Abstract
Only a limited number of techniques are available for assessing the effect of different coating materials on cell adherence to screws. In this study, we describe a simple and inexpensive method for evaluation of cell adhesion on irregular surfaces such as the surgical or implant screws. For this purpose, we prepared semi-submerged screws in the petri dishes using agar. Using BSA- or HA-coated screws, we tested whether BSA or HA could improve cell adherence when used as coating materials. Agar-coated screws were used as internal control. Then the “ratio of cell adherence” was calculated by subtracting the reference RCA value obtained from the agar coated screws (internal control). When compared to that of the non-coated screws both the HA- and BSA-coating improved cell adherence on the screws by 2.34 and 2.72 fold respectively. Similarly, MTT assay data revealed that the metabolic capacities of cells on HA- or BSA-coated screws were improved by 2.36 and 2.86 fold respectively. These findings suggest that this protocol can be used for comparing the ability of cells to attach on irregular surfaces such as dental or orthopedic screws and assessing their viability.
Keywords: Irregular surfaces, Dental, Orthopedics, Cell culture
Introduction
Implants are used in various clinical applications including the areas of orthopedics or dentistry. Studies in implant technology have expanded significantly over the last decades. Cellular adhesion is essential for the successful integration of implants into the bone tissue. Therefore, biocompatible surfaces that promote a favorable environment both fort the cells and host tissues are necessary for dental implants to successfully modulate the adhesion, proliferation and phenotypic expression of osteoblastic cells (Osman and Swain 2015).
Several different experimental approaches exist for evaluation of cell adhesion efficiency. For example, the “wash off” technique is a simple method for the determination of short term adherent cells where the number of the detached cells from the implant surfaces can be counted (García and Gallant 2003). Additionally, as reviewed by Khalili and Ahmad (2015), several different methods such as the cytodetachment technique, micropipette aspiration technique, force spectroscopy measurement methods, biomembrane force probe, optical tweezers, spinning disc methods, etc. can also be employed (Khalili and Ahmad 2015). However, these techniques are not easily accessible in every laboratory and they may not allow for an effective determination of cell adhesion and proliferation rates on irregular surfaces such as the grooves of implant screws. Therefore, we have developed a modified version of the wash-of method as a simple and inexpensive alternative which can allow for comparison of cell adhesion and proliferation rates on irregular surfaces such as the implant screws.
Materials and methods
Cell culture
Human osteoblast cell line (HOB, 406-05F Sigma-Aldrich) were grown in Dulbecco’s Modified Eagle’s medium (Sigma, 5546) supplemented with 10% heat-inactivated fetal bovine serum (Biowest, S1810-500), and the cells were incubated at 37 °C with 5% CO2. For MTT and trypan blue assays, the trypsinized cells were seeded out in 96-well flat-bottom culture plates.
Preparation of screws
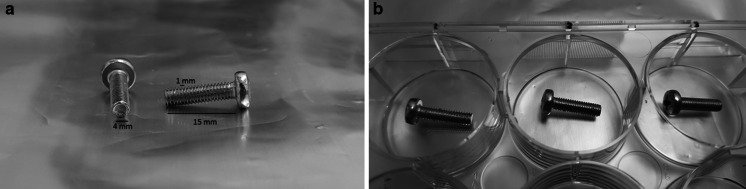
We used chrome-plated 15 × 4 mm metal screws with 1 mm of groove width (Fig. 1a). and all experiments were carried out using identical screws. The metal screws were sterilized in an autoclave. Three different types of coating materials were tested: BSA (10%) (Sigma-Aldrich., A9418), agar (0.2%) (Multicell-800-010-LG) or HA (10%) (Orthovisc-2 ml Sodium Hyaluronate). BSA and HA coatings were used as positive controls since they are known to improve cell adherence on the surfaces (Horváthy et al. 2016; Schulz et al. 2014). On the other hand, agar coating was used as a negative control since the cells are not able to attach on agar-coated surfaces (Tanaka et al. 2016). The results were compared to screws without any coating material. The coating was performed by inserting sterile coverslips (24-well) and screws (6-well) (sterilized with 95% EtOH and flamed) into each well of a well plate. BSA (10%), agar (0.2%) or HA (10%) were prepared and added 0.5 ml or 3 ml of the solutions to each well. Gently sank the floating coverslips with a sterile pipette tip. Incubated at room temperature for 3 h or overnight at 4 °C. Discarded the solutions and wash each well once with 1–3 ml of 1X PBS (Fig. 1b).
Fig. 1.
Representative images of metal screws with chrome plating. a 15 × 4 mm metal screws with 1 mm of groove width, b grooves of the screw does not disappear after the coating
Preparation of agar petri dishes
To stabilize the screws at the bottom of the plate, screws were fixed with a drop of a molten candle (Fig. 2a). Screws (with or without coating) were placed in a single 10 mm petri dish side by side. And liquid agar (%1.5) was poured until the screws were semi-submerged in agar (Fig. 2b) and then the agar allowed to solidify (Fig. 2b) Since, the cells are not able to attach on the agar surface, semi-submerged screws provided the only surface for the cells to attach.
Fig. 2.
Representative images of metal screws with chrome plating. a Screws were fixed with a drop of a molten candle, b semi-submerged (half-buried) screw in solid agar, c fully covered the surface of the screws with DMEM
Seeding cells on agar plates containing screws
Then 5 ml of medium (DMEM; 10% FBS, 1% P/S) containing 4 × 106 cells/ml poured on the agar plate until the medium fully covered surface of the screws (Fig. 2c). All the exposed agar surfaces, as well as the screws, are expected to receive an almost equal number of cells since cells were already suspended in the medium before adding on the plates. This type of design, where screws with different coating materials are placed into the same plate, allows for simultaneous comparison of the effects of different coating materials on cell adherence in the same medium.
Additional controls
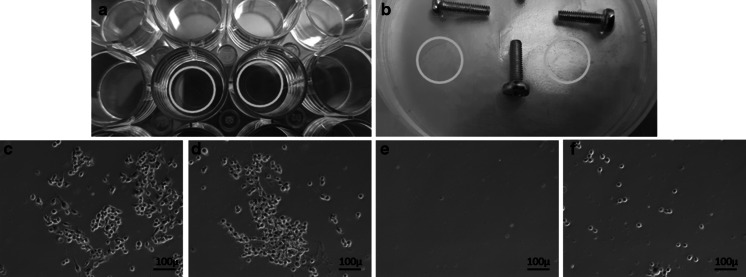
To test whether agar coating works as a non-adherent surface and to ensure that the adequate number of cells are seeded into the dishes we have also used coverslips (with 1 cm2 area) as additional controls. For this purpose, we have prepared agar coated coverslips, 10% hyaluronic acid-coated or 10% BSA coated coverslips and non-coated coverslips served as a control (Fig. 3a). One of each coverslip was placed on the empty regions of the agar (Fig. 3b). Then, coverslips were removed from agar and examined under the microscope for assessing the number and adherence of cells (Fig. 3c).
Fig. 3.
a Coating coverslips, b coverslips on agar, c microscope image of BSA coated coverslip (Magnification ×10), d microscope image of HA-coated coverslip (Magnification ×10), e microscope image of agar coated coverslip (Magnification ×10), f microscope image of the non-coated coverslip (Magnification ×10)
In vitro analysis of cell attachment performance of cells
First incubation period
After the addition of cell suspension, petri dishes were incubated for 24 h under optimal culturing conditions (37 °C, 5% CO2) to allow the cells to adhere. Then, non-adherent cells are washed away by rinsing the petri dishes with washing buffer (PBS) three times and the wash buffer is discarded. At this stage, the cells that are loosely attached on the agar surfaces are washed away and only the cells that are attached to the grooves of the screw are left.
Second incubation period
To be able to specifically determine the ability of the cells to adhere to the surface of the screws an additional incubation period of 48 h (second incubation period) was included in the protocol. Since the cells on the agar surfaces were already washed away in the first incubation period, only the cells that were able to attach to the screws were allowed to further adhere to the screws.
Determination of the number of detached cells (DC values)
At the end of the second incubation period, petri dishes are washed two times in PBS buffer. But this time wash buffer is collected (is nor discarded) and centrifuged to pellet the detached cells (DC). The number of detached cells (DC) were later determined by Trypan Blue staining.
Determination of the number of adherent cells (AC values)
After washing the petri dishes with PBS and collecting detached cells at the and of the second incubation period, the screws were transferred into 6-well plates. For this purpose, the agar surrounding the screw was carefully cut with a sterile cell scraper (SPL-90020., 1/Sleeve, Length 230 mm, Blade wide 13 mm) and removed together with the screw (Fig. 4a). Then the screw still buried in agar is transferred into 6-well plates containing 800 μl of trypsin–EDTA and incubated for 5 min at 37 °C in an incubator (Fig. 4b). Since cells cannot attach to agar surfaces, variations in agar dimensions surrounding the screws are not expected to affect calculations. For inactivation of trypsin–EDTA, 1600 μl of complete medium was added on trypsin–EDTA and then the cells were collected by centrifugation. Then cell pellet was resuspended in 200 μl of the full medium. To determine the number of adherent cells (AC) 15 μl of this cell suspension was used for Trypan blue staining. The remaining 185 μl of cell suspension was used for MTT assay.
Fig. 4.
a Cutting with a sterile cell scraper, b screw still buried in agar transferred to 6-well plate for trypsin
Determination of the ratio of adherent cells (RAC)
The “ratio of the adherent cells” (RAC) was calculated using the following formula: RAC (%) = AC/(AC + DC) *100. Therefore, the ability of cells to adhere to irregular surfaces can be compared between different materials, surfaces or surface coatings using this formula.
Data interpretation and calculation of relative RCA values
RCA value obtained from agar coated screws (RCAagar) was used as the reference value and RCAagar was subtracted from RCA values obtained from different treatments using the following formula: Δ%RCAX = (%RCAX − %RCAagar).
Relative changes in cell adherence in response to different coating materials were calculated by dividing each ΔRCA value by Δ %RCAnon-coated. For HA: (Δ%RCAHA/Δ%RCAnon-coated), or BSA: (Δ%RCABSA/Δ%RCAnon-coated).
Note 1: Since all the screws were placed in the same petri dish along with the agar-coated screw, agar-coated screws were used as the internal control for the elimination of nonspecific attachments or aggregations that might occur in the grooves. Using internal control might especially be important if data from more than one petri dish must be used. This can prevent variations that might arise from technical differences.
Trypan blue staining
0.4% trypan blue prepared in PBS was mixed in a 1:1 ratio with 15 μl of the cell suspension. The number of blue-colored (dead) cells and non-stained cells was determined by counting the hemocytometer in 5–10 min (Strober 2015).
MTT assay
MTT test was performed as described by Scudiere et al. (1988). The 24-well plate format was adapted to the 96-well plate. Briefly, cell pellets obtained by trypsinization were suspended in 200 μl full medium and seeded into 96-well plates. The cells were incubated overnight to adhere to the surface. Cell surface was gently washed once with PBS and MTT (Biofroxx, 3580GR001, Lot: 5A13FBF0, CAS: 298-93-1) reagent (0.5 mg/ml) prepared in DMEM was added on the cells with 200 μl/well. After incubation for 4 h at 37° C in the dark, the MTT solution was discarded from the wells. DMSO was added (200 μl/well) as a solvent of formazan crystals. Absorbance at 540 nm was recorded using a spectrophotometer.
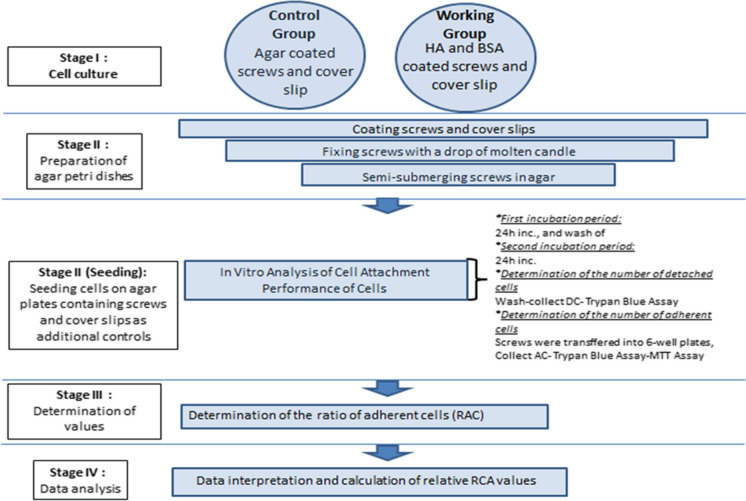
Simplified schematic diagram
A schematic representation of the step by step protocol is provided in Fig. 5.
Fig. 5.
Schematic representation of protocol
Statistical analysis
Statistical analysis was performed using GraphPad (Prism 5) software. Multiple comparisons were made using Tukey’s procedure. P < 0.05 was considered statistically significant. Analysis of variance was used for statistical analysis of the viability and adherence index among the groups. For statistical analysis results were expressed as mean values obtained from at least three independent experiments.
Results and discussion
In this study, we aimed to develop a simple and inexpensive method for evaluating the effect of different coating materials on cell adhesion to screws such as dental or orthopedic screws. To test the efficiency and the reliability of this protocol we have compared the ability of HOB mammalian bone cells to adhere to the screws coated with HA, BSA or agar. Non-coated screws were also included.
Control experiments: testing the effect of coating materials
Agar was used as negative control while HA or BSA was used as positive controls. As seen in Fig. 3e, microscope images confirmed that cells were not able to attach to surfaces coated with agar. However, BSA- or HA-coated coverslips provided surfaces on which cells could attach (Fig. 3c, d). Moreover, different coverslips had a similar amount of cells, suggesting that an even cell distribution on the petri dishes was achieved during seeding.
Data interpretation and calculations
To test the reliability and the sensitivity of this technique, changes in the number of the attached and the detached cells were compared between identical metal screws with or without BSA, agar, HA surface coatings. BSA and HA coatings were used as positive controls since they are known to improve cell adherence on the surfaces (Horváthy et al. 2016; Schulz et al. 2014). On the other hand, agar coating was used as a negative control since the cells are not able to attach on agar-coated surfaces.
In line with previous reports (Tanaka et al. 2016), data obtained from cover-slip experiments clearly demonstrated that HOB cells were not able to attach to flat surfaces coated with agar (Fig. 3e). However, when the screws were coated with agar we detected cell attachment (RCAagar 23.6% ± 1) although the ratio of the attached cells was lower than the non-coated group (RCAnon-coated 25.4% ± 3). This observation suggests that the grooves of the screws can provide gaps and irregular spaces where cells can get stuck and cannot be washed away during the rinse steps. Therefore, it is very important to set a reference (baseline) which represents the ratio for the non-specific bound cells. Our observations suggest that RCA value obtained from agar coated screws (RCAagar) can be used as the reference value (Table 1).
Table 1.
RCA values
| RAC (%) = AC/(AC + DC) *100 | |||
|---|---|---|---|
| %RCAHA | %RCABSA | %RCAagar | %RCAnon-coated |
| 25.85 | 24.82 | 25.08 | 28.99 |
| 31.44 | 27.04 | 24.04 | 24.56 |
| 26.35 | 33.93 | 21.58 | 22.69 |
Since all the screws were placed in the same petri dish along with the agar-coated screw, agar-coated screws were used as the internal control for the elimination of nonspecific attachments or aggregations that might occur in the grooves. Using internal control might especially be important if data from more than one petri dish must be used. This can prevent variations that might arise from technical differences.
Thus, RCA data obtained from RCAagar was subtracted from RCA values obtained from different treatments (RCAX) to achieve a more accurate and reliable relative comparison, using the following formula: Δ%RCAX = (%RCAX − %RCAagar).
For this experiment, the following RCA values were obtained RCABSA: 28.6% ± 4 and RCAHA: 27.9% ± 3. In this context relative changes in cell adherence in response to different coating materials was calculated as follows (Table 2).
Table 2.
Relative changes in cell adherence
| Δ%RCAX | %RCAX | %RCAagar | ||
|---|---|---|---|---|
| Δ%RCAnon-coated | 25.4 | − | 23.6 | = 1.85 |
| Δ%RCAHA | 27.9 | − | 23.6 | = 4.31 |
| Δ%RCABSA | 28.6 | − | 23.6 | = 5.03 |
When compared to that of the non-coated screws, HA-coating improved adherence by app. 2.34 fold (Δ%RCAHA/Δ%RCAnon-coated), while BSA-coated improved adherence ratio by 2.72 fold (Δ%RCABSA/Δ%RCAnon-coated) (Fig. 6). Cell adherence fold of RCA values was found to be significantly higher in coated screws when compared with the non-coated screws (p < 0.05).
Fig. 6.

Comparing to that of the non-coated screws adherence ratio
Additionally, the metabolic capacity of the adherent cells (AC) was also compared using MTT assay after the cells were detached from the surfaces of the screw by trypsinization.
Moreover, cell viability rates of adherent cells (AC) were found to be significantly higher in coated screws when compared with the non-coated screws (p < 0.0001).
Thus, AC data obtained from ACagar was subtracted from AC values obtained from different treatments (ACX) to achieve a more accurate and reliable relative comparison, using the following formula: Δ%ACX = (%ACX − %ACagar).
For this experiment, the following AC values were obtained ACBSA: 88.3% ± 7 and ACHA: 84.7% ± 6.
In this context relative changes in cell viability in response to different coating materials was calculated as follows (Table 3).
Table 3.
Relative changes in cell viability
| Δ%ACX | %ACX | %ACagar | ||
|---|---|---|---|---|
| Δ%ACnon-coated | 74.7 | − | 67.3 | = 7.33 |
| Δ%ACHA | 84.7 | − | 67.3 | = 17.33 |
| Δ%ACBSA | 88.3 | − | 67.3 | = 21 |
When compared to that of the non-coated screws, HA-coating improved viability by app. 2.36 fold (Δ%ACHA/Δ%ACnon-coated), while BSA-coated improved adherence ratio by 2.86 fold (Δ%ACBSA/Δ%ACnon-coated) (Fig. 7).
Fig. 7.

Comparing the viability of the attached cells
Conclusions
Our findings clearly showed that this protocol can efficiently discriminate between different coating materials as well as negative and positive controls. Therefore, we suggest that this method can be used as a simple and inexpensive technique for the evaluation of cell adherence to irregular surfaces such as surgical or dental screws. Although similar simple methods exist (Rosa et al. 2003) an accurate analysis may not be possible due to lack of proper reference values which can be used in comparisons.
RCA values obtained from the agar-coated screws suggest that the grooves of the screws can provide gaps and irregular spaces where cells can get stuck and cannot be washed away during the rinse steps (Table 1). Therefore, it is very important to use internal control which represents the ratio for the non-specific bound cells and therefore can be used for data normalization. In the experimental design, all the screws were placed in the same petri dish along with the agar-coated screws. Thus, the agar-coated screw can serve as an internal control for the elimination of nonspecific attachments or aggregations that might occur in the grooves. Using an internal control might especially be necessary if data from more than one petri dish should be compared (i.e., if data obtained from 3 different petri dishes, then 3 different reference values which represent 3 different dishes are required). This can prevent variations that might arise from technical differences.
In summary, using this simple method, we were able to successfully compare the effects of different surface coating materials on the ability of cells to adhere to metal screws and asses the influence of these surfaces on the metabolic capacity of the adherent cells.
Acknowledgements
This project would have been impossible without the support of Assoc. Prof. Dr. Cenk KIG.
Authors’ contributions
Gundogan G. I. carried out the experiments. Gundogan G. I. designed the model of the study and provided literature search. Gundogan G. I. contributed to processing, analysis, and collection of data. The manuscript was written by Gundogan G. I.
Funding
Not applicable.
Data and/or code availability
All the cell lines, raw experimental data, data on statistical analysis and/or detailed explanations regarding Materials and methods are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflicts of interest
The authors declare that she has no competing interests.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- García AJ, Gallant ND. Stick and grip: measurement systems and quantitative analyses of integrin-mediated cell adhesion strength. Cell Biochem Biophys. 2003;39:61–73. doi: 10.1385/CBB:39:1:61. [DOI] [PubMed] [Google Scholar]
- Horváthy DB, Vácz G, Szabõ T, Szigyártõ IC, Torõ I, Vámos B, Hornyák I, Renner K, Klára T, Szabõ BT, Dobõ-Nagy C, Doros A, Lacza Z. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J Biomed Mater Res Part B Appl Biomater. 2016;104:126–132. doi: 10.1002/jbm.b.33359. [DOI] [PubMed] [Google Scholar]
- Khalili AA, Ahmad MR. A review of cell adhesion studies for biomedical and biological applications. Int J Mol Sci. 2015;16:18150. doi: 10.3390/ijms160818149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman RB, Swain MV. A critical review of dental implant materials with an emphasis on titanium versus Zirconia. Materials. 2015;8:932–958. doi: 10.3390/ma8030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AL, Beloti MM, Van Noort R. Osteoblastic differentiation of cultured rat bone marrow cells on hydroxyapatite with different surface topography. Dent Mater. 2003;19:768–772. doi: 10.1016/S0109-5641(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Schulz MC, Korn P, Stadlinger B, Range U, Möller S, Becher J, Schnabelrauch M, Mai R, Scharnweber D, Eckelt U, Hintze V. Coating with artificial matrices from collagen and sulfated hyaluronan influences the osseointegration of dental implants. J Mater Sci Mater Med. 2014;25:247–258. doi: 10.1007/s10856-013-5066-3. [DOI] [PubMed] [Google Scholar]
- Scudiere DA, Shoemaker RH, Paul KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines1. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111:A3.B.1–A3.B.3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Moriguchi H, Sato A, Kawai T, Shimba K, Jimbo Y, Tanaka Y. Microcasting with agarose gel via degassed polydimethylsiloxane molds for repellency-guided cell patterning. RSC Adv. 2016;6:54754–54762. doi: 10.1039/C6RA11563B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the cell lines, raw experimental data, data on statistical analysis and/or detailed explanations regarding Materials and methods are available from the corresponding author on reasonable request.