Abstract
Background
Parkinson's disease is a neurodegenerative disorder, and a major cause of disability. Levodopa, a prodrug of dopamine, remains the gold standard in the pharmacological management of Parkinson's disease. Despite several attempts to improve the clinical efficacy of levodopa, new oral levodopa formulations are needed to overcome irregular absorption and variable plasma concentrations.
Objective
The aim of this study was to evaluate the in vitro and in vivo kinetic properties of chitosan-coated hydroxypropylmethyl cellulose microparticles of levodopa (and carbidopa).
Methods
Microparticles were formulated by encapsulating levodopa powder in chitosan-coated hydroxypropylmethyl cellulose using the spray-drying method. Levodopa microparticles were evaluated for size, zeta potential, drug loading capacity, encapsulation efficiency and in vitro release. In evaluating in vivo pharmacokinetics, Sprague Dawley rats were administered either levodopa/carbidopa powder, levodopa/carbidopa microparticles, or Sinemet CR (a controlled release formulation of levodopa/carbidopa). The dose of respective formulations administered was 20/5 mg/kg; 20 mg levodopa combined with 5 mg carbidopa per kilogram body weight of animals. Treatments were administered via the oral route every 12 hours. Blood samples were collected after predetermined times following the third dose. Plasma was obtained from blood collected, and levodopa levels determined by HPLC. Pharmacokinetic parameters, including Cmax, Tmax, AUC, and t½ of the various formulations, were estimated.
Results
The mean (SD) size of levodopa microparticles was 0.5 (0.05) µm with polydispersity index of 0.41 and a zeta potential of 10.8 mV. Of the expected 20% drug loading, the actual drug loading capacity of levodopa microparticles was found to be 19.1%, giving an encapsulation efficiency of 95.7%. The in vitro release kinetics of levodopa microparticles showed a controlled and sustained release, with about 80% release occurring after 12 hours. In vivo pharmacokinetic studies showed that rats administered levodopa/carbidopa microparticles had greater AUC (612.7 [17.42] ng.h/mL) and higher Cmax (262.4 [38.86] ng/mL) compared with Sinemet CR: AUC 354.7 (98.09) ng.h/mL and Cmax 95.5 (20.87) ng/mL. However, Sinemet CR had a much longer half-life (6.1 [2.58] hours) compared with levodopa/carbidopa microparticles (2.0 [0.31] hours).
Conclusions
Findings from this study suggest that chitosan-coated hydroxypropylmethyl cellulose microparticles of levodopa/carbidopa may give relatively high levels of levodopa in circulation. (Curr Ther Res Clin Exp. 2020; 81:XXX–XXX)
© 2020 Elsevier HS Journals, Inc.
Key words: levodopa, microparticles, Parkinson's disease, pharmacokinetics
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder that influences the ability to control the skeletal muscular system. PD mostly presents with tremor, stiffness, and slowed movements, with postural instability appearing in some patients as the disease progresses.1 PD affects more than 1% of the population older than age 55 years, and nearly 3% of the population older than age 70 years.2 In Africa, there is paucity of data on prevalence of PD; however, reports suggest a prevalence of 7 out of 100,000 in Ethiopia and 67 out of 100,000 in Nigeria.3
PD is pathologically characterized by the loss of dopamine-producing neurons in the basal ganglia of the brain. The low dopamine levels in the brain leads to fundamental motor symptoms such as bradykinesia, distal tremor, and muscle rigidity. Comorbidities known to occur with PD include sleep disturbances, depression, dementia, falls, and fractures. PD has no cure, and this may be due to the idiopathic nature of the disease. As such, current therapies neither slowdown nor stop the progression of the disease.4 Treatment is therefore aimed at relieving symptoms and improving the quality of life of patients. Of all drugs available for the management of PD, 1 found to be clinically efficacious is the biological precursor of dopamine, levodopa.5 Although levodopa is regarded as the gold standard, adverse effects that come with its prolonged use are potentially disabling.6 Some of these adverse effects include motor fluctuations and levodopa-induced dyskinesia.7
Furthermore, a challenge with the use of levodopa is its peripheral (gut wall, liver, and kidney) decarboxylation, by dopa decarboxylase, to dopamine.8 For this reason, levodopa is coadministered with a peripheral dopa decarboxylase inhibitor, carbidopa. This approach increases levels of levodopa reaching the brain and also reduces adverse effects such as nausea, vomiting, cardiac arrhythmias, and hypotension. Although levodopa/carbidopa combination is effective, there may be dyskinesia and severe on-off motor fluctuations.9 These potentially disabling motor complications are associated with variable drug absorption and fluctuating plasma concentrations of levodopa due to its short half-life.10 Sometimes, management of PD may include the use of catechol-O-methyl transferase inhibitors (eg, entacapone).11 Combination of levodopa/carbidopa with catechol-O-methyl transferase inhibitors is known to improve levodopa bioavailability.12
Recent studies have formulated agents that try to maintain a near-constant levodopa plasma concentration to maximize therapeutic effect. Some of these new approaches include controlled release formulations and improved drug delivery systems of levodopa.13,14 Polymer-based delivery systems have been shown to improve the pharmacokinetics of drugs, decrease side effects, and increase efficacy.15 One unique polymer for oral drug delivery is chitosan, which is pH sensitive, and has remarkable physicochemical (easily modified chemically and reactive side groups) and biological (biocompatible, nontoxic, and biodegradable) properties that makes it a promising candidate for drug delivery in the gastrointestinal tract.15 Furthermore, microparticles are known to have effective drug entrapment and controlled release profiles. Microparticles are useful drug delivery systems that are successful in encapsulating both water insoluble and sparingly soluble agents to improve their therapeutic efficacy.16 The current study, therefore, sought to formulate chitosan-coated hydroxypropylmethyl cellulose (HPMC) microparticles of levodopa (and carbidopa) and evaluate in vitro and in vivo kinetic characteristics.
Materials and Methods
Materials
Levodopa and carbidopa powders were purchased from Sigma-Aldrich (St Louis, Missouri). Glutaraldehyde was purchased from VWR International (Radnor, Pennsylvania). HPMC was a gift from Ernest Chemist Limited, Ghana. All other reagents used for experiments were of analytical grade and purchased from approved suppliers.
Preparation of levodopa microparticles
Preparation of levodopa microparticles was done according to the spray drying technique described by Nettey et al,17 with minor modifications. To prepare microparticles with 20% drug loading, 1 part levodopa was added to 4 parts polymer. The polymer (HPMC) solution was prepared by weighing 10 g HPMC powder into 200 mL deionized water. Levodopa solution was prepared separately, by weighing 2.5 g levodopa powder into 100 mL deionized water, followed by the dropwise addition of 10 M hydrochloric acid until the drug was completely dissolved. The levodopa solution was slowly added to HPMC solution with stirring. This was followed by the addition of deionized water to make a final volume of 400 mL. The homogenous mixture obtained was stirred on a magnetic stir plate for 1 hour, after which 2 mL glutaraldehyde (50%) solution was added to cross-link HPMC molecules. To quench the cross-linking, 2 mL of a 1% sodium bisulfite solution was added to the mixture and stirring continued for 1 hour. After that, 50 mL of a 0.15% chitosan solution was then added to the solution containing crosslinked HPMC with levodopa. This was to allow for the adsorption of the chitosan unto the polymer surface. The solution was left to stir on the magnetic stir plate for 1 hour. The resulting solution was fed into the Bilon-6000Y laboratory spray-dryer (Shanghai Bilon Instrument Co Ltd, Shanghai, China) to obtain levodopa microparticles. Samples were protected from light in the entire process.
Carbidopa microparticles were also prepared in a similar way as described.
Size and zeta potential determination of levodopa microparticles
Size and zeta potential was determined as described by Gomes et al,18 with slight modification. One milligram of levodopa microparticles was weighed and suspended in 20 mL deionized water. The suspension was further diluted with deionized water and transferred into a cuvette. To determine the average size and zeta potential of the microparticles, the cuvette was placed into the cell holder of the Malvern Nano Zetasizer (Malvern Instruments Inc, Westborough, Massachusetts). The intensity fluctuations in the scattered light was analyzed and used to calculate the size of microparticles by an inbuilt digital correlator.
Content analysis of levodopa microparticles
The actual drug content of microparticles was determined using a reverse-phase HPLC method described by Bhatnagar et al,19 with minor modifications. A mass of 25 mg levodopa microparticles (weighed in triplicate) was crushed in a mortar, and suspended in 10 mL deionized water. The pH was adjusted to 2.6 with hydrochloric acid to allow for the dissolution of levodopa. The suspension was transferred into Eppendorf tubes, and centrifuged at 3000 rpm for 10 minutes at 25°C. A portion of the supernatant was diluted to a final concentration of 100 µg/mL from which further serial dilutions were made to obtain the concentrations 50, 25, and 12.5 µg/mL. A tenfold dilution was further made from the 50 µg/mL and 12.5 µg/mL to obtain 5 µg/mL and 1.25 µg/mL solutions respectively. The above solutions (100, 50, 25, 12.5, 5, and 1.25 µg/mL) were pipetted into new Eppendorf tubes and 20 µL each was injected into an HPLC system (Agilent Technologies System, Hamburg, Germany). The HPLC had the following chromatographic conditions: mobile phase composed of 0.01 M phosphate buffer (adjusted to a pH of 2.68 with hydrochloric acid) and methanol (20:80 v/v). An injection volume of 20 µL, a flow rate of 1.0 mL/min for 10 minutes and a wavelength of 280 nm were used. A Vertex Plus C18 (Chromline Equipment Company (Mumbai, Maharashtra)), 150 × 4 mm column was used for all analyses. The HPLC method showed good linearity with coefficient of determination (R2) of 0.996 for the calibration curve of levodopa. Mobile phase run periodically contained no detectable amount of drug. Coefficient of variation was <15% for replicates and also for intra- and interday precision. The sample recovery in the formulation was high, showing values of 92% (0.58%), 95% (0.94%), and 98% (1.35%), respectively, for samples spiked at 1, 5, and 10 ppm of drug.
Encapsulation efficiency of levodopa microparticles
Encapsulation efficiency of levodopa microparticles was done to determine how effective the HPMC polymer was in entrapping the drug. After the determination of the actual amount of drug in 25 mg microparticles, the theoretical amount of drug supposed to be in 25 mg microparticles was also calculated. The encapsulation efficiency was determined using the formula:
In vitro drug release of levodopa microparticles
Samples of levodopa microparticles (75 mg) were weighed and transferred into empty size 0 gelatin capsule shells. The filled capsules were placed in the baskets of USP Dissolution Apparatus 1 (Distek Inc, North Brunswick, New Jersey), and each of the vessel flasks was filled with 500 mL phosphate buffered saline (PBS) of pH 6.8. After the temperature reached 37°C, the baskets were lowered into the vessels and allowed to rotate at 100 rpm. After, 5 mL buffer (receiver fluid) was drawn from the vessel flask at the following time points: 0, 0.25, 0.5, 1, 2, 4, 8, 12, and 24 hours, for analysis and replaced with an equal amount of fresh PBS (temperature of 37°C). The amount of drug released was determined by HPLC, as described by Bhatnagar et al19 and reviewed above. The release experiment was performed in triplicate.
Formulations parameters for carbidopa microparticles
For levodopa microparticles, the size (with polydispersity index) was estimated using the same methods as described earlier for levodopa microparticles.
In vivo pharmacokinetic evaluation of levodopa/carbidopa microparticles
Animal acquisition and housing
Male Hsd:Sprague-Dawley rats weighing 150 to 200 g and aged 6 to 8 weeks, were obtained from the Department of Animal Experimentation, School of Biomedical and Allied Health Sciences, University of Ghana, Korle-Bu. The animals were housed in stainless steel cages. Each rat occupied a minimum space of 2 ft3 (61 cm × 31 cm) with soft wood shavings as bedding. Rats were fed with normal pellet diet (Agrimat, Kumasi, Ghana), given water ad libitum, and maintained under optimal laboratory conditions (temperature 25°C [±1°C], relative humidity 60% to 70%, and 12-hour light–dark cycle). All feeding and water troughs were cleaned regularly to prevent contamination. Animals were housed in an inspected and approved facility. The animals were acclimatized to this environment for 2 weeks before experimentation. By simple random sampling, the Sprague-Dawley rats were put into 3 groups consisting of 5 animals each.
Drug administration and blood sample collection
Animals were fasted overnight before administration of treatments. Animals in Group 1 received the positive control drug (Sinemet CR (Merck Pharmaceutical, Kenilworth, New Jersey) [levodopa/carbidopa controlled release]), Group 2 received levodopa/carbidopa powder (combined in a ratio of 4:1), and rats in Group 3 were given microparticles of levodopa/carbidopa (combined in a ratio of 4:1). The dose of the respective formulations administered was 20/5 mg/kg. That is, 20 mg (levodopa) combined with 5 mg (carbidopa) per kilogram body weight of the animals. Treatments were administered via the oral route every 12 hours. Both powder and microparticles were suspended in water before administration (via an oral gavage). After administration of the third dose, rat tails were snipped and blood samples collected into EDTA tubes at time intervals 0.25, 0.5, 1, 2, 4, and 12 hours. A solution of 25% sodium metabisulfite in water was made and added to rat blood samples collected in a 1:10 v/v ratio of 1 part of 25% sodium metabisulfite solution in water to 10 parts of rat blood sample collected. This was to minimize oxidation of levodopa in blood. Blood samples were immediately centrifuged at 10,000 rpm for 5 minutes at 25°C to separate plasma.
Determination of plasma levodopa levels
Levodopa in plasma was analysed using HPLC, as described by Elbarbry et al,20 with minor modifications. Stock solution of levodopa (4.6 mg/mL) was freshly prepared by dissolving the analytical standard in 0.5 M perchloric acid. Working solutions with concentrations at 1840, 920, 460, and 46 µg/mL were obtained by serial dilution of the stock solution with methanol containing 0.05% v/v perchloric acid.
A stock of the internal standard solution was prepared by dissolving 20 mg methyldopa in 15 mL deionized water to obtain a concentration of 1.3 mg/mL. Working internal standard solution was prepared by making 1:100 dilution of the stock (1 µL methyldopa standard in 99 µL methanol containing 0.05% perchloric acid).
All stock and working standards were stored at –20°C until analysis using HPLC. Briefly, levodopa in plasma was extracted using protein precipitation method with perchloric acid as the precipitating agent. To 50 µL rat plasma, 100 µL of the working internal standard solution and 25 µL of 0.5 M perchloric acid were added. The mixture was vortexed for 2 minutes and centrifugation was done at 10,000 rpm at 4°C. Supernatant was then transferred into an auto-sampler vial. A Cecil Adept HPLC system (Cecil Instrumentation Services Ltd, Cambridge, United Kingdom) consisting of an auto-sampler, a binary pump, and a model diode-array detector was used. The chromatographic analysis was performed using the Zorbax (Sigma-Aldrich (St Louis, Missouri)) C18 column (4.6 mm × 300 mm) with a particle size of 5 µm and column temperature of 40°C. The mobile phase consisted of 20 mM phosphate buffer (pH 2.5) and methanol HPLC grade. This was run at a flow rate of 1 mL/min for 10 minutes. An injection of 20 µL of the supernatant was made directly into the analytical column for immediate HPLC analysis. Absorbance of levodopa was detected at 280 nm. Quantitation of the unknown (levodopa in plasma) was by interpolation from the weighted linear regression line of the ratios of the peak areas of the analytical and internal standard. Lower limit of detection of levodopa was 10 ng/mL. The coefficient of variation over the entire calibration range during assay process was <4 %.
Ethical Issues
The study protocol was approved by the Ethics and Protocol Review Committee of the College of Health Sciences, University of Ghana, with a protocol identification number: CHS-Et/M.8 - 5.14/2018-2019.
Data analysis
Data were expressed as mean (SD). Statistical test of significance was taken as P < 0.05, and performed on all continuous data using unpaired t test for comparison of 2 independent sample means, and 1-way ANOVA for comparison of more than 2 independent sample means. Pharmacokinetic parameters for levodopa in the 3 groups were determined by noncompartmental analysis. Cmax and Tmax of levodopa were extrapolated from concentration-time curves. The ke was determined by linear regression analysis of the terminal-linear part of the log plasma concentration-time curves. AUC and area under the first moment curve were calculated by the linear trapezoidal rule. Mean residence time (MRT) was calculated as area under the first moment curve/AUC. Pharmacokinetic analysis was conducted using GraphPad Prism 7.0 software (San Diego, California).
Results
Characteristics of chitosan-coated HPMC microparticles of levodopa and carbidopa
Levodopa microparticles were characterized for their size, polydispersity index (PDI), zeta potential, drug loading, and encapsulation efficiency. These results are summarized in Table 1. Carbidopa microparticles had an average size of 0.67 µm with a PDI of 0.29.
Table 1.
Characteristics of chitosan-coated hydroxypropylmethyl cellulose microparticles of levodopa
| Characteristic | Value |
|---|---|
| Average size (µm) | 0.5 |
| Polydispersity index | 0.41 |
| Zeta potential (mV) | 10.8 |
| Drug loading (%) | 19.1 |
| Encapsulation efficiency (%) | 95.7 |
In vitro release of levodopa from microparticles
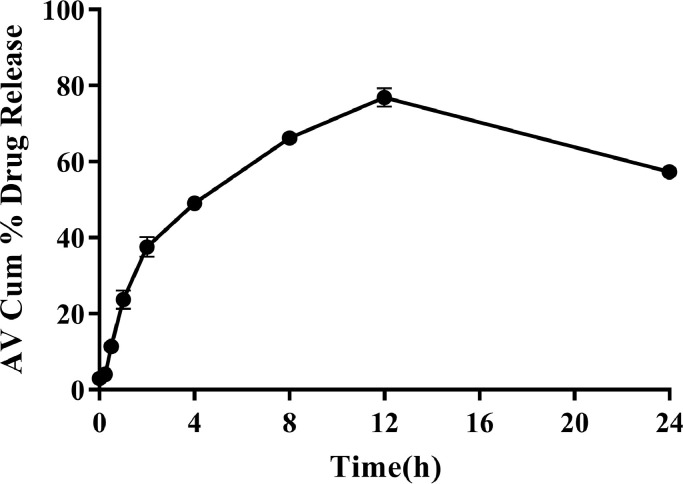
Release of levodopa from the HPMC-chitosan matrix was studied over a 24-hour period. The release of the drug increased steadily over time with maximum release of about 80% occurring at 12 hours. The in vitro release of levodopa is as shown in Fig. 1.
Fig. 1.
Mean cumulative (%) release of levodopa from microparticles (n = 3) in phosphate buffered saline (pH = 6.8) at 37°C. Error bars indicate SD.
In vivo pharmacokinetic evaluation of levodopa/carbidopa microparticles in Sprague-Dawley rats
Concentration-time curve
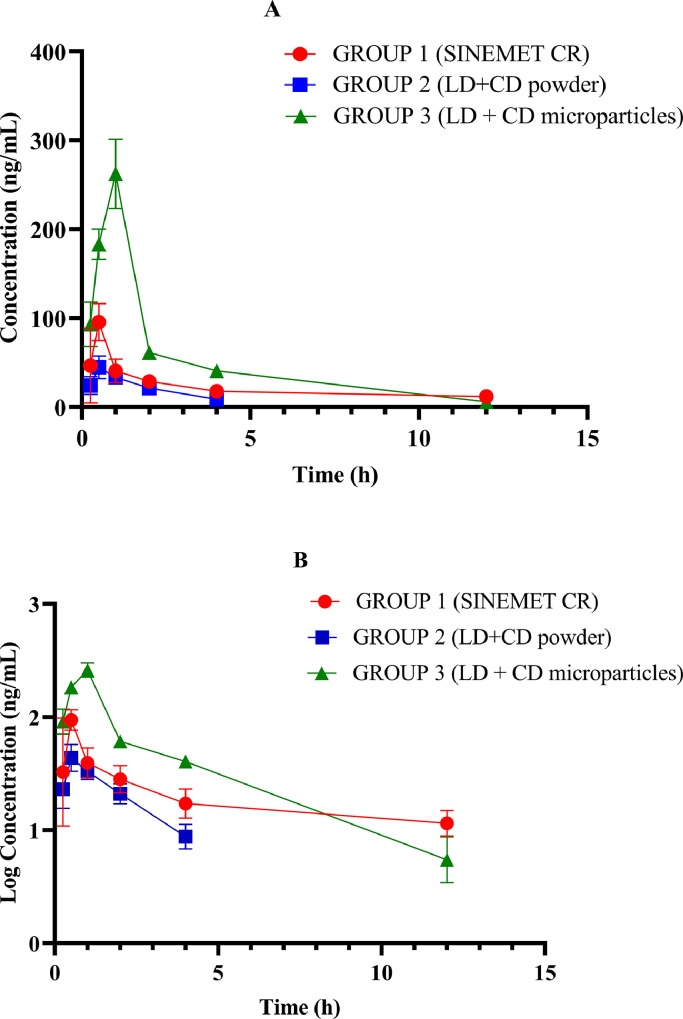
The concentration-time curves of levodopa in plasma for Sprague-Dawley rats in the 3 groups; Group 1 (positive control: Sinemet CR), Group 2 (levodopa/carbidopa powder) and Group 3 (levodopa/carbidopa microparticles) are shown in Fig. 2. Cmax was highest in the group administered levodopa/carbidopa microparticles.
Fig. 2.
Plasma concentration-time (A) and semi-log concentration-time (B) curves of levodopa for the 3 Sprague Dawley rat treatment groups (n = 5) following respective formulations administered at 20/5 mg/kg. Group 1 = Sinemet CR (a controlled release formulation of levodopa/carbidopa). Group 2 = levodopa/carbidopa powder (combined in a ratio of 4:1). Group 3 = levodopa/carbidopa (combined in a ratio of 4:1). Error bars indicate SD. LD + CD = levodopa plus carbidopa.
Pharmacokinetic parameters of levodopa in treatment groups
Comparison with 1-way ANOVA showed that Tmax, Cmax, AUC0–∞, ke, t½, and MRT of levodopa differed significantly (P < 0.05) among the 3 treatment groups, as shown in Table 2.
Table 2.
Pharmacokinetic (PK) parameters of levodopa in the 3 treatment groups (n = 5).
| PK parameter | Treatment group* |
P value | ||
|---|---|---|---|---|
| Sinemet CR† | LD + CD powder | LD + CD MP | ||
| Tmax (h) | 0.51 (0.12) | 0.70 (0.17) | 1.00 (0.35)‡ | 0.027 |
| Cmax (ng/mL) | 95.52 (20.87) | 44.70 (12.81) | 262.43 (38.86)|| | 0.0001 |
| AUC0–∞ (ng.h/mL) | 354.69 (98.09) | 105.82 (7.85)§ | 612.70 (17.42)§ | 0.0001 |
| ke (1/h) | 0.11 (0.05) | 0.44 (0.13)§ | 0.35 (0. 06)‡ | 0.0079 |
| t½ (h) | 6.14 (2.58) | 1.57 (0. 43)‡ | 2.00 (0. 31)‡ | 0.0095 |
| AUMC (ng.h2/mL) | 2018.10 (127.35) | 324.53 (45.00)|| | 1763.47 (59.39)‡ | 0.0001 |
| MRT (h) | 5.70 (0.26) | 3.07 (0.01)‡ | 2.88 (0.03)‡ | 0.032 |
AUMC = area under the first moment curve; LD + CD = levodopa plus carbidopa; MP = microparticles; MRT = mean residence time.Cmax = maximum observed plasma concentration; Tmax = time to Cmax; Ke = elimination rate constant; t1/2 = elimination half-life; AUC0–∞ = area under the concentration–time curve from time zero extrapolated to infinity.
Values are presented as mean (SD). Post hoc analysis are comparisons made with Sinemet CR (Merck Pharmaceutical, Kenilworth, New Jersey) formulation
Manufacturer name and location.
P < 0.05 for comparison with Sinemet CR formulation.
P < 0.01 for comparison with Sinemet CR formulation.
P < 0.001 for comparison with Sinemet CR formulation.
Post hoc analysis; where Sprague-Dawley rats administered Sinemet CR is compared with rats administered levodopa/carbidopa microparticles and levodopa/carbidopa powder, is also shown in Table 2. Comparison showed that Tmax differed significantly (P = 0.024) between Sprague-Dawley rats administered Sinemet CR and those administered levodopa/carbidopa microparticles. However, a comparison of Tmax between the Sinemet CR group and levodopa/carbidopa powder group showed no statistically significant difference (P > 0.05).
Cmax of levodopa concentration after administration did not differ significantly (P > 0.05) between the Sinemet CR group and levodopa/carbidopa powder group. However, Cmax was found to differ significantly (P < 0.001) between Sinemet CR group and the levodopa/carbidopa microparticles group.
A comparison of total drug exposure (AUC) between group administered Sinemet CR and levodopa/carbidopa powder, and between Sinemet CR group and levodopa/carbidopa microparticles group all differed significantly (P < 0.01).
Levodopa t½ in the group administered Sinemet CR was longer compared with groups administered levodopa/carbidopa microparticles and levodopa/carbidopa powder. This difference in t½ was statistically significant (P < 0.05).
Additionally, MRT of levodopa in the group administered Sinemet CR was longer than the other formulations (P < 0.05). A comparison of pharmacokinetic parameters of levodopa in current study and previously reported studies is also shown in Table 3.
Table 3.
Comparison of pharmacokinetic (PK) parameters of levodopa in current and previous studies.
| Parameter | Current study | Baek et al21 | Hsu et al22 |
|---|---|---|---|
| Formulation | Chitosan-coated HPMC LD-CD microparticles | Floatable spray- coated LD-CD-ENT microparticles | Extended release capsules of LD-CD |
| Drug ratio | LD:CD (20:5 mg) | LD:CD:ENT (30:7.5:60 mg) | LD:CD (390:97.5 mg) |
| In vivo model | Sprague-Dawley rats | C57BL6 mice | Healthy humans |
| Levodopa dose (mg) | 20 | 30 | 390 |
| HED (mg/m2) | 120 | 90 | 240.5 |
| PK parameters | |||
| Tmax (h) | 1 | 4 | 4.5 |
| Cmax (ng/mL) | 262.4 | 5038.20 | 1326 |
| AUC0–∞ (ng.h/mL) | 612.7 | 70492.05 | 7244 |
| t1/2 (h) | 2.0 | 5.4 | 1.9 |
| Normalized PK parameters | |||
| Tmax (h) | 0.01 | 0.04 | 0.02 |
| Cmax (ng/mL) | 2.19 | 55.98 | 5.51 |
| AUC0–∞ (ng.h/mL) | 5.11 | 783.25 | 30.12 |
| t1/2 (h) | 0.02 | 0.06 | 0.01 |
CD = carbidoba; ENT = entacapone; HED = human equivalent dose; HPMC = hydroxypropylmethyl cellulose; LD = levodopa.
Discussion
It is established that physical properties of active ingredients and excipients such as particle size can influence processes like dissolution and subsequently absorption of a drug. For these reasons, it is important to measure and control drug particle size during drug design or formulation. The size of microparticles, for instance, can influence biodistribution and pharmacokinetic properties of a drug. In the current study, the average size of the prepared levodopa microparticles was 0.5 µm with a PDI of 0.41. The spray drying method has traditionally been used to produce particles with a narrow size, usually ranging from 0.1 to 10 µm. It is known that the nozzle used as well as some parameters such as pump rate and compressed spray air flows can affect size of a resultant microparticle.23 Small nozzle diameter and increased air flow rate produces microparticles with small sizes. A small-sized microparticle confers an extra advantage of a large surface area to volume ratio. Thus, for a given rate of drug diffusion through the microparticle, the rate of flux of drug per mass of formulation will increase with decreasing particle size. In addition to a large surface area to volume ratio, water penetration into small particles may be quicker (due to shorter distance from the surface to the center of the particle), hence ensuring efficient drug release.24
Zeta potential of the formulated microparticles was positive with a magnitude of 10.8. The stability of a suspension is usually enhanced as the zeta potential increases in magnitude, but as it approaches zero the stability is reduced. Thus, the formulated microparticles will be stable when reconstituted into a suspension before administration.
The percent drug loading of levodopa microparticles was measured to determine the actual mass of active ingredient. With a drug load of 19.1%, the weighed 20 mg of microparticles contained an actual drug content of 4.788 mg out of the expected 5 mg, giving an encapsulation efficiency of 95.7%. The relatively high encapsulation efficiency obtained gives an indication of how effective the HPMC polymer entrapped levodopa. These findings are consistent with work done by Choudhary et al,25 in which preparative variables for floating microspheres of levodopa/carbidopa were evaluated and optimized as a gastro-retentive drug delivery system. Choudhary et al25 showed that high drug entrapment efficiency of prepared floating microspheres of levodopa (resulting from high polymer ratio) exhibited a prolonged drug release of about 10 hours and buoyancy of more than 12 hours.19 Arica et al26 also prepared and evaluated injectable biodegradable carbidopa/levodopa microparticles. Likewise, optimization of formulation variables was done and increasing entrapment efficiency further increased drug yield and extended duration of drug release.
In the current study, in vitro release of levodopa from microparticles was controlled, showing a steady increase for first 12 hours, followed by a dip in the curve. The increase in drug release was sustained: with about 80% release of levodopa occurring after 12 hours. The high entrapment effect reduced the adsorption of levodopa to the surface of microparticles, and thus minimized a burst release and ensured a more controlled drug release from the microparticles. However, the dip in the curve after 12 hours could be as a result of degradation of levodopa after its release into the dissolution medium.27 The controlled/delayed release of drug from microparticles occurs naturally due to increased distance from surface with time and also decreased drug concentration within the particle. In actual fact, drug degradation was occurring the whole time, but drug release exceeded the rate of degradation in the first 12 hours. For the comparison drug, Sinemet CR, previous studies have reported a release of about 90% of the active ingredient in about 3 hours.28
Levodopa in solution can undergo autoxidation, a reaction accelerated by high pH.29 A study by Di Stefano et al30 supports the claim that levodopa is unstable and undergoes chemical hydrolysis at high pH. The pH of the PBS was used as the dissolution medium in the present study was 6.8. This could have increased the degradation of levodopa in the dissolution medium. Additionally, the degradation of the drug due to chemical hydrolysis in the dissolution medium over time could account for the dip in the overall concentration observed after the 12 hour time point, hence the dip in the curve as shown in the in vitro kinetic study.
With the in vivo pharmacokinetic characteristics of the 3 formulations (levodopa/carbidopa powder, levodopa/carbidopa microparticles, and Sinemet CR), it was evident that the highest plasma levodopa level (Cmax) was achieved with levodopa/carbidopa microparticles. These results are similar to other studies that have compared microparticles with traditional dosage forms.17,21 The small size of microparticles may enhance the extent of absorption from the site of administration into circulation due to the large surface area to volume ratio. Additionally, coating microparticles with chitosan aids adherence of the formulation to intestinal mucosal surface, thereby enhancing contact time and allowing for gradual release of the drug from the polymer for absorption. The aforementioned properties of microparticles may ultimately lead to a high Cmax.31 Cmax of a drug is often related to pharmacological response. Cmax should ideally be above minimum effective concentration but less than what would lead to adverse drug reactions.32
Levodopa Tmax in plasma was also evaluated in the current study. A comparison of Tmax between rats administered Sinemet CR and levodopa/carbidopa powder showed no significant difference. However, there was a significant difference between the Tmax of rats administered levodopa/carbidopa microparticles and Sinemet CR. Cmax for Sinemet CR was reached at 0.5 hours, whereas for levodopa/carbidopa microparticles time to reach Cmax was 1 hour. At time 0.5 hours on the concentration-time curve, the plasma levels of levodopa/carbidopa microparticles was higher than that of both Sinemet CR and levodopa/carbidopa powder. It could be inferred that this initial high plasma level of levodopa with the levodopa/carbidopa microparticles may be advantageous in achieving rapid onset of drug action.
Total drug exposure (AUC0–∞) was found to be highest with the levodopa/carbidopa microparticles. The microparticles had an AUC about 2-fold greater than Sinemet CR. This high AUC of the microparticles could be attributed to the small particle size and the mucoadhesive property conferred by the chitosan. This may have enhanced levodopa absorption across the intestinal lumen. There is often a direct relationship between AUC and bioavailability.33 Thus, it can be inferred from the results that levodopa/carbidopa microparticles may have had greatest bioavailability than Sinemet CR and levodopa/carbidopa powder. Additionally, Bredberg et al34 quoted levodopa AUC after intravenous administration as 42 µg/mL/min/dose. Based on this AUC intravenous administration value, the calculated absolute bioavailability for chitosan-coated HPMC levodopa (carbidopa) microparticles after unit reconciliation was 44%.
The t½ of levodopa for the various formulations were also estimated. The half-life for Sinemet CR (6.1 hours) was longer than that of levodopa/carbidopa powder (1.6 hours), and this difference was found to be statistically significant. This difference could be attributed to the fact that Sinemet CR has been designed to release levodopa over a long period of time (controlled release), whereas levodopa/carbidopa powder does not have any such modification. A comparison of the half-life of levodopa for rats administered Sinemet CR and levodopa/carbidopa microparticles showed that the half-life of Sinemet CR (6.1 hours) was longer than levodopa/carbidopa microparticles (2.0 hours). Sinemet CR, a drug already on the market, most likely has undergone many formulation modifications in order to prolong its release. It is noteworthy that although levodopa/carbidopa microparticles had a larger AUC than Sinemet CR, its decline at the terminal part of the concentration-time curve was sharper. Data from this study suggests that levodopa was eliminated faster in Sprague-Dawley rats that received levodopa/carbidopa microparticles than Sinemet CR, and as reflected in the ke of the 2 formulations: 0.35 h−1 versus 0.11 1/h.
Conclusions
Compared with a prolonged/extended release product already on the market; that is, the Sinemet CR that was used in this study, levodopa/carbidopa microparticles coated with chitosan aids adherence of this formulation to intestinal mucosal surface, thereby enhancing contact time and ultimately leading to high Cmax of levodopa.31 Despite the aforementioned improved pharmacokinetic characteristics of the chitosan-coated HPMC microparticles of levodopa (and carbidopa), we suggest that a more improved controlled release profile can be achieved by use of high molecular weight HPMC and combinations of 2 to 3 different polymers. Future studies should be conducted to ascertain the biodistribution of this formulation; that is, the microparticles of levodopa/carbidopa, in the brain.
Acknowledgments
CRediT authorship contribution statement
S. K. Amponsah, H. Nettey, B. O. Dankyi and G. L. Allotey-Babington designed the study. Laboratory work was done by B. O. Dankyi and N. A. Goode. Data analyses were performed by B. O. Dankyi, N. A. Goode and I. Adams, under the supervision of S. K. Amponsah, H. Nettey and G. L. Allotey-Babington.
Acknowledgments
The authors thank William Appau, Kwame Nkrumah University of Science and Technology, Ghana, for his expertise in the HPLC analysis and to Nana Asare, Department of Pharmacology and Toxicology, School of Pharmacy, University of Ghana, for his assistance in the pharmacokinetic sampling.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
- 1.Kouli A, Torsney MK, Kuan W. Codon Publications; UK: 2018. Parkinson's disease: Etiology, Neuropathology, and Pathogenesis; pp. 3–18. Chapter 1Pages. [PubMed] [Google Scholar]
- 2.Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 3.Williams U, Bandmann O, Walker R. Parkinson's Disease in Sub-Saharan Africa: A Review of Epidemiology, Genetics and Access to Care. J Mov Disord. 2018;11(2):53–64. doi: 10.14802/jmd.17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson's disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 5.LeWitt PA. Levodopa for the treatment of Parkinson's disease. N Engl J Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 6.Gunay MS, Ozer AY, Chalon S. Drug Delivery Systems for Imaging and Therapy of Parkinson's disease. Curr Neuropharmacol. 2016;14(4):376–391. doi: 10.2174/1570159X14666151230124904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guridi J, González-Redondo R, Obeso JA. Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson's disease. Parkinsons Dis. 2012;2012 doi: 10.1155/2012/943159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waller DG, Sampson AP. Fifth Edition. Elsevier Publishers; UK: 2018. Medical Pharmacology and Therapeutics; pp. 325–336. Chapter 24. Pages. [Google Scholar]
- 9.Salat D, Tolosa E. Levodopa in the treatment of Parkinson's disease: current status and new developments. J. Parkinsons Dis. 2013;3(3):255–269. doi: 10.3233/JPD-130186. [DOI] [PubMed] [Google Scholar]
- 10.Senek M, Sten-Magnus A, Askmark H, Bergquist F, Radu C, Anders E, Lycke S, Medvedev A, Memedi M, Ojisson F, Spira J, Westin J, Nyholm D. Levodopa/carbidopa microtablets in Parkinson's disease: a study of pharmacokinetics and blinded motor assessment. Eur J Clin Pharmacol. 2017;73:563–571. doi: 10.1007/s00228-017-2196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korczyn DA. Drug treatment of Parkinson's Disease. Dialogues Clin Neurosci. 2004;6(3):315–322. doi: 10.31887/DCNS.2004.6.3/akorczyn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer RF. A promising new technology for Parkinson's disease. Neurol. 2005;65:S6–10. doi: 10.1212/wnl.65.2_suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 13.Ngwuluka N, Pilay V, DuToit LC, Ndesendo V, Choonara Y, Modi G, Naidoo D. Levodopa Delivery Systems: advancement in delivery of the gold standard. Expert Opin. Drug Deliv. 2010;7(2):203–224. doi: 10.1517/17425240903483166. [DOI] [PubMed] [Google Scholar]
- 14.LeWitt PA, Hauser RA, Grosset DG, Stocchi F, Saint-Hilaire MH, Ellenbogen A, Leinonen M, Hampson NB, DeFeo-Fraulini T, Freed MI, Kieburtz KD. A randomized trial of inhaled levodopa (CVT-301) for motor fluctuations in Parkinson's disease. Mov Disord. 2016;31(9):1356–1365. doi: 10.1002/mds.26611. [DOI] [PubMed] [Google Scholar]
- 15.Saikia C, Gogoi P, Maji TK. Chitosan: A promising biopolymer in drug delivery applications. J Mol Genet Med. 2015;S4:006. [Google Scholar]
- 16.Bale S, Khurana A, Reddy AS, Singh M, Godugu C. Overview on therapeutic applications of microparticlulate drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2016;33(4):309–361. doi: 10.1615/CritRevTherDrugCarrierSyst.2016015798. [DOI] [PubMed] [Google Scholar]
- 17.Nettey H, Allotey-Babington GL, Somuah I, Banga BN, Afrane B, Amponsah SK, Annor H, Darko H, Hanson K, Aidoo A, Broni MN, Sasu C, Nyarko A. Assessment of formulated amodiaquine microparticles in Leishmania donovani infected rats. J Microencapsul. 2017;34(1):21–28. doi: 10.1080/02652048.2017.1280094. [DOI] [PubMed] [Google Scholar]
- 18.Gomes AJ, Faustino AS, Machado AE, Zaniquelli ME, de Paula Rigoletto T, Lunardi CN, Lunardi LO. Characterization of PLGA microparticles as a drug carrier for 3-ethoxycarbonyl-2h-benzofuro[3,2-f]-1-benzopyran-2-one. Ultrastructural study of cellular uptake and intracellular distribution. Drug Deliv. 2006;13(6):447–454. doi: 10.1080/10717540600640369. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar P, Vyas D, Sinha SK, Chakrabarti T. Stability indicating HPLC method for simultaneous estimation of entacapone, levodopa and carbidopa in pharmaceutical formulation. J Chromatogr Sep Tech. 2015;6(304):2. [Google Scholar]
- 20.Elbarbry F, Nguyen V, Mirka A, Zwickey H, Rosenbaum R. A new validated HPLC method for the determination of levodopa: Application to study the impact of ketogenic diet on the pharmacokinetics of levodopa in Parkinson's participants. Biomed Chromatogr. 2019;33(1):e4382. doi: 10.1002/bmc.4382. [DOI] [PubMed] [Google Scholar]
- 21.Baek JS, Tee JK, Pang YY, Tan EY, Lim KL, Ho HK, Loo SCJ. Improved Bioavailability of Levodopa Using Floatable Spray-Coated Microcapsules for the Management of Parkinson's Disease. Neuromolecular Med. 2018;20(2):262–270. doi: 10.1007/s12017-018-8491-0. [DOI] [PubMed] [Google Scholar]
- 22.Hsu A, Yao HM, Gupta S, Modi NB. Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet(®)), sustained-release carbidopa-levodopa (Sinemet(®) CR), and carbidopa-levodopa-entacapone (Stalevo(®)) J Clin Pharmacol. 2015;55(9):995–1003. doi: 10.1002/jcph.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsarov PD, Pilicheva BA, Manev HM, Lukova PK, Kassarova MI. Optimization of Chitosan Microspheres Spray Drying via 32 Full Factorial Design. Folia Med (Plovdiv) 2017;59(3):310–317. doi: 10.1515/folmed-2017-0037. [DOI] [PubMed] [Google Scholar]
- 24.Singh MN, Hemant KS, Ram M, Shivakumar HG. Microencapsulation: A promising technique for controlled drug delivery. Res Pharm Sci. 2010;5(2):65–77. [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhary H, Agrawal AK, Malviya R, Yadav SK, Jaliwala YA, Patil UK. Evaluation and Optimization of preparative variables for controlled-release floating microspheres of levodopa/carbidopa. Pharmazie. 2010;65:194–198. [PubMed] [Google Scholar]
- 26.Arica B, Kaş HS, Moghdam A, Akalan N, Hincal AA. Carbidopa/levodopa-loaded biodegradable microspheres: in vivo evaluation on experimental Parkinsonism in rats. J Control Release. 2005;102(3):689–697. doi: 10.1016/j.jconrel.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Bozdag SS, Calis S, Kas HS, Ercan MT, Peksoy I, Kincal AA. In vitro evaluation and intraarticular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul. 2001;18(4):443–456. doi: 10.1080/02652040010018641. [DOI] [PubMed] [Google Scholar]
- 28.Han CH, Hsu L, Hsu AF. U.S. Patent No. 7,094,427. 2006. Washington, DC: U.S. Patent and Trademark Office.
- 29.Umek N, Gersak B, Vintar N, Sostaric M, Mavri J. Dopamine autoxidation is controlled by acidic pH. Front Mol Neurosci. 2018;11:467. doi: 10.3389/fnmol.2018.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Stefano A, Sozio P, Cocco A, Iannitelli A, Santucci E, Costa M, Pecci L, Nasuti C, Cantalamessa F, Pinnen F. L-dopa- and dopamine-(R)-alpha-lipoic acid conjugates as multifunctional codrugs with antioxidant properties. J Med Chem. 2006;49(4):1486–1493. doi: 10.1021/jm051145p. [DOI] [PubMed] [Google Scholar]
- 31.Raval JA, Patel JK, Patel MM. Formulation and in vitro characterization of spray-dried microspheres of amoxicillin. Acta Pharm. 2010;60:455–465. doi: 10.2478/v10007-010-0034-7. [DOI] [PubMed] [Google Scholar]
- 32.Rizk ML, Zou L, Savic RM, Dooley KE. Importance of drug pharmacokinetics at the site of action. Clin Transl Sci. 2017;10(3):133–142. doi: 10.1111/cts.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson AJ, Kushner W. Clinical Pharmacokinetics. Ann Rev Pharmacol Toxicol. 1979;19:105–127. doi: 10.1146/annurev.pa.19.040179.000541. [DOI] [PubMed] [Google Scholar]
- 34.Bredberg E, Lennernäs H, Paalzow L. Pharmacokinetics of levodopa and carbidopa in rats following different routes of administration. Pharm Res. 1994;11(4):549–555. doi: 10.1023/a:1018970617104. [DOI] [PubMed] [Google Scholar]