Abstract
Background
Oxidative damages contributes to age-related macular degeneration (AMD) caused vision blindness, but the molecular mechanisms are still largely unknown.
Objectives
This study managed to investigate this issue by conducting in vitro experiments.
Methods
Oxidative stress were evaluated by L-012 dye, DHE staining and MDA assay. CCK-8 and colony formation assay were conducted to examine cell proliferation. Cell death was evaluated by trypan blue staining and Annexin V-FITC/PI double staining method through flow cytometry (FCM). The binding sites of miR-23a and GLS1 mRNA were predicted by online miRDB database and validated by dual-luciferase reporter gene system. Real-Time qPCR for miR-23a levels and Western Blot for protein expressions.
Results
The retinal pigment epithelial (RPE) cells (ARPE-19) were subjected to hydrogen peroxide (H2O2) stimulation to simulate AMD progression in vitro, and we identified a novel miR-23a/glutaminase-1 (GLS1) pathway that regulated H2O2 induced oxidative damages in ARPE-19 cells. Mechanistically, H2O2 induced oxidative stress, inhibited cell proliferation and induced cell death in ARPE-19 cells in a dose- and time-dependent manner. Also, H2O2 stimulation hindered cell invasion, migration and glutamine uptake in ARPE-19 cells. Interestingly, we proved that H2O2 increased miR-23a levels, while downregulated glutaminase-1 (GLS1) in ARPE-19 cells, and miR-23a targeted 3′ untranslated region (3′UTR) of GLS1 mRNA for GLS1 degradation. Finally, our data suggested that silencing miR-23a upregulated GLS1 to reverse the detrimental effects of H2O2 treatment on ARPE-19 cells.
Conclusions
In general, analysis of the data suggested that miR-23a ablation upregulated GLS1 to attenuate H2O2 stimulation induced oxidative damages in ARPE-19 cells in vitro, and this study broadened our knowledge in this field, which might help to provide novel theranostic signatures for AMD.
Keywords: Age-related macular degeneration, Oxidative stress, ARPE-19, miR-23a, GLS1
Introduction
Age-related macular degeneration (AMD) is the most frequent senile ophthalmic disease that leads to substantial vision degeneration and even blindness (O’Neill et al. 2020; Sato et al. 2019), and retinal pigment epithelial (RPE) cells are commonly used as in vitro models for AMD investigations (Cui et al. 2019; Simmons et al. 2020). Recent studies reported that AMD pathogenesis was closely associated with heredity (Gorin and daSilva 2020), inflammation (Kauppinen et al. 2016) and environmental stress (Dalvi et al. 2019). Aside from the above factors, aging related oxidative stress contributed a lot to AMD pathogenesis (Datta et al. 2017; Jarrett and Boulton 2012; Donato et al. 2018a,b, c). For example, oxidative stress induced pentraxin 3 expressions in RPE cells involved in RPE cell dysfunction (Ji Cho et al. 2019) and AMD pathogenesis (Hwang et al. 2019). Therefore, elimination of oxidative damages helped to slow down AMD progression and researchers found that the anti-oxidative agents, including Exendin-4 (EX4) (Cui et al. 2019) and phospholipid complex of quercetin (Q-PC) (Shao et al. 2019), alleviated H2O2 induced oxidative damages in RPE cells. However, the detailed molecular mechanisms of oxidative stress induced AMD are still not fully delineated, and the present study established H2O2-induced cellular models in ARPE-19 cells to mimic AMD development in vitro according to the previous work (Li et al. 2016).
MicroRNAs (miRNAs) are small non-coding RNAs composed of about 15–25 nucleotides (Fujii et al. 2019; Jing et al. 2020; Sastre etal. 2019), which regulated biological functions of cells by targeting associated downstream genes (Gulinaer et al. 2019; Yan et al. 2020). Recent studies identified that multiple miRNAs involved in the regulation of AMD progression, such as miR-340 (Yan et al. 2018), miR-150 (Lin et al. 2018) and miR-23a (Li et al. 2016). Specifically, researchers found that upregulated miR-23a was observed in patients and animal models with AMD (Romano et al. 2017). In addition, miR-23a participated in the regulation of oxidative stress, and miR-23a attenuated oxidative stress induced injury in mice models with focal cerebral ischemia-reperfusion (Zhao et al. 2014). Notably, inhibition of miR-23a protected RPE cells from oxidative stress-induced apoptosis by promoting glutaminase and glutamine uptake (Li et al. 2016). Therefore, we focused on investigating the regulating mechanisms of miR-23a in AMD pathogenesis.
Extracellular glutamine uptake was essential for cell survival and proliferation, and glutamine was catalyzed into glutamate in mitochondria to release adenosine triphosphate (ATP) for sustaining normal cell functions (Ansari et al. 2020; Zhang et al. 2020). Glutaminase-1 (GLS1) played an important role in regulating glutamine uptake and catabolism, a lack of GLS1 abrogated the conversion of glutamine into glutamate in cells (Reynolds et al. 2014; Heuvel et al. 2012). Interestingly, GLS1 also participated in the regulation of oxidative stress, specifically, deficiency of GLS1 triggered oxidative stress in glioma cells (Martín-Rufián et al. 2014) and GLS1 overexpression alleviated H2O2 induced RPE cell death (Li et al. 2016). In addition, knock-down of GLS1 contributed to H2O2-induced ARPE-19 cell death by regulating glutamine uptake (Li et al. 2016), and GLS1 could be negatively regulated by miR-23a (Li et al. 2016). The above information enlightened us that miR-23a/GLS1 axis might be crucial for AMD progression.
Based on the above publications, we aimed to explore the role of miR-23a/GLS1 axis in the regulation of H2O2 modulated RPE cell functions. This study will give some insights into the underlying mechanisms of AMD pathogenesis and provide potential therapeutic agents for AMD treatment in clinic.
Materials and methods
Cell culture
Human retinal pigment epithelial cell line (ARPE-19 cells) and HEK-293T cells were procured from American Type Culture Collection (ATCC, USA). The above cells were cultured in RPMI medium (GIBCO, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO, USA) and 1% penicillin/streptavidin (GIBCO, USA), and maintained in a humidified atmosphere of 5% CO2 at 37 °C. The ARPE-19 cells were treated with different concentrations of H2O2 (0, 10, 20, 40, 80 µmol/L) for 0 h, 24 h, 48 h, 72 h and 96 h, respectively.
Vectors transfection
The ARPE-19 cells were transfected with miR-23a mimics, anti-miR-23a or GLS1-siRNA using LipoFiterTM liposome transfection kit (Hanbio Biotechnology, Shanghai, China) according to the manufacturer’s instructions and experimental procedures provided by the previous study (Donato et al. 2018a, b, c). After 8 h of incubation at 37 °C, RPMI-1640 complete medium containing FBS and antibiotics was adopted for incubation for 24 h.
Real-Time qPCR
Real-Time qPCR was used to determine the levels of miR-23a in ARPE-19 cells in keeping with the experimental procedures provided by the previous study (Kauppinen et al. 2016). Primer sequences: miR-23a (Forward: 5′-GTGGTGAGGTGGACCTACAATC-3′, Reverse: 5′-GCGGAACTTAGCCACTGTGAAC-3′); U6: (Forward: 5′-CTCGCTTCGGCAGCACA-3′, Reverse: 5′-AACGCTTCACGAATTTGCGT-3′).
Western Blot
Western Blot was conducted to detect the expressions of proteins in ARPE-19 cells according to the previous study (Kauppinen et al. 2016). The antibodies against GLS1 (1:1000, Abcam, UK), β-actin (1:1000, Abcam, UK) were obtained. Finally, the enhanced chemiluminescent (ECL) system was employed to visualize protein bands, which were quantified by the Image J software and normalized to β-actin.
Cell counting kit-8 (CCK-8) assay
The ARPE-19 cells were pre-transfected with the mentioned vectors and incubated with different concentrations of H2O2 (0, 10, 20, 40, 80 µmol/L) for 0 h, 24 h, 48 h, 72 h and 96 h respectively, the cell proliferation abilities were evaluated by using the CCK-8 assay kit (YEASEN, Shanghai, China) in keeping with the manufacturer’s protocol.
Colony formation assay
The ARPE-19 cells were plated onto the 24-well plates and cultured for 14 days at the standard culture conditions. Then, cells were fixed with paraformaldehyde and stained with crystal violet purchased from Beyotime Biotechnology (Shanghai, China) for 10 min at room temperature. The colonies containing at least 10 cells were counted under the light microscope.
Annexin V-FITC/PI double staining assay
Cell apoptosis was examined by using the Annexin V-FITC/PI apoptosis detection kit (BD Bioscience, USA) according to the manufacturer’s protocol. The ARPE-19 cells were double-stained with Annexin V-FITC and PI for 35 min at room temperature without light exposure, respectively. After that, a flow cytometer (FCM, ThermoFisher Scientific, MA, USA) was used to measure cell apoptosis ratio.
Dihydroethidium (DHE) staining
The DHE reaction solution (MKbio, Shanghai, China) was added to the ARPE-19 cells according to the experimental instructions provided by the producer. Cells were washed with phosphate buffer saline (PBS), stained with 50 µM DHE staining solution for 1 h at room temperature, and 10 random fields from the sample were captured by using the fluorescence microscope at the magnification of 400× and the fluorescence intensity was quantified by Image J software.
Glutamine uptake assay
The ARPE-19 cells lysed through ultrasonic lysis. The protein concentration was determined using a BCA protein assay kit (Thermo Scientific, USA) according to the manufacturer’s instructions. The quantity of glutamine was analyzed by the release of ammonia (NH3) using the Glutamine Detection Kit according to the instructions.
Transwell assay
ARPE-19 cells were placed in the top chamber with a Matrigel-coated membrane (BD Biosciences, USA). Cells were plated in serum-free medium with Matrigel, while 10% FBS supplementing culture medium was used as a chemo-attractant in the lower chamber. After 24 h of incubation, the filter membrane was fixed with ethanol, stained with 0.1% crystal violet (Beyotime, Shanghai, China), and photographed under a microscope.
Wound scratch assay
The ARPE-19 cells were seeded onto the 6-well plates and cultured for 24 h until the cell confluency reached about 90%. After that, the 200 µL pipette tips were used to create the scratches in the plates. Then, the plates were placed into the incubator and the cells were cultured under the standard culture conditions. The photographs were taken by using the microscope purchased from Olympus (Tokyo, Japan) at 0 h to 24 h post-incubation. The cell migration abilities were evaluated by assessing the changes of the scratch area.
Dual-luciferase reporter gene system
The online miRDB database (http://mirdb.org/) predicted that miR-23a could bind to the 3′ untranslated regions (UTR, position 199–205) of GLS1 mRNA, which were cloned into the pGL3 vectors. The wild-type GLS1 (pGL3-GLS1 WT), mutant plasmid (pGL3-GLS1 MUT) and pGL3-basic control plasmid (pGL3-control) were synthesized from Guangzhou Borui Biological Co., Ltd. The above-mentioned plasmids, miR-23a mimic and NC mimic were then transiently co-transfected into 293T cells (ATCC, Manassas, Virginia) using LipoFiterTM liposome transfection reagent. After 24 h of transfection, the luciferase activity was assayed using the Dual-Luciferase Reporter Assay System (Promega) on an InfiniteM200 luminometer (Tecan) according to the manufacturer’s protocol and the experimental procedures provided by the previous work (Donato et al. 2018a, b, c).
Statistical analysis
All the data were collected and analyzed by using the SPSS 18.0 software. The data were represented as Means ± Standard Deviation (SD). The Student’s t-test was used to compare the means of two groups. The analysis of variance (ANOVA) method was conducted to compare the means among multiple groups, and the post hoc tukey’s test was used for multiple comparison correction. “NS” represented “No statistical significance”. “**” represented “P < 0.01”. Each experiment had at least 3 repetitions.
Results
The effects of H2O2 treatment on ROS production, cell proliferation, cell apoptosis migration and glutamine uptake in ARPE-19 cells
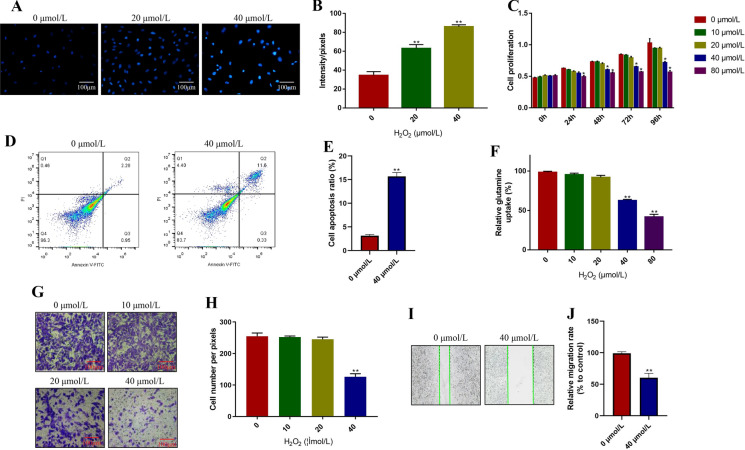
The ARPE-19 cells were exposed to different concentrations of H2O2 (0, 10, 20, 40, 80 µmol/L) for 0 h, 24 h, 48 h, 72 h and 96 h, respectively. The ROS levels were determined by the DHE staining assay and the results showed that we successfully inducted oxidative stress cell models in ARPE-19 cells after 48 h H2O2 stimulation (P < 0.05, Fig. 1a, b). Additionally, the CCK-8 assay results showed that H2O2 inhibited ARPE-19 cell proliferation in a dose and time-dependent manner (P < 0.05, Fig. 1c). Specifically, the proliferation abilities of ARPE-19 cells were significantly decreased by treating cells with high-dose H2O2 (40, 80 µmol/L), instead of low-dose H2O2 (0, 10, 20 µmol/L) (P < 0.05, Fig. 1c). Consistently, the Annexin V-FITC/PI double staining assay results showed that high-dose H2O2 (40 µmol/L, 48 h) significantly promoted cell apoptosis in ARPE-19 cells (P < 0.05, Fig. 1d, e). Of note, previous study reported that H2O2 induced oxidative stress influenced glutamine uptake (Yan et al. 2020), and this study proved that H2O2 inhibited glutamine uptake in ARPE-19 cells in a concentration-dependent manner (P < 0.05, Fig. 1f). Furthermore, the transwell assay results showed that ARPE-19 cell invasion abilities were significantly inhibited by high-dose H2O2 (40 µmol/L, 48 h), while low-dose H2O2 (0, 10, 20 µmol/L, 48 h) had little impacts on ARPE-19 cell invasion (P < 0.05, Fig. 1g, h), which were validated by the wound scratch assay results (P < 0.05, Fig. 1i, j), and suggested that high-dose H2O2 (40 µmol/L, 48 h) inhibited cell migration in ARPE-19 cells.
Fig. 1.
Hydrogen peroxide (H2O2) influenced ROS generation, cell proliferation, apoptosis, mobility and glutamine uptake in ARPE-19 cells. The cells were treated with 0, 10, 20, 40 and 80 µmol/L H2O2 for 0 h, 24 h, 48 h, 72 h and 96 h, respectively. a, b DHE staining assay was performed to measure ROS levels in ARPE-19 cells. c CCK-8 assay was conducted to detect cell proliferation. d, e Annexin V-FITC/PI double staining assay was used to examine cell apoptosis ratio in ARPE-19 cells. f The relative glutamine uptake in ARPE-19 cells was determined. g, h Transwell assay was used to detect ARPE-19 cell invasion. i, j The wound scratch assay was performed to determine cell migration abilities in ARPE-19 cells. Each experiment repeated at least 3 times. **P < 0.01
Targeting MiR-23a reversed the effects of H2O2 stimulation on ARPE-19 cells
Previous study found that miR-23a was closely related with H2O2 regulated RPE cell functions (Yan et al. 2020), which were also verified in this study. Specifically, after treating ARPE-19 cells with different concentrations of H2O2 (0, 10, 20, 40, 80 µmol/L) for 48 h, Real-Time qPCR was conducted to determine the levels of miR-23a, which were normalized to the internal reference U6 (Fig. 2a). The results showed that H2O2 significantly increased the levels of miR-23a in ARPE-19 cells in a dose-dependent manner (P < 0.05, Fig. 2a). Next, the miR-23a mimic and inhibitor were delivered into ARPE-19 cells to overexpress and knock-down miR-23a, respectively (P < 0.05, Fig. 2b). The results showed that both high-dose H2O2 (40 µmol/L, 48 h) and miR-23a overexpression alone increased ROS levels in ARPE-19 cells to a limit extent, and upregulation of miR-23a aggravated the promoting effects of H2O2 (40 µmol/L, 48 h) induced ROS production, which were abrogated by silencing miR-23a (P < 0.05, Fig. 2c, d). In addition, miR-23a overexpression alone inhibited cell proliferation (P < 0.05, Fig. 2e) and promoted cell apoptosis (P < 0.05, Fig. 2f, g) in ARPE-19 cells, and upregulated miR-23a aggravated the inhibiting effects of high-dose H2O2 on cell viability, which were reversed by knocking down miR-23a (P < 0.05, Fig. 2e–g). Of note, miR-23a had similar effects on glutamine uptake (Fig. 2h), cell invasion (Fig. 2i, j) and migration (Fig. 2k, l). Specifically, both overexpressed miR-23a and H2O2 (40 µmol/L, 48 h) alone inhibited cell mobility (P < 0.05, Fig. 2i–l) and glutamine uptake (P < 0.05, Fig. 2h) in ARPE-19 cells, and the effects of H2O2 (40 µmol/L, 48 h) on the above cell functions were enhanced by overexpressing miR-23a and reversed by downregulating miR-23a (P < 0.05, Fig. 2h–l).
Fig. 2.
MiR-23a affected H2O2 regulated oxidative stress, cell proliferation, apoptosis, invasion, migration and glutamine uptake in ARPE-19 cells. a H2O2 increased miR-23a levels in ARPE-19 cells in a dose-dependent manner. b MiR-23a was overexpressed and knocked down in ARPE-19 cells. c, d DHE staining assay was conducted to measure ROS levels in ARPE-19 cells. e CCK-8 assay was used to determine cell proliferation. f, g Annexin V-FITC/PI double staining assay was conducted to examine cell apoptosis ratio in ARPE-19 cells. h H2O2 inhibited glutamine uptake in ARPE-19 cells by upregulating miR-23a. i, j Cell invasion was determined by transwell assay. k, l Cell migration abilities were evaluated by using the wound scratch assay. Each experiment repeated at least 3 times. **P < 0.01
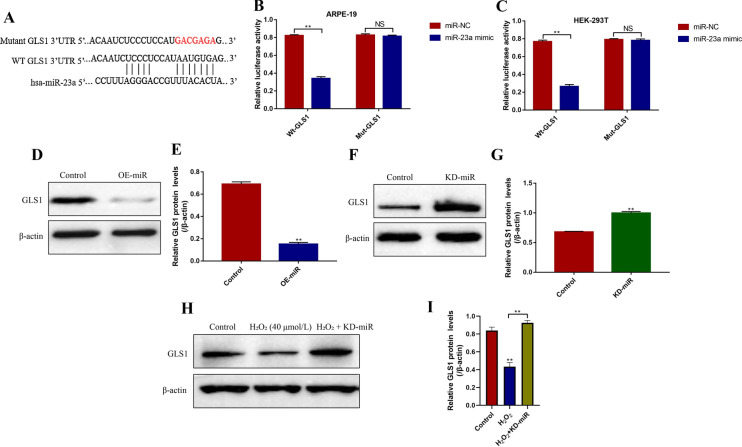
H2O2 negatively regulated GLS1 expressions in ARPE-19 cells by upregulating miR-23a
GLS1 could be negatively regulated by miR-23a in RPE cells (Yan et al. 2020), which were validated in this study. Specifically, the binding sites of miR-23a and 3′ untranslated region (UTR, position 199–205) of GLS1 mRNA were predicted by using the online miRDB database (http://mirdb.org/) (Fig. 3a). The dual-luciferase reporter gene system assay results showed that miR-23a mimic significantly decreased the luciferase activity in both ARPE-19 cells (P < 0.05, Fig. 3b) and HEK-293T cells (P < 0.05, Fig. 3c) co-transfected with wild-type GLS1 (Wt-GLS1) instead of mutant GLS1 (Mut-GLS1). Further experiments validated that the expression levels of GLS1 were decreased by overexpressing miR-23a (P < 0.05, Fig. 3d, e), and increased by knocking down miR-23a (P < 0.05, Fig. 3f, g) in ARPE-19 cells. In addition, we proved that high-dose H2O2 (40 µmol/L, 48 h) inhibited GLS1 expressions through upregulating miR-23a (P < 0.05, Fig. 3h, i). Mechanistically, the results showed that H2O2 (40 µmol/L, 48 h) treatment decreased the expression levels of GLS1 in ARPE-19 cells, which were reversed by knocking down miR-23a (P < 0.05, Fig. 3h, i).
Fig. 3.
H2O2 inhibited GLS1 expressions in ARPE-19 cells by upregulating miR-23a. a The binding sites of miR-23a and 3′UTR regions of GLS1 mRNA were predicted by using the online miRDB database (http://mirdb.org/). Dual-luciferase reporter gene system was employed to validate the binding sites of miR-23a and 3′UTR regions of GLS1 mRNA in b ARPE-19 cells and c HEK-293T cells respectively. d–g Western Blot was employed to determine the expressions of GLS1 in ARPE-19 cells. h, i H2O2 inhibited GLS1 expressions by upregulating miR-23a. Each experiment repeated at least 3 times. **P < 0.01. “NS” represented “no statistical significance”
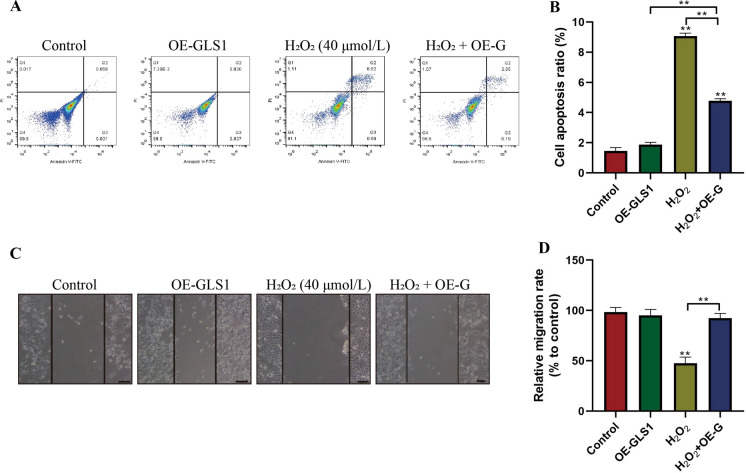
Upregulation of GLS1 reversed the detrimental effects of H2O2 on ARPE-19 cell viability and mobility
Since GLS1 could be inhibited by H2O2 in ARPE-19 cells, we next explored whether H2O2 influenced cell functions by targeting GLS1. To achieve this, GLS1 was overexpressed in ARPE-19 cells, and cells were then treated with high-dose H2O2 (40 µmol/L) for 48 h. The Annexin V-FITC/PI double staining results showed that GLS1 overexpression alone did not influence cell apoptosis, while upregulation of GLS1 abrogated the promoting effects of H2O2 on ARPE-19 cell death (P < 0.05, Fig. 4a, b). In addition, H2O2 affected cell migration abilities by targeting GLS1 (P < 0.05, Fig. 4c, d). Specifically, the wound scratch assay results showed that GLS1 overexpression had little effects on cell migration, and high-dose H2O2 (40 µmol/L, 48 h) slowed down cell migration, which were reversed by upregulating GLS1 (P < 0.05, Fig. 4c, d). The above results suggested that H2O2 inhibited cell viability and mobility in ARPE-19 cells through downregulating GLS1.
Fig. 4.
The effects of GLS1 on the regulation of ARPE-19 cell apoptosis and migration. The ARPE-19 cells were treated with high-dose H2O2 (40 µmol/L) for 48 h. a, b Apoptotic cell death was evaluated by using the Annexin V-FITC/PI double staining assay. c, d Wound scratch assay was employed to measure cell migration abilities. Each experiment repeated at least 3 times. **P < 0.01. “NS” represented “no statistical significance”
Knock-down of miR-23a protected ARPE-19 cells from H2O2 induced oxidative damages by upregulating GLS1
Further experiments were conducted to investigate whether silencing miR-23a reversed H2O2 induced oxidative damages in ARPE-19 cells by targeting GLS1. To achieve this, the ARPE-19 cells were transfected with miR-23a inhibitor and sh-GLS1 vectors to downregulate miR-23a and GLS1, respectively. The colony formation assay showed knock-down of miR-23a promoted colony formation abilities in H2O2 (40 µmol/L, 48 h) treated ARPE-19 cells, which were abrogated by knocking down GLS1 (P < 0.05, Fig. 5a, b). Consistently, H2O2 (40 µmol/L, 48 h) inhibited ARPE-19 cell proliferation, which were aggravated by silencing GLS1 and rescued by knocking down miR-23a (P < 0.05, Fig. 5c). Of note, the protective effects of miR-23a ablation on cell proliferation were abolished by downregulating GLS1 in H2O2 (40 µmol/L, 48 h) treated ARPE-19 cells (P < 0.05, Fig. 5c). In addition, knock-down of miR-23a promoted high-dose H2O2 (40 µmol/L, 48 h) treated ARPE-19 cell invasion, which were also abrogated by downregulating GLS1 (P < 0.05, Fig. 5e, f). Furthermore, H2O2 (40 µmol/L, 48 h) promoted ROS production (P < 0.05, Fig. 5g, h) and impeded glutamine uptake (P < 0.05, Fig. 5d) in ARPE-19 cells by regulating miR-23a/GLS1 axis in a similar manner. Specifically, high-dose H2O2 (40 µmol/L) promoted ROS production (P < 0.05, Fig. 5g, h) and decreased glutamine levels (P < 0.05, Fig. 5d) in ARPE-19 cells, which were reversed by knocking down miR-23a and enhanced by downregulating GLS1.
Fig. 5.
H2O2 regulated ARPE-19 cell functions through miR-23a/GLS1 axis. a, b Colony formation assay was conducted to determine ARPE-19 cell colony formation abilities. c CCK-8 assay was used to detect cell proliferation. d H2O2 hindered glutamine uptake in ARPE-19 cells through miR-23a/GLS1 axis. e, f Cell invasion abilities were measured by transwell assay. g, h DHE staining was performed to detect ROS levels in ARPE-19 cells. Each experiment repeated at least 3 times. **P < 0.01
Discussion
Oxidative damages contributed to the development of age-related macular degeneration (AMD) (Datta et al. 2017; Jarrett and Boulton 2012), but the underlying mechanisms are still largely unknown. Therefore, the present study aimed to investigate this issue. According to the previous work (Li et al. 2016), hydrogen peroxide (H2O2) treated ARPE-19 cell models were established to mimic oxidative stress mediated AMD progression in vitro. Notably, recent data indicated that that oxidative stress played a biphasic role in the regulation of cell functions (Chan et al. 2016, 2019). On the one hand, moderate oxidative protected ARPE-19 cells from death through triggering intrinsic protective mechanisms (Chan et al. 2019), on the other, excessive oxidative damages directly induced ARPE-19 cell death (Chan et al. 2016). The present study validated that H2O2 affected cell functions in a concentration dependent manner. Specifically, high-dose H2O2 induced cell death and inhibited cell mobility in ARPE-19 cells, while low-dose H2O2 had little effects on the above cell functions, which were in consistent with the previous publications (Chan et al. 2016, 2019) and indicated that excessive oxidative damages brought detrimental effects to ARPE-19 cells. Additionally, extracellular glutamine uptake was pivotal for sustaining normal cell functions (Ansari et al. 2020; Zhang et al. 2020), and we found that H2O2 hindered glutamine uptake in ARPE-19 cells, which were in accordance with the previous study (Li et al. 2016).
Previous work provided evidences to support that miR-23a was closely related with AMD pathogenesis (Romano et al. 2017), hence we selected miR-23a as a candidate gene in this study. As expected, we found that H2O2 elevated miR-23a levels in a concentration-dependent manner, indicating that excessive oxidative stress significantly induced miR-23a upregulation in ARPE-19 cells, which were in line with the previous work (Li et al. 2016). Further gain-and loss-function experiments validated that high-dose H2O2 regulated ARPE-19 cell functions through miR-23a. Mechanistically, both miR-23a overexpression and H2O2 stimulation triggered ROS production, inhibited cell proliferation, mobility and glutamine uptake, and promoted cell apoptosis in ARPE-19 cells to a very limited extent, and overexpression of miR-23a aggravated the detrimental effects of H2O2 on the above cell functions, which were attenuated by downregulating miR-23a, indicating that knock-down of miR-23a helped to sustain normal biological functions in H2O2-stimulated ARPE-19 cells and in consistent with the previous publication (Li et al. 2016).
Furthermore, based on the previous work (Li et al. 2016) and information from the online miRDB database (http://mirdb.org/), glutaminase-1 (GLS1) was identified as the downstream target and could be negatively regulated by miR-23a in ARPE-19 cells in this study. Interestingly, we also evidenced that H2O2 inhibited GLS1 expressions by upregulating miR-23a. Previous literatures reported that GLS1 regulated oxidative stress mediated ARPE-19 cell death (Li et al. 2016; Martín-Rufián et al. 2014), which were validated in our study. Specifically, silencing GLS1 increased ROS levels in ARPE-19 cells, and GLS1 overexpression abrogated the effects of H2O2 on cell apoptosis and migration in ARPE-19 cells. Additionally, we proved that knock-down of miR-23a rescued cell functions in H2O2-stimulated ARPE-19 cells by targeting GLS1. Mechanistically, miR-23a ablation promoted cell proliferation, mobility and glutamine uptake, and inhibited ROS generation in ARPE-19 cells treated with H2O2, which were all reversed by knocking down GLS1.
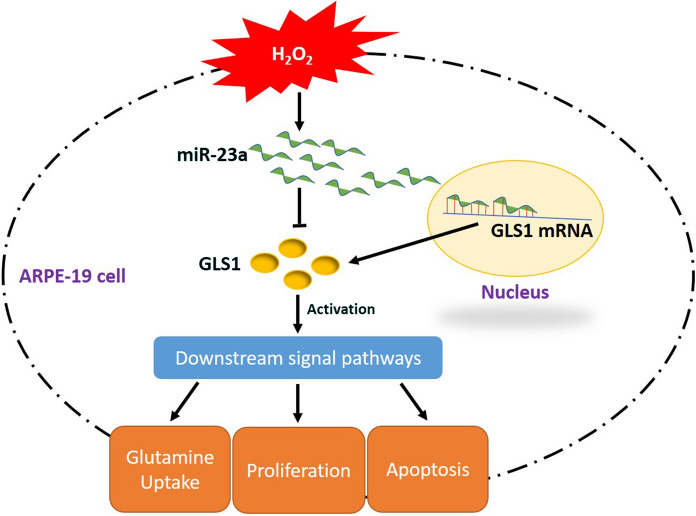
Taken together, this in vitro study proved that knock-down of miR-23a alleviated H2O2-induced oxidative damages in ARPE-19 cells through upregulating GLS1 (Fig. 6) and provided potential therapeutic agents for AMD treatment in clinic. However, according to the previous work (Ayaz and Dinç 2018), multiple miRNAs involved in the regulation of oxidative stress mediated alterations of ARPE-19 cell functions, further RNA-Seq experiments are still needed to investigate this issue in our future work.
Fig. 6.
The graphical abstract of this research. In brief, H2O2 inhibited GLS1 expressions by upregulating miR-23a in ARPE-19 cells, which led to oxidative damages mediated inhibition of glutamine uptake, cell proliferation and apoptosis in ARPE-19 cells
Authors’ contributions
YZ and MY designed and conducted all the experiments in this study, besides, they wrote the draft. TZ was responsible for data collection and analysis, figures design and language modification. JW financially supported this study, and provide guidance for this work.
Funding
This work was supported by the Youth Science and Innovation Research Fund of Xinjiang Province (No. WJWY-202039).
Availability of data and materials
All the raw data and material involved in this study are available from the corresponding author upon appropriate request.
Compliance with ethical standards
Conflict of interest
All the co-authors declared that there are no conflicts of interest in this study.
Ethics approval
All the experiment procedures involved in this study were approved by the Research Ethics Committee of the Fifth Affiliated Hospital of Xinjiang Medical University, Urumchi, Xinjiang Province, China.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yang Zhou, Email: zhouyangdoctor@sina.com.
Meilibanu Yusufu, Email: 95319915@qq.com.
Ting Zhang, Email: zhang_ting442@163.com.
Jing Wang, Email: jing_wang1106@163.com.
References
- Ansari RE, Craze ML, Althobiti M, Alfarsi L, Ellis IO, Rakha EA, Green AR. Enhanced glutamine uptake influences composition of immune cell infiltrates in breast cancer. Br J Cancer. 2020;122(1):94–101. doi: 10.1038/s41416-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz L, Dinç E. Evaluation of microRNA responses in ARPE-19 cells against the oxidative stress. Cutan Ocul Toxicol. 2018;37(2):121–126. doi: 10.1080/15569527.2017.1355314. [DOI] [PubMed] [Google Scholar]
- Chan CM, Huang DY, Huang YP, Hsu SH, Kang LY, Shen CM, Lin WW. Methylglyoxal induces cell death through endoplasmic reticulum stress-associated ROS production and mitochondrial dysfunction. J Cell Mol Med. 2016;20(9):1749–1760. doi: 10.1111/jcmm.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CM, Huang DY, Sekar P, Hsu SH, Lin WW. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J Biomed Sci. 2019;26(1):40. doi: 10.1186/s12929-019-0531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Tian L, Lu D, Li H, Cui J. Exendin-4 Protects Human Retinal Pigment Epithelial Cells from H2O2-Induced Oxidative Damage via Activation of NRF2 Signaling. Ophthalmic Res. 2019;63:404–412. doi: 10.1159/000504891. [DOI] [PubMed] [Google Scholar]
- Dalvi S, Galloway CA, Winschel L, Hashim A, Soto C, Tang C, MacDonald LA, Singh R. Environmental stress impairs photoreceptor outer segment (POS) phagocytosis and degradation and induces autofluorescent material accumulation in hiPSC-RPE cells. Cell Death Discov. 2019;5:96. doi: 10.1038/s41420-019-0171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato L, Scimone C, Nicocia G, Denaro L, Robledo R, Sidoti A, D’Angelo R. GLO1 gene polymorphisms and their association with retinitis pigmentosa: a case-control study in a Sicilian population. Mol Biol Rep. 2018;45(5):1349–1355. doi: 10.1007/s11033-018-4295-4. [DOI] [PubMed] [Google Scholar]
- Donato L, Bramanti P, Scimone C, Rinaldi C, Giorgianni F, Beranova-Giorgianni S, Koirala D, D’Angelo R, Sidoti A. miRNAexpression profile of retinal pigment epithelial cells under oxidative stress conditions. FEBS Open Bio. 2018;8(2):219–233. doi: 10.1002/2211-5463.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato L, Scimone C, Rinaldi C, Aragona P, Briuglia S, D’Ascola A, D’Angelo R, Sidoti A. Stargardt Phenotype Associated With Two ELOVL4 Promoter Variants and ELOVL4 Downregulation: New Possible Perspective to Etiopathogenesis? Invest Ophthalmol Vis Sci. 2018;59(2):843–857. doi: 10.1167/iovs.17-22962. [DOI] [PubMed] [Google Scholar]
- Fujii R, Yamada H, Yamazaki M, Munetsuna E, Ando Y, Ohashi K, Ishikawa H, Shimoda H, Sakata K, Ogawa A, Kobayashi S, Suzuki K. Circulating microRNAs (miR-126, miR-197, and miR-223) are associated with chronic kidney disease among elderly survivors of the Great East Japan Earthquake. BMC Nephrol. 2019;20(1):474. doi: 10.1186/s12882-019-1651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin MB, daSilva MJ. Predictive genetics for AMD: Hype and hopes for genetics-based strategies for treatment and prevention. Exp Eye Res. 2020;191:107894. doi: 10.1016/j.exer.2019.107894. [DOI] [PubMed] [Google Scholar]
- Gulinaer AJ, Ju AN, Gao M, Luo Y, Bo YL. Over-expression of miR-187 inhibited cell proliferation and metastasis of glioma via down-regulating SMAD1. Eur Rev Med Pharmacol Sci. 2019;23(24):10908–10917. doi: 10.26355/eurrev_201912_19794. [DOI] [PubMed] [Google Scholar]
- Hwang N, Kwon MY, Woo JM, Chung SW. Oxidative Stress-Induced Pentraxin 3 Expression Human Retinal Pigment Epithelial Cells is Involved in the Pathogenesis of Age-Related Macular Degeneration. Int J Mol Sci. 2019;20(23):6028. doi: 10.3390/ijms20236028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Cho M, Yoon SJ, Kim W, Park J, Lee J, Park JG, Cho YL, Kim JH, Jang H, Park YJ, Lee SH, Min JK. Oxidative stress-mediated TXNIP loss causes RPE dysfunction. Exp Mol Med. 2019;51(10):1–13. doi: 10.1038/s12276-019-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Zhang X, Luo K, Luo Q, Yin M, Wang W, Zhu Z, Zheng J, He X. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231:119682. doi: 10.1016/j.biomaterials.2019.119682. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Zhong BW, Zhang HX, Zhou HY, Luo J, Liu Y, Xu GC, Luan CS, Fang J. Inhibition of the oxidative stress-induced miR-23a protects the human retinal pigment epithelium (RPE) cells from apoptosis through the upregulation of glutaminase and glutamine uptake. Mol Biol Rep. 2016;43(10):1079–1087. doi: 10.1007/s11033-016-4041-8. [DOI] [PubMed] [Google Scholar]
- Lin JB, Moolani HV, Sene A, Sidhu R, Kell P, Lin JB, Dong Z, Ban N, Ory DS, Apte RS. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight. 2018;3(7):e120157. doi: 10.1172/jci.insight.120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Rufián M, Nascimento-Gomes R, Higuero A, Crisma AR, Campos-Sandoval JA, Gómez-García MC, Cardona C, Cheng T, Lobo C, Segura JA, Alonso FJ, Szeliga M, Albrecht J, Curi R, Márquez J, Colquhoun A, Deberardinis RJ, Matés JM. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J Mol Med. 2014;92(3):277–290. doi: 10.1007/s00109-013-1105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HC, Limnios IJ, Barnett NL. Advancing a Stem Cell Therapy for Age-Related Macular Degeneration. Curr Stem Cell Res Ther. 2020;15(2):89–97. doi: 10.2174/1574888X15666191218094020. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33(5):556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano GL, Platania CBM, Drago F, Salomone S, Ragusa M, Barbagallo C, Di Pietro C, Purrello M, Reibaldi M, Avitabile T, Longo A, Bucolo C. Retinal and Circulating miRNAs in Age-Related Macular Degeneration: An In vivo Animal and Human Study. Front Pharmacol. 2017;8:168. doi: 10.3389/fphar.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre D, Baiochi J, de Souza Lima IM, Canto de Souza F, Corveloni AC, Thomé CH, Faça VM, Schiavinato J, Covas DT, Panepucci RA. Focused screening reveals functional effects of microRNAs differentially expressed in colorectal cancer. BMC Cancer. 2019;19(1):1239. doi: 10.1186/s12885-019-6468-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Takeuchi M, Karasawa Y, Takayama K, Enoki T. Comprehensive expression patterns of inflammatory cytokines in aqueous humor of patients with neovascular age-related macular degeneration. Sci Rep. 2019;9(1):19447. doi: 10.1038/s41598-019-55191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Yu H, Yang Y, Li M, Hang L, Xu X. A Solid Dispersion of Quercetin Shows Enhanced Nrf2 Activation and Protective Effects against Oxidative Injury in a Mouse Model of Dry Age-Related Macular Degeneration. Oxid Med Cell Longev. 2019;2019:1479571. doi: 10.1155/2019/1479571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons KT, Mazzilli JL, Mueller-Ortiz SL, Domozhirov AY, Garcia CA, Zsigmond EM, Wetsel RA. Complement Receptor 1 (CR1/CD35)-expressing retinal pigment epithelial cells as a potential therapy for age-related macular degeneration. Mol Immunol. 2020;118:91–98. doi: 10.1016/j.molimm.2019.11.007. [DOI] [PubMed] [Google Scholar]
- van den Heuvel AP, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13(12):1185–1194. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Qin Y, Yu J, Peng Q, Chen X. MiR-340/iASPP axis affects UVB-mediated retinal pigment epithelium (RPE) cell damage. J Photochem Photobiol B. 2018;186:9–16. doi: 10.1016/j.jphotobiol.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Yan L, Zhang Y, Li K, Wang M, Li J, Qi Z, Wu J, Wang Z, Ling L, Liu H, Wu Y, Lu X, Xu L, Zhu Y, Zhang Y. miR-593-5p inhibit cell proliferation by targeting PLK1 in non small cell lung cancer cells. Pathol Res Pract. 2020;216(2):152786. doi: 10.1016/j.prp.2019.152786. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu R, Shuai Y, Huang Y, Jin R, Wang X, Luo J. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br J Cancer. 2020;122(1):82–93. doi: 10.1038/s41416-019-0637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Tao Z, Wang R, Liu P, Yan F, Li J, Zhang C, Ji X, Luo Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res. 2014;1592:65–72. doi: 10.1016/j.brainres.2014.09.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the raw data and material involved in this study are available from the corresponding author upon appropriate request.