Abstract
Objectives
In 2018, we surveyed investigators conducting HIV cure-related clinical research, drawing on information from the online listing established by Treatment Action Group (TAG). The purpose of the survey was to facilitate a landscape analysis of the field. In 2019, we fielded a second survey in order to provide updated information and assess any shifts in the landscape.
Methods
Trials and observational studies listed as of August 16, 2019 formed the sample set. Survey questions addressed funding, trial development, recruitment, enrollment, participant demographics, antiretroviral therapy status, HIV reservoir assays, invasive procedures, study completion, data sharing and dissemination plans. A survey was sent to the contact(s) for each study. Supplemental information was collected from clinicaltrials.gov and available presentations/publications of study results.
Results
A total of 97 interventional trials and 36 observational studies were identified, with 30 including analytical treatment interruptions. Total projected enrollment is 13,732 participants, with observational studies contributing the majority (8,325). Most interventional trials are in early phases. The majority of current research is located in the USA, involves predominately male participants and is limited in racial and ethnic diversity. Prespecified demographic enrollment targets are rare. Two thirds of respondents to our previous survey reported that enrollment is progressing more slowly than anticipated.
Conclusions
A diverse range of interventions are being evaluated in HIV cure research, but participant diversity is far from optimal with a continuing underrepresentation of women. Broadening inclusion and geographic reach will be necessary to achieve the goal of developing widely effective, safe and accessible curative interventions.
Keywords: HIV cure research, Participant diversity, Clinical trials registries, Analytical treatment interruptions
Introduction
In 2019, the HIV cure research field received encouragement from reports that two additional individuals may have been cured of the virus after receiving stem cell transplants from donors homozygous for the CCR5Δ32 mutation.1,2 At the time of the last public presentation, HIV viral load had remained undetectable after stopping antiretroviral therapy (ART) for 22 months in one case and 8 months in the other.3 Monitoring is ongoing, and the hope is that they will join Timothy Ray Brown as examples that an HIV cure is possible. For more than a decade, Brown has been the only person considered cured, having received stem cell transplants from a CCR5Δ32 homozygote in 2007.
While it’s good news that Brown’s cure may be reproducible, the cases also emphasize that confirming the possibility of achieving HIV cures does not necessarily equate to creating a practical curative approach. Stem cell transplants carry a significant risk of mortality and are only appropriate when medically indicated for life-threatening cancer diagnoses.
A recent review by Thumbi Ndung’u and colleagues highlights the importance of developing curative strategies that have the potential to be broadly efficacious among all populations, easy to administer and globally accessible.4 The authors note that efforts are now underway to develop an ideal “target product profile” (TPP).
One means of assessing the progress of the HIV cure research field is to track clinical research via online trial registries. The community-based organization Treatment Action Group (TAG) has collated information from registries and used it to maintain an online listing of HIV cure-related research since 2014.5 The primary aim is to provide a resource for HIV-positive people who might be interested in participating in studies. The listing divides interventional trials into broadly defined therapeutic categories for the purposes of improving comprehensibility and giving a sense of the diversity of approaches under study. “Combinations” refers to trials that combine interventions from different categories; trials involving multiples of the same type of approach are included within those categories.
In August 2018, the Bill & Melinda Gates Foundation contracted TAG to perform a landscape analysis of HIV cure-related clinical research by drawing on the online listing to survey study investigators. Results were published in November 2019.6 In 2019, the Bill & Melinda Gates Foundation contracted TAG to conduct a second survey in order to generate updated information on the field and assess any shifts in the landscape. This paper presents a summary of our findings.
Methods
TAG’s ‘Research Towards a Cure Trials’5 provided the starting point for this landscape analysis. The listing is populated through regular searches on clinicaltrials.gov and the World Health Organization WHO International Clinical Trials Registry Platform, and at the time of the analysis also included studies originally sourced from the UK Central Portfolio Management System (CPMS) (since changed to the UK Clinical Trials Gateway7) and the websites for the FRESH cohort and IciStem study.8,9 Criteria for designating a trial or observational study as being HIV cure-related include any of the following:
-
•
Any explicit articulation in the registry entry that the study is related to HIV cure research
-
•
Inclusion of relevant endpoints, such as measures of the HIV reservoir or other parameters connected to HIV persistence
-
•
Evaluations of immune responses that may have a role in controlling viral replication
-
•
Assessments of viral load rebound after antiretroviral therapy (ART) interruption
HIV cure-related clinical trials and observational studies listed as of August 16, 2019 that met these criteria formed the sample set for this landscape analysis. The researchers developed survey questions in Qualtrics (Provo, UT) during July and August 2019. Questions centered on study development and design, funding, study recruitment and enrollment, participant demographics and compensation, study completion, data sharing and dissemination plans, and progress questions for repeat responders. The survey included multiple choice and text entry questions. Respondents had the option to elaborate on most questions with open-ended comments. We collected responses from September 9, 2019–October 31, 2019.
The survey link was sent to all study contacts in September 2019. Study contacts were defined as the person listed on the registry entry or the person who responded to the 2018 survey. Non-responders received up to three reminder emails, a link to the published results of the 2018 survey, and an offer to forward the survey to the contact’s designee (e.g., study coordinator, clinical trials specialist, administrator) for completion.
The authors reviewed registry listings for invasive procedures, anticipated total enrollment, inclusion/exclusion criteria, study location(s), projected completion date, and observational model (for observational studies). We also gathered demographic information (sex, gender, race, and ethnicity) from articles and abstracts of studies that had presented or published interim results by the time of this analysis.
We prepared a master dataset in Excel that contained survey responses, demographic data, and registry data. Qualtrics saved incomplete responses two weeks after respondents began the survey. Incomplete surveys were reviewed and included in the master dataset if a respondent had answered all of the questions in at least one domain.
The authors performed summary analysis on the full dataset including descriptive statistics to identify frequencies, counts, and trends in the following domains: geographic distribution of studies, enrollment, participant demographics, projected trial completion, study development and trial design, study funding, participant compensation, trial details (participant ART status, reservoir assays, invasive procedures), data sharing plans, and community input.
Ethics statement: Study procedures were reviewed by the University of Maryland, Baltimore County (UMBC) Office of Research Protections and Compliance and were determined not to be human subjects research as defined by DHHS regulations 46.102(7)(l) or 46.102(e)(1).
Results
Description of data
The sample set contained 133 studies representing 24 categories. Of the 133 studies that were contacted, 73 responded. Sixty-five of those 73 completed the survey, 37 of whom had also completed the 2018 survey. Seven studies declined to complete the survey. Reasons for declining were unwillingness to comment on an ongoing study (N = 2), results of study have already been published (N = 2), and a stated belief that the survey was not relevant to their study (N = 3). An additional study closed during the survey response window due to bankruptcy of the study drug provider. The overall response rate was 54.8%, comparable to the response rate for the 2018 survey.6 Twenty-two of 24 categories were represented in survey responses. Supplemental Table 1 provides a breakdown of responses received by study categories.
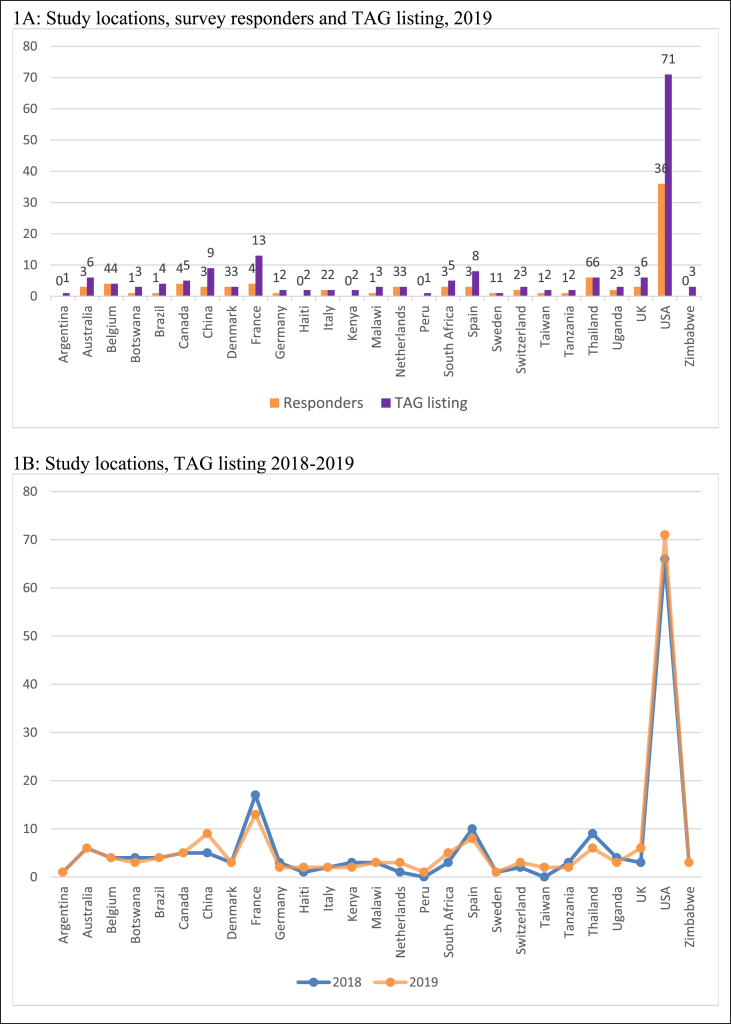
Survey responses were received for studies that plan to enroll in 23 countries. As in the 2018 survey, the US remains the most common location, consistent with the US National Institutes of Health (NIH) being the source of the majority of global HIV cure research funding.10 A total of 36 respondents indicated their study plans to enroll in the US. The study locations represented in survey responses are similar to the study locations represented in the TAG listing and have remained stable from 2018 to 2019 (Fig. 1).
Fig. 1.
Study locations.
1A: Study locations, survey responders and TAG listing, 2019
1B: Study locations, TAG listing 2018-2019.
Of the 133 studies in the sample set, 97 studies are interventional trials and 36 are observational studies. Thirty-three observational studies listed their observational model in the registry entry. The majority of observational studies (N = 22) are designed with a cohort model. The remaining observational studies listed as interventional single group assignment studies (N = 5) or designed using a case-only observational model (N = 3), case control model (N = 2), or using an ecologic/community registry model (N = 1).
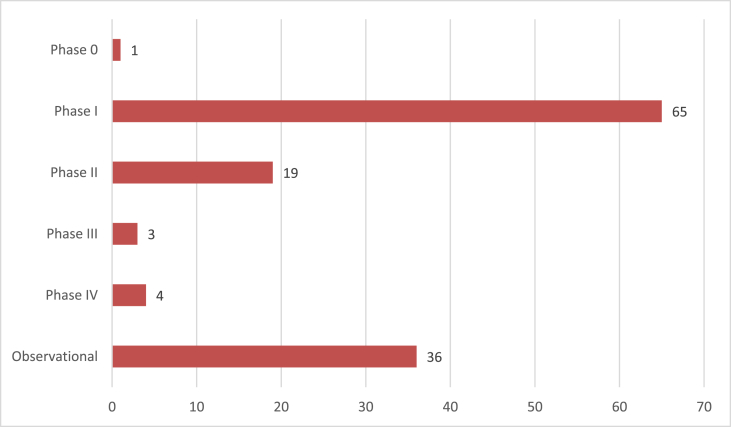
The overwhelming majority of cure-related studies are still in early Phases of the clinical trials process: 1 study is Phase 0 (a designation used for a gene therapy trial in the Chinese registry), 65 studies are Phase I (including Phase Ib, Phase I/II and Phase I/IIa), and 19 studies are Phase II (including Phase IIa and II/III). Only 7 studies are listed as Phase III or IV (see Fig. 2 for study distribution).
Fig. 2.
Distribution of studies on TAG listing.
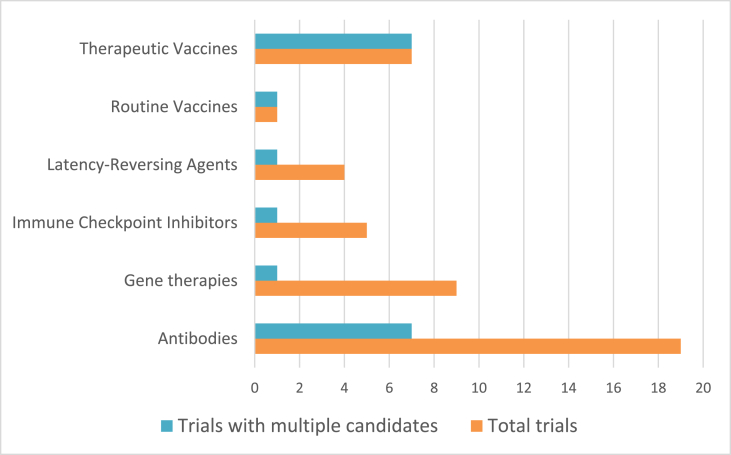
“Combinations” are defined as interventions from multiple categories. Trials combining multiple interventions of the same type (inter-category combinations) are listed within that category. Fig. 3 illustrates the proportion of trials in a category that combine multiple interventions of the same type (e.g., two therapeutic vaccines). Supplemental figure 1 shows the different categories represented in combination trials.
Fig. 3.
Inter-category combinations.
Enrollment
Across the cure landscape, total projected enrollment is 13,732 participants, with observational studies accounting for the majority of this total (8325 participants). Projected enrollment information was available for 33 of 36 observational studies. Two studies that plan to enroll >/ = 2000 participants contribute significantly to this total, as mean projected enrollment for observational studies is 252 (median = 66).
Most interventional studies will enroll small numbers of participants: mean projected enrollment for interventional studies is 56 (median 29; range 3–905). Only three interventional studies will enroll more than 150 participants. When those three studies are excluded, mean projected enrollment for interventional studies becomes 32 (median 24; range 3–150). Table 1 presents planned enrollment by category. Enrollment targets for two trials of HIV treatment in newborns (totaling 1505 participants) represent the number of pregnant HIV-positive women at risk for mother-to-child transmission that will be enrolled. The aim of these studies is to treat the small subset of newborns diagnosed with HIV infection, which is likely to approximate 5–10% of the enrollment target total. The majority of the newborns will be uninfected and receive standard preventive HIV drug regimens, exiting the trials 4–6 weeks postpartum. Since the 2018 analysis one of these trials, IMPAACT P1115, has been moved from “Treatment Intensification/Early Treatment” category to “Combinations” due to the addition of the experimental broadly neutralizing antibody VRC01 to the protocol.
Table 1.
Planned enrollment by category.
| Category | Mean | Median | Range | Total |
|---|---|---|---|---|
| Adoptive immunotherapy (N = 1) | 12 | – | – | 12 |
| Anti-Inflammatory (N = 2) | 87 | 87 | 64–110 | 174 |
| Anti-Proliferative (N = 1) | 5 | – | – | 5 |
| Antibodies (N = 19) | 40 | 40 | 8–75 | 767 |
| Antiretroviral therapy (N = 1) | 40 | – | – | 40 |
| Cannabinoids | 26 | – | – | 26 |
| Combinations (N = 17) | 88 | 34 | 8–905 | 1507 |
| Cytokines (N = 2) | 15 | 15 | 10–20 | 30 |
| Dual-Affinity Re-Targeting (DART) Molecules (N = 1) | 26 | – | – | 26 |
| Gene Therapies (N = 9) | 16 | 12 | 6–40 | 152 |
| Gene Therapies for HIV-Positive People with Cancers (N = 6) | 8 | 7 | 3–18 | 51 |
| Gonadotropin-Releasing Hormone (GnRH) Agonists (N = 1) | 52 | – | – | 52 |
| Hormones (N = 1) | 22 | – | – | 22 |
| Imaging Studies (N = 4) | 15 | 14 | 5–30 | 63 |
| Immune Checkpoint Inhibitors (N = 5) | 48 | 45 | 20–96 | 241 |
| Latency-Reversing Agents (N = 4) | 29 | 24 | 9–60 | 117 |
| Observational (N = 33) | 252 | 66 | 3–2550 | 8325 |
| Proteasome Inhibitors (N = 1) | 18 | – | – | 18 |
| Retinoids (N = 1) | 12 | – | – | 12 |
| Stem Cell Transplantation (N = 3) | 36 | 25 | 5–80 | 110 |
| Stimulants (N = 1) | 10 | – | – | 10 |
| Therapeutic Vaccines (N = 7) | 38 | 40 | 24–60 | 268 |
| Toll-Like Receptor Agonists (N = 1) | 28 | – | – | 28 |
| Treatment Intensification/Early Treatment (N = 7) | 239 | 101 | 60–621 | 1676 |
| Total | 13,732 |
Sixty-five studies answered survey questions related to enrollment. When asked if study enrollment has begun, 45/65 studies (69%) indicated enrollment is ongoing; 17/65 studies (26%) indicated enrollment is complete; and 4/65 (6%) answered no. Of studies that are not yet enrolling, 3/4 anticipated enrollment would begin by the end of 2019. Supplemental Table 2 presents a summary of current enrollment by category, based on survey responses received.
Participant demographics
The survey included several questions about demographic targets. Respondents were asked if their study had any enrollment targets related to sex, gender, older age (>50 years) or race/ethnicity. Over three-quarters of respondents indicated there were no formal or informal targets for any of these demographic categories.
The proportion of respondents who did note targets are as follows:
-
•
Sex: 17% (N = 11) had an informal target, 6% (N = 4) had a formal target.
-
•
Gender: 10% (N = 6) had an informal target, 3% (N = 2) had a formal target.
-
•
Age over 50: 3% (N = 2) had an informal target, no respondents indicated a formal target.
-
•
Hispanic descent: 3% (N = 2) had an informal target, 1% (N = 1) had a formal target.
-
•
Black or African descent: 5% (N = 3) had an informal target, 5% (N = 3) had a formal target.
-
•
Asian descent: 5% (N = 3) had an informal target, no studies reported a formal target.
Survey respondents were also questioned about the demographics of currently enrolled participants and 60 respondents provided this information. Of these 60, 31 provided information on sex of current participants, 20 provided information on gender of current participants, 20 indicated if any participants had identified as transgender, 21 provided information on race or ethnicity, and 16 provided information on number of participants over 50 years old. Table 2 summarizes responses related to sex, gender, and age of current participants.
Table 2.
Demographics of current participants (sex, gender, and age).
| Category (N = respondents) | #/total | % | Mean | Median |
|---|---|---|---|---|
| Total participants (N = 60) | 2754 | – | 45 | 12 |
| Female participants-sex (N = 31) | 260/1549 | 16.7% | 8 | 1 |
| Women participants-gender (N = 20) | 230/1241 | 18.5% | 11 | 1 |
| Transgender (N = 20) | 18/1233 | 1.4% | 1 | 0 |
| Participants over 50 (N = 16) | 49/731 | 6.7% | 3 | 2 |
Two large, related studies that provided racial and ethnic data on current enrollment (total N = 624 participants) are taking place exclusively in Thailand. Since inclusion of these numbers might present a misleading picture of current demographic averages, a summary of reported totals excluding these studies is as follows (representing 19 studies with a total of 219 participants):
-
•
White (N = 117, 53.4%)
-
•
Black (N = 71, 32.4%)
-
•
Hispanic (N = 26, 11.9%)
-
•
Asian (N = 12, 5.5%)
Information on participant demographics was also sourced from an additional 42 studies that have presented or published results over the past year (see supplemental Tables 3 and 4). Out of a total of 1165 participants, 163 (14%) were female and 1002 were male (including one transgender person). Race and/or ethnicity was reported for 29 of these studies, representing 768 participants. A majority (N = 328, 42.7%) were white Non-Hispanic while 260 (33.8%) were Black or African American, 95 (12.4%) Asian, 31 (4%) Hispanic, 11 (1.4%) Indigenous Canadians, 11 (1.4%) “Other,” 6 (0.78%) Native American, and 4 (0.5%) more than one race. The remainder were either unreported or specifically cited as “Unknown or not reported.”
Trial completion
The TAG listing includes the estimated completion dates that are reported in trial registry entries. Based on this information, 80 studies project completion by the end of 2020. Supplemental figure 2 summarizes projected completion dates from survey responses and TAG listing data, by quarter.
Funding
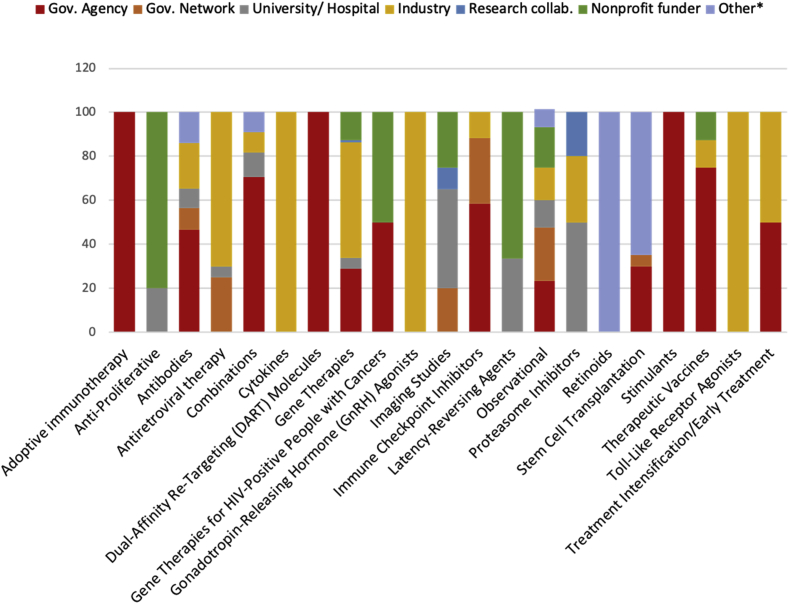
Of the 67 respondents who provided a breakdown of funders/sponsors for their study, 57% listed government agencies or network, 18% industry, 11% a nonprofit, 7% university/hospital, and 7% “Other” (including self-funded, the European Commission, and a combination of other funders). Most studies (45 of 67) received 100% of their funding from one category of funder. Seven study categories receive 100% of their funding from one category of funder (Adoptive Immunotherapy; Cytokines; DART Molecules; GnRH Agonists; Retinoids; Stimulants; and Toll-Like Receptor Agonists). The remaining study categories represented in survey responses receive funding from 2 or more funders. Fig. 4 provides a breakdown of the mean per-study-category funding by funder category.
Fig. 4.
Mean per-category funding by funder category.
Trial details
Questions were posed regarding requirements for participant ART use, the HIV reservoir assay being employed and any invasive procedures (including analytical treatment interruptions).
The vast majority of respondents (51/65, 78%) reported that their studies will enroll participants who are on ART with suppressed HIV viral loads. In most of the remaining cases (8/65, 12%), ART is being initiated after enrollment.
The most commonly used assays to measure the HIV reservoir were the quantitative virus outgrowth assay (qVOA) and polymerase chain reaction (PCR) to measure HIV DNA, which were cited by 29/65 (44.6%) and 20/65 (31%) respondents respectively.
Leukapheresis was the most ubiquitous invasive procedure, noted by 30/65 (46%) of respondents, followed by gut-associated lymphoid tissue (GALT) biopsy (18/65, 27.7%) and analytical treatment interruptions (ATIs) (15/65, 23%). In many instances these procedures were optional rather than mandatory. See supplemental figure 3 for complete information on reported invasive procedures.
Drawing on the information included in study registry entries, a total of 30 out of the 133 studies (22.5%) in the TAG listing as of August 16, 2019 feature an ATI, although in some cases ART will only be interrupted if certain criteria are met.
Data sharing plans
On the subject of data sharing, nearly half of the respondents (46%) reported that their funder required a data sharing agreement as a condition of financial support. Even if that was not the case, almost all were either making data available at the behest of the study team (36%) or expressed willingness to share if there were opportunities to do so (16%). Only 4% of respondents were not interested in sharing data.
Community input
Approximately two-thirds of respondents stated that there was some form of community input into the development of their study. In most cases (34%) this involved a research network or regional project community advisory board (CAB), such as the AIDS Clinical Trials Group (ACTG) Global CAB or a Martin Delaney Collaboratory CAB. Local or institutional CABs were cited by the majority of respondents who selected “other” (18%) and then reported the specific type of community input. For 17% of respondents, community members provided informal input in the absence of a formal CAB review. About a third of respondents (31%) stated that community input was not required during the development of their study.
Repeat responders
We included two questions specifically for study contacts who completed our 2018 survey. Out of 37 repeat responders, 67% reported that enrollment has progressed slower than previously anticipated, 23% that it has progressed as anticipated, and 10% faster than anticipated. In 2018, 31 respondents provided an estimated timeline for presentation or publication of their results, and approximately two-thirds (20/31, 64.5%) had been able to meet this timeline.
Discussion
The HIV cure research field has been fueled by the hope of additional cases of cures achieved by stem cell transplantation, but surveying the current landscape of clinical studies emphasizes that there are significant challenges ahead when it comes to developing broadly effective, safe and accessible curative approaches.
The recent initiation of efforts to develop target product profiles, with particular focus on the African continent where the burden of HIV remains highest,4 is a salutary step. But as we show here, HIV cure-related clinical research is still largely in the earliest phases and very little is occurring in Africa or other under-resourced areas of high prevalence. While limited, there are already data indicating that both HIV persistence and prospects for immune control of the virus could vary in the African setting compared to Western countries.11,12
The profile of current research participation remains skewed heavily toward men, both based on responses to our survey and analysis of study results that have been presented or published over the past year. At the same time, evidence continues to accumulate for notable sex differences in key parameters relevant to HIV cure research.[13], [14], [15], [16] This disconnect has profound implications for developing interventions applicable to Africa, where young females bear the brunt of the epidemic.
Analyses from the ACTG indicate that the paucity of women in studies is not due to a higher rate of screening out (at least in the USA),17 highlighting the importance of advocacy, education, support and outreach to facilitate increased screening for studies. The NIH policy on considering SABV (sex as a biological variable) in all vertebrate animal and human studies, which was instituted relatively recently, also has the potential to enhance this critical area of HIV cure research.18
Racial and ethnic diversity also remains far from optimal, with Hispanic underrepresentation being particularly stark in the studies we assessed. Participation by transgender individuals is extremely limited (at best) although one exception is the productive HIV cure-related research program being conducted in Thailand, which has been able to involve a proportion of transgender women.19
The use of ATIs in HIV cure research continues to raise thorny issues,20 with the past year seeing increasing attention to the risk of transmission associated with allowing periods of viral load rebound. Concerns about this issue have been thrown into sharp relief by the publication of two case reports describing HIV transmission from participants in ATI trials to HIV-negative partners.21,22
Best practices for reducing the risk of transmission during ATIs have yet to be fully formulated, but there is broad consensus that access to pre-exposure prophylaxis (PrEP) for HIV-negative partners or sexual contacts should be offered or facilitated when appropriate.23 Frustratingly, this is another area where women will be underserved, as the newest option for PrEP—Descovy—was not studied for efficacy in women and is only approved for use by men (a situation that is likely to persist for at least several years).24
Based on our analyses, we outline several recommendations for the field in Table 3. Despite the potential shortcomings in the current research landscape and the challenges that persist, there are an encouragingly diverse collection of interventions under study, offering reason for optimism that promising leads will emerge and draw us closer to the horizon where an effective, affordable and convenient HIV cure becomes available.
Table 3.
Recommendations for researchers pursuing HIV cure-related research.
| Recommendation 1: Demographic targets |
We strongly encourage researchers to set formal enrollment targets (sex, gender, age, race, ethnicity, location) wherever possible. When formal targets are unfeasible or impractical, informal targets can be used. Formal or informal targets should be coupled with an enrollment strategy, and success in achieving demographic targets should be reported in publications and presentations. |
| Recommendation 2: Consensus workshop on addressing sex and gender in HIV cure research | Known (and unknown) sex differences in HIV reservoirs,25 the potential impact of exogenous hormones (used for gender affirming therapy, contraception, or menopausal hormone therapy) on pharmacokinetics of ART, and gender differences in attitudes towards HIV cure26 are key sex and gender considerations in HIV cure research. Cure related research is still in early stages, but the field is developing quickly. A consensus workshop focused on sex and gender in cure research (similar to the 2018 ATI consensus workshop27) could lay the groundwork for future pooled analyses. As nearly all survey respondents indicated either plans for or interest in data sharing, establishing a consensus on scientific priorities now can facilitate future data sharing. |
| Recommendation 3: Invest in, and engage with, communities in geographically affected areas | Engaging community members in sub-Saharan Africa and other under-resourced areas of high HIV prevalence is both an ethical and scientific imperative for HIV cure-related research. Research programs should develop broad and comprehensive engagement strategies during the formative stages of cure-related research to ensure the field is responsive to the priorities of all affected populations. |
| Recommendation 4: Incorporate behavioral and social sciences in cure research | In light of the diversity of potentially curative approaches under investigation, incorporating behavioral and social sciences (BSSR) into cure studies may illuminate psychosocial aspects of HIV cure, reveal motivations for participating in clinical studies, and elucidate participants’ perceptions of risk and benefits in cure-related research.28,29 Knowledge gained from BSSR will be crucial when researchers seek to enroll larger numbers of participants in later-Phase trials and can inform future health literacy and health communication efforts. |
| Recommendation 4: Transparency of reporting | The major limitation of this and the previous analysis was data availability. We encourage researchers to report and disaggregate data by sex, gender, race, ethnicity, and age whenever possible. We are buoyed by the field’s receptiveness to data sharing and are hopeful this analysis underscores the value of entering study information and results on clinicaltrials.gov (even when doing so is not mandated). |
Limitations
As noted in our previous publication,6 the use of clinical trial registries as a source of information has potentially significant limitations due to the reliance on researchers or sponsors (who may already be overburdened) to register their work appropriately. Furthermore, there is no requirement to register Phase I trials in clinicaltrials.gov.
The therapeutic categories employed in the TAG “Research Toward a Cure” trials listing used as the basis for this analysis are primarily intended to make the information more manageable and comprehensible for users, and reflect the subjective judgment of TAG staff rather than any independent adjudication of which category an intervention belongs to.
Our survey response rate of 54.8% was not optimal and limits the amount of detail we were able to provide on the overall HIV cure research field at the current time. Several respondents did not complete the full survey which further limits the amount of detail we were able to provide in this analysis. Although it is difficult to know why study contacts chose not to complete the survey, potential explanations include hesitance to provide information about a study that is still in development/enrolling or has not yet been presented, busy schedules/competing priorities/time constraints, reticence to report sub-optimal participant diversity, or unfamiliarity with/lack of personal connection to the author who sent the survey.
Authors’ contributions
RJ initiated the TAG Research Toward an HIV Cure online listing. LB designed and conducted the survey and performed the data collection and analyses. LB and RJ collaborated on writing the manuscript.
Funding
Bill & Melinda Gates Foundation.
Declaration of competing interest
All authors declare that they have no competing interests.
Acknowledgements
The authors thank all the respondents who took time to complete our survey and made this report possible. We also thank Mike McCune and Emily Turner at the Bill & Melinda Gates Foundation for their guidance on the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2020.100010.
Contributor Information
Liz Barr, Email: barrlizbarr@gmail.com.
Richard Jefferys, Email: richard.jefferys@treatmentactiongroup.org.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gupta R.K., Abdul-Jawad S., McCoy L.E. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019 Apr;568(7751):244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen B, Knops E, Lübke N et al. Analytic treatment interruption (ATI) after allogeneic CCR5-D32 HSCT for AML in 2013. Conference on Retroviruses and Opportunistic Infections. March 2019. Seattle, WA, USA. Abstract 394.
- 3.Nijhuis M. 10th IAS Conference on HIV Science (IAS2019) July 2019. HIV cure by stem cell transplantation. Mexico City, Mexico. Abstract MOSY0706. [Google Scholar]
- 4.Ndung’u T., McCune J.M., Deeks S.G. Why and where an HIV cure is needed and how it might be achieved. Nature. 2019 Dec;576(7787):397–405. doi: 10.1038/s41586-019-1841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treatment Action Group Research toward a cure trials. http://www.treatmentactiongroup.org/cure/trials Available at: (accessed December 2019)
- 6.Barr L., Jefferys R. A landscape analysis of HIV cure-related clinical trials and observational studies in 2018. J Virus Erad. 2019 Nov 4;5(4):212–219. [PMC free article] [PubMed] [Google Scholar]
- 7.UK clinical trials Gateway. https://www.ukctg.nihr.ac.uk/ Available at: (accessed December 2019)
- 8.Ragon Institute of MGH, MIT and Harvard The FRESH study (females rising through education, support, and Health) http://www.ragoninstitute.org/international/fresh/ Available at: (accessed December 2019)
- 9.International collaboration to guide and investigate the potential for HIV cure by stem cell transplantation (IciStem) https://www.icistem.org/ Available at: (accessed December 2019)
- 10.AVAC . July 2019. Global Investment in HIV Cure Research and Development in 2018.https://www.avac.org/resource/global-investment-hiv-cure-research-and-development-2018 Available at: (accessed December 2019) [Google Scholar]
- 11.Prodger J.L., Lai J., Reynolds S.J. Reduced frequency of cells latently infected with replication-competent human immunodeficiency virus-1 in virally suppressed individuals living in rakai, Uganda. Clin Infect Dis. 2017 Oct 15;65(8):1308–1315. doi: 10.1093/cid/cix478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossez M., Martin G.E., Pace M. Virological remission after antiretroviral therapy interruption in female African HIV seroconverters. AIDS. 2019 Feb 1;33(2):185–197. doi: 10.1097/QAD.0000000000002044. [DOI] [PubMed] [Google Scholar]
- 13.Scully E.P., Gandhi M., Johnston R. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J Infect Dis. 2019 Mar 15;219(7):1084–1094. doi: 10.1093/infdis/jiy617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantero-Pérez J., Grau-Expósito J., Serra-Peinado C. Resident memory T cells are a cellular reservoir for HIV in the cervical mucosa. Nat Commun. 2019 Oct 18;10(1):4739. doi: 10.1038/s41467-019-12732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le C.N., Britto P., Brummel S.S. Time to viral rebound and safety after antiretroviral treatment interruption in postpartum women compared with men. AIDS. 2019 Nov 15;33(14):2149–2156. doi: 10.1097/QAD.0000000000002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn T.C., Prodger J., Capoferri A.M. 9th HIV Persistence during Therapy Workshop. December 2019. Sex differences in the latent reservoir of virally suppressed HIV-1 infected individuals living in Rakai, Uganda. Miami, FL, USA. Abstract OP 7.4. [Google Scholar]
- 17.Smeaton L.M., Kacanek D., Mykhalchenko K. Screening and enrollment by sex in HIV clinical trials in the United States. Clin Infect Dis. 2019 Aug 22;71(5):1300–1305. doi: 10.1093/cid/ciz959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton J.A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol Behav. 2018 Apr 1;187:2–5. doi: 10.1016/j.physbeh.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Muccini C., Crowell T.A., Kroon E. Leveraging early HIV diagnosis and treatment in Thailand to conduct HIV cure research. AIDS Res Ther. 2019 Sep 6;16(1):25. doi: 10.1186/s12981-019-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau J.S.Y., Smith M.Z., Allan B. Perspectives on analytical treatment interruptions in people living with HIV and their Health care providers in the landscape of HIV cure-focused studies. AIDS Res Hum Retrovir. 2019 Apr;36(4):260–267. doi: 10.1089/aid.2019.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lelièvre J.D., Hocqueloux L. Unintended HIV-1 transmission to a sex partner in a study of a therapeutic vaccine candidate. J Infect Dis. 2019 Jul 2;220(Supplement_1):S5–S6. doi: 10.1093/infdis/jiz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ugarte A., Romero Y., Tricas A. Unintended HIV-1 infection during analytical therapy interruption. J Infect Dis. 2020 Apr 27;221(10):1740–1742. doi: 10.1093/infdis/jiz611. [DOI] [PubMed] [Google Scholar]
- 23.Dawson L. Human immunodeficiency virus transmission risk in analytical treatment interruption studies: relational factors and moral responsibility. J Infect Dis. 2019 Jul 2;220(Supplement_1):S12–S15. doi: 10.1093/infdis/jiz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackstock O. Barring cisgender women from the Descovy trials was a bad call. Stat News. November 25, 2019 https://www.statnews.com/2019/11/25/descovy-trials-excluded-cisgender-women-bad-call/ Available at: (accessed December 2019) [Google Scholar]
- 25.Gianella S., Tsibris A., Barr L., Godfrey C. Barriers to a cure for HIV in women. J Int AIDS Soc. 2016;19(1):20706. doi: 10.7448/IAS.19.1.20706. Published 2016 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubé K., Eskaf S., Evans D. The dose response: perceptions of people living with HIV in the United States on alternatives to oral daily antiretroviral therapy. AIDS Res Hum Retroviruses. 2020 Apr;36(4):324–348. doi: 10.1089/aid.2019.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julg B., Dee L., Ananworanich J. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV. 2019;6(4):e259–e268. doi: 10.1016/S2352-3018(19)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubé K., Auerbach J.D., Stirratt M.J., Gaist P. Applying the behavioural and social sciences research (BSSR) functional framework to HIV cure research. J Int AIDS Soc. 2019;22(10) doi: 10.1002/jia2.25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubé K., Taylor J., Sylla L. ’Well, it’s the risk of the unknown… right?’: a qualitative study of perceived risks and benefits of HIV cure research in the United States. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0170112. Published 2017 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.