-5p on MDA-MB-231 breast cancer cell. Kim et al. (2019) demonstrated that quercetin as a natural sources, the therapeutic potential of quercetin on cancer by targeting pathways and related miRNAs (Kim et al. 2019). In this context, it has been shown that molecules of biological origin derived from nature have regulatory effects of miRNAs that play a role in cancerogenesis.
In this study, we aimed to compare the miRNAs formed against the drugs used in routine treatment in the SMiR database (https://lmmd.ecust.edu.cn/database/smir-nbi/) and the miRNAs that respond to usnic acid in breast cancer. Li et al. developed novel computational tool, namely the SMiR-NBI model for demonstrates to miRNAs and target genes profile of 17 A-BCDM for used as potential biomarkers in breast cancer (Li et al. 2016). The common and different miRNA profiles were evaluated to understand the molecular characterization of drugs and usnic acid for treatment of breast cancer. This study revealed that usnic acid and Letrozole, Mitoxantrone, Capecitabine and Vincristine had the most different miRNA profile with usnic acid. The reason is that these four anti-cancer drugs contain very few experimentally verified miRNAs in SMiR tool. It is thought that the similarity level is low due to the low number of miRNAs experimentally confirmed in the SMiR Tool.
Creighton et al. developed that the software computes (https://sigterms.sourceforge.net.) which included results from PicTar, TargetScan, and miRanda algorithms for determining to implicate roles for specific microRNAs and microRNA-regulated genes (Creighton et al. 2008). Wen-Tsong et al. established a web based platform and this platform can be easily accessed at https://ppi.bioinfo.asia.edu.tw/pathway/ for cancer biology research which contain integrate transcription factors, microRNAs, miRNA targets and network motifs information (Hsieh et al. 2015). Kedaigle and Fraenkel found integrative analyses tool for determining relationships between molecules and pathways (Kedaigle and Fraenkel 2018). Mejia-Pedroza et al. developed a tool that compares information obtained from biological and pharmacological databases containing data on pathways and drugs in breast subtypes and disease-specific experimental transcriptomic results (Mejia-Pedroza et al. 2018). We chose the SMiR tool in our study because it is a database containing small molecule drugs such as usnic acid, a specific tool containing breast cancer data and contains data from 17 different anti-cancer breast cancer drugs.
The most similar anti-cancer drugs with usnic acid are 5-Fluorouracil with 19.69% and Sulindac Sulfide(13,60%), while Tamoxifen, Paclitaxel and Docetaxel are the least similar drugs with usnic acid. Subsequently, we investigated similarities of usnic acid and anti-cancer drugs at miRNA level and we were revealed 20 common miRNAs. As a result of study, compared with usnic acid and anti-cancer drugs was formed 20 different category. Usnic acid has shown the most common association with 5- Fluorouracil with a similarity rate in 13 category within these different 20 category. Thus, the most similar drug to usnic acid in terms of miRNA characterization is 5-fluorouracil. When we compared the chemical molecular structure of usnic acid and 17 anti-breast cancer drugs, we identified 5 different groups. 1. Group: Tertiary, secondary and primary amine group containing molecules: Tamoxifen, Metformin, Topotecan, Vincristine, Paclitaxel, Docetaxel. 2. Group: Fluorouracil-based molecules: Fluorouracil, Capecitabine and Gemcitabine. 3. Group: Sulfo or sulfone group containing molecules: Fulvestrant, Bicalutamide, Sulindac. 4. Group: Molecular structure is completely not similar to other molecules: Cisplatin and Letrozole. 5. Group: Phenolic molecules containing Acyl group: Curcumin, Doxoruicin, Mitoxantrone. Usnic acid shows structural similarity with Fluorouracil anti-cancer drug with fluoracil group. Similarly, the sulfo and sulfone groups of usnic acid has common similarity with sulindac sulfate. In SMIR tool, the reason why usnic acid shows high similarity with Fluorouracil and sulindac sulfate may be similar in chemical structure and functional group. The Curcumin molecular structure has a functional part that is common to Usnic acid molecules, which are likely groups that can make similar hydrogen bonds and molecular interactions. Usnic acid and Tamoxifen are similar in that they have radical absorber and planar structure. Shah et al. (2011) showed 5-Fluorouracil significantly changes the expression level of miRNAs. After 48 h of treatment with a 0.01 µM, 42 miRNAs were significantly expressed in MCF-7 cell. Of these miRNAs, 23 miRNAs were up‐regulated, but 19 were down‐regulated (Shah et al. 2011). In this study, the expression profiles of miRNAs co-expressed with usnic acid and 17 anti-cancer drugs were evaluated. miR-1275 showed up-expression profile and 16 miRNAs determined down-expressed compared with miRNAs response usnic acid. However, one of the 5 up-regulated miRNAs, mir-326 showed a similar profile with other drugs. The common miRNAs will provide a new perspective into cancer chemotherapy and designing of novel anticancer drugs.
We performed pathway analysis of miRNAs of A-BCDM and thus we determined the pathway analysis of miRNAs specific to breast cancer. As a result of the pathway analysis, common pathways of miRNAs were selected. The common pathways were determined as apoptosis, p53 signaling, MAPK signaling, drug metabolism cytochrome P450, VEGF signaling and cell cycle pathways. The target genes of miRNAs were also investigated. It was observed that these miRNAs have important roles in the control of cellular processes by targeting transcripts in the range of 415 to 5000. Many studies indicated that usnic acid inhibits growth by suppressing VEGFR-2 mediated AKT and ERK ½ signaling pathways (Song et al. 2012), apoptosis and cell cycle arrest (Singh et al. 2013; Dinçsoy and Cansaran-Duman 2017). In a previous study determined that usnic acid response miRNAs played role in five pathways which were cell carcinoma, neurotrophin signaling, gap junction, Hedgehog signaling and apoptosis pathways (Kılıç et al. 2019). According to the results, a similar pathways was found between 17 anti-cancer drugs and usnic acid. These results revealed usnic acid molecule may have effected on breast cancer by the same pathways as other 17 anti-cancer drugs.
In order to observe the effect of usnic acid on TNBC, the antiproliferative effect of usnic acid was performed with tamoxifen which is one of the most used drugs in breast cancer treatment in this study. As a result of this analysis, IC50 values for triple negative breast cancer cells were 1.26E−05 M for tamoxifen and 1.21E−005 M for usnic acid. Tamoxifen determined to be an effective therapeutic option for ER-positive breast cancer, but it was also founded response in ER-negative breast cancer in recent years due to ERβ may serve as a the steroid hormone receptor family as predictive factor in of tamoxifen sensitivity in TNBC (Yan et al. 2013; Manna et al. 2015). Therefore, usnic acid has been shown to be as effective as tamoxifen which is one of the most commonly used drugs for the treatment of TNBC.
Kim et al. evaluated the possible effects of chemotherapeutic drug therapy on the expression profile of miRNAs. They have determined the biological and pharmacological potential of quercetin by combining different data sets and increasing the use of bioinformatics tools that include miRNA-mediated gene editing networks (Kim et al. 2019). Thus, functional experiments should be performed strictly to verify the miRNAs and its targets in the further studies. Researchers determined some of the significantly dysregulated miRNAs and perform verification experiments to confirm their targets and then point out the functional role of miRNAs and the underlying mechanisms in miRNA and breast cancer.
In addition, the expression level of miRNA was determined by applying usnic acid on MDA-MB-231 breast cancer and MCF-12A non-cancerous epithelial cell. We aimed to determine the gene expression of miR-26a-5p in this study since miR-26a-5p is specific for 5 anticancer drugs (Tamoxifen, Gemcitabine, Metformin, Curcumin, Sulindac Sulfide) and usnic acid. miR-26a-5p was up-regulated in cancer cells compared to normal cells, but the level of expression miR-26a-5p decreased in breast cancer cells by application of usnic acid. miR-26a-5p targets important genes such as CDK6, MYC in cell cycle process and FOXO3 in apoptotic process. In breast cancer, 29 miRNAs were found to be different from normal cell. For example, miR-21 and miR-155 have been found to be over-expressed in breast tumor tissues, and the presence of miR-195 in circulation has been potentially to be an ideal breast tumor marker. Some articles have indicated that the role of miR-26a-5p in breast cancer. Huang et al. was reported miR-26a-5p has a complementary role in cells proliferation, invasion and apoptosis of rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS) (Huang et al. 2019). Lv et al. determined snoRNAs and their host genes (SNHG6) contributed to malignant breast cancer behaviors by regulating miR-26a-5p/ MAPK6 (Lv et al. 2019). It was evaluated suppression of the expression of genes in apoptotic and cell cycle process by usnic acid and in re-arranging the disrupted cell cycle process in the cancer cell and directing them to apoptosis.
Conclusion
It is the most important step in the development of miRNA-based therapeutics to identify the best miRNA candidates or miRNA targets for cancer disease. Experimental results can be supported by data from bioinformatics tools and the role of miRNA therapeutics in therapy can be activated. It is clear that usnic acid has potential in the treatment of breast cancer. As an alternative to drugs used in routine treatment, the effect of usnic acid and usnic acid response miRNAs in breast cancer should be supported by further research.
Acknowledgements
We thank Ankara University, Management of Scientific Research Projects (Project no. 15B0415001 and 16H0415002), for the financial support.
Compliance with ethical standarts
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bartel D. MicroRNAs. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Comprehensive molecular portraits of human breast tumours (2012) Nature 490:61–70 [DOI] [PMC free article] [PubMed]
- Creighton C, Nagaraja A, Hanash S, Matzuk M, Gunaratne P. A bioinformatics tool for linking gene expression profiling results with public databases of microRNA target predictions. RNA. 2008;14:2290–2296. doi: 10.1261/rna.1188208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard K, Hanna W, Kahn H, Sawka C, Lickley L, Rawlinson E, Sun P, Narod S. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Dinçsoy A, Cansaran Duman D. Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite. Turk J Biol. 2017;41:484–493. [Google Scholar]
- Esquela-Kerscher A, Slack F. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Fornander T, Cedermark B, Mattsson A, Skoog L, Theve T, Askergren J, Rutqvist L, Glas U, Silfverswärd C, Somell A, Wilking N, Hjalmar M. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;333:117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- Fyles A, McCready D, Manchul L, Trudeau M, Merante P, Pintilie M, Weir L, Olivotto I. Tamoxifen with or without Breast Irradiation in Women 50 Years of Age or Older with Early Breast Cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- Geng X, Zhang X, Zhou B, Zhang C, Tu J, Chen X, Wang J, Gao H, Qin G, Pan W. Usnic acid induces cycle arrest, apoptosis, and autophagy in gastric cancer cells in vitro and in vivo. Med Sci Monit. 2018;24:556–566. doi: 10.12659/MSM.908568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W, Liu L. The role of traditional chinese herbal medicines in cancer therapy—from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- Hsieh W, Tzeng K, Ciou J, Tsai J, Kurubanjerdjit N, Huang C, Ng K. Transcription factor and microRNA-regulated network motifs for cancer and signal transduction networks. BMC Syst Biol. 2015;9:S5. doi: 10.1186/1752-0509-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Warner M, Gustafsson J. Estrogen receptors in breast carcinogenesis and endocrine therapy. Mol Cell Endocrinol. 2014;418:240–244. doi: 10.1016/j.mce.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Huang Z, Xing S, Liu M, Deng W, Wang Y, Huang Z, Huang Y, Huang X, Wu C, Guo X, Pan X, Jiang J, Feng F, Li T. MiR-26a-5p enhances cells proliferation, invasion, and apoptosis resistance of fibroblast-like synoviocytes in rheumatoid arthritis by regulating PTEN/PI3K/AKT pathway. Biosci Rep. 2019;39:BSR20182192. doi: 10.1042/BSR20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedaigle A, Fraenkel E. Turning omics data into therapeutic insights. Curr Opin Pharmacol. 2018;42:95–101. doi: 10.1016/j.coph.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kılıç N, Aras S, Cansaran-Duman D. Determination of vulpinic acid effect on apoptosis and mRNA expression levels in breast cancer cell lines. Anti-Cancer Agents Med Chem. 2018;18(14):2032–2041. doi: 10.2174/1871520618666180903101803. [DOI] [PubMed] [Google Scholar]
- Kılıç N, Islakoğlu Y, Büyük İ, Gür-Dedeoğlu B, Cansaran-Duman D. Determination of usnic acid responsive miRNAs in breast cancer cell lines. Anticancer Agents Med Chem. 2019;19:1463–1472. doi: 10.2174/1871520618666181112120142. [DOI] [PubMed] [Google Scholar]
- Kim D, Khan H, Ullah H, Hassan S, Šmejkal K, Efferth T, Mahomoodally M, Xu S, Habtemariam S, Filosa R, Lagoa R, Rengasamy K. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol Res. 2019;147:104346. doi: 10.1016/j.phrs.2019.104346. [DOI] [PubMed] [Google Scholar]
- Kinghorn A, de Blanco E, Lucas D, Rakotondraib H, Orjala J, Soejarto D, Oberlies N, Pearce C, Wani M, Stockwell B, Burdette J, Swanson S, Fuchs J, Phelps M, Xu L, Zhang X, Shen Y. Discovery of anticancer agents of diverse natural origin. Anticancer Res. 2016;36:5623–5638. doi: 10.21873/anticanres.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Mishra J, Singh R. Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and γ-H2A.X phosphorylation. Chem Biol Interact. 2019;315:108898. doi: 10.1016/j.cbi.2019.108898. [DOI] [PubMed] [Google Scholar]
- Li J, Lei K, Wu Z, Li W, Liu G, Liu J, Cheng F, Tang Y. Network-based identification of microRNAs as potential pharmacogenomic biomarkers for anticancer drugs. Oncotarget. 2016;7:45584. doi: 10.18632/oncotarget.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C, Mazouni C, Hess K, André F, Tordai A, Mejia J, Symmans W, Gonzalez-Angulo A, Hennessy B, Green M, Cristofanilli M, Hortobagyi G, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- Lv P, Qiu X, Gu Y, Yang X, Xu X, Yang Y. Long non-coding RNA SNHG6 enhances cell proliferation, migration and invasion by regulating miR-26a-5p/MAPK6 in breast cancer. Biomed Pharmacother. 2019;110:294–301. doi: 10.1016/j.biopha.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Manna S, Bostner J, Sun Y, Miller L, Alayev A, Schwartz N, Lager E, Fornander T, Nordenskjöld B, Yu J, Stål O, Holz M. ERRα is a marker of tamoxifen response and survival in triple-negative breast cancer. Clin Cancer Res. 2015;22:1421–1431. doi: 10.1158/1078-0432.CCR-15-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía-Pedroza R, Espinal-Enríquez J, Hernández-Lemus E. Pathway-based drug repositioning for breast cancer molecular subtypes. Front Pharmacol. 2018;9:905. doi: 10.3389/fphar.2018.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, Ito Y, Tokudome N, Toi M, Kuroi K, Suzuki E. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2010;17:199–204. doi: 10.1007/s12282-009-0139-3. [DOI] [PubMed] [Google Scholar]
- Nguyen VK, Sichaem J, Nguyen HH, Nguyen XH, Huynh TTL, Nguyen TP, Niamnont N, Mac DH, Pham DD, Chavasiri W (2019) Synthesis and cytotoxic evaluation of usnic acid benzylidene derivatives as potential anticancer agents. Nat Prod Res 1–10 [DOI] [PubMed]
- Perou C, Sørlie T, Eisen M, van de Rijn M, Jeffrey S, Rees C, Pollack J, Ross D, Johnsen H, Akslen L, Fluge Ø, Pergamenschikov A, Williams C, Zhu S, Lønning P, Børresen-Dale A, Brown P, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pyrczak-Felczykowska A, Narlawar R, Pawlik A, Guzow-Krzemińska B, Artymiuk D, Hać A, Ryś K, Rendina L, Reekie T, Herman-Antosiewicz A, Kassiou M. Synthesis of usnic acid derivatives and evaluation of their antiproliferative activity against cancer cells. J Nat Prod. 2019;82:1768–1778. doi: 10.1021/acs.jnatprod.8b00980. [DOI] [PubMed] [Google Scholar]
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: indexing natural products for their anticancer bioactivity. PLoS ONE. 2017;12:e0187925. doi: 10.1371/journal.pone.0187925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole R, Slack F. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- Shah M, Pan X, Fix L, Farwell M, Zhang B. 5-fluorouracil drug alters the microrna expression profiles in MCF-7 breast cancer cells. J Cell Physiol. 2011;226:1868–1878. doi: 10.1002/jcp.22517. [DOI] [PubMed] [Google Scholar]
- Simpson P, Reis-Filho J, Gale T, Lakhani S. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- Singh N, Nambiar D, Kale R, Singh R. Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr Cancer. 2013;65:36–43. doi: 10.1080/01635581.2013.785007. [DOI] [PubMed] [Google Scholar]
- Song Y, Dai F, Zhai D, Dong Y, Zhang J, Lu B, Luo J, Liu M, Yi Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis. 2012;15:421–432. doi: 10.1007/s10456-012-9270-4. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou C, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen M, van de Rijn M, Jeffrey S, Thorsen T, Quist H, Matese J, Brown P, Botstein D, Lonning P, Borresen-Dale A. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanman Ü, Yangın S, Cansaran-Duman D (2019) Determination of dysregulated mirna expression levels by qRT-pcr after the application of usnic acid to breast cancer. Anticancer Agents Med Chem 19 [DOI] [PubMed]
- van Rooij E, Purcell A, Levin A. Developing MicroRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- Venkata Mallavadhani U, Vanga N, Balabhaskara Rao K, Jain N (2019) Synthesis and antiproliferative activity of novel (+)- usnic acid analogues. J Asian Nat Prod Res 1–16 [DOI] [PubMed]
- Weigelt B, Reis-Filho J. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- White I. The tamoxifen dilemma. Carcinogenesis. 1999;20:1153–1160. doi: 10.1093/carcin/20.7.1153. [DOI] [PubMed] [Google Scholar]
- Yan Y, Li X, Blanchard A, Bramwell V, Pritchard K, Tu D, Shepherd L, Myal Y, Penner C, Watson P, Leygue E, Murphy L. Expression of both Estrogen Receptor-beta 1 (ER-β1) and its co-regulator Steroid Receptor RNA Activator Protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with Estrogen Receptor-alpha (ER-α)-Negative Early Breast Cancer (EBC) Ann Oncol. 2013;24:1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- Yangın S, Tanman Zıplar Ü, Cansaran-Duman D. Pharmaceutical applications based on next generation sequencing technology in oncologic drug development. Turk Hij Den Biyol Derg. 2019;76:473–486. [Google Scholar]
- Yu L, Ma X, Zhang L, Zhang J, Gao L. Prediction of new drug indications based on clinical data and network modularity. Sci Rep. 2016;6:32530. doi: 10.1038/srep32530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Su R, Wang B, Zhang L, Zou Y, Zhang J, Gao L. Prediction of novel drugs for hepatocellular carcinoma based on multi-source random walk. IEEE/ACM Trans Comput Biol Bioinform. 2017;14:966–977. doi: 10.1109/TCBB.2016.2550453. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhao J, Gao L. Predicting potential drugs for breast cancer based on miRNA and tissue specificity. Int J Biol Sci. 2018;14:971–982. doi: 10.7150/ijbs.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurdacan B, Egeli U, Guney Eskiler G, Eryilmaz I, Cecener G, Tunca B. Investigation of new treatment option for hepatocellular carcinoma: a combination of sorafenib with usnic acid. J Pharm Pharmacol. 2019;71:1119–1132. doi: 10.1111/jphp.13097. [DOI] [PubMed] [Google Scholar]