Abstract
Introduction
The real-world effectiveness of belimumab for systemic lupus erythematosus (SLE) in six countries was evaluated in the OBSErve program. The aim of this post hoc analysis (GSK study 206351) was to pool individual patient OBSErve data to further evaluate the effectiveness of belimumab in a large sample of patients with SLE.
Methods
OBSErve (Argentina, Canada, Germany, Spain, Switzerland, and the USA) enrolled adults ≥ 18 years of age with SLE, who were prescribed belimumab as part of standard therapy (index: date of belimumab initiation). Endpoints (month 6 vs. index) included physician-assessed overall clinical response to belimumab in the overall population (primary) and high disease activity subgroups (secondary; patients with a SLEDAI-2K/SELENA-SLEDAI score ≥ 10 or patients with high anti-dsDNA or low complement at index); other secondary endpoints included changes in glucocorticosteroid (GCS) use and changes in disease activity. Factors associated with physician-assessed overall clinical response were also evaluated.
Results
In total, 830 patients were included in the overall population (mean [standard deviation (SD)] age: 41.9 [12.57] years; female: 89.3%; 60.4% from the USA). Nearly half (48.1%) of belimumab-treated patients experienced a ≥ 50% physician-assessed improvement in their overall manifestations, and 13% achieved a near normalization of their condition (equal to ≥ 80% improvement). Initiating belimumab while on high-dose (> 7.5 mg/day) GCS use was associated with ≥ 50% clinical improvement at month 6 (OR: 1.9, p = 0.003). Most (78.1%; n = 518/663) patients were able to reduce or discontinue their oral GCS dose after 6 months of belimumab, with a mean (SD) change of − 8.5 (10.74) mg/day prednisone-equivalent. The mean (SD) change from belimumab initiation in disease activity score (SLEDAI-2K/SELENA-SLEDAI) was − 5.7 (4.5; n = 344).
Conclusions
Belimumab improves clinical manifestations of SLE and is associated with GCS dose reductions in a real-world clinical setting, supporting the real-world effectiveness of belimumab for SLE.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-020-00243-2) contains supplementary material, which is available to authorized users.
Keywords: Belimumab, Glucocorticosteroid sparing, Real-world effectiveness, Real-world experience, SLE, Systemic lupus erythematosus
Key Summary Points
| Why carry out this study? |
| The efficacy and safety of belimumab in patients with systemic lupus erythematosus (SLE) have been demonstrated in several randomized clinical trials. However, strict eligibility criteria and inflexible concomitant medication management can narrow study populations, limiting the generalizability and interpretation of these findings to real-world clinical practice populations. |
| The real-world effectiveness of belimumab in patients with SLE in six countries was evaluated in the OBSErve program. |
| The aim of this post hoc analysis was to pool individual patient data from these six OBSErve studies to further evaluate the real-world effectiveness of belimumab in a large sample of patients with SLE. |
| What was learned from the study? |
| Belimumab treatment for at least 6 months improves clinical manifestations of SLE in the majority of patients and enables glucocorticosteroid dose reductions, with over half of patients achieving doses ≤ 7.5 mg, and is well tolerated in a real-world clinical setting. |
| These results support the real-world effectiveness of belimumab in the management of SLE. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13020566.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease with various clinical presentations and typically a relapsing–remitting course [1]. To manage disease activity, patients with SLE often require lifelong medications such as antimalarials, and immunosuppressive agents, in addition to oral glucocorticosteroids (GCS) to prevent and treat flares [2]. Repeated cumulative treatment with these therapies, particularly high-dose (≥ 7.5 mg/day) GCS, is associated with significant morbidity and irreversible damage accrual, and a reduction in GCS exposure is an important treatment goal in SLE [2].
Patients with SLE have elevated levels of B-cell–activating factor (BAFF; also known as B-lymphocyte stimulator [BLyS]), which promotes abnormal B-cell activation and differentiation [3]. Belimumab is a human immunoglobulin G1λ monoclonal antibody that inhibits the activity of BAFF [4] and is licensed for the treatment of adult and pediatric patients with active autoantibody-positive SLE who are receiving standard SLE therapy [5, 6]. The efficacy and safety of belimumab in patients with SLE has been demonstrated in several randomized clinical trials [7–10]. However, the strict inclusion and exclusion criteria and requirements for consistent concomitant medication use can limit the generalizability and interpretation of the findings of clinical trials to the real-world SLE patient population treated in everyday clinical practice. Furthermore, determining whether improvements in clinical trial composite endpoints translate into benefits in routinely assessed clinical outcomes based on physician judgment provides valuable information for physicians and patients when making treatment decisions. Consequently, the real-world effectiveness and utilization patterns of intravenous (IV) belimumab in patients with SLE have been evaluated in several countries in the OBSErve (evaluation Of use of Belimumab in clinical practice SEttings) program [11–16]. In these observational studies, adult patients with SLE treated with belimumab in a clinical practice setting demonstrated consistent clinical improvements. However, with the exception of the USA, which included ≥ 500 patients, sample sizes were small (50–100 patients), [11–16] limiting the ability to explore patient subgroups or factors associated with treatment effects.
The aim of this post hoc analysis was to pool individual patient data from the OBSErve studies [11–16] to further evaluate the real-world effectiveness and utilization of belimumab in a large sample of patients with SLE.
Methods
Study Design
This was a post hoc pooled analysis (GSK study 206351) of patient-level data pooled from the six retrospective, observational cohort OBSErve studies from 97 study centers based in Argentina (GSK study 201282), Canada (GSK study 117300), Germany (GSK study 117214), Spain (GSK study 200883), Switzerland (GSK study 201232), and the USA (GSK study 117295) [11–17]. Follow-up times varied between the original studies (OBSErve Canada, Germany, Spain, and Switzerland: 6 months; OBSErve Argentina and USA: up to 24 months), for the current analysis, patient data collected at belimumab start (index date, between September 2012 and March 2016) and at 6 months after index, or at time of discontinuation, were analyzed, because this was the longest duration of follow-up time for which all countries could contribute data (Supplementary Fig. 1). As the studies were retrospective, study protocols had no influence on the physicians’ treatment decisions or collection of patient data.
Ethics committee/institutional review board approval was not required for this analysis, as it used previously published data. All procedures performed in the original OBSErve studies were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the original OBSErve studies. The reporting of this study conforms to the CONsolidated Standards of Reporting Trials (CONSORT) 2010 guideline [18].
Physicians
Complete physician eligibility criteria for each individual OBSErve study have been published [11–16]. In brief, rheumatologists and/or internal medicine specialists who prescribed belimumab to patients with SLE as part of their usual clinical practice were invited to participate. Physicians extracted patient information about demographics, and disease and treatment characteristics. All patient data were anonymized.
Patients
The current analysis included all patients from the individual OBSErve studies. The following patient eligibility criteria were used in the primary studies: adults ≥ 18 years of age with a diagnosis of SLE, who were prescribed belimumab as part of standard SLE therapy, who initiated their belimumab therapy ≥ 6 months prior to study enrollment (Argentina, Germany, Spain, Switzerland), or had received ≥ 8 belimumab infusions during the ≥ 6 months prior to study enrollment (Canada, USA), and for whom reasons for belimumab initiation could be identified. Patients enrolled in an SLE clinical trial or who had initiated belimumab as part of a clinical trial were not included.
In OBSErve Argentina, Canada, Germany, Spain, and Switzerland, physicians examined their patient records to identify all patients with SLE receiving belimumab. To avoid selection bias, all patients who met the eligibility criteria (according to each country’s indication) were selected for inclusion. In OBSErve US, a random sample of eligible patients was selected by retrospective chart review of all available patients for each individual study center.
Endpoints and Assessments
The primary endpoint (physician-assessed overall clinical response to belimumab at month 6) was evaluated as worse, no improvement, < 20% (minimal) improvement, 20–49% (clear but moderate) improvement, 50–79% (large) improvement, or ≥ 80% (near normalized) improvement, compared with index.
Patient demographics (e.g., age, gender, country of enrollment), and clinical (disease severity and duration, number and type of SLE manifestations, number of comorbidities) and treatment characteristics (e.g., concomitant medications) at belimumab initiation were described as part of the secondary endpoint. Secondary endpoints (at month 6 vs. date of belimumab initiation) also included physician-assessed overall clinical response among patients in high disease activity subgroups; patient characteristics associated with a ≥ 50% improvement in physician-assessed overall clinical response to belimumab; change in SLE Disease Activity Index-2000 (SLEDAI-2K)/Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) score (choice based on center preference for each individual patient); reasons for belimumab initiation and discontinuation (physician-reported) and changes in concomitant GCS use (i.e., mean change in dose, proportion of patients experiencing dose change, and proportion of patients achieving dose reduction to ≤ 7.5 mg/day (prednisone-equivalent); and reasons for belimumab initiation and discontinuation (physician-reported).
Exploratory endpoints were also analyzed at month 6, including physician-assessed overall clinical response and concomitant GCS use based on immunomodulator (azathioprine, cyclophosphamide, cyclosporine, methotrexate, mycophenolate mofetil, thalidomide, leflunomide, or tacrolimus) use at belimumab initiation; and physician-assessed improvement in specific clinical manifestations reported as present at belimumab initiation.
For the secondary endpoint of physician-assessed overall clinical response, patients were assigned to one of two high disease activity subgroups to reflect the indicated population in Europe [5]: Subgroup 1, patients with a SLEDAI-2K/SELENA-SLEDAI score ≥ 10; and Subgroup 2, patients with high anti-dsDNA or low complement (C3 and/or C4, < lower limit of normal [based on physician’s assessment of the medical record]).
Sample Size and Statistical Analysis
Pre-specified primary and secondary endpoints of the current study were selected based on consistent data reporting/availability across the original OBSErve studies. Thus, except for SLEDAI-2K/SELENA-SLEDAI scores, which are not routinely assessed in clinical practice, the extent of missing data was expected to be minimal. Where data were missing, it has been clearly indicated throughout the results, and no imputation was performed for missing data.
Descriptive statistics were used to summarize continuous data (mean, median, standard deviation [SD], 95% confidence intervals [CIs], minimum and maximum, and range) and categorical data (counts and frequencies). The overall clinical response to belimumab was compared between the patients exposed to one immunosuppressant (IM) versus those exposed to at least two IMs at belimumab initiation, using the Wilcoxon rank-sum test. A logistic regression model was fitted to estimate the predictors of ≥ 50% improvement (combined responses of 50–79% and ≥ 80% improvement) physician-assessed overall clinical response to belimumab at month 6. The response categories were dichotomized (≥ 50% or < 50% improvement) to facilitate interpretation by using a generalized linear model specifying a binomial distribution rather than ordinal regression incorporating multiple response levels; ≥ 50% was selected as this represents a large improvement and used to differentiate patients likely to derive a large benefit from belimumab treatment. The following covariates were included in the model based on clinical understanding of characteristics that influence treatment response: age (reference case: 18–27 years); gender (reference case: female); ethnicity (reference case: Caucasian/white); disease severity at belimumab initiation (reference case: mild); prior IM use (reference case: no prior use); SLE disease duration (reference case: ≤ 5 years); GCS dose at belimumab initiation (reference case: ≤ 7.5 mg/day); presence of SLE manifestations at belimumab initiation (reference case: no manifestations at belimumab initiation); presence of high anti-dsDNA and/or low complement at belimumab initiation based on physician judgment (reference case: no anti-dsDNA and/or high complement at belimumab initiation). SLEDAI-2K/SELENA-SLEDAI score was not included in the final model due to missing data (n = 484 patients at baseline), which would substantially reduce the number of observations that could be included. Prior to fitting the model, multicollinearity of covariates was assessed using the variance inflation factor (VIF), with VIF > 10 indicative of one covariate being a linear combination of other covariates. A general rule of one covariate for every 20 observations in the smallest response group was applied to eliminate overfitting the model. Odds ratios (OR) and p values were reported for each covariate included in the model. The Hosmer–Lemeshow test was used to assess whether the observed rates matched the expected rates in the population subgroups (i.e., how well the model predicted overall clinical response to belimumab treatment). A p value (< 0.05) indicated a poor fit.
Results
Patient Demographics and Clinical Characteristics
In total, 830 patients were included in this pooled analysis, with the majority (n = 501/830, 60.4%) being from the USA, female (n = 741/830, 89.3%) and Caucasian/white (n = 540/830, 65.1%); mean (SD) age was 41.9 (12.57) years (Table 1). Most patients were classified as having high disease activity with either a SLEDAI-2K/SELENA-SLEDAI score of ≥ 10 (n = 208/346 of patients with available SLEDAI-2K/SELENA-SLEDAI score at belimumab initiation, 60.1%) or were positive for anti-dsDNA or low complement (n = 687/830, 82.8%). Patients presented with a range of clinical manifestations at belimumab initiation, with musculoskeletal, mucocutaneous, and immunologic being the most common (Table 1).
Table 1.
Patient demographics and clinical characteristics at date of belimumab initiation (index)
| N = 830 | |
|---|---|
| Agea, years, mean (SD) | 41.9 (12.6) |
| Female, n (%) | 741 (89.3) |
| Country of enrollment, n (%) | |
| Argentina | 58 (7.0) |
| Canada | 52 (6.3) |
| Germany | 102 (12.3) |
| Spain | 64 (7.7) |
| Switzerland | 53 (6.4) |
| USA | 501 (60.4) |
| Race/ethnicity, n (%) | |
| Caucasian/white | 540 (65.1) |
| African American/African origin/West Indian/Black | 134 (16.1) |
| Hispanic | 91 (11.0) |
| Asian | 36 (4.3) |
| Mixed | 17 (2.1) |
| Other | 7 (0.8) |
| American Indian/Native American | 5 (0.6) |
| Disease severityb, n (%) | |
| Mild | 58 (7.1) |
| Moderate | 593 (72.1) |
| Severe | 171 (20.8) |
| Disease durationc, n (%) | |
| ≤ 5 years | 377 (45.5) |
| 6–10 years | 199 (24.0) |
| > 10 years | 252 (30.4) |
| Low C3 (< lower limit of normal), n (%) | 474 (57.1) |
| Low C4 (< lower limit of normal), n (%) | 479 (57.7) |
| High anti-dsDNA, n (%) | 540 (65.1) |
| SLEDAI-2K/SELENA-SLEDAI scored, n (%) | |
| < 10 | 138 (39.9) |
| ≥ 10 | 208 (60.1) |
| SLEDAI-2K/SELENA-SLEDAI score, mean (SD) | 11.0 (5.3) |
| Clinical manifestations by organ system involvement, n (%) | |
| Musculoskeletal | 598 (72.1) |
| Mucocutaneous | 490 (59.0) |
| Immunologic | 468 (56.4) |
| Constitutional (fatigue, fever, and/or weight loss) | 402 (48.4) |
| Hematologic | 231 (27.8) |
| Renal | 137 (16.5) |
| Cardiopulmonary | 127 (15.3) |
| Central nervous system | 111 (13.4) |
| Headache | 81 (9.8) |
| Organic brain damage | 17 (2.0) |
| Seizure | 13 (1.6) |
| Cranial nerve neuropathy | 8 (1.0) |
| Cerebrovascular accident | 7 (0.8) |
| Psychosis | 7 (0.8) |
| Vasculitis | 37 (4.5) |
| Gastrointestinal | 4 (0.5) |
| Visual/ocular | 1 (0.1) |
| Other | 42 (5.1) |
| Mucocutaneous and/or musculoskeletal | 719 (86.6) |
| No other organ involvement | 81 (9.8) |
| With renal involvement | 114 (13.7) |
| Without renal involvement | 524 (63.1) |
| No mucocutaneous or musculoskeletal | 111 (13.4) |
| SLE medications, n (%) | |
| Oral GCS | 663 (79.9) |
| Antimalarial | 579 (74.0) |
| Immunosuppressant | 488 (58.8) |
| Azathioprine | 171 (35.0) |
| Cyclophosphamide | 2 (0.4) |
| Cyclosporine | 13 (2.7) |
| Methotrexate | 151 (30.9) |
| Mycophenolate mofetil | 165 (33.8) |
| Mycophenolate sodium | 1 (0.2) |
| Thalidomide | 1 (0.2) |
| Leflunomide | 2 (0.4) |
| Tacrolimus | 1 (0.2) |
C complement, GCS glucocorticosteroids, SD standard deviation, SELENA-SLEDAI Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000
an = 778, excluding Canada, in which individual patient data for age were reported as a categorical outcome
bn = 822
cn = 828
dn = 346, data available for a subset of patients in each OBSErve study
On a country level, demographic and clinical characteristics at belimumab initiation were broadly similar. However, patients enrolled from OBSErve Switzerland and USA had a shorter disease duration (45.3 and 55.7% < 5 years, respectively) compared with 23.1% in Canada and 39.7% in Argentina, and more patients from Switzerland had mild disease (43.4%) at belimumab initiation compared with 2.8% in the USA and 10.5% in Argentina. Patients from Switzerland also had the lowest mean number of comorbidities, and lowest proportion of patients with SELENA-SLEDAI scores ≥ 10 (32.1%), whereas the USA had the highest proportion of patients with SELENA-SLEDAI scores ≥ 10 (77.9%). The majority of patients of Black race were from the USA (Supplementary Table 1).
Physician-Assessed Clinical Improvements After 6 Months of Belimumab Treatment
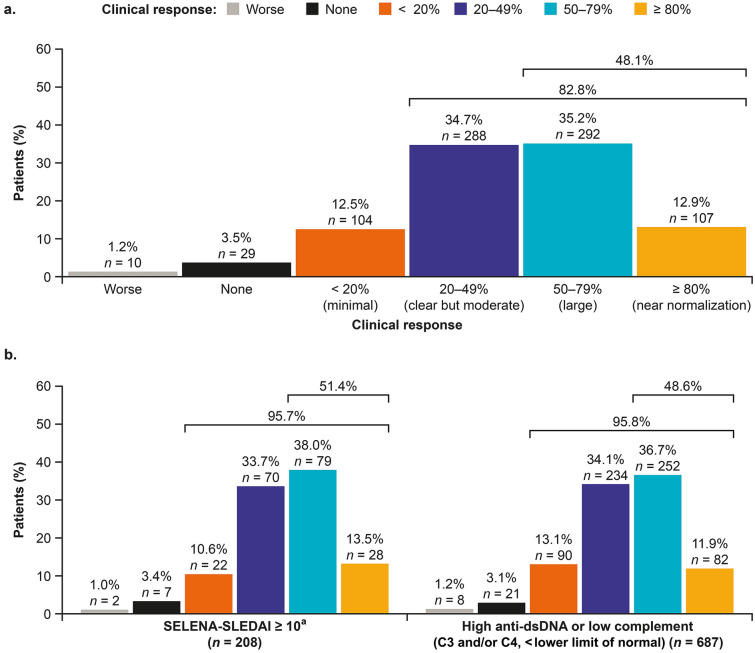
At month 6, most (n = 791/830, 95.3%) patients had an improvement from the date of belimumab initiation in their overall condition. A minimal improvement (< 20%) was observed for 12.5% (n = 104/830) of patients, with a further 34.7% (n = 288/830), 35.2% (n = 292/830), and 12.9% (n = 107/830) experiencing an improvement of 20–49%, 50–79%, and ≥ 80%, respectively. Worsening was reported for ten patients (1.2%; Fig. 1a). Similarly, 95.7% of patients with high disease activity defined as SLEDAI-2K/SELENA-SLEDAI ≥ 10 and 95.8% of those with high disease activity defined as high anti-dsDNA or low complement based on physician judgment (C3 and/or C4, < lower limit of normal) demonstrated an improvement in their overall condition, with 11.9–13.5% achieving a near normalization of their condition (≥ 80% improvement from date of belimumab initiation) (Fig. 1b).
Fig. 1.
Physician-assessed overall clinical response at month 6 in the overall population (a) and patients with SLEDAI-2K/SELENA-SLEDAI score ≥ 10 and those with serologically active disease (b). C complement, dsDNA double-stranded deoxyribonucleic acid, SLEDAI-2K/SELENA-SLEDAI Safety of Estrogens in Lupus National Assessment-SLE Disease Activity Index, SLE systemic lupus erythematosus
aSLEDAI-2 K/SELENA-SLEDAI scores were only available for 42% (n = 346) of the pooled cohort
Characteristics Associated with Physician-Assessed Clinical Improvements
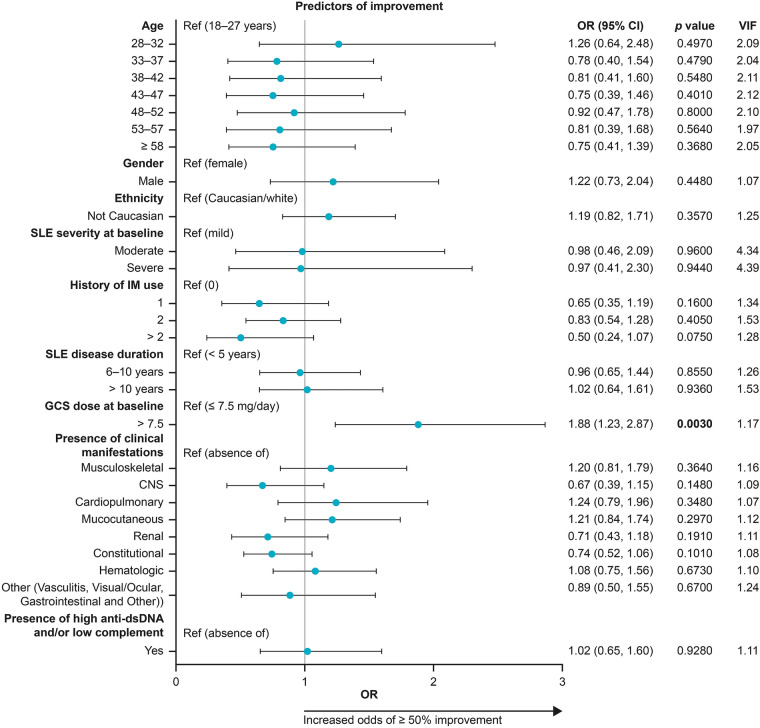
Use of high-dose GCS (> 7.5 mg/day prednisone-equivalent) at belimumab initiation was the only factor significantly associated with ≥ 50% overall clinical improvement (OR: 1.88 [95% CI: 1.23, 2.87]; p = 0.003). No other trends in other patient or treatment characteristics were observed. Numerical differences were observed amongst patients with varying manifestations at baseline; the sample size was low for several organ systems as the majority of patients had mucocutaneous (59%) and musculoskeletal (72%) involvement (Fig. 2).
Fig. 2.
Predictors of a large (≥ 50%) physician-assessed overall clinical improvement at month 6 based on patient characteristics at date of belimumab initiation (index). CI confidence interval, CNS central nervous system, dsDNA double-stranded deoxyribonucleic acid, GCS glucocorticosteroid, OR odds ratio, SLE systemic lupus erythematosus, VIF variance initiation factor. The OR represents the odds of achieving an overall clinical improvement of ≥ 50% will occur given a particular characteristic, compared with the odds of an overall clinical improvement of ≥ 50% in the reference case OR > 1: characteristic is associated with higher odds of a large (≥ 50%) improvement; OR < 1: the characteristic is associated with lower odds of a large (≥ 50%) improvement. Black horizontal lines represent 95% CIs
Country-Specific Analysis of Clinical Improvement at Month 6
The distribution of the degree of physician-assessed clinical improvement in response to belimumab from date of belimumab initiation at month 6 between countries was statistically significant (p < 0.0001; Supplementary Table 2). Patients from Switzerland had a smaller clinical improvement from the date of belimumab initiation to month 6 compared with other countries, with 17.0% (n = 9/53) having no improvement compared with 0.6% from the USA and 9.4% from Spain. Spain and Argentina had the largest proportion of patients with ≥ 80% clinical improvement from date of belimumab initiation (n = 17/64, 26.6% and n = 12/58, 20.7%, respectively), compared with 8.8% from Germany and 17.3% from Canada.
Change from Date of Belimumab Initiation in SLEDAI-2K/SELENA-SLEDAI at Month 6
In total, 346 patients had a SLEDAI-2K/SELENA-SLEDAI score at belimumab initiation (mean [SD]: 11.0 [5.3]) and 349 patients at month 6 (5.3 [3.8]). The mean (SD) change between these two time points, calculated for 344 patients, was − 5.7 (4.5).
Treatment Patterns Associated with Belimumab
The most common reason for belimumab initiation was due to the previous treatment regimen not being effective (n = 617/830, 74.3%), followed by the desire to decrease GCS use (n = 460/830, 55.4%) and the patient’s condition worsening (n = 451/830, 54.3%; Table 2). In total, 28.3% of patients in Switzerland initiated treatment due to condition worsening versus 50% from both Canada and Argentina, and 60.8% from Germany. Of those from whom discontinuation data were available (n = 277), only 12 (4.3%) patients discontinued belimumab during the 6 months of treatment (Table 2). The most common reasons for belimumab discontinuation were patient request and ineffective treatment (both n = 4, 33.3%), and adverse event and disease progression (both n = 3, 25.0%).
Table 2.
Reasons for belimumab initiation and discontinuation
| Reason for initiating belimumaba, n (%) | N = 830 |
|---|---|
| Previous treatment regimen not effective | 617 (74.3) |
| To decrease use of GCS | 460 (55.4) |
| Patient condition worsening | 451 (54.3) |
| Previous treatment regimen not well tolerated | 186 (22.4) |
| Patient request | 58 (7.0) |
| Previous treatment regimen inconvenient | 29 (3.5) |
| Drug-to-drug interactions with previous medication | 9 (1.1) |
| Other | 5 (0.6) |
| Reasons for discontinuing belimumabab, n (%) | n = 277 |
|---|---|
| Total number of discontinuations | 12 (4.3) |
| Medication not effective | 4 (33.3) |
| Patient request | 4 (33.3) |
| Disease progression | 3 (25.0) |
| Adverse event | 3 (25.0) |
| Loss of insurance or reimbursement | 2 (16.7) |
| Lack of compliance | 1 (8.3) |
| Death | 1 (8.3) |
| Other | 2 (16.7) |
GCS glucocorticosteroids
aCategories are not mutually exclusive
bData from Argentina, Germany, Spain, and Switzerland only
At belimumab initiation, 663 (79.9%) patients were receiving oral GCS (Table 3) at a mean (SD) prednisone-equivalent dose of 16.7 (12.67; median, 15.0 [range, 2, 100]) mg/day. The majority (n = 518/663, 78.1%) of patients were able to reduce or discontinue their oral GCS dose after 6 months of belimumab treatment (Table 3). At month 6, the mean (SD) prednisone-equivalent dose was 8.3 (6.16) mg/day, a decrease from the date of belimumab initiation of − 8.5 (10.74) mg/day. Among the 526 patients receiving > 7.5 mg/day dose of oral GCS at belimumab initiation, 52.6% of those with data for month 6 (n = 258/491) reduced their dose to ≤ 7.5 mg/day at month 6 (Table 3).
Table 3.
Oral GCS use at date of belimumab initiation (index) and during 6 months of belimumab treatment
| N = 830 | |
|---|---|
| Oral GCS use at index, n (%) | 663 (79.9) |
| Oral GCS dose (prednisone-equivalent) at index, n (%) | |
| ≤ 7.5 mg/day | 137 (20.7) |
| > 7.5 mg/day | 526 (79.3) |
| Oral GCS use at month 6, n (%) | |
| Discontinued | 50 (7.5) |
| Reduced dose | 468 (70.6) |
| No change in dose | 124 (18.7) |
| Increased dose | 21 (3.2) |
| Oral GCS dose (prednisone-equivalent), mg/day; mean (SD) | |
| Index | 16.7 (12.7) |
| Month 6 | 8.3 (6.2) |
| Change between index and month 6 | − 8.5 (10.7) |
| Oral GCS dose (prednisone-equivalent) at month 6, n (%) | n = 613 |
| ≤ 7.5 mg/day | 375 (61.2) |
| > 7.5 mg/day | 238 (38.8) |
| Reduction to ≤ 7.5 mg/daya | 258 (52.6) |
GCS glucocorticosteroids
aFrom > 7.5 mg/day at index; n = 491
Patient Response to Belimumab Based on Patient History of IM Use
Physician-assessed response to belimumab at month 6 was similar in patients with prior and/or concomitant exposure to 0, 1, 2, or > 2 IMs during the 6 months prior to the date belimumab initiation (inclusive) (disease-modifying anti-rheumatic drugs, excluding hydroxychloroquine; Supplementary Table 3). There was no clear trend in the changes in GCS use or dose based on patient history of IM use (Supplementary Table 3).
Physician-Assessed Clinical Improvements in Organ-Specific Manifestations
Of the 598 and 490 patients with musculoskeletal and mucocutaneous manifestations at belimumab initiation, 311 (51.8%) and 269 (54.7%) patients experienced ≥ 50% improvement from the date of belimumab initiation at month 6, respectively. Similarly, of the 127 patients with cardiopulmonary manifestations at belimumab initiation, 70 (55.1%) experienced improvement from belimumab initiation to month 6 of ≥ 50% and 37 (29.1%) had improvement ≥ 80% (data not shown).
The most common (≥ 100 patients) individual manifestations are shown in Supplementary Fig. 2. Of these, over half of the patients demonstrated at least a 50% improvement in manifestations of arthritis and mucosal ulcer. In the 137 patients with renal manifestations at belimumab initiation, 132 (15.9%) had proteinuria and 11 (1.3%) had pyuria; the degree of improvement in these specific manifestations was evenly distributed amongst the response categories; similar results were seen for alopecia and leukopenia (Supplementary Fig. 2).
Discussion
This post hoc pooled analysis of real-world medical data pooled from six OBSErve studies provides important insights into clinical outcomes and concomitant medication use in a large global sample of patients with SLE treated with belimumab. Following 6 months of belimumab IV treatment, most patients demonstrated clinical improvements in SLE, and reductions in GCS dose, consistent with the primary studies conducted in Argentina, Canada, Germany, Spain, Switzerland, and the USA [11–16]. As assessed by their physicians, nearly half of belimumab-treated patients experienced ≥ 50% improvement in their overall manifestations, and 13% achieved a near normalization of their condition (equal to ≥ 80% improvement). The results were consistent between the overall population and subgroups of patients with a SLEDAI-2K/SELENA-SLEDAI score of ≥ 10 and those positive for anti-dsDNA or low complement. These results are also consistent with a post hoc analysis of the BLISS IV studies, which defined high disease activity as a SELENA-SLEDAI score of ≥ 10, low complement levels, anti-dsDNA 30 IU/ml, and GCS use [19].
Similar to the original OBSErve studies, this pooled analysis confirmed improvements in SLEDAI scores and reductions in GCS use after 6 months of belimumab treatment. As noted in the 2019 update of the European League Against Rheumatism recommendations for the management of SLE, GCS should be discontinued or daily dose reduced to ≤ 7.5 mg to avoid organ damage accrual associated with prolonged high-dose GCS intake [2]. A steroid-sparing effect of belimumab has previously been indicated in post hoc analyses and long-term continuation studies of belimumab clinical trials [19–21]. In the current study, 71% of patients were able to reduce and 8% to discontinue their oral GCS during the first 6 months of belimumab treatment, in addition to 53% reducing their dose to ≤ 7.5 mg/day. Furthermore, the mean GCS dose reduction of 8.5 mg/day in this study was similar to that observed in an independent, investigator-initiated, prospective study in Italy (7.6 mg/day) of patients with active SLE [22].
In the current study, compared with patients receiving ≤ 7.5 mg/day dose at belimumab initiation (21%), a high (> 7.5 mg/day) GCS dose at date of belimumab initiation was significantly (p = 0.003) associated with almost twice the odds of a patient achieving an overall clinical improvement ≥ 50% at month 6. This is similar to a post hoc analysis of the BLISS-52 and BLISS-76 randomized clinical trials, which included a more restricted group of patients and found that there was a significant association between GCS dose at baseline and response to belimumab [19]. This post hoc analysis also found that SELENA-SLEDAI scores ≥ 10 also predicted better belimumab responses; investigation of this relationship was precluded in the current study by more than half of the patients having missing SLEDAI-2K/SELENA-SLEDAI scores [19]. Together, these results suggest that real-world patients who are receiving high-dose oral GCS (> 7.5 mg/day), which indicates that they have a higher degree of disease activity, are likely to have a greater degree of improvement from belimumab treatment than those on lower doses.
OBSErve did require patients to have a previous treatment history and/or active disease. Consequently, differences were observed in the characteristics of the patients included in the individual OBSErve studies, which likely contributed to between-country differences in degree of improvement since belimumab treatment. For example, many more patients in Switzerland than in any of the other countries were initiated with belimumab when their disease severity was considered mild (as assessed by the physician). The capacity of patients with already mild disease to have a large degree of improvement is lower than those with severe disease, and accordingly this was reported in OBSErve Switzerland [14]. Indeed, of all the countries assessed in OBSErve, Switzerland had the highest proportion of patients with no or < 20% improvement, which reflects a desirable outcome for patients whose mild, and presumably well-controlled, disease is sought to be maintained rather than further improved. In the primary OBServe Switzerland study, reductions from baseline in SELENA-SLEDAI score and GCS dose (− 5.7 mg/day reduction from baseline at 6 months) were also observed [14]. More mild patients receiving belimumab in Switzerland compared with other countries may suggest that belimumab is being used to prevent future flares and damage over the long term rather than to intervene when patients are progressing or are less well controlled. This is further supported by the finding that only 28% of patients in Switzerland compared with 50 to 61% in other countries initiated belimumab due to disease worsening. The largest proportion of patients experiencing near normalization of their SLE was in Spain, where > 25% had a ≥ 80% improvement since belimumab initiation. The highest proportion of patients with severe disease at belimumab initiation was also observed in Spain. Consequently, these data also provide insights into the differing treatment approaches between countries, although it should be noted that sample sizes for individual countries were small, meaning between-country comparisons should be interpreted with caution.
Physician-assessed improvement in specific clinical manifestations was broadly consistent with their assessment of their overall response. In this cohort, 72% of patients had musculoskeletal involvement at date of belimumab initiation and more than half of these patients were reported to have ≥ 50% improvement in this organ system by month 6. Improvement at the level of individual, specific clinical manifestations is dependent upon the baseline severity of that manifestation, which has not been analyzed here, and the inherent ability of each to improve. For example, patients with proteinuria may be expected to have stable, low levels of proteinuria given that patients with active nephritis and uncontrolled proteinuria were less likely to receive belimumab prior to initiation of the BLISS-LN study in 2012 (NCT01639339) [23]. However, this cannot be confirmed in the absence of renal laboratory parameters, and without a definition/cutoff for proteinuria, which were not collected and defined in the OBSErve studies, respectively. The results of the regression analysis may imply that there are lower odds of a large (≥ 50%) improvement in patients with renal, central nervous system, and constitutional manifestations at date of belimumab initiation. However, this does not suggest that these patients are achieving no or a poor response since patients assessed as having had 20–49% improvement at month 6, pre-defined as a clear but moderate improvement are not included in the outcome variable of the binomial regression. Therefore, results of the regression analysis should be considered conservative.
This study has a number of limitations inherent to this type of observational study. Although validated SLE assessment scores were reported where available from patient medical records, the primary outcome of the overall clinical response was based on the subjective clinical physician judgment. As such, the accuracy of the physician judgment cannot be compared with validated disease activity measures such as the SELENA-SLEDAI or British Isle Lupus Assessment Group instruments, nor was it verified by another physician, which poses a potential for misclassification bias. Selection bias may be present, introduced by the use of convenience sampling by most of the individual OBSErve country studies, although some studies included random sampling of patients. Additionally, treatment patterns, clinical outcomes, and resource use data represent only the medical sites of physicians participating in the study and may vary from those of non-participating physicians. The study population was predominantly Caucasian/white with other races and ethnicities in whom SLE is generally more severe with higher disease activity and more damage accrual (i.e., people of Hispanic, Black, and Asian race) [24] largely underrepresented. Subgroup analysis by race/ethnicity was not conducted as part of our pooled analysis; however, because 92% of the patients of Black race were in OBSErve US, the results published in that specific individual study are of note [15]. In OBSErve US, the change in overall clinical response at 0–6 months did not significantly differ between ethnic subgroups (p = 0.2622), with 46.8% and 45.5% of patients of Caucasian or African-American origin, respectively, reporting a ≥ 50% improvement in clinical response. Neither the subgroup analysis according to IM use nor the regression analysis were specified in the individual OBSErve studies and should be interpreted with some caution. Additionally, data on SLEDAI score were missing for over half of the included patients, which may have increased or decreased mean changes from baseline, although it should be noted that improvements from the date of belimumab initiation in SLEDAI score are in line with previous clinical trials [7, 9, 10].
As the current study analyzed outcomes only following 6 months of belimumab treatment, the results of this study will not fully reflect belimumab’s clinical effectiveness. Both the USA and Argentina OBSErve studies included follow-up to 24 months after belimumab initiation. Additional analysis with a longer follow-up time could be considered on a pooled cohort of these patients to explore predictors of clinical outcomes over a longer belimumab treatment period. Patient-reported outcomes were not collected in the individual OBSErve studies, which would have provided further insights into belimumab treatment outcomes from the patients’ perspective. While cross-sectional survey studies provide important confirmation of treatment satisfaction [25], real-world assessment of improvements in health-related quality of life or fatigue are limited. Finally, as was the case with the individual OBSErve studies, the results of this pooled analysis may not be generalizable outside the countries included. Nonetheless, this study provides important additional validation of the real-world effectiveness of belimumab in a large sample of patients with SLE in a real-world setting.
Conclusions
In conclusion, this pooled analysis demonstrated that belimumab improves clinical manifestations of SLE and enables a reduction in GCS ≤ 7.5 mg/day in over half of the patients in a real-world clinical setting. The results of this study corroborate the conclusions of the individual OBSErve studies and confirm the real-world effectiveness of belimumab IV in the management of SLE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Shannon Ferrante of GSK for her contribution to the study.
Funding
Sponsorship for this study and the Rapid Service Fee were funded by GlaxoSmithKline (GSK), Middlesex, UK. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing Assistance
Medical writing assistance was provided by Gosia Carless, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
The data in this manuscript have been presented at the 2019 EULAR Annual European Congress of Rheumatology, June 12–15, 2019, Madrid, Spain.
Disclosures
Christopher E. Collins has received grant/research support from Exagen; was a member of the speakers’ bureau for GSK, Exagen, AbbVie, and Novartis; and has acted as a consultant for GSK, Exagen, and AbbVie. Christopher E. Collins was affiliated with MedStar Washington Hospital Center at the time of study and is now affiliated with Aurinia Pharmaceuticals Inc. Josefina Cortes-Hernández has received grant/research support and is on the speakers’ bureau for GSK. Mercedes A. Garcia has received grant/research support and is on the speakers’ bureau for GSK. Johannes von Kempis has nothing to disclose. Andreas Schwarting has received grant/research support from GSK, Pfizer, AbbVie, Novartis, and Roche and speaker’s fee from GSK and Novartis. Zahi Touma has received research support from GSK Canada and is a consultant with UCB, Pfizer, and Janssen Inc. Milena Kurtinecz and Kerry Gairy are employees of GSK and hold stocks and shares in the company.
Compliance with Ethics Guidelines
An ethics committee/institutional review board approval was not required for this analysis, as it used previously published data. All procedures performed in the original OBSErve studies were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the original OBSErve studies. The reporting of this study conforms to the CONsolidated Standards of Reporting Trials (CONSORT) 2010 guideline [18].
Data Availability
Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com.
Footnotes
Christopher E. Collins: at the time of study MedStar Washington Hospital Center.
References
- 1.D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 2.Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–2459. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 4.Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 5.EMA. Benlysta summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/benlysta-epar-product-information_en.pdf. Accessed July 2020.
- 6.FDA. News release. FDA approves first treatment for pediatric patients with lupus. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-pediatric-patients-lupus. Accessed July 2020.
- 7.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Bae S-C, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. 2018;77:355–363. doi: 10.1136/annrheumdis-2017-211631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, Placebo-Controlled Study. Arthritis Rheumatol. 2017;69:1016–1027. doi: 10.1002/art.40049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Touma Z, Sayani A, Pineau CA, Fortin I, Matsos M, Ecker GA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada Study. Rheumatol Int. 2017;37:865–873. doi: 10.1007/s00296-017-3682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarting A, Schroeder JO, Alexander T, Schmalzing M, Fiehn C, Specker C, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany Study. Rheumatol Ther. 2016;3:271–290. doi: 10.1007/s40744-016-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortés J, Andreu JL, Calvo J, García-Aparicio AM, Coronell CG, Díaz-Cerezo S. Evaluation of use of belimumab in clinical practice settings (observe study) in Spain: health resource utilization and labour absenteeism. Value Health. 2014;17:A534. doi: 10.1016/j.jval.2014.08.1703. [DOI] [PubMed] [Google Scholar]
- 14.von Kempis J, Duetsch S, Reuschling N, Villiger R, Villiger PM, Vallelian F, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly. 2019;149:w20022. doi: 10.4414/smw.2019.20022. [DOI] [PubMed] [Google Scholar]
- 15.Collins CE, Dall'Era M, Kan H, Macahilig C, Molta C, Koscielny V, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3:e000118. doi: 10.1136/lupus-2015-000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babini A, Garcia MA, Barreira JC, Pons-Estel B, Iglesias M, Streger G. Evaluation of use of belimumab in clinical practice settings: results in Argentina. Arthritis Rheumatol. 2016;68(Suppl 10):1–4550. [Google Scholar]
- 17.Babini A, Cappuccio AM, Caprarulo C, Casado G, Eimon A, Figueredo H, et al. Evaluation of belimumab treatment in patients with systemic lupus erythematosus in a clinical practice setting: results from a 24-month OBSErve study in Argentina. Lupus. 2020;29(11):1385–1396. doi: 10.1177/0961203320947814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71:1343–1349. doi: 10.1136/annrheumdis-2011-200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy-six-week phase iii parent study in the united states. Arthritis Rheumatol. 2018;70:868–877. doi: 10.1002/art.40439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol (Hoboken, NJ) 2019;71:1125–1134. doi: 10.1002/art.40861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017;69:115–123. doi: 10.1002/acr.22971. [DOI] [PubMed] [Google Scholar]
- 23.Clinicaltrials.gov. Efficacy and safety of belimumab in patients with active lupus nephritis (BLISS-LN). https://clinicaltrials.gov/ct2/show/NCT01639339. Accessed July 2020.
- 24.González LA, Toloza SM, McGwin G, Jr, Alarcón GS. Ethnicity in systemic lupus erythematosus (SLE): its influence on susceptibility and outcomes. Lupus. 2013;22:1214–1224. doi: 10.1177/0961203313502571. [DOI] [PubMed] [Google Scholar]
- 25.Pascoe K, Lobosco S, Bell D, Hoskin B, Chang DJ, Pobiner B, et al. Patient- and physician-reported satisfaction with systemic lupus erythematosus treatment in US clinical practice. Clin Ther. 2017;39:1811–1826. doi: 10.1016/j.clinthera.2017.07.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com.