Abstract
The potassium transporter high-affinity K+ transporter/K+ uptake permease/K+ transporter (HAK/KUP/KT) family plays a vital role in potassium uptake, and potassium ion (K+)-mediated environmental stress. In the present study, we identified 22 IbHAK/KUP/KT (HAK) genes in sweet potato [Ipomoea batata (L.) Lam] and the same number of HAK genes from sweet potato wild relative Ipomoea trifida. Phylogeny analysis indicated that the HAKs can be divided into five clades. Chromosomal distribution and genome synteny analyses revealed two tandem-duplicated gene pairs IbHAK16/17 and IbHAK17/18 on chromosomes 13 and eight segmental-duplicated gene pairs on chromosomes 1, 3, 5, 8, 10, 12, 14 among the IbHAK gene family. Eleven orthologous HAK gene pairs between I. batata and I. trifida were involved in the duplication of genomic blocks based on comparative genomic analysis. The Ka/Ks ratios of these IbHAK genes ranged from 0.02 to 0.55(< 1), further indicated that purifying selection was the primary force driving the evolution of HAKs in Ipomoea. A heat map based on RNA-seq data showed that 13 HAKs in Xushu32 (a K+-tolerant sweet potato genotype) and 10 HAKs in Ningzi1 (a K+-sensitive sweet potato genotype) in response to K+ deficiency stress. Quantitative real-time PCR (qRT-PCR) analysis revealed IbHAK2, -3, -8, -10, -11, -18, -19, and -21 were induced in both Xushu32 and Ningzi1 under low K+ stress. Compared with other IbHAK genes, IbHAK8 showed more strongly upregulation after exposure to drought and salt stress. Furthermore, co-expression analysis showed that only IbHAK8 of 22 IbHAK genes involved in network interactions with 30 genes related to abiotic and biotic stresses. Taken together, these results are helpful for further functional studies on IbHAK and molecular breeding of sweet potato.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-020-02552-3.
Keywords: Sweet ptotato, HAK/KUP/KT family, IbHAK, Identification, Gene expression
Introduction
Potassium ions (K+) are essential nutrients in plants and are involved in a range of biochemical processes and responses to various environmental stresses (Hedrich 2012). Plant potassium deficiency can result in weak stems, easy lodging, and decreased tolerance to abiotic and biotic stresses because of degradation of proteins and chlorophyll (Stewart et al. 2005; Ashley et al. 2006). K+ also contribute to the quality and production of sweet potato [Ipomoea batata (L.) Lam], which is the seventh-largest crop in the world. As the typical “K+ favoring” food crop, it needs significant supplementation of potassium fertilizer during the swelling stage of tuberous roots (Chen et al. 2013). The K+ concentration in the soil of sweet potato-growing areas in southern China, however, is usually low (< 2%), which in turn reduces sweet potato quality and production, such as sucrose conversion, starch synthesis, and root dry weight (Tang et al. 2014).
Two mechanisms for potassium uptake operate through the potassium transport system- that is, at low (< 0.2 mM) or high (> 0.5 mM) potassium concentrations (Gierth and Maser 2007). K+ transporters in plants can be classified into four major families: HAK/KUP/KT family (HAK family), Trk/HKT family, KEA (K + efflux anti-porter) family, and CHX (cation/hydrogen exchanger) family (Szczerba et al. 2009; Véry and Sentenac 2003; Maser et al. 2002). Among these, the HAK/KUP/KT family is the largest K + transporter family in plants and has been identified in bacteria, fungi, and plants (Li et al. 2018). HAK/KUP/KT K+ transporters play versatile roles in K+ acquisition and transport (Nieves-Cordones et al. 2016). AtKUP1 in Arabidopsis and HvHAK1 in barley were first identified based on their homologs in fungal HAK and bacterial KUP, and yeast complement experiments revealed that these function in K+ uptake (Kim et al. 1998; Santa-María et al. 1997). A total of 13 AtKUPs with K+ transport domains have been identified in Arabidopsis and most of the AtKUPs (KUP1, 2, 4, 5–7, 10, and 11) complement an Escherichia coli mutant that lacks a K+ uptake gene (Ahn et al.2004; Adams et al. 2019; Fu et al.1998; Maser et al. 2001).
HAK/KUP/KT family members are located on the inner membrane, mediate ion transport, play roles in membrane systems, and are expressed in various tissues. AtHAK5 was detected in membrane fractions and can be induced by K+ deprivation to relocate from the endoplasmic reticulum (ER) to vacuolar membranes (Gierth et al. 2005). AtKUP2 has been localized to the plasma membrane and mitochondria through the expression of AtKUP2-GFP fusion protein in Nicotiana benthamiana leaf epidermal cells (Rajappa et al. 2020). A total of 27 ZmHAKs have been identified from maize, of which 25 were predicted to be located on the plasma membrane and two others were located on the chloroplast and ER. Approximately 27 OsHAKs in rice, which possesses 11–15 transmembrane domains, are expressed in the roots, seeds, leaves, and stems, which play important roles in K+ transport and regulate growth and development (Gupta et al. 2008).
Phylogenetic analysis has grouped plant HAK/KUP/KT genes into four to five clades with functional divergence (Gomez-Porras et al. 2012; Very et al. 2014; Nieves-Cordones et al. 2016). The members of HAK genes in each clade are not evenly distributed between dicotyledons and monocotyledons, and clades Ib and IV are absent in cruciferae (Nieves-Cordones et al. 2016). HAK genes family members are highly diverse in terms of tissue-specific expression patterns, which may support their role in different K+ affinity and K+-mediated developmental processes (Gupta et al. 2008). Clade I, which includes AtHAK5, OsHAK1, and OsHAK5, has been associated with high-affinity K+ uptake and mostly expressed in root parts, which can help plant roots absorb K+ under K+ deficiency stress environment (Gierth et al. 2005). AtHAK5 was the only system capable of taking up K+ at extremely low K+ concentrations (< 10 μM) in Arabidopsis (Nieves-Cordones et al. 2010). OsHAK5 is mainly expressed in root epidermal cells, the pericycle, and vascular bundle tissues and plays an important role in K+ accumulation in rice during growth. Moreover, other ions also may mediate the regulatory mechanism of high-affinity K+ uptake. NH4+ can inhibit the expression of HvHAK1 (Santa-Maria et al. 2000), whereas Na+ increases the expression of AtHAK5 under K+-deficient conditions. AtHAK5 and AtKUP9 also contribute to high-affinity Cs+ uptake, even when it is toxic to plants (Alemán et al. 2014; Kobayashi 2010). Clade II, including AtKUP2, is involved in low-affinity K+ uptake, regulated plant growth, and cell size, and expressed in the leaves and shoot vasculature, flowers, stems, siliques, and root steles (Elumalai et al. 2002; Grabov 2007). Expression of OsHAK2, OsHAK7, and HvHAK2 rescued the sensitive phenotype of mutant E. coli strain with defective K+ uptake under low K+ supply (Horie et al. 2011; Senn et al. 2001; Shen et al. 2015).
HAK/KUP/KT family is also involved in K+-mediated abiotic stress responses (Hamamoto et al. 2015; Horie et al. 2009; Liang et al. 2020). OsHAK5/21 in rice and ZmHAK1/4 in maize are involved in salt stress by regulating the K+/Na+ ratio (Yang et al. 2014; Horie et al. 2011; Shen et al. 2015; Sun et al. 2018; Zhang et al. 2019). HvHAK1 confers drought and salt tolerance in barley by enhancing leaf mesophyll H+ homoeostasis and improving K+-nutrition (Fulgenzi et al. 2008; Mangano et al. 2008; Feng et al. 2020a). AtKUP6/8 in Arabidopsis regulates osmotic and drought stress tolerance (Gierth et al. 2005). The transcription factors bHLH122 and WRKY33 can bind with the AtKUP2 promoter and regulate the expression of AtKUP2, leading to increased salt tolerance in Arabidopsis (Rajappa et al. 2020; Osakabe et al. 2013). Hormones and their intricate crosstalk are also regulated by the HAK family, resulting in modulating plant growth in response to diverse environment stresses (Elumalai et al. 2002; Han et al. 2016). AtKUP6 and AtKUP8 are involved in the control of lateral root formation by effecting crosstalk between auxin and ABA (Gierth et al. 2005; Qi et al. 2008; Chen et al. 2018, 2019). Similarly, AtKUP2 and AtKUP4 play a role in turgor-driven cell expansion by contributing to auxin homeostasis (Elumalai et al. 2002; Vicente-Agullo et al. 2004; Rigas et al. 2013).
To date, numerous studies have revealed the function of the HAK family, which has been identified in several plants, such as tobacco (Song et al. 2019), poplar (He et al. 2012), cassava (Ou et al. 2018), wheat (Cheng et al. 2018b), and rice (Banuelos et al. 2002). Limited information is available on HAK genes in sweet potato, however, because of its complex genetic background. The recently available genome sequences of diploid Ipomoea trifida G. Don (2 n = 2 x = 30), with 462 Mb genome sizes, which is considered to be an ancestral species of hexaploid cultivated sweet potato (Feng et al. 2018; Wu et al. 2018), and the continuous development of genome sequences in sweet potato provide the opportunity to study the HAK family in Ipomoea. Here, 22 IbHAK genes were identified in sweet potato cv. Taizhou6 and the same number of HAK genes was identified in I. trifida. Then, we analyzed their phylogenetic relationship, gene structure, cis-activity, gene duplication, synteny analysis, and expression profiles in response to K+ deficiency and abiotic stress treatment. These analyses provide a solid foundation for further functional dissection of the HAK genes and several candidate HAK genes that potentially may be utilized in the molecular breeding of sweet potato.
Materials and methods
Plant materials and treatments
We used the tolerance of K+ deficiency sweet potato cv. Xushu32 and the sensitivity to K+-deficiency sweet potato cv. Ningzi1 screened in our previous studies to study the expression of IbHAK genes in response to K+-deficiency stress (Liu et al. 2017). Cuttings of 12–13 mm in length and with similar features in the two varieties were cultured in water for three days of recovery and then transferred into modified Hoagland’s nutrient solution without potassium ion (-K treatment). The root tips were collected after 10 days of -K treatment or normal condition treatment (with 10 mM KCl). We selected Pushu32, a widely planted cultivar with high and stable yield, high commodity rate, and wide adaptability, to investigate the expression patterns of the IbHAK genes under drought and salt stress. Two-week-old seedlings of Pushu32 were cut and transformed into hydroponic treatment. After five days, the seedlings were subjected to drought treatment (without nutrient solution) and salt treatment (300 mM NaCl in nutrient solution) for 1 h, 3 h, 6 h, 12 h, 24 h, and 48 h.
Identification of HAK genes in Ipomoea
We obtained the genome sequence of sweet potato cv. Taizhou6 from the sweet potato genome database (http://ipomoea-genome.org) and the genome sequence of I. trifda was downloaded from Genomics Resource (http://sweetpotato.plantbiology.msu.edu/). We used the Hidden Markov Model (HMM) profile of the K+ transporter domain (PF02705) to query the candidate HAKs from sweet potato genomic database using HMMER3.0 with a cutoff of 0.01 (Eddy 2011; Sun and Buhler 2007). Then, we examined the candidate HAK protein sequences, which include the K+ transporter domain, using the Pfam database (http://pfam.xfam.org) and SMART program (http://smart.embl-heidelberg.de/) (Bateman et al. 2004; Punta et al. 2012). The nonredundant and confident genes were gathered and assigned as HAK genes. Finally, we predicted the molecular weight (MW) and isoelectric points (pI) of the HAK proteins by ExPASy (http://web.expasy.org/protparam/) and predicted subcellular localization using CELLO (http://cello.life.nctu.edu.tw/) and Wolf PSORT ( https://wolfpsort.hgc.jp/).
Sequence alignment and phylogenetic reconstruction
We downloaded the whole genome of other species, including Arabidopsis, rice (Oryza sativa), and maize (Zea mays), from Phytozome Database (https://phytozome.jgi.doe.gov/pz/portal.html) and identified HAK/KUP/KT family genes. Multiple sequences were aligned by ClustalX 2.0 (Larkin et al. 2007). We constructed phylogenetic trees using MEGA7.0 according to the maximum likelihood method and performed bootstrap testing with 1000 iterations (Tamura et al. 2011).
Sequence analysis and function prediction
We determined the exon–intron structure of the HAK genes by the TBtools program and identified the conserved motifs of HAK proteins by MEME (http://meme.nbcr.net/meme/intro.html), with the motif number set to 15. We analyzed the cis-elements in the 1500-bp sequences upstream of the coding sequences by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Chromosomal distribution and gene collinearity analysis
We obtained the distribution of the IbHAK genes in the chromosome from the sweet potato database. We used MCScanX to detect duplicated genes. The main configuration parameters were set as follows: the default of match score, max gaps, E-value are 50, 25 and 1e-05. The synonymous substitution rates (Ks), nonsynonymous substitution rates (Ka), and Ks/ Ka of each duplicated gene pair were determined using KaKs_Calculator. The divergence time was computed as T = Ks/(2 × 1.5 × 10 − 8) × 10 − 6 million years ago (MYA) (Koch et al. 2000). We visualized synteny blocks between sweet potato and I. trifida genomes by TBtools (Chen et al. 2020).
Transcriptome and qRT-PCR analysis
We extracted total RNA of each sample with a plant RNA extraction kit and synthesized cDNA using TIANScript II RT kit (TIANGEN, China). We employed reads per kilobase of exon model per million mapped reads (RPKM) to calculate gene expression levels. The transcriptomic data on the expression of IbHAK genes in two sweet potato cultivars under K+-deficiency stress was retrieved from our in-house RNA-seq data (unpublished). We downloaded global gene expression profiles over various tissues in I. trifida from I. trifda Genomics Resource (http://sweetpotato.plantbiology.msu.edu/).
To further analyze the expression pattern of IbHAK genes under K+ deficiency stress and abiotic stresses, we selected eight IbHAK genes based on the RNA-seq data, analyzed their expression level using qRT-PCR, and designed primers by Primer5 (Table S1). IbARF was used as internal reference gene (forward primer: 5′-CTTTGCCAAGAAGGAGATG-3′; reverse primer: 5′-TCTTGTCCTGACCACCAACA-3′). SYBR@ Green Real-time PCR Master Mix (ToYoba, Japan) was used for qRT-PCR amplification in a OneStep Real-Time System (Applied Biosystem, USA). The qRT-PCR conditions were as follows: 95 °C for 5 min; followed by 40 cycles of 95 °C for 15 s, 57 °C for 15 s, and 72 °C for 30 s; and a final extension at 72 °C for 5 min. We used three biological replicates for each gene. The relative expression levels of the IbHAKs were calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001). We analyzed the data and compared the means using LS at a 0.01 level of significance.
Co-expression analysis of IbHAK genes
The co-expression network of the IbHAK genes was performed by the R package of the weighted gene co-expression network analysis (WGCNA). The predicted networks were generated by setting the minimum required interaction score > 0.6, and then visualized in Cytoscape. Functional enrichment and annotation analysis of co-expression genes were performed based on the Nr database (https://ftp.ncbi.nlm.nih.gov/blast/db/FASTA/).
Results
Identification of HAK genes in Ipomoea
We identified 22 IbHAK genes in sweet potato after removing redundant sequences, which were assigned names from IbHAK1 to IbHAK22 based on their position on the chromosomes (Table 1). Of the 22 IbHAK proteins, IbHAK11 was the smallest protein with 437 amino acids (1311 bp), and IbHAK3 was the largest one with 1200 amino acids (3600 bp). The MW of the proteins ranged from 48.6 to 135.0 KDa, and the pI ranged from 5.54 (IbHAK1) to 9.31 (IbHAK14). All IbHAK proteins were located in the plasma membrane and possessed 6–13 transmembrane segments (Fig. S1). The identified IbHAK proteins also were subjected to BLAST analysis with the Arabidopsis genome, and the similarity of IbHAK sequences to their best-match known genes varied from 39% (IbHAK15 related to AtKUP1) to 79% (IbHAK1 related to AtKUP7). Additionally, 22 HAK genes also were identified from a wild relative of sweet potato, I. trifida. Their CDS size, isoelectric point (IP), molecular weight (MV), and chromosome location are listed in Table 2.
Table 1.
Information of HAK/KUP/ KT gene family in sweetpotato
| Number | Gene name | Gene ID | Chromosome location | CDS size/bp | Length/aa | PI | Molecular weight (KDa) | Subcellular location | TMSa | Arabidopsis thaliana | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Percent identity/% | ||||||||||
| 1 | IbHAK1 | g402.t1 | Chr 1 | 2319 | 773 | 5.54 | 86.1 | PM | 8 | AT5G09400.1 | AtKUP7 | 79 |
| 2 | IbHAK2 | g5429.t1 | Chr 1 | 2313 | 771 | 9.01 | 85.1 | PM | 10 | AT3G02050.1 | AtKUP3 | 69.56 |
| 3 | IbHAK3 | g11459.t1 | Chr 3 | 3600 | 1200 | 8.69 | 135.0 | PM | 13 | AT5G14880.1 | AtKUP8 | 69.34 |
| 4 | IbHAK4 | g13452.t1 | Chr 3 | 2379 | 793 | 6.71 | 87.9 | PM | 11 | AT2G40540.5 | AtKT2 | 79.55 |
| 5 | IbHAK5 | g21499.t1 | Chr 4 | 2121 | 707 | 8.86 | 78.3 | PM | 11 | AT2G30070.1 | AtKUP1 | 66.24 |
| 6 | IbHAK6 | g23059.t1 | Chr 5 | 2415 | 805 | 9.25 | 91.2 | PM | 9 | AT2G40540.5 | AtKT2 | 72.86 |
| 7 | IbHAK7 | g25426.t1 | Chr 5 | 2307 | 769 | 7.15 | 85.9 | PM | 12 | AT2G40540.5 | AtKT2 | 74.44 |
| 8 | IbHAK8 | g36689.t1 | Chr 7 | 2970 | 990 | 9.03 | 110.3 | PM | 12 | AT4G13420.1 | AtHAK5 | 58.13 |
| 9 | IbHAK9 | g40272.t1 | Chr 8 | 2349 | 783 | 8.55 | 86.9 | PM | 7 | AT1G70300.1 | AtKUP6 | 59.16 |
| 10 | IbHAK10 | g41926.t1 | Chr 9 | 2445 | 815 | 7.5 | 91.8 | PM | 10 | AT5G09400.1 | AtKUP7 | 74.12 |
| 11 | IbHAK11 | g48527.t1 | Chr 10 | 1311 | 437 | 8.32 | 48.6 | PM | 8 | AT2G35060.4 | AtKUP11 | 78.56 |
| 12 | IbHAK12 | g50289.t1 | Chr 10 | 2280 | 760 | 7.79 | 85.0 | PM | 11 | AT2G35060.4 | AtKUP11 | 72.72 |
| 13 | IbHAK13 | g56804.t1 | Chr 12 | 2337 | 779 | 8.99 | 86.8 | PM | 6 | AT3G02050.1 | AtKUP3 | 66.47 |
| 14 | IbHAK14 | g56832.t1 | Chr 12 | 2202 | 734 | 9.31 | 81.1 | PM | 9 | AT3G02050.1 | AtKUP3 | 62.47 |
| 15 | IbHAK15 | g60399.t1 | Chr 12 | 2217 | 739 | 8.91 | 82.1 | PM | 12 | AT2G30070.1 | AtKUP1 | 39.39 |
| 16 | IbHAK16 | g64039.t1 | Chr 13 | 2316 | 772 | 8.99 | 87.2 | PM | 10 | AT4G13420.1 | AtHAK5 | 54.18 |
| 17 | IbHAK17 | g64040.t1 | Chr 13 | 2160 | 720 | 8.91 | 81.1 | PM | 9 | AT4G13420.1 | AtHAK5 | 57.73 |
| 18 | IbHAK18 | g64041.t1 | Chr 13 | 2268 | 756 | 5.95 | 84.8 | PM | 11 | AT4G13420.1 | AtHAK5 | 53.28 |
| 19 | IbHAK19 | g64043.t1 | Chr 13 | 2166 | 722 | 8.73 | 80.6 | PM | 10 | AT4G13420.1 | AtHAK5 | 61.23 |
| 20 | IbHAK20 | g65642.t1 | Chr 14 | 2118 | 706 | 8.66 | 79.2 | PM | 11 | AT1G70300.1 | AtKUP6 | 66.67 |
| 21 | IbHAK21 | g65645.t1 | Chr 14 | 2421 | 807 | 8.68 | 88.6 | PM | 12 | AT1G60160.1 | AtKUP5 | 72.48 |
| 22 | IbHAK22 | g66077.t1 | Chr 14 | 2385 | 795 | 8.35 | 89.0 | PM | 13 | AT2G40540.5 | AtKT2 | 78.88 |
PM plasma membrane
aTMS number of transmembrane segments posses
Table 2.
Information of HAK/KUP/ KT gene family in I. trifida
| Gene ID | Chromosome location | CDS size/bp | Length/aa | PI | Molecular weight (KDa) | Subcellular location | TMSa |
|---|---|---|---|---|---|---|---|
| itf10g07360.t1 | Chr10 | 999 | 333 | 8.13 | 36,915.1 | PM | 6 |
| itf13g16550.t1 | Chr13 | 1023 | 341 | 9.12 | 38,797.26 | PM | 3 |
| itf12g25840.t1 | Chr12 | 2241 | 747 | 8.73 | 81,287.03 | PM | 11 |
| itf05g02410.t1 | Chr5 | 2424 | 808 | 7.16 | 88,894.71 | PM | 13 |
| itf01g29030.t1 | Chr1 | 2478 | 826 | 8.93 | 90,067.26 | PM | 13 |
| itf04g28620.t1 | Chr4 | 1950 | 650 | 8.76 | 71,393.84 | PM | 10 |
| itf10g17120.t1 | Chr10 | 2403 | 801 | 7.56 | 88,350.14 | PM | 11 |
| itf12g08530.t1 | Chr12 | 2442 | 814 | 9.09 | 89,235.51 | PM | 12 |
| itf09g03480.t1 | Chr9 | 4980 | 1660 | 6.25 | 183,527.11 | PM | 10 |
| itf13g05200.t1 | Chr13 | 1683 | 561 | 8.7 | 61,110.06 | PM | 9 |
| itf10g07370.t1 | Chr10 | 1089 | 363 | 8.43 | 40,561.5 | PM | 3 |
| itf01g01920.t1 | Chr1 | 2589 | 863 | 5.86 | 94,405.97 | PM | 10 |
| itf14g04440.t1 | Chr14 | 2337 | 779 | 8.58 | 86,014.6 | PM | 12 |
| itf14g04480.t1 | Chr14 | 2559 | 853 | 6.69 | 92,051.3 | PM | 12 |
| itf13g16500.t1 | Chr13 | 1635 | 545 | 9.19 | 61,247.9 | PM | 6 |
| itf13g16560.t1 | Chr13 | 1086 | 362 | 7.06 | 39,457.6 | PM | 7 |
| itf13g16520.t1 | Chr13 | 2442 | 814 | 6.25 | 90,132.87 | PM | 11 |
| itf08g11870.t1 | Chr8 | 2391 | 797 | 8.34 | 87,130.84 | PM | 12 |
| itf03g00870.t1 | Chr3 | 2403 | 801 | 7.87 | 88,665.73 | PM | 12 |
| itf13g16490.t1 | Chr13 | 2097 | 699 | 8.58 | 78,055.67 | PM | 9 |
| itf03g14180.t1 | Chr3 | 2496 | 832 | 6.55 | 90,740.96 | PM | 11 |
| itf07g18670.t1 | Chr7 | 2370 | 790 | 8.74 | 87,295.83 | PM | 12 |
PM plasma membrane
aTMS number of transmembrane segments posses
Phylogenetic and classification analyses of HAK proteins
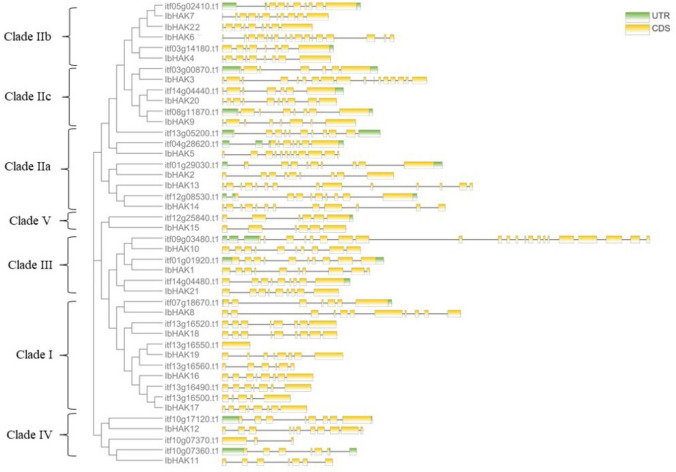
To analyze the phylogenetic relationship and evolution of the HAK family in sweet potato and different plants, we used 98 genes from several plants, including A. thaliana, O. sativa, and Z. mays, to construct a phylogenetic tree (Fig. 1; Table S2). These HAK proteins could be divided into five clades based on a previous report (Nieves-Cordones et al. 2016). Clade I consisted of five IbHAKs and six ItHAKs and was further divided into subclades IA and IB and a divergent member (AtHAK5). We identified the largest numbers of HAK genes in clade II, including 11 IbHAKs and 9 ItHAKs, which could be separated into three subclades IIa, IIb, and IIc. Clade III was composed of three IbHAKs and three ItHAKs; clade IV included two IbHAKs and three ItHAKs; and clade V was composed of only one IbHAK and one ItHAK (Fig. 1).
Fig. 1.
The phylogenetic tree of sweet potato, I.trifda, Arabidopsis, maize and rice HAK potassium transporters. Nit Ninety-eight HAK proteins including 22 of the IbHAK proteins (marked with red solid circles) and 22 of ItHAK protein (marked with red hollow circles) were used to construct the phylogenetic tree. Genes from different clusters (I–V) were indicated with different colors zones. The joint unrooted tree was generated by the maximum likelihood method. Bootstrap values from 1000 replicates were indicated at each branch. The protein loci of rice, maize, and Arabidopsis HAK family proteins are listed in Table S1. Os, Oryza sativa; Zm, Zea mays; At, Arabidopsis thaliana
Gene structure and conserved motif analyses of HAKs
To provide the evolutionary relationship of HAKs in Ipomoea, we constructed a phylogenetic tree based on the citrus full-length protein sequences (Fig. 2). The structural features have been shown to play critical roles in the evolution of multiple gene families. Moreover, to gain insights into the structural evolution of HAK genes in Ipomoea, we compared the genomic DNA sequences to analyze the conserved motifs and the composition of introns/exons. We identified 15 conserved motifs by the MEME database, which were annotated as K+ potassium transporter motif (Table S3). Most of the HAK genes shared common motif compositions and all of the identified IbHAKs contained motifs 3 and 4. The 15 conserved motifs were arranged from 33 to 40 times among the 44 HAK genes. A total of 15 (34%) HAK genes were found to have 15 motifs, whereas 23 (52%) HAKs possessed 10–14 motifs, and 6 (14%) HAKs had 5 to 8 motifs (Fig. 2). Next, the HAK genes contained 1–22 exons and the majority of the HAK genes harbored 7–12 exons. Most closely related members shared similar exon/intron structures, such as 8–11 exons of IbHAKs in clade I, 7–17 exons of IbHAKs in clade II, and 9–10 exons of IbHAKs in clade III (Fig. 3). Together, the common K+ potassium transporter motifs and similar gene structure in the same cluster supported the phylogenetic classification of the HAK family and implied functional similarities among these HAK genes.
Fig. 2.
Putative motif distribution of HAKs in sweet potato and I.trifida. Motifs were detected used the MEME tools and identified by different colors numbered 1–15
Fig. 3.
Gene structure of IbHAK gene family in sweet potato and I.trifida. The green boxes, yellow boxes, and the black lines were indicated upstream/downstream, exons, and introns, respectively. Gene models are drawn to scale as indicated at right of the figure. The direction is from 5′-3′
Chromosome location and evolution of IbHAK genes
A total of 22 IbHAK genes were unevenly distributed across the 15 chromosomes (Chr1–Chr15; 2 n = 90), except for chromosomes 2, 6, 11, and 15 (Fig. S2). Chromosome 13 contained the largest number of IbHAK genes (4 genes), followed by chromosome 14 (3 genes). Chromosomes 1, 3, 5, and 10 each had 2 IbHAK genes, whereas chromosomes 4 and 7 each contained one IbHAK gene (Fig. S2). Fragment duplication genes were the most common on chromosomes 14 and 5, followed by chromosomes 3 and 12 (Fig. S2). A total of eight pairs of segmental duplications and only two gene pairs (on chromosome 13) apparently evolved from a tandem duplication event.
To further explore the evolutionary relationship of the sweet potato HAK gene family, we computed the Ks and Ka of these gene pairs. We also estimated the Ka/Ks to determine which type of codon selection operated during evolution. Our results showed that the Ka/Ks ratios of these IbHAKs ranged from 0.07 to 0.61(< 1), suggesting that the purifying selection might have operated on the codons during the evolution and expansion of the IbHAK genes. The paralogous IbHAK genes seemed to have undergone segmental duplication from 4.40 to 91.76 MYA, with an average of 46.32 MYA, and the tandemly duplicated gene pair seemed to have emerged 19.28 MYA (Table 3).
Table 3.
Ka/Ks calculation and estimated divergence time for the duplicated IbHAK gene pairs
| Duplicated gene pair | Ks | Ka | Ka/Ks | Duplicated type | Purify selection | Divergence time(MYA) |
|---|---|---|---|---|---|---|
| IbHAK9/IbHAK20 | 0.88 | 0.15 | 0.17 | Segmental duplication | Yes | 58.51 |
| IbHAK4/IbHAK6 | 1.29 | 0.21 | 0.16 | Segmental duplication | Yes | 85.89 |
| IbHAK4/IbHAK22 | 1.38 | 0.10 | 0.07 | Segmental duplication | Yes | 91.76 |
| IbHAK6/IbHAK22 | 0.23 | 0.14 | 0.60 | Segmental duplication | Yes | 15.52 |
| IbHAK6/IbHAK7 | 0.23 | 0.14 | 0.61 | Segmental duplication | Yes | 15.15 |
| IbHAK7/IbHAK1 | 0.61 | 0.13 | 0.22 | Segmental duplication | Yes | 40.86 |
| IbHAK20/IbHAK9 | 0.88 | 0.15 | 0.17 | Segmental duplication | Yes | 58.51 |
| IbHAK22/IbHAK7 | 0.07 | 0.01 | 0.17 | Segmental duplication | Yes | 4.40 |
| IbHAK16/IbHAK17 | 0.14 | 0.04 | 0.31 | Trandem duplication | Yes | 9.13 |
| IbHAK17/IbHAK18 | 0.44 | 0.13 | 0.31 | Trandem duplication | Yes | 29.43 |
Moreover, we identified the genome-wide collinear duplicated blocks in the I. batata and I. trifida genomes and the orthologous collinear blocks between two genomes. A total of 15 HAK pairs were involved in duplicated genomic blocks, and 11 HAK pairs were accounted as orthologous genes according to the results of synteny analysis (Fig. 4). To understand the evolutionary constraints acting on the orthologous HAK genes, we calculated the Ka/Ks values for these gene pairs. The Ka/Ks values between the pairwise comparisons ranged from 0.02 to 0.55 (Table 4), suggesting that the purifying selection acted on the orthologous HAKs.
Fig. 4.
Syntenic relationship of HAK genes shown on chromosome map between I. batata and I.trifida. Red lines between the chromosomes in sweet potato (top) and I.trafida (bottom) indicated orthologous gene pairs
Table 4.
Ka/Ks ration of IbHAK and their orthologous genes in I. trifida
| IbHAK | Orthologs | Ka | Ks | Ka/Ks | MYA |
|---|---|---|---|---|---|
| IbHAK1 | itf01g01920.t1 | 0.01 | 0.04 | 0.35 | 1.214343434 |
| IbHAK2 | itf01g29030.t1 | 0.00 | 0.01 | 0.11 | 0.358429079 |
| IbHAK3 | itf03g00870.t1 | 0.03 | 0.07 | 0.43 | 2.304105535 |
| IbHAK4 | itf03g14180.t1 | 0.01 | 0.05 | 0.19 | 1.809055342 |
| IbHAK8 | itf07g18670.t1 | 0.05 | 0.14 | 0.35 | 4.668284578 |
| IbHAK11 | itf10g07360.t1 | 0.04 | 0.08 | 0.55 | 2.599742838 |
| IbHAK12 | itf10g17120.t1 | 0.00 | 0.04 | 0.02 | 1.286457432 |
| IbHAK14 | itf12g08530.t1 | 0.04 | 0.08 | 0.44 | 2.771665588 |
| IbHAK15 | itf12g25840.t1 | 0.01 | 0.10 | 0.15 | 3.334939961 |
| IbHAK19 | itf13g16490.t1 | 0.03 | 0.09 | 0.37 | 3.016024925 |
| IbHAK20 | itf14g04440.t1 | 0.00 | 0.04 | 0.03 | 1.265039373 |
Tissue-specific expression profile analysis of I. trifida
To study the expression profiles and protein functions of HAKs in I. trifida, we performed transcriptome analysis of five different tissues (flowers, flower buds, leaves, roots, and stems) by RNA-seq data (Table S4). The majority of genes in clades I and III had higher expression levels in the roots or stems, except for itf13g16500, which showed very low or undetected expression levels in all examined tissues (Fig. 5). The genes in clade IV were strongly expressed in the flowers and roots, whereas the only gene that was classified into clade V showed strong expression levels in flower buds. Clade II had three types of expression patterns based on subclade classification. Clade IIa, including 4 HAK genes and 3 that were highly expressed in stems; clade IIb, including 2 HAK genes and that were highly expressed in flower buds; and clade IIc, including 3 HAK genes and that were highly expressed in flowers and flower buds (Fig. 5). The results showed that each clade of HAK genes shared similar tissue-specific expression patterns in I. trifida, suggesting that these genes from different clades may have different roles.
Fig. 5.

Tissue-specific expression levels of HAK genes in I. trifida. A heat map with clustering is created based on the RPKM value of 22 ItHAK genes. The relative expression abundance of each gene in flower, flower bud, leaf, root and stem. The coloured scale varies from green to red, which indicates low or high expression
Cis-element prediction of IbHAK genes
To identify IbHAK cis-acting elements, a number of cis-regulatory elements were investigated in the region 2000 bp upstream of the genes (Fig. 6). The four main types of the cis-regulatory elements are as follows: core promoter region (CAAT-box, TATA-box), light-induced reactive-related elements, environmental stress response elements, and hormone response elements. The detailed information on the environmental stress response elements and hormone response elements are summarized in Tables S4 and S5, including low-temperature response elements (LTR), drought response elements (MBS), defense and stress response elements (TC-rich repeats), and wound response elements (WUN-motifs), as well as abscisic acid responsiveness (ABRE), auxin responsiveness (AuxRR-core, TGA-element), gibberellin responsiveness (P-box), salicylic acid responsiveness (TCA-element), and MeJA responsiveness (CGTCA-motif) elements. Various hormone and environmental response element in the promoter of HAK genes, provide a key foundation for further functional research of IbHAK gene family.
Fig. 6.
Predicted cis-elements in the promoter regions of IbHAK genes. The IbHAK genes were shown on the left side of the figure. The scale bars at the bottom were indicated the length of the promoter sequence. Color boxes were indicated cis-elements in promoter regions. The predicted cis-elements were shown at the right of the figure
Differential expression of IbHAK genes in response to K+-deficiency stress
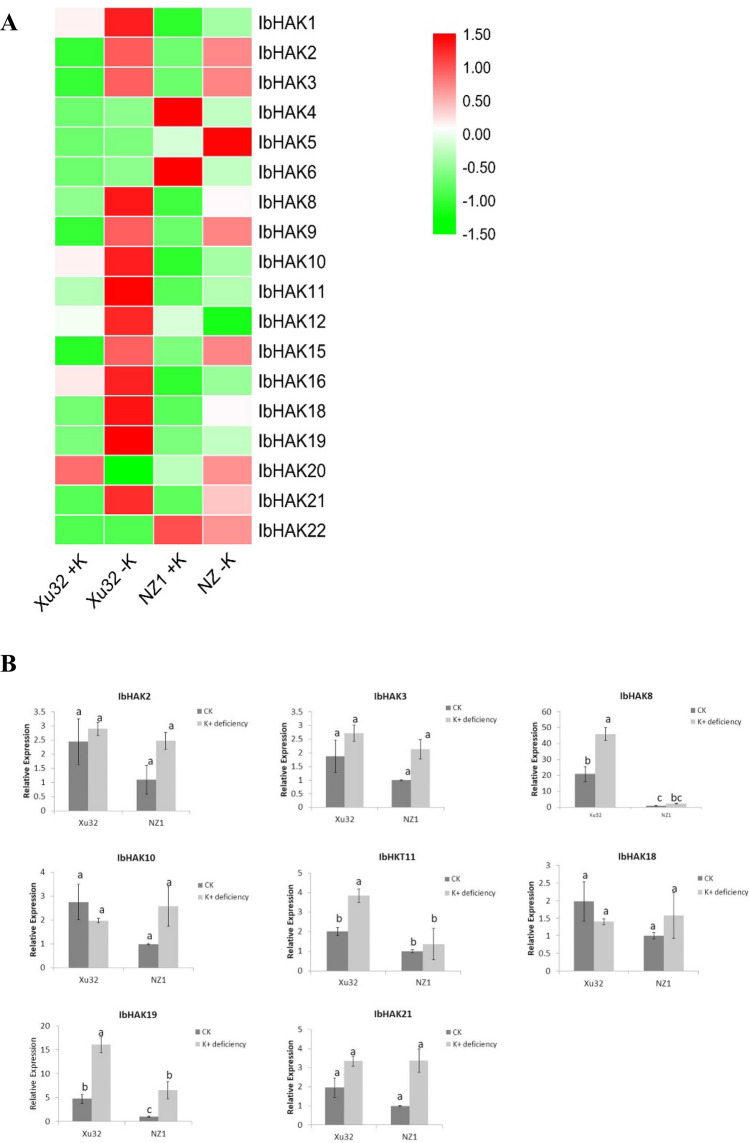
The HAK gene family functions in K+ transport. Thus, we analyzed the expression profiles of the IbHAK genes in sweet potato cv. Xushu32 (Xu32) and cv. Ningzi1 (NZ1) under K+ deficiency stress derived from the RNA-Seq dataset and qRT-PCR. Of the examined 22 genes, we obtained 18 genes from RNA-seq databases, but information on the other 4 genes (IbHAK7, -13, -14 and -17) was missing. A heat map showed that the majority of the IbHAK genes were upregulated in two cultivars, and the total number of upregulated IbHAK genes was higher in Xu32 than in NZ1 (13 in Xu32 and 10 in and NZ1). Eight genes (IbHAK2, -3, -8, -10, -11, -18, -19, and -21) were induced in both Xu32 and NZ1. Four genes (IbHAK4, -6, -12, and -22) were downregulated in NZ1, whereas only IbHAK20 was downregulated in Xu32 (Fig. 7a).
Fig. 7.
Expression profiles of IbHAKs genes in Xushu32 and Ningzi1 under K + deficiency stress. a The heatmap of RNA-seq expression data for IbHAKs. The coloured scale varies from green to red, indicating relatively low or high expression. Scale bars represent the log2 transformations of the RPKM values. b Expression patterns of eight selected IbHAKs genes in response to K+ deficiency stress were examined by a qPCR assay. The mean fold changes of each gene between treated and control samples in Xushu32 and Ningzi1 were used to calculate its relative expression levels. Data are means ± SD of three biological replicates. Means denoted by the same letter do not significantly differ at P < 0.05 as determined by Duncan’s multiple range test
In addition, based on the RNA-seq data, we selected the eight induced IbHAK genes in the two cultivars for qRT-PCR analysis. The results showed that it was different from the expression of the IbHAK gene family in the two sweet potato cultivars. For example, IbHAK19 was significantly upregulated under K+-deficiency stress treatment in both cultivars; however, the expression level and rising range of the gene was higher in Xu32 than in NZ1. IbHAK8 and IbHAK11 were strongly upregulated in Xu32, whereas we did not observe any significant increase in NZ1 under K+-deficiency stress (Fig. 7b).
Differential expression of IbHAK genes under salt and drought stress
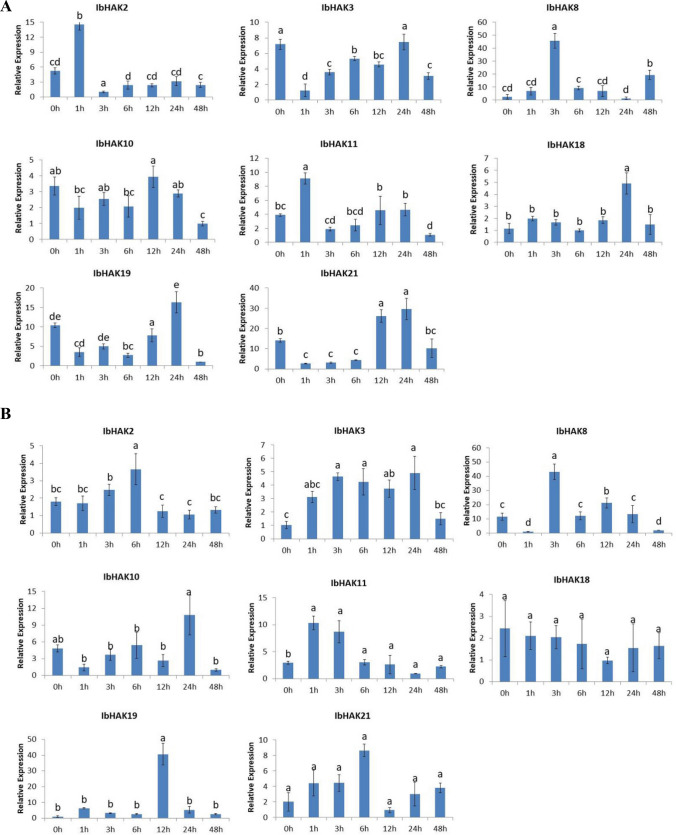
Members of HAK family also participate in stress-related responses. To investigate the response of IbHAK genes to environmental stresses in sweet potato, these eight selected genes were used for further study of the expression profiling after exposure to drought and high salinity stresses. Under drought stress, the expression of IbHAK8 in leaves and three IbHAK genes (IbHAK2, -11, -19) in roots were noticeably increased. The expression of two IbHAK genes (IbHAK18 and IbHAK19) in leaves and IbHAK10 in roots were initially decreased and then increased. Two IbHAK genes (IbHAK3 and IbHAK11) in leaves and IbHAK8 in roots were expressed at a similar level at all time point. Under salt stress, four IbHAK genes (IbHAK2, -8, -11) in leaves and 3 IbHAK genes (IbHAK-3, -8, -11) in roots were rapid upregulated and the expression levels peaked at 1 h to 3 h, while the expression levels of three IbHAK genes (IbHAK18, -19, -21) in leaves and two IbHAK genes (IbHAK3 and IbHAK11) in roots peaked at 12 h to 24 h. IbHAK10 in leaves and IbHAK18 in roots could not regulate at all time point.
Interestingly, we found that IbHAK8 were strongly upregulated by drought and salt stress. The expression level of IbHAK8 increased 15 times than control after 6 h exposures to dehydration in leaves, and upregulated more than 45 times in leaves after stress treatment.
Co-expression analysis of IbHAK genes
To uncover the possible roles that IbHAKs played in sweet potato, the co-expression analysis was performed based on the expression profile of K+-deficiency stress treatment between two sweet potato genotype (Xushu32 and NZ1). We found only IbHAK8 was involved in co-expression network interactions. 30 genes related with environmental stresses were predicated co-expressing with IbHAK8, such as zinc finger protein, NAC (no apical meristem (NAM), ATAF1/2, and cup-shaped cotyledon (CUC2)) transcription factor, nicotinamide adenine dinucleotide (NADH) dehydrogenase, peroxidase (POD), proline-rich receptor-like protein kinase 1 (PERK1) and receptor-like serine/threonine-protein kinase (LRR) (Fig. 10). The function predication of all co-expressing genes was listed in Table S9.
Fig. 10.

Co-expression network analyses of IbHAK8. The solid red circles indicated the co-expressed genes inferred from expression profiles. WGCNA program was used to produce association network and Cytoscape software was used to visualize co-expression network
Discussion
The plant HAK/KUP/KT family has a major function in root K+ acquisition and also plays a vital role in environmental stress adaptation and regulation of cell size (Li et al. 2018). The recently released genomes of sweetpotato cv. Taizhong6 and its probable progenitor I. trifida have allowed us to study HAK genes in sweetpotato. We identified a total of 22 IbHAK genes in sweetpotato (I. batata) and 22 ItHAK genes in I. trifida. Five clades were classifications of HAKs based on phylogenetic tree analysis. Further analyses found that each clade of the HAK protein shared similar gene structures and specific tissue expression pattern, which indicated function diversity in different clades of HAKs.
Gene duplication events, including whole genome duplication (WGD), segmental duplications, and tandem duplications, are the main driving forces in species evolution by providing raw materials for evolution of new functions (Soltis and Soltis 2016). The ε WGD event in angiosperms occurred at around 220 MYA and the σ WGD event for monocots occurred at 130 MYA, which resulted in functional novelties and complex regulatory network (Jiao et al. 2011; Clark and Donoghue 2017). The ancient WGD contributed to the expansion of the HAK gene family in both dicots and monocots (Feng et al. 2020b; Grabov 2007). In this study, the estimated divergence time of segmental and tandem duplication of IbHAK genes pairs occurred less than 100 MYA, indicating preferential gene retention and frequent chromosomal arrangements after ancient WGD (Table 3). A previous report showed an initial crossing between a tetraploid progenitor and a diploid progenitor (mostly as I. trifida) in sweetpotato hexaploidization (Yang et al. 2017). This study showed that around 68.2% (15/22) of IbHAKs and 59.1% HAKs (13/22) in I. trifida were involved in duplicated genomic blocks, and the Ka/Ks values of 11 HAK orthologous gene pairs were < 0.6 (Fig. 4; Table 4). This suggests that a strong purifying selection acted on the Ipomoea orthologous HAKs to remove deleterious mutations at the protein level.
HAK/KUP/KT proteins always exhibit great diversity in terms of various expression patterns in a number of plant species. Plants acquire K+ from soil through root tissues and then are transported among compartments within cells and from roots to shoots (Sustr et al. 2019). Different affinity K+ uptake of HAKs mainly is due to tissue-specific expression. Approximately 14 of 27 OsHAKs in rice and 10 of 13 AtHAK genes in Arabidopsis are highly expressed in root tissues (Ahn et al. 2004; Okada et al. 2018). In I. trifida, 11 HAK genes in clades I, IIa, and III that are mostly highly expressed in the roots or stems may function in high-affinity K+ uptake, whereas other clades are involved in low-affinity K+ transport (Fig. 5). This result may help elucidate the function of orthologue IbHAK genes in sweetpotato. Moreover, transcriptional regulation of K+ transporter genes represents a major mechanism in plant responses to low-K+ stress. In our study, K+-starvation upregulated the expression pattern of eight HAKs (IbHAK2, -3, -8, -10, -11, -18, -19, and -21) in both K+-tolerant and K+-sensitive sweetpotato genotypes (Fig. 7). The number and the upregulated expression levels of IbHAK genes induced by K+-deficiency stress was higher in Xu32 than in NZ1, which may have resulted in different K+-deficiency tolerance levels and mechanisms of K+ uptake in the two different sweetpotato genotypes.
K+ transporter HAK is essential to regulating water potential and turgor during osmotic adjustment through the control of K+ influx and efflux (Li et al. 2018). HAK gene family responses to salt and drought stresses according to their transcript levels are upregulated by osmotic adjustment in Arabidopsis (Ahn et al. 2004), wheat (Cheng et al. 2018a), and cassava (Ou et al. 2018). Similar results were found in our study—for example, IbHAK8 was homologous and closely related to AtHAK5, which was strongly induced by K+ starvation, drought, and salt stress. Transcriptome analysis revealed that its ortholog in I. trifida, itf07g18670.t1, was upregulated by various hormone and environmental stresses (Table S8). In Arabidopsis, AtHAK5 positively regulated K+-mediated drought and osmotic resistances (Brauer et al. 2016; Huang et al. 2019). Additionally, several cis-elements associated with environmental stresses and hormone-mediated gene signaling of AtHAK5 genes may have played significant roles in its regulation during K+ deficiency (Hong et al. 2013). AtHAK5 and its homologs from tomato could activate the calcium-regulated protein kinase system CIPK23/CBL complex and interact with potassium channel AKT1, resulting in K+ homeostasis under adverse environmental conditions (Pyo et al. 2010; Lara et al. 2020; Ragel et al. 2015; Bacha et al. 2015). IbHAK8 may have mediated plant adaptive response to environmental stresses similar to AtHAK5 based on its expression pattern (Figs. 8 and 9). Moreover, several genes associated with abiotic and biotic stresses including protein kinase (LRR and PERK), transcription factor (NAC and ZIC) and oxidoreductase (cytochrome oxidase, NADH dehydrogenase and peroxidase) were predicated co-expression with IbHAK8 (Fig. 10). The results further suggest that IbHAK8 may be involved in a wide range of environmental stress related signaling pathway.
Fig. 8.
Expression profiles of IbHAKs genes in response to drought stress. a Expression patterns of selected IbHAKs genes in response to drought stress in leaves of Pushu32 were examined by a qPCR assay. b Expression patterns of selected IbHAKs genes in response to drought stress in roots of Pushu32 were examined by a qPCR assay. The samples were obtained at 0, 1, 3, 6, 12, 24, 48 h after drought stress. The mean fold changes of each gene between treated and control samples in Xushu32 and Ningzi1 were used to calculate its relative expression levels. Data are means ± SD of three biological replicates. Means denoted by the same letter do not significantly differ at P < 0.05 as determined by Duncan’s multiple range test
Fig. 9.
Expression profiles of IbHAKs genes in response to salt stress. a Expression patterns of selected IbHAKs genes in response to salt stress in leaves of Pushu32 were examined by a qPCR assay. b Expression patterns of selected IbHAKs genes in response to salt stress in roots of Pushu32 were examined by a qPCR assay. The samples were obtained at 0, 1, 3, 6, 12, 24, 48 h after salt stress. The mean fold changes of each gene between treated and control samples in Xushu32 and Ningzi1 were used to calculate its relative expression levels. Data are means ± SD of three biological replicates. Means denoted by the same letter do not significantly differ at P < 0.05 as determined by Duncan’s multiple range test
In conclusion, we identified 22 IbHAK genes in sweetpotato and 22 ItHAK genes in I. trifida and analyzed their classification, evolutionary relationship, protein motif, and gene structure. Stress- and hormone-related cis-element, RNA-Seq data, and qRT-PCR analyses indicated that these genes may play important roles in K+ uptake mechanism and stress response regulation. This study provided a theoretical basis for the application of IbHAK genes in molecular breeding of sweetpotato and provided key insight into the functions of HAK genes in sweetpotato. For example, the expression pattern and co-expression analysis of IbHAK8 is required to elucidate its precise role in sweetpotato.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- LTR
Low-temperature response element
- WUN
Wound responsive element
- ABRE
Abscisic acid-responsiveness
- WGD
Whole-genome duplications
- MYA
Million years ago
- NMT
Noninvasive microtest system
- HAK/KUP/KT
High-affinity K+ transporter/K+ uptake permease/ K+ transporter
- qRT-PCR
Quantitative real-time PCR
- NAC
No apical meristem (NAM), ATAF1/2, and cup-shaped cotyledon (CUC2)
- WGCNA
Weighted gene co-expression network analysis
- NADH
Nicotinamide adenine dinucleotide
- PERK1
Proline-rich receptor-like protein kinase 1
- LRR
Receptor-like serine/threonine-protein kinase
Author contributions
RJ and MY contributed to the design of the work. RJ and ZT gave final approval of this version to be published. RJ and WJ performed all the experiments. RJ, ML and PZ contributed to the data analysis. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- Adams E, Miyazaki T. Shin R (2019) Contribution of KUPs to potassium and cesium accumulation appears complementary in Arabidopsis. Plant Signal Behav. 2018;10(1080/15592324):1554468. doi: 10.1080/15592324.2018.1554468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Shin R, Schachtman DP. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004;134(3):1135–1145. doi: 10.1104/pp.103.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemán F, Caballero F, Rodenas R, Rivero RM, Martínez V, Rubio F. The F130S point mutation in the Arabidopsis high-affinity K+ transporter AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front Plant Sci. 2014;5:1–11. doi: 10.3389/fpls.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MK, Grant M, Grabov A. Plant responses to potassium deficiencies: a role for potassium transport proteins. J Exp Bot. 2006;57(2):425–436. doi: 10.1093/jxb/erj034. [DOI] [PubMed] [Google Scholar]
- Bacha H, Ródenas R, López-Gómez E, García-Legaz MF, Nieves-Cordones M, Rivero RM, Martínez V, Botella M, Rubio F. High Ca2+ reverts the repression of high-affinity K+ uptake produced by Na+ in Solanum lycopersycum L. (var. microtom) plants. J Plant Physiol. 2015;180:72–79. doi: 10.1016/j.jplph.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Banuelos MA, Garciadeblas B, Cubero B, Rodriguez-Navarro A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol. 2002;130(2):784–795. doi: 10.1104/pp.007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer ELL, Studholme DJ, Yeats C, Eddy SR. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer EK, Ahsan N, Dale R, Kato N, Coluccio AE, Piñeros MA, Kochian LV, Thelen JJ, Popescu SC. The raf-like kinase ILK1 and the high affinity K+ transporter HAK5 are required for innate immunity and abiotic stress response. Plant Physiol. 2016;171(2):1470–1484. doi: 10.1104/pp.16.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools–an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020 doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Chen G, Hu J, Lian J, Zhang Y, Zhu L, Zeng DL, Guo LB, Yu L, Xu GH, Qian Q. Functional characterization of OsHAK1 promoter in response to osmotic/drought stress by deletion analysis in transgenic rice. Plant Growth Regul. 2019;88(3):241–251. doi: 10.1007/s10725-019-00504-3. [DOI] [Google Scholar]
- Chen G, Zhang Y, Ruan BP, Guo LB, Zeng DL, Gao ZY, Zhu L, Hu J, Ren DY, Yu L, Xu GH, Qian Q. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism. Plant Sci. 2018;274:261–270. doi: 10.1016/j.plantsci.2018.05.034. [DOI] [PubMed] [Google Scholar]
- Chen XG, Shi CY, Li HM, Zhang AJ, Shi XM, Tang ZH, Wei M. Effects of potassium fertilization period on photosynthetic characteristics and storage root starch accumulation of edible sweetpotato. J Appl Ecol. 2013;24(3):759–763. [PubMed] [Google Scholar]
- Cheng X, Liu X, Mao W, Zhang X, Chen S, Zhan K, Bi H, Xu H. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L) Int J Mol Sci. 2018 doi: 10.3390/ijms19123969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XY, Liu XD, Mao WW, Zhang XR, Chen SL, Zhan KH, Bi HH, Xu HX. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L.) Int J Mol Sci. 2018 doi: 10.3390/ijms19123969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JW, Donoghue PCJ. Constraining the timing of whole genome duplication in plant evolutionary history. Proc Biol Sci. 2017 doi: 10.1098/rspb.2017.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. Accelerated Profile HMM Searches. Plos Comput Biol. 2011 doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elumalai RP, Nagpal P, Reed JW. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell. 2002;14(1):119–131. doi: 10.1105/tpc.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JY, Li M, Zhao S, Zhang C, Yang ST, Qiao S, Tan WF, Qu HJ, Wang DY, Pu ZG. Analysis of evolution and genetic diversity of sweetpotato and its related different polyploidy wild species I. trifida using RAD-seq. BMC Plant Biol. 2018;18(1):181. doi: 10.1186/s12870-018-1399-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Liu W, Qiu CW, Zeng F, Wang Y, Zhang G, Chen ZH, Wu F. HvAKT2 and HvHAK1 confer drought tolerance in barley through enhanced leaf mesophyll H+ homoeostasis. Plant Biotechnol J. 2020;18(8):1683–1696. doi: 10.1111/pbi.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Wang Y, Zhang N, Wu Z, Zeng Q, Wu J, Wu X, Wang L, Zhang J, Qi Y. Genome-wide systematic characterization of the HAK/KUP/KT gene family and its expression profile during plant growth and in response to low-K+ stress in Saccharum. BMC Plant Biol. 2020;20(1):20. doi: 10.1186/s12870-019-2227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HH, Luan S. AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgenzi FR, Peralta ML, Mangano S, Danna CH, Vallejo AJ, Puigdomenech P, Santa-María GE. The ionic environment controls the contribution of the barley HvHAK1 transporter to potassium acquisition. Plant Physiol. 2008;147:252–262. doi: 10.1104/pp.107.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Maser P. Potassium transporters in plants—Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 2007;581(12):2348–2356. doi: 10.1016/j.febslet.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Gierth M, Maser P, Schroeder JI. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137(3):1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Porras JL, Riano-Pachon DM, Benito B, Haro R, Sklodowski K, Rodriguez-Navarro A, Dreyer I. Phylogenetic analysis of K+ transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Front Plant Sci. 2012;3:167. doi: 10.3389/fpls.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A. Plant KT/KUP/HAK potassium transporters: single family—multiple functions. Ann Bot. 2007;99(6):1035–1041. doi: 10.1093/aob/mcm066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Qiu XH, Wang L, Xie WB, Zhang CJ, Xiong LZ, Lian XM, Zhang QF. KT/HAK/KUP potassium transporters gene family and their whole-life cycle expression profile in rice (Oryza sativa) Mol Genet Genom. 2008;280(5):437–452. doi: 10.1007/s00438-008-0377-7. [DOI] [PubMed] [Google Scholar]
- Hamamoto S, Horie T, Hauser F, Deinlein U, Schroeder J, Uozumi N. HKT transporters mediate salt stress resistance in plants: from structure and function to the field. Curr Opin Biotechnol. 2015;32:113–120. doi: 10.1016/j.copbio.2014.11.025. [DOI] [PubMed] [Google Scholar]
- Han M, Wu W, Wu WH, Wang Y. Potassium Transporter KUP7 Is Involved in K+ acquisition and translocation in Arabidopsis root under K+-limited conditions. Mol Plant. 2016;9(3):437–446. doi: 10.1016/j.molp.2016.01.012. [DOI] [PubMed] [Google Scholar]
- He CY, Cui K, Duan AG, Zeng YF, Zhang JG. Genome-wide and molecular evolution analysis of the Poplar KT/HAK/KUP potassium transporter gene family. Ecol Evol. 2012;2(8):1996–2004. doi: 10.1002/ece3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R. Ion channels in plants. Physiol Rev. 2012;92(4):1777–1811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- Hong JP, Takeshi Y, Kondou Y, Schachtman DP, Matsui M, Shin R. Identification and characterization of transcription factors regulating Arabidopsis HAK5. Plant Cell Physiol. 2013;54(9):1478–1490. doi: 10.1093/pcp/pct094. [DOI] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder J. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci. 2009;14:660–668. doi: 10.1016/j.tplants.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Sugawara M, Okada T, Taira K, Kaothien-Nakayama P, Katsuhara M, Shinmyo A, Nakayama H. Rice sodium-insensitive potassium transporter, OsHAK5, confers increased salt tolerance in tobacco BY2 cells. J Biosci Bioeng. 2011;111(3):346–356. doi: 10.1016/j.jbiosc.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cao H, Yang L, Chen C, Shabala L, Xiong M, Niu M, Liu J, Zheng Z, Zhou L, Peng Z, Bie Z, Shabala S. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J Exp Bot. 2019;70(20):5879–5893. doi: 10.1093/jxb/erz328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473(7345):97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10(1):51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Haubold B, Mitchell-Olds T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, arabis, and related Genera (Brassicaceae) Mol Biol Evol. 2000;17(10):1483–1498. doi: 10.1093/oxfordjournals.molbev.a026248. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Uozumi N, Hisamatsu S, Yamagami M. AtKUP/HAK/KT 9, a K+ transporter from Arabidopsis thaliana, mediates Cs+ uptake in escherichia coli. J Agric Chem Soc Japan. 2010;74(1):203–205. doi: 10.1271/bbb.90638. [DOI] [PubMed] [Google Scholar]
- Lara A, Ródenas R, Andrés Z, Martínez V, Quintero FJ, Nieves-Cordones M, Botella MA, Rubio F. AtHAK5-mediated root high-affinity K+ uptake is regulated by the protein kinases AtCIPK1 and AtCIPK9. J Exp Bot. 2020 doi: 10.1093/jxb/eraa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Li W, Xu G, Alli A, Yu L. Plant HAK/KUP/KT K+ transporters: Function and regulation. Semin Cell Dev Biol. 2018;74:133–141. doi: 10.1016/j.semcdb.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Liang M, Gao YC, Mao TT, Zhang X, Song ZZ. Characterization and expression of KT/HAK/KUP transporter family genes in willow under potassium deficiency, drought, and salt stresses. Bio Med Res Int. 2020;2020(6):1–12. doi: 10.1155/2020/2690760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang A, Chen X, Jin R, Li HM, Tang ZH. The effect of potassium deficiency on growth and physiology in sweetpotato [Ipomoea batatas (L.) Lam] during Early Growth. HortScience. 2017;52(7):1020–1028. doi: 10.21273/HORTSCI12005-17. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mangano S, Silberstein S, Santa-Marıa GE. Point mutations in the barley HvHAK1 potassium transporter lead to improved K+ -nutrition and enhanced resistance to salt stress. FEBS Lett. 2008;582:3922–3928. doi: 10.1016/j.febslet.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Maser P, Gierth M, Schroeder JI. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil. 2002;247(1):43–54. doi: 10.1023/a:1021159130729. [DOI] [Google Scholar]
- Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126(4):1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Cordones M, Alemán F, Martínez V, Rubio F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol Plant. 2010;3(2):326–333. doi: 10.1093/mp/ssp102. [DOI] [PubMed] [Google Scholar]
- Nieves-Cordones M, Rodenas R, Chavanieu A, Rivero RM, Martinez V, Gaillard I, Rubio F. Uneven HAK/KUP/KT protein diversity among angiosperms: species distribution and perspectives. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Yamane S, Yamaguchi M, Kato K, Shinmyo A, Tsunemitsu Y, Iwasaki K, Ueno D, Demura T. Characterization of rice KT/HAK/KUP potassium transporters and K+ uptake by HAK1 from Oryza sativa. Plant Biotechnol. 2018;35(2):101–111. doi: 10.5511/plantbiotechnology.18.0308a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Ohiraki H, Yamada K, Seo SU, Abo M, Yoshimura E, Shinozaki K, Yamaguchi-Shinozaki K. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell. 2013;25(2):609–624. doi: 10.1105/tpc.112.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou W, Mao X, Huang C, Tie W, Yan Y, Ding Z, Wu C, Xia Z, Wang W, Zhou S, Li K, Hu W. Genome-wide identification and expression analysis of the KUP family under abiotic stress in cassava (Manihot esculenta Crantz) Front Physiol. 2018;9:17. doi: 10.3389/fphys.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer ELL, Eddy SR, Bateman A, Finn RD. The Pfam protein families database. Nucleic Acids Res. 2012;40(D1):D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo YJ, Gierth M, Schroeder JI, Cho MH. High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010;153(2):863–875. doi: 10.1104/pp.110.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Hampton CR, Shin R, Barkla BJ, White PJ, Schachtman DP. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in Arabidopsis. J Exp Bot. 2008;59(3):595–607. doi: 10.1093/jxb/erm330. [DOI] [PubMed] [Google Scholar]
- Ragel P, Ródenas R, García-Martín E, Andrés Z, Villalta I, Nieves-Cordones M, Rivero RM, Martínez V, Pardo JM, Quintero FJ, Rubio F. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis Roots. Plant Physiol. 2015;169(4):2863–2873. doi: 10.1104/pp.15.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajappa S, Krishnamurthy P, Kumar PP. Regulation of AtHUP2 expression by bH1H and WRKY transcription factors helps to confer increased salt tolerance to arabidopsis thaliana plants. Front Plant Ence. 2020 doi: 10.3389/fpls.2020.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S, Ditengou FA, Ljung K, Daras G, Tietz O, Palme K, Hatzopoulos P. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013;197(4):1130–1141. doi: 10.1111/nph.12092. [DOI] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9(12):2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Maria GE. High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiol. 2000;123(1):297–306. doi: 10.1104/pp.123.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn ME, Rubio F, Bañuelos MA, Rodríguez-Navarro A. Comparative functional features of plant potassium HvHAK1 and HvHAK2 transporters. J Biol Chem. 2001;276:44563–44569. doi: 10.1074/jbc.M108129200. [DOI] [PubMed] [Google Scholar]
- Shen Y, Shen LK, Shen ZX, Jing W, Ge HL, Zhao JZ, Zhang WH. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015;38(12):2766–2779. doi: 10.1111/pce.12586. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. Ancient WGD events as drivers of key innovations in angiosperms. Curr Opin Plant Biol. 2016;30:159–165. doi: 10.1016/j.pbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Song ZB, Wu XF, Gao YL, Cui X, Jiao FC, Chen XJ, Li YP. Genome-wide analysis of the HAK potassium transporter gene family reveals asymmetrical evolution in tobacco (Nicotiana tabacum) Genome. 2019;62(4):267–278. doi: 10.1139/gen-2018-0187. [DOI] [PubMed] [Google Scholar]
- Stewart WM, Dibb DW, Johnston AE, Smyth TJ. The contribution of commercial fertilizer nutrients to food production. Agron J. 2005;97(1):1–6. doi: 10.2134/agronj2005.0001. [DOI] [Google Scholar]
- Sun Y, Buhler J. Designing patterns for profile HMM search. Bioinformatics. 2007;23(2):E36–E43. doi: 10.1093/bioinformatics/btl323. [DOI] [PubMed] [Google Scholar]
- Sun YL, Mu CH, Zheng HX, Lu SP, Zhang H, Zhang XC, Liu X. Exogenous Pi supplementation improved the salt tolerance of maize (Zea mays L.) by promoting Na+ exclusion. Sci Rep. 2018 doi: 10.1038/s41598-018-34320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustr M, Soukup A, Tylova E. Potassium in root growth and development. Plants (Basel, Switzerland) 2019 doi: 10.3390/plants8100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. K+ transport in plants: physiology and molecular biology. J Plant Physiol. 2009;166(5):447–466. doi: 10.1016/j.jplph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ZH, Zhang YG, Wei M, Chen XG, Shi XM, Zhang AJ, Ding YF. Screening and evaluation indicators for low potassium-tolerant and potassium efficient sweetpotato (Ipomoea batatas L.) Varieties (Lines) Acta Agronomica Sinica. 2014;40(3):542–549. doi: 10.3724/SP.J.1006.2014.00542. [DOI] [Google Scholar]
- Very AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J Plant Physiol. 2014;171(9):748–769. doi: 10.1016/j.jplph.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Véry AA, Sentenac H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol. 2003;54:575–603. doi: 10.1146/annurev.plant.54.031902.134831. [DOI] [PubMed] [Google Scholar]
- Vicente-Agullo F, Rigas S, Desbrosses G, Dolan L, Hatzopoulos P, Grabov A. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant J Cell Mol Biol. 2004;40(4):523–535. doi: 10.1111/j.1365-313X.2004.02230.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Lau KH, Cao QH, Hamilton JP, Sun H, Zhou C, Eserman L, Gemenet DC, Olukolu BA, Wang HY, Crisovan E, Godden GT, Jiao C, Wang X, Kitavi M, Manrique-Carpintero N, Vaillancourt B, Wiegert-Rininger K, Yang XS, Bao K, Schaff J, Kreuze J, Gruneberg W, Khan A, Ghislain M, Ma DF, Jiang JM, Mwanga ROM, Leebens-Mack J, Coin LJM, Yencho GC, Buell CR, Fei ZJ. Genome sequences of two diploid wild relatives of cultivated sweetpotato reveal targets for genetic improvemen. Nat Commun. 2018;9(1):1–12. doi: 10.1038/s41467-018-06983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Moeinzadeh MH, Kuhl H, Helmuth J, Xiao P, Haas S, Liu G, Zheng J, Sun Z, Fan W, Deng G, Wang H, Hu F, Zhao S, Fernie AR, Boerno S, Timmermann B, Zhang P, Vingron M. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat plants. 2017;3(9):696–703. doi: 10.1038/s41477-017-0002-z. [DOI] [PubMed] [Google Scholar]
- Yang TY, Zhang S, Hu YB, Wu FC, Hu QD, Chen G, Cai J, Wu T, Moran N, Yu L, Xu GH. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014;166(2):945–U757. doi: 10.1104/pp.114.246520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liang XY, Wang LM, Cao YB, Song WB, Shi JP, Lai JS, Jiang CF. A HAK family Na+ transporter confers natural variation of salt tolerance in maize. Nat Plants. 2019;5(12):1297. doi: 10.1038/s41477-019-0565-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.