Abstract
Human activities are transforming grassland biomass via changing climate, elemental nutrients, and herbivory. Theory predicts that food-limited herbivores will consume any additional biomass stimulated by nutrient inputs (‘consumer-controlled’). Alternatively, nutrient supply is predicted to increase biomass where herbivores alter community composition or are limited by factors other than food (‘resource-controlled’). Using an experiment replicated in 58 grasslands spanning six continents, we show that nutrient addition and vertebrate herbivore exclusion each caused sustained increases in aboveground live biomass over a decade, but consumer control was weak. However, at sites with high vertebrate grazing intensity or domestic livestock, herbivores consumed the additional fertilization-induced biomass, supporting the consumer-controlled prediction. Herbivores most effectively reduced the additional live biomass at sites with low precipitation or high ambient soil nitrogen. Overall, these experimental results suggest that grassland biomass will outstrip wild herbivore control as human activities increase elemental nutrient supply, with widespread consequences for grazing and fire risk.
Subject terms: Community ecology, Grassland ecology
It is unclear whether terrestrial herbivores are able to consume the extra plant biomass produced under nutrient enrichment. Here the authors test this in grasslands using a globally distributed network of coordinated field experiments, finding that wild herbivore control on grassland production declines under eutrophication.
Introduction
Grasslands are a critical sink for atmospheric carbon, a key energy source for terrestrial food webs, and a vital resource for human food and fuel production1–3. Grassland plant biomass is controlled by interdependent factors that vary in space and time4, including climate3,5, the availability of growth-limiting resources, such as nitrogen and phosphorus6,7, and plant interactions with herbivores8. However, human activities are altering these processes9–11. For example, regional signatures of temperature and precipitation are changing12, and anthropogenic nitrogen deposition has increased dramatically since the start of the 20th century6,13,14. Growing evidence suggests that widespread, but regionally variable, eutrophication of terrestrial ecosystems6, and alteration of climate are changing global grassland productivity15–17. Yet, estimates of nutrient limitation of biomass production are commonly performed in ecosystems without accounting for the effects of herbivores18,19. This hinders our ability to evaluate the generality and magnitude of herbivore control of plant biomass. Notably, while grassland biomass production is critically important for services, including animal forage, soil health, and atmospheric carbon capture3, reduced consumer control of biomass in a eutrophic world could, for example, reduce plant biodiversity20 or increase fuel load and fire severity21.
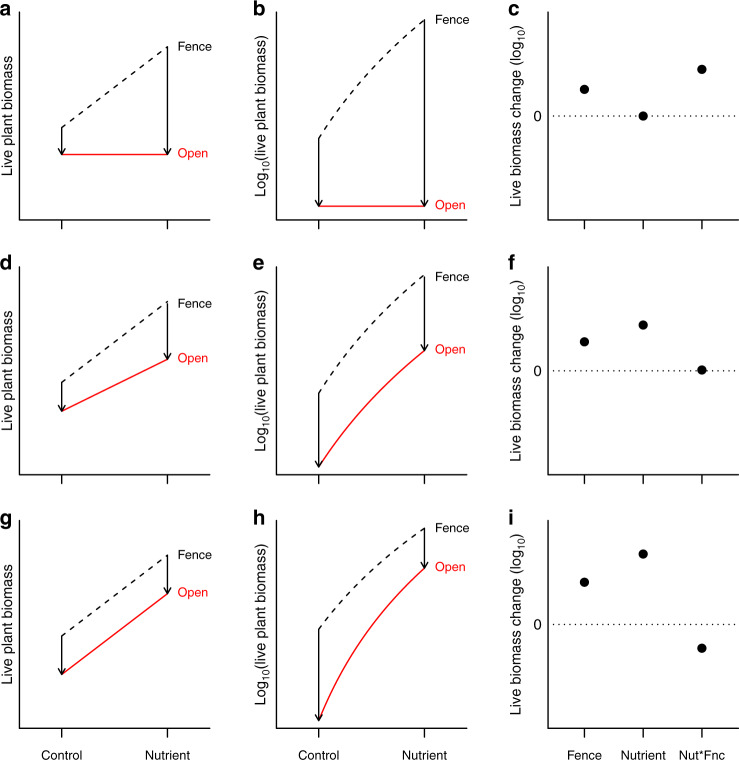
Simple equilibrium theory suggests that herbivores should be able to consume the additional plant production stimulated by elevated nutrient supply22–24. In particular, “consumer-controlled” theory predicts that when consumers are limited by their food resources, consumption will increase to counter any additional production, leading to no net change in plant biomass22 (Fig. 1a). Although a wealth of experiments in marine and freshwater ecosystems demonstrate that herbivory can counterbalance increased primary production due to eutrophication25–27, few studies have simultaneously manipulated terrestrial soil resources and herbivory by large vertebrates to test these predictions28. In a comprehensive meta-analysis summarizing nearly 200 experiments that concurrently manipulated both nutrient supply and herbivores, only 4% (eight studies) were in herbaceous-dominated terrestrial ecosystems and, of these, only four studies examined the effects of vertebrate herbivores25. These few grassland studies, generally lasting <3 years, suggest that herbivores have little impact on biomass, and fertilization can increase biomass even in the presence of herbivores18,25. Although data poor, this trend is more consistent with alternative “resource-controlled” theory that predicts increasing plant biomass along a productivity gradient even in the presence of herbivores29–31. Importantly, this theory predicts that herbivores will consume a constant proportion of plant biomass, regardless of environmental productivity (Fig. 1d).
Fig. 1. Predictions for plant biomass with increasing environmental productivity.
Arrows indicate the predicted difference in biomass at ambient (Control) and elevated (Nutrient addition) productivity in the presence (Open) and absence (Fence) of herbivores. Predictions for plant mass with increasing productivity when herbivores a consume all additional biomass produced (see ref. 24); d consume a constant proportion of biomass (see ref. 29); g consume a constant amount of biomass. Panels b, e, h show these predictions for log(biomass). Panels c, f, i show the difference (arrow lengths (fence and nutrient) and difference of arrow lengths (Nut*Fnc) in b, e, h), and visualize these hypotheses as they would look in the factorial experimental test.
A large body of theory has examined the consequences of a variety of realistic mechanisms that alter herbivore–plant interactions under eutrophic conditions25,31, potentially shifting plants from resource control to consumer control25,31,32. For example, fast-growing, highly nutritious plant species with low investment in defense often dominate in high resource environments33, which could lead to greater herbivore control of biomass in high resource environments than in conditions of low nutrient supply, as predicted by consumer-controlled theory (Fig. 1a)25. In contrast, theory that considers herbivore dietary specialization or selectivity suggests that herbivores will reduce the abundances of preferred plant species more in high resource environments. Compositional turnover due to this selective feeding can lead to reduced herbivore control of biomass with increasing productivity, ultimately resulting in proportional consumption (Fig. 1d) or even reduced consumption (Fig. 1g)25,31,32,34–36. In addition, the high among-study variability observed in plant biomass control by vertebrate herbivores25,34 is likely governed by context-dependent responses, varying with precipitation31,34, or other regional climatic, edaphic, or biotic gradients that could alter plant biomass control, but are not effectively characterized in a meta-analysis. Thus, there is a clear gap in existing data, but theory provides a guide for testing whether—and under what conditions—herbivores will control the accumulation of biomass in modern, eutrophic grasslands, where most wild grazer communities have been extensively modified and often driven to reduced population sizes by humans11.
We test the “consumer-control” hypothesis that vertebrate herbivores in grasslands consume the additional primary production due to eutrophication24,25, using an experiment replicated at 58 grassland sites spanning six continents. Our factorial experiment manipulates elemental nutrients (nitrogen, phosphorus, potassium, and micronutrients) and vertebrate herbivores larger than ~50 g (see “Methods” section and ref. 37), allowing us to test for the hypothesized interaction between nutrients and herbivores in controlling grassland biomass25. Each year in each plot, we used standard methods37 to measure aboveground live biomass. We test for non-additivity in log space, or a nonproportional dependence of herbivory on nutrient addition. Thus, a positive interaction indicates that herbivores consume proportionally more biomass under fertilized conditions compared to control, the hypothesis arising from the simplest consumer-resource models22,24 (Fig. 1a–c). No interaction in log space indicates herbivores track increased biomass production with a proportional increase in consumption29–31 (Fig. 1d–f), and a negative interaction indicates herbivores consume proportionally less under fertilized conditions25 (Fig. 1g–i). We test whether a variety of biotic and abiotic factors are associated with the strength of the interaction between nutrients and herbivores. Importantly, this includes determining the role of herbivore impact by additionally testing whether plant biomass control under eutrophication increases with increasing wild herbivore abundance and diversity or is greater at sites with domestically managed livestock than those with only wild herbivores.
This distributed experimental work demonstrates that, on average, herbivores in grasslands around the world remove a constant proportion of fertilized biomass. Thus, grassland biomass accumulates with fertilization, even with wild herbivores present. However, these results are context dependent. In particular, in locations where wild herbivores remain abundant or domestic livestock are also present, herbivores tend to keep up; they remove proportionally more biomass under fertilized conditions than under ambient conditions. Herbivore effects also vary along biogeographic gradients. For example, with increasing ambient soil nitrogen, plant biomass shifts from resource- to consumer-controlled, and herbivores keep up with fertilized biomass production in sites with low precipitation.
Results
Testing for an interaction between fertilization and fencing
This distributed experiment, performed at 58 grasslands sites on six continents, and including sites with wild vertebrate herbivores where fencing more than doubled aboveground live biomass (Supplementary Fig. 2), provided a strong test of the ability of vertebrate herbivores to consume the additional plant biomass produced in response to environmental eutrophication. Nonetheless, the experiment provided no evidence for an overall interaction between fertilization and fencing for most of the study duration (P > 0.05), indicating constant proportional biomass removal under both ambient and elevated nutrient conditions (Figs. 1d–f and 2).
Fig. 2. Treatment effects on aboveground grassland biomass were similar for sites with 2–4 years of response data (n = 58, open circles), at least 5 years (n = 42, gray), and at least 8 years (n = 24, black).
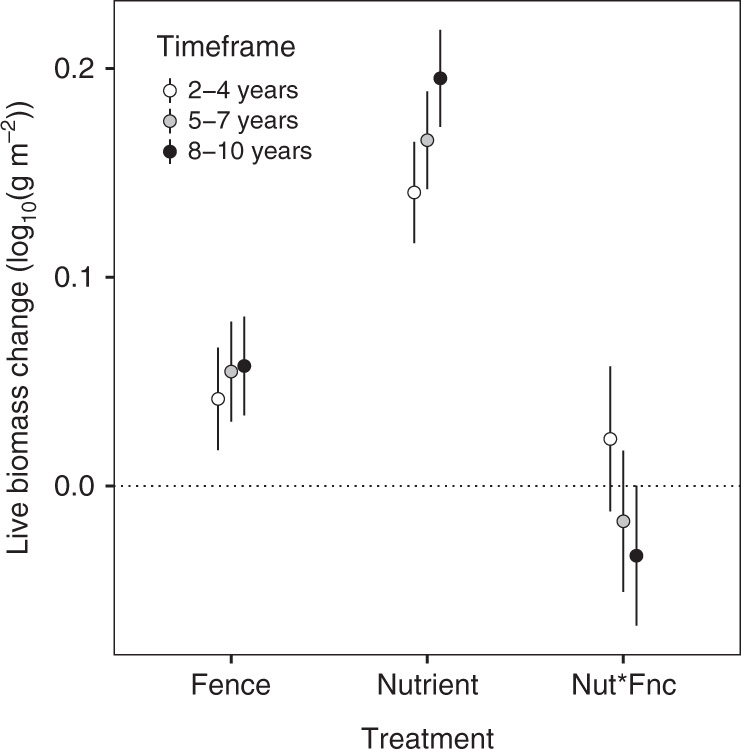
Error bars represent 95% confidence intervals. Main effect values represent model mean differences of the experimental treatment from the control after controlling for site and year as random effects. The nutrient by fence interaction (“Nut*Fnc”) is compared to the sum of the effect of nutrients alone and fencing alone, such that zero indicates additivity.
The fertilizer-induced increase in biomass inside fences was marginally smaller than the increase induced in the presence of large vertebrate herbivores in the longest-running sites (6.6% reduction at 8–10 years, Fig. 2 and Supplementary Table 3c), pointing to a slightly larger proportional impact of herbivores under ambient than fertilized conditions (Fig. 1g). However, across all sites, herbivore impacts on plant biomass with elevated nutrient supply were predicted by their impacts under unfertilized, ambient conditions (slope = 0.85; r2 = 0.20, Supplementary Fig. 2).
Mean effects of fencing and fertilization
In spite of the absence of support for an interaction between fencing and fertilization in controlling grassland biomass, both treatments altered grassland biomass. Across sites spanning six continents, both exclusion of vertebrate herbivores (P < 0.001, Supplementary Table 3a) and fertilization (P < 0.001, Supplementary Table 3a) increased aboveground biomass, with vertebrate herbivore exclusion leading to a 12% average increase in biomass by year 2, and fertilization leading to an average 58% biomass increase (Fig. 2 and Supplementary Fig. 1).
Temporal trends in fertilization and fencing
Because our experiment was replicated for 2–10 years at all sites, we tested the hypothesis that the variation in herbivore effects observed in past studies can be explained by study duration25, by comparing our full range of sites to the subset of 42 sites with 5 or more years and the subset of 24 sites with 8 or more years of continuous experimental manipulations. The subset of 24 sites with 8 or more years of data demonstrated that nutrients (P < 0.001) and herbivore exclusion (P < 0.001) led to a persistent increase in biomass of similar magnitude to the shorter-term effect across all sites (Fig. 2 and Supplementary Table 3b).
Testing for an interaction contingent on herbivore type, herbivore biomass, or herbivory intensity
Vertebrate herbivores consumed much more of the fertilization-induced biomass at sites where domestic and wild herbivores were both present. In particular, for the subset of eight sites with a mix of domestic livestock and wild herbivores (Supplementary Table 1), the biomass increase due to fertilization inside fences was 41% greater than expected from the independent effects of these treatments (P = 0.006, Supplementary Table 3d and Supplementary Fig. 3).
We additionally tested whether herbivore control of fertilized biomass increased either with an herbivore index based on site-level expert knowledge38 that quantified herbivore impact intensity and frequency or with modeled herbivore biomass23,32,39. Across all sites, the additional fertilized plant biomass was removed where the herbivore index was very high (P = 0.01, Fig. 3a and Supplementary Table 4a). However, at most sites, represented by low to intermediate herbivore index values, herbivores did not keep up with the additional fertilization-induced biomass. Modeled herbivore biomass did not predict the site-level biomass response (P = 0.18, Supplementary Table 4A). Because of covariation between the intensity and frequency of herbivores (herbivore index) and other site-level characteristics34, the impact of herbivores on fertilized biomass was less apparent in models just including site-level climate data (Supplementary Table 4b), but was clear when ambient site-level nutrients also were included (Supplementary Table 4c). Although the herbivore index results were consistent with the analysis of sites with domestic livestock (Supplementary Table 3d and Supplementary Fig. 3), the sites with the largest herbivore index values were distinct from those with domestic grazers; only two of the top ten sites with the greatest index values had domestic grazers (Supplementary Table 1).
Fig. 3. Herbivore control of grassland biomass varies with the biotic and abiotic environment.
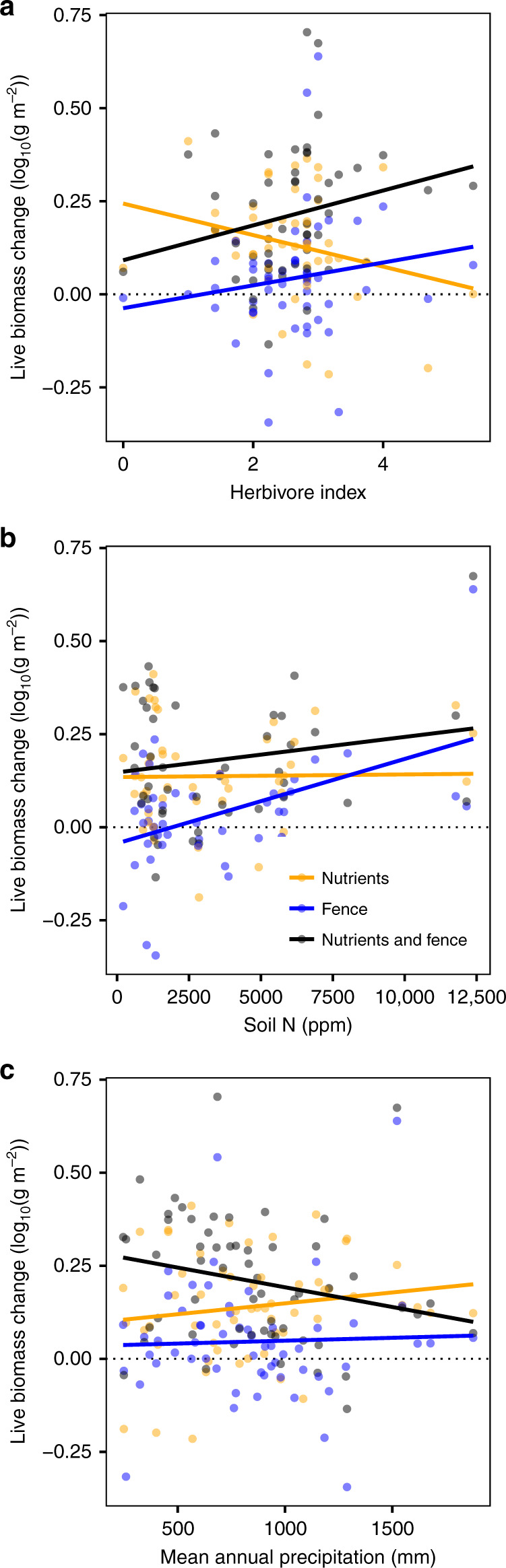
Plots show the average site-level difference of each treatment from the unfertilized, unfenced control across a gradient of a herbivore impact intensity and frequency, b ambient soil nitrogen, and c mean annual precipitation. Gradients in panels a–c were identified from statistical models presented in Supplementary Tables 4a–c.
Testing for an interaction contingent on abiotic and biotic characteristics
The sites in this experiment spanned a wide range of ambient edaphic (e.g., 90–17,160 p.p.m. soil N), climatic (e.g., 246–1,877 mm mean annual precipitation), and biotic (e.g., 1–31 plant species per m2) characteristics, providing insights into the contingency of fence effects on local abiotic and biotic factors. This experiment demonstrated that the impact of fencing varied in strength with edaphic characteristics and climate. In particular, nutrients increased biomass in the presence and absence of herbivores, whereas excluding herbivores had little effect at sites with low soil N, but had an increasingly positive effect on plant biomass with increasing soil N (P = 0.006, Fig. 3b and Supplementary Table 4c). Herbivores also had the greatest effects in fertilized plots at low precipitation sites (Fig. 3c and Supplementary Table 4b). In contrast, the magnitude of herbivore effects on biomass was not associated with either plant species richness or species temporal turnover (P >> 0.05).
Discussion
In contrast to earlier syntheses of existing data25,28, the results of this multisite replicated experiment demonstrate that grassland biomass around the world is limited by vertebrate herbivores, as well as nutrients, revealing striking similarity to the main effects of consumers and resources in more extensively studied marine and freshwater ecosystems18,25–27,40. However, in spite of a very wide range of biomass responses among sites—including sites with wild herbivores where fencing more than doubled biomass—vertebrate herbivores did not, in general, keep up with fertilized biomass production. Thus, the results of this study provide little support for the central hypothesis that wild terrestrial herbivores remove the additional biomass produced in fertilized plots, for example, due to greater nutritional quality or palatability41. Instead, evidence from this experiment points to constant proportional biomass reduction by herbivores, indicating that herbivores consume more plant biomass under fertilized conditions; nonetheless, fertilized grassland biomass accumulation outpaces herbivory.
Although wild herbivores did not, on average, consume the additional biomass produced with fertilization, the interaction between nutrients and herbivores increased with site-level intensity of herbivory23,24,34, highlighting the context-dependence of resource and consumer control of plant biomass22,29–31. In particular, these globally distributed experimental results suggest that herbivores will, on average, consume a constant proportion of biomass (Fig. 1d–f). In contrast, in environments where wild herbivores remain abundant11 and in human-managed agricultural settings, herbivores can remove the additional biomass produced in future, eutrophic grasslands, consistent with simple consumer-controlled theory22,24 (Fig. 1a–c). However, given that wild herbivore population sizes in many regions of the world are limited by factors such as habitat loss, hunting, and disease11 rather than food supply, the increased plant biomass from eutrophication in grasslands without domestic grazing is likely to remain mostly unconsumed. Further, the observation that long-term nutrient deposition has led to increased live plant biomass in the presence of herbivores across a wide range of global grasslands15 is consistent with the prediction that arises from this experiment: in most grasslands, herbivores are not keeping up with increased biomass production. While unavailable for the current study, site- and plot-scale measurements of the rates of plant productivity and consumption rates by vertebrate herbivores would provide additional insights into the global variation—and likely future trends—in herbivore control of grassland biomass.
Although studies in some terrestrial ecosystems have found very long timescales necessary to detect plant biomass responses to herbivore exclusion42, we found that 2–4 years of treatments led to similar conclusions about the overall effects of herbivores on grassland biomass, as did study durations of up to a decade. Thus, our extensively replicated experimental results additionally demonstrate that herbivore exclusion leads to a rapid increase and persistently elevated biomass through time, in contrast to previous data syntheses that found no overall effect of herbivores across studies and a general decline in the effects of herbivores on grassland biomass with time25.
This multisite study, spanning a globally relevant range of edaphic and climatic characteristics, supports hypotheses that abiotic factors also determine the conditions under which herbivores consume the additional biomass from eutrophication4,15,19,38. Along biogeographic gradients of soil nitrogen, our results demonstrated that plant biomass shifted from resource- to consumer-controlled. These results are consistent with the theoretical prediction that biomass control will change along a gradient of environmental productivity24,31 and that the plant species that dominate in high resource environments33,38 are particularly susceptible to herbivore control of biomass24. This result from our multisite experiment clarifies that the impact of herbivores on plant biomass is contingent on the abiotic characteristics of a site, providing explanation for the high variability in herbivore control of biomass that has been observed in meta-analyses25,34.
The impact of herbivores also varied with climate, with greatest effects of vertebrate herbivores in fertilized plots at low precipitation sites. Taken together with the consistent proportional impact of fencing on biomass across the precipitation gradient, these results do not support the hypothesis of an increasing rate of predator control of herbivores or plant regrowth along a precipitation-induced productivity gradient31,34. Rather, the marked impact of herbivores on fertilized, but not unfertilized, biomass at low precipitation sites is consistent with differential impacts of herbivores on plant biomass due to palatability. In particular, a general pattern that has been documented in this experiment38 is that when plant growth is limited by water, nutrients accumulate in plant tissues, whereas with increasing water availability, the nutrient to carbon content of tissues decreases with plant growth, even when nutrients are added43. Thus, the nutrient content of fertilized biomass tends to be higher than control conditions in dry sites, but similar to control in mesic sites38, making fertilized plots in dry regions markedly more attractive to herbivores44. This result likely reflects a preference response of herbivores to fertilized plots in regions with low precipitation. However, with nutrient addition at larger spatial scales, only if herbivore population growth is limited by the nutritional quality of resources in dry sites could this effect amplify with time. While population dynamics of herbivores in some low precipitation systems can vary with the quality of their resources45, elevated nitrogen inputs can lead to increased standing biomass even in dryland systems46, suggesting that this preference response in low precipitation regions may be weaker at the landscape scale. However, while decreased fertilization effects on biomass production in arid systems are often attributed to nutrient immobilization or water resource limitation47, these results suggest that herbivory also may play a role. Taken together, these directly comparable experimental results, spanning nearly an eightfold range of precipitation, point to variation in resource quality as a likely mechanism underpinning the strength of herbivore control of eutrophic grassland biomass31,38.
Measures of plant composition allowed us to test whether herbivore control of biomass production declined with increasing plant species diversity or compositional turnover in response to the treatments31. Although herbivores can increase compositional turnover toward dominance by grazing-resistant or tolerant plant species48, leading to reduced impacts of herbivores on plant biomass25,31, our data demonstrate that neither plant species richness nor species temporal turnover was associated with the magnitude of the fencing effects. Past studies have found conflicting effects of herbivores on live biomass, including strongly increasing49,50 or decreasing50–52 biomass, possibly due to variable responses by the species present at the site. Even within sites, plant species turnover in response to eutrophication often is not predictable by functional group, frequently responding in a species-specific manner to the combination of consumers and plants present at the site42,53,54. This extreme context-dependence likely explains why species turnover rates do not predict biomass responses to treatments across the wide range of grasslands included in this experiment31.
This work fills a key knowledge gap: previous syntheses have bemoaned the scarcity of manipulative experimental studies testing hypotheses about the generality of effects of vertebrate herbivores and nutrient supply on terrestrial biomass production25,28,42. This experiment demonstrates that both nutrient supply and vertebrate herbivory control aboveground biomass in the world’s grasslands, with treatment effects appearing rapidly, and persisting or increasing for up to a decade. It also underscores the importance of context-dependent impacts of herbivores along biotic, climatic, and edaphic gradients31, with herbivores counteracting the effects of nutrient addition, particularly at sites with low precipitation and those with high ambient soil nitrogen or high grazing intensity. Thus, while the grassland biomass response to fertilization shifted to control by consumers at very high site-level abundance and diversity of herbivores, this was only one among several biotic and abiotic factors associated with aboveground plant biomass control in grasslands around the world.
These results reconcile the high variability of grassland responses to consumer and resource perturbations that has been documented among sites and studies25,28,49–52, and demonstrate that these forces most often operate independently to control grassland biomass. In an era in which the challenges of climate change and catastrophic wildfires, driven by high fuel loads, are omnipresent3,20,21, understanding the controls on grassland biomass is crucial. This distributed experiment provides a powerful demonstration that the stimulation of plant biomass production associated with increasing nutrient supply generally exceeds herbivores’ consumption capacity in non-agricultural grasslands, with implications for future grazing, biodiversity, and fire risk management strategies in the face of continued anthropogenic perturbation of global nutrient cycles.
Methods
A full factorial combination of large herbivore exclusion via fencing (“control” or “fenced”) and addition of nutrients (“control” or “all nutrients”) was applied to 5 × 5 m plots at 58 sites spanning six continents, as part of the Nutrient Network experimental collaboration (www.nutnet.org; Supplementary Table 1)37. Most sites had three replicate blocks, and all sites had collected 1 year of pretreatment data and 2–10 consecutive years of posttreatment data (Supplementary Table 1). All sites were located in the herbaceous vegetation (“grassland”) representative of the region.
Treatments
Experimental design and treatments are detailed in ref. 37. Nutrient treatments (“NPK”) received: 10 g N m−2 yr−1 as time-release urea [(NH2)2CO], 10 g P m−2 yr−1 as triple-super phosphate, [Ca(H2PO4)2], 10 g K m−2 yr−1 as potassium sulfate [K2SO4] and 100 g m−2 of a micronutrient mix of Fe (15%), S (14%), Mg (1.5%), Mn (2.5%), Cu (1%), Zn (1%), B (0.2%), and Mo (0.05%). Macronutrients (N, P, and K) were applied annually; micronutrients were applied once at the start of the experiment (year 1).
Exclosure treatments (“fence”) were 230 cm tall. The lower 90 cm was surrounded by 1 cm woven wire mesh with a 30 cm outward-facing flange stapled to the ground to exclude digging animals (e.g., rabbits and voles), though not fully subterranean ones (e.g., gophers and moles). The upper 90 cm had three evenly spaced barbless wires to restrict larger vertebrate access (e.g., bison, elk, reindeer, or kangaroos). A few sites deviated from this fence design (Supplementary Table 2). While all sites had a wild herbivore community (e.g., a mix of rodents, lagomorphs, ungulates, marsupials, etc), domestic livestock (e.g., sheep, yak, goat, and cattle) were present at ten sites (Supplementary Table 1), allowing us to compare herbivore effects at sites with managed herds.
Vegetation sampling
We analyzed annual peak season live biomass by measuring aboveground biomass of all plants rooted within two 0.1 m2 (10 × 100 cm) strips in each experimental plot. Clipped vegetation was separated into live and dead components, dried at 60 °C for 48 h, and weighed to the nearest 0.01 g. We collected all leaves and current year’s woody growth from shrubs and subshrubs occurring in plots. We visually estimated the percent cover of each species to the nearest 1% in a randomly designated, but permanently marked, 1 × 1 m subplot within each 25 m2 plot to quantify species richness and composition.
Herbivory
We quantified potential vertebrate herbivore impact in two ways. First, we used a published empirical metric of herbivore impact intensity and frequency (“herbivore index”)38. In brief, all herbivore species that consume grassland biomass throughout the year were documented by the PI at each site (>2 kg), and PIs assigned an importance value for each species that reflected the impact or frequency of encounter, from 1 (present, but low impact and frequency) to 5 (high impact and frequency). An index value was calculated for each site as the sum of herbivore importance values for all herbivores38. This empirical herbivore index, based on a standardized rubric completed for each site, accounts for site-level variation in herbivore abundance and diversity, integrated across seasons and years. Second, we extracted the modeled terrestrial potential wild grazer biomass from a published dataset39 (“modeled herbivore biomass”) using site-level latitude and longitude values. We included the model-estimated value of herbivore biomass for each site location as a second standardized metric of potential herbivore impact among our experimental sites. While each of these provides information about potential and actual grazing intensity, neither is a direct site- or treatment-scale measure. Finally, although studies in some grasslands have shown arthropods can control plant biomass, we did not include insect herbivory in this study because previous work in this experiment suggests that arthropods increase in biomass with increasing plant biomass, but they do not strongly suppress plant biomass in any of the treatments55. Thus, we focus here on the impacts of fences—and vertebrate herbivory—because evidence suggests that arthropod herbivores are impacted by the treatments, but have little overall effect on the treatments.
Soils
We collected two 2.5 cm diameter by 10 cm depth soil cores, free of litter and vegetation, from each plot prior to initiation of the experiment (year 0—“Y0”). We composited cores from each plot, homogenized through a 2 mm sieve, air dried, and assayed for %N and %C, using dry combustion GC analysis (COSTECH ESC 4010 Element Analyzer, University of Nebraska, Lincoln, NE, USA) and also assayed for soil phosphorus, potassium, and micronutrients, soil pH, organic matter, and texture (A&L Analytical Laboratory, Memphis, TN, USA). Because the site-scale correlation between the ambient soil %N and %C was high (0.96, P < 0.001), we included %N in our models.
Climate
We characterized site-level climate and seasonality over 10–30 year timespans using the WorldClim database (version 1.4; http://www.worldclim.org/bioclim)56 associated to sites via latitude and longitude. We included mean annual temperature (°C; “BIO1” in the WorldClim database), mean annual precipitation (mm per year; BIO12), precipitation variability (coefficient of variation in precipitation among months; BIO15), rainfall-potential evapotranspiration (mm per month, PET data from CGIAR)57, temperature variability (standard deviation of temperature among months; BIO4), and mean precipitation in the warmest quarter (mm; BIO18).
Atmospheric nitrogen deposition
We characterized site-level atmospheric nitrogen input using modeled N-deposition (kg N ha−1 y−1) associated with study sites via latitude and longitude. Model input included measurements and future projections using a global three-dimensional chemistry-transport model TM3 (ref. 58). Because our study sites span continents, the 5-degree longitude by 3.75-degree latitude model and output grid resolution (50 × 50 km sub-grids) were sufficient to differentiate N-deposition rates among sites.
Statistical analyses
To test the hypothesis that vertebrate herbivores in grasslands can counterbalance the increased primary production due to eutrophication, we assessed the effect of each experimental treatment on the change in plot-scale biomass starting with a mixed-effects model (lmer function in the lme4 R library), with site and treatment year (number of years treatments had been applied) nested within site as random intercepts. We used the Satterthwaite’s degrees of freedom method to estimate P values for the mixed-effects models. Fertilization and fencing were fixed effects, and live biomass was log10 transformed. Biomass change in response to fencing was estimated as log(Bf+) − log (Bf−), where Bf+ was average site-level live biomass in fenced plots and Bf− was average site-level live biomass in control plots. For the other treatments, we performed the analogous calculations. We examined residuals for homogeneity of variance.
To test whether treatment effects decline with time, we analyzed the dataset of 58 sites with pretreatment and 2 or more years of experimental data, as well as the subset of 42 sites with 5 or more years of data and 24 sites with 8 or more years of data. We additionally tested whether the same main effects, interactions, and time-independence were obtained with only the subset of sites with 8–10 years of data; models were qualitatively similar to the full dataset, so we do not present this analysis. Two additional desert sites had no live biomass in a substantial proportion of the plots across all sampling dates (45% of ethamc.au plots and 33% of ethass.au plots). The numerous zeros destabilized statistical models; all model results were sensitive to the statistical choices for addressing the zeros at these two sites (e.g., adding a small value). Thus, we excluded these two sites from the current analyses for statistical reasons. All analyses were performed using R (version 3.2.3; R Foundation for Statistical Computing).
To determine the conditions under which herbivory most strongly counteracted the effect of nutrients on aboveground biomass, we fit mixed-effects models that included all interactions between the fertilization, fencing, and environmental covariates. We included site-level species richness (all taxa in all plots and years from each site), species turnover estimated as mean community distance between each posttreatment year’s composition and pretreatment composition (Jaccard distance), site-level N-deposition, climate factors, and plot-scale soil chemistry (soil N, soil P, and pH). We did not have a complete set of soil chemistry and N-deposition data for all sites, so we separately examined the effects of soil N, P, and pH and N-deposition in a regression that included the 36 sites for which these data were available.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was generated using data from the Nutrient Network (http://www.nutnet.org) experiment, funded at the site scale by individual researchers. Author contributions are detailed in the “Author contributions” section and Supplementary Table 5; Supplementary Table 6 lists all data contributors who are not authors. Coordination and data management have been supported by funding to E.T.B and E.W.S. from the National Science Foundation Research Coordination Network (NSF-DEB-1042132) and Long-Term Ecological Research (NSF-DEB-1234162 and NSF-DEB-1831944 to Cedar Creek LTER) programs, and the Institute on the Environment (DG-0001-13). We also thank the Minnesota Supercomputer Institute for hosting project data and the Institute on the Environment for hosting Network meetings. The authors dedicate this paper to the memory of Enrique Chaneton who contributed data and early ideas to this work.
Source data
Author contributions
E.T.B. and E.W.S. developed and framed the research question, analyzed the data, and coordinate the Nutrient Network collaboration. W.S.H. contributed to analyses. E.T.B., W.S.H., P.B.A., M.N.B., M.W.C., S.C., M.C.C., C.A.A., C.R.D., T.L.D., I.D., A.E., J.L.F., D.S.G., R.W.H., K.J.K., L.S.L., A.S.M., J.P.M., J.L.M., B.M., H.O.V., S.A.P., J.N.P., A.C.R., M. Sankaran, M. Schütz, C.J.S., R.V., P.A.W., and E.W.S. contributed data. E.T.B. wrote the paper and all other authors (W.S.H., P.B.A., C.A.A., M.N.B., M.W.C., M.C.C., C.R.D., T.L.D., I.D., A.E., J.L.F., P.G., D.S.G., R.W.H., A.M.K., K.J.K., L.S.L., A.S.M., J.P.M., J.L.M., B.M., R.O.H., H.O.V., S.A.P., J.N.P., A.C.R., M. Sankaran, M. Schütz, J.S., C.J.S., R.V., P.A.W., and E.W.S.) contributed to paper writing.
Data availability
Source data (plant, herbivore, and soil nitrogen) are provided with this paper. The plant, herbivore, and soil nitrogen data presented in the current study are also available in the Environmental Data Initiative (EDI) repository59 with the identifier 10.6073/pasta/a318fe0fb11eb43c1a2c8233b2e3494f. The WorldClim database (version 1.4) is available at http://www.worldclim.org/bioclim. The modeled herbivore mass data by Zhu et al.39 are available in the PANGAEA repository at 10.1594/PANGAEA.884853.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Lauchlan Fraser and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-19870-y.
References
- 1.Running SW. A measurable planetary boundary for the biosphere. Science. 2012;337:1458–1459. doi: 10.1126/science.1227620. [DOI] [PubMed] [Google Scholar]
- 2.Haberl H, et al. Quantifying and mapping the human appropriation of net primary production in Earth’s terrestrial ecosystems. Proc. Natl Acad. Sci. USA. 2007;104:12942–12945. doi: 10.1073/pnas.0704243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, et al. Spatio-temporal patterns and climate variables controlling of biomass carbon stock of global grassland ecosystems from 1982 to 2006. Remote Sens. 2014;6:1783. doi: 10.3390/rs6031783. [DOI] [Google Scholar]
- 4.Grace JB, et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature. 2016;529:390–393. doi: 10.1038/nature16524. [DOI] [PubMed] [Google Scholar]
- 5.Del Grosso S, et al. Global potential net primary production predicted from vegetation class, precipitation, and temperature. Ecology. 2008;89:2117–2126. doi: 10.1890/07-0850.1. [DOI] [PubMed] [Google Scholar]
- 6.Galloway JN, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70:153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 7.Chadwick OA, Derry LA, Vitousek PM, Huebert BJ, Hedin LO. Changing sources of nutrients during four million years of ecosystem development. Nature. 1999;397:491–497. doi: 10.1038/17276. [DOI] [Google Scholar]
- 8.McNaughton SJ, Oesterheld M, Frank DA, Williams KJ. Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature. 1989;341:142–144. doi: 10.1038/341142a0. [DOI] [PubMed] [Google Scholar]
- 9.Foley JA, et al. Solutions for a cultivated planet. Nature. 2011;478:337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 10.White, R. P., Murray, S. & Rohweder, M. Pilot Analysis of Global Ecosystems (PAGE): Grassland Ecosystems, 70 (World Resources Institute, Washington, DC, 2000).
- 11.Ripple, W. J. et al. Collapse of the world’s largest herbivores. Sci. Adv.1, e1400103 (2015). [DOI] [PMC free article] [PubMed]
- 12.Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 151 (Geneva, Switzerland, 2014).
- 13.Canfield DE, Glazer AN, Falkowski PG. The evolution and future of earth’s nitrogen cycle. Science. 2010;330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- 14.Stevens CJ. Nitrogen in the environment. Science. 2019;363:578–580. doi: 10.1126/science.aav8215. [DOI] [PubMed] [Google Scholar]
- 15.Stevens CJ, et al. Anthropogenic nitrogen deposition predicts local grassland primary production worldwide. Ecology. 2015;96:1459–1465. doi: 10.1890/14-1902.1. [DOI] [Google Scholar]
- 16.Reyer CPO, et al. A plant’s perspective of extremes: terrestrial plant responses to changing climatic variability. Glob. Change Biol. 2013;19:75–89. doi: 10.1111/gcb.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knapp AK, et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience. 2008;58:811–821. doi: 10.1641/B580908. [DOI] [Google Scholar]
- 18.LeBauer DS, Treseder KK. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology. 2008;89:371–379. doi: 10.1890/06-2057.1. [DOI] [PubMed] [Google Scholar]
- 19.Fay PA, et al. Grassland productivity limited by multiple nutrients. Nat. Plants. 2015;1:15080. doi: 10.1038/nplants.2015.80. [DOI] [PubMed] [Google Scholar]
- 20.Borer ET, et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature. 2014;508:517–520. doi: 10.1038/nature13144. [DOI] [PubMed] [Google Scholar]
- 21.Knapp AK, Seastedt TR. Detritus accumulation limits productivity of tallgrass prairie: the effects of its plant litter on ecosystem function make the tallgrass prairie unique among North American biomes. BioScience. 1986;36:662–668. doi: 10.2307/1310387. [DOI] [Google Scholar]
- 22.Volterra V. Variations and fluctuations of the numbers of individuals in animal species living together. Nature. 1926;118:558–560. doi: 10.1038/118558a0. [DOI] [Google Scholar]
- 23.Crawley, M. J. Herbivory: The Dynamics of Animal-Plant Interactions, Vol. 10, 437 (University of California Press, 1983).
- 24.Oksanen L, Oksanen T. The logic and realism of the hypothesis of exploitation ecosystems. Am. Nat. 2000;155:703–723. doi: 10.1086/303354. [DOI] [PubMed] [Google Scholar]
- 25.Gruner DS, et al. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 2008;11:740–755. doi: 10.1111/j.1461-0248.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 26.Hillebrand H, et al. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proc. Natl Acad. Sci. USA. 2007;104:10904–10909. doi: 10.1073/pnas.0701918104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worm B, Lotze HK, Hillebrand H, Sommer U. Consumer versus resource control of species diversity and ecosystem functioning. Nature. 2002;417:848–851. doi: 10.1038/nature00830. [DOI] [PubMed] [Google Scholar]
- 28.Turkington R. Top-down and bottom-up forces in mammalian herbivore - vegetation systems: an essay review. Botany. 2009;87:723–739. doi: 10.1139/B09-035. [DOI] [Google Scholar]
- 29.DeAngelis, D. L. Dynamics of Nutrient Cycling and Food Webs (Chapman and Hall, London, 1992).
- 30.Arditi R, Ginzburg LR. Coupling in predator-prey dynamics: ratio-dependence. J. Theor. Biol. 1989;139:311–326. doi: 10.1016/S0022-5193(89)80211-5. [DOI] [Google Scholar]
- 31.Chase JM, Leibold MA, Downing AL, Shurin JB. The effects of productivity, herbivory, and plant species turnover in grassland food webs. Ecology. 2000;81:2485–2497. doi: 10.1890/0012-9658(2000)081[2485:TEOPHA]2.0.CO;2. [DOI] [Google Scholar]
- 32.Leibold MA. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am. Nat. 1989;134:922–949. doi: 10.1086/285022. [DOI] [Google Scholar]
- 33.Endara MJ, Coley PD. The resource availability hypothesis revisited: a meta-analysis. Funct. Ecol. 2011;25:389–398. doi: 10.1111/j.1365-2435.2010.01803.x. [DOI] [Google Scholar]
- 34.Milchunas DG, Lauenroth WK. Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecol. Monogr. 1993;63:327–366. doi: 10.2307/2937150. [DOI] [Google Scholar]
- 35.Polis GA, Strong DR. Food web complexity and community dynamics. Am. Nat. 1996;147:813–846. doi: 10.1086/285880. [DOI] [Google Scholar]
- 36.Murdoch W. Community structure, population control, and competition - a critique. Am. Nat. 1966;100:219–226. doi: 10.1086/282415. [DOI] [Google Scholar]
- 37.Borer ET, et al. Finding generality in ecology: a model for globally distributed experiments. Methods Ecol. Evolution. 2014;5:65–73. doi: 10.1111/2041-210X.12125. [DOI] [Google Scholar]
- 38.Anderson, T. M. et al. Herbivory and eutrophication mediate grassland plant nutrient responses across a global climatic gradient. Ecology99, 822–831 (2018). [DOI] [PubMed]
- 39.Zhu D, et al. The large mean body size of mammalian herbivores explains the productivity paradox during the Last Glacial Maximum. Nat. Ecol. Evol. 2018;2:640–649. doi: 10.1038/s41559-018-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillebrand H. Top-down versus bottom-up control of autotrophic biomass - a meta-analysis on experiments with periphyton. J. North Am. Benthol. Soc. 2002;21:349–369. doi: 10.2307/1468475. [DOI] [Google Scholar]
- 41.Frank R, Merle LF. Effects of annual applications of low N fertilizer rates on a mixed grass prairie. J. Range Manag. 1983;36:359–362. doi: 10.2307/3898487. [DOI] [Google Scholar]
- 42.Olofsson J, et al. Long-term experiments reveal strong interactions between lemmings and plants in the Fennoscandian highland tundra. Ecosystems. 2014;17:606–615. doi: 10.1007/s10021-013-9740-6. [DOI] [Google Scholar]
- 43.Lemaire G, Jeuffroy M-H, Gastal F. Diagnosis tool for plant and crop N status in vegetative stage: theory and practices for crop N management. Eur. J. Agron. 2008;28:614–624. doi: 10.1016/j.eja.2008.01.005. [DOI] [Google Scholar]
- 44.Hillebrand H, et al. Herbivore metabolism and stoichiometry each constrain herbivory at different organizational scales across ecosystems. Ecol. Lett. 2009;12:516–527. doi: 10.1111/j.1461-0248.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 45.Hempson GP, Illius AW, Hendricks HH, Bond WJ, Vetter S. Herbivore population regulation and resource heterogeneity in a stochastic environment. Ecology. 2015;96:2170–2180. doi: 10.1890/14-1501.1. [DOI] [PubMed] [Google Scholar]
- 46.Sickman JO, et al. Quantifying atmospheric N deposition in dryland ecosystems: a test of the Integrated Total Nitrogen Input (ITNI) method. Sci. Total Environ. 2019;646:1253–1264. doi: 10.1016/j.scitotenv.2018.07.320. [DOI] [PubMed] [Google Scholar]
- 47.Yahdjian L, Gherardi L, Sala OE. Nitrogen limitation in arid-subhumid ecosystems: a meta-analysis of fertilization studies. J. Arid Environ. 2011;75:675–680. doi: 10.1016/j.jaridenv.2011.03.003. [DOI] [Google Scholar]
- 48.Koerner SE, et al. Plant community response to loss of large herbivores differs between North American and South African savanna grasslands. Ecology. 2014;95:808–816. doi: 10.1890/13-1828.1. [DOI] [PubMed] [Google Scholar]
- 49.Frank DA, McNaughton SJ, Tracy BF. The ecology of the Earth’s grazing ecosystems: profound functional similarities exist between the Serengeti and Yellowstone. Bioscience. 1998;48:513–521. doi: 10.2307/1313313. [DOI] [Google Scholar]
- 50.Augustine DJ, McNaughton SJ. Interactive effects of ungulate herbivores, soil fertility, and variable rainfall on ecosystem processes in a semi-arid savanna. Ecosystems. 2006;9:1242–1256. doi: 10.1007/s10021-005-0020-y. [DOI] [Google Scholar]
- 51.Ritchie ME, Tilman D, Knops JMH. Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology. 1998;79:165–177. doi: 10.1890/0012-9658(1998)079[0165:HEOPAN]2.0.CO;2. [DOI] [Google Scholar]
- 52.Pastor J, Dewey B, Naiman RJ, McInnes PF, Cohen Y. Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology. 1993;74:467–480. doi: 10.2307/1939308. [DOI] [Google Scholar]
- 53.Grellmann D. Plant responses to fertilization and exclusion of grazers on an Arctic tundra heath. Oikos. 2002;98:190–204. doi: 10.1034/j.1600-0706.2002.980202.x. [DOI] [Google Scholar]
- 54.Hartley SE, Mitchell RJ. Manipulation of nutrients and grazing levels on heather moorland: changes in Calluna dominance and consequences for community composition. J. Ecol. 2005;93:990–1004. doi: 10.1111/j.1365-2745.2005.01025.x. [DOI] [Google Scholar]
- 55.Lind EM, et al. Increased grassland arthropod production with mammalian herbivory and eutrophication: a test of mediation pathways. Ecology. 2017;98:3022–3033. doi: 10.1002/ecy.2029. [DOI] [PubMed] [Google Scholar]
- 56.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 57.Trabucco A, Zomer RJ, Bossio DA, van Straaten O, Verchot LV. Climate change mitigation through afforestation/reforestation: a global analysis of hydrologic impacts with four case studies. Agric. Ecosyst. Environ. 2008;126:81–97. doi: 10.1016/j.agee.2008.01.015. [DOI] [Google Scholar]
- 58.Dentener, F. J. Global Maps of Atmospheric Nitrogen Deposition, 1860, 1993, and 2050. Oak Ridge National Laboratory Distributed Active Archive Center. 10.3334/ORNLDAAC/830 (2006).
- 59.Borer, E. T. et al. Environmental Data Initiative10.6073/pasta/a318fe0fb11eb43c1a2c8233b2e3494f (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data (plant, herbivore, and soil nitrogen) are provided with this paper. The plant, herbivore, and soil nitrogen data presented in the current study are also available in the Environmental Data Initiative (EDI) repository59 with the identifier 10.6073/pasta/a318fe0fb11eb43c1a2c8233b2e3494f. The WorldClim database (version 1.4) is available at http://www.worldclim.org/bioclim. The modeled herbivore mass data by Zhu et al.39 are available in the PANGAEA repository at 10.1594/PANGAEA.884853.