Abstract
Epithelial cells in the liver (known as hepatocytes) are high-performance engines of myriad metabolic functions and versatile responders to liver injury. As hepatocytes metabolize amino acids, alcohol, drugs, and other substrates, they produce and are exposed to a milieu of toxins and harmful byproducts that can damage themselves. In the healthy liver, hepatocytes generally divide slowly. However, after liver injury, hepatocytes can ramp up proliferation to regenerate the liver. Yet, on extensive injury, regeneration falters, and liver failure ensues. It is therefore critical to understand the mechanisms underlying liver regeneration and, in particular, which liver cells are mobilized during liver maintenance and repair. Controversies continue to surround the very existence of hepatic stem cells and, if they exist, their spatial location, multipotency, degree of contribution to regeneration, ploidy, and susceptibility to tumorigenesis. This review discusses these controversies. Finally, we highlight how insights into hepatocyte regeneration and biology in vivo can inform in vitro studies to propagate primary hepatocytes with liver regeneration-associated signals and to generate hepatocytes de novo from pluripotent stem cells.
Keywords: Liver Stem Cells, Regeneration, Zonation, Differentiation, Hepatocyte Expansion, Ploidy, Tumorigenesis
Abbreviations used in this paper: 3D, three-dimensional; EGF, epidermal growth factor; HCC, hepatocellular carcinoma; HepLC, hepatocyte-like cell; HGF, hepatocyte growth factor; HIF, hypoxia-inducible factor; PI3K/Akt, phosphoinositide-3-kinase/protein kinase B; PSC, pluripotent stem cell; Skp2, S-phase kinase associated protein 2; Tert, telomerase reverse transcriptase; TNF, tumor necrosis factor; Yap, Yes-associated protein
Summary.
This review discusses controversies regarding hepatic stem cells and their roles in liver maintenance and regeneration. Insights in liver regeneration helped advance in vitro studies to propagate primary hepatocytes and to generate hepatocytes from pluripotent stem cells.
The liver is essential to life and performs diverse critical roles as a metabolic workhorse and guardian. Akin to a 24-hour biochemical factory, the liver synthesizes voluminous amounts of key proteins (eg, coagulation factors and carrier proteins) and detoxifies harmful substances (eg, ammonia and bilirubin).1,2 For details on liver functions, we refer the reader to other reviews.3, 4, 5, 6, 7, 8, 9
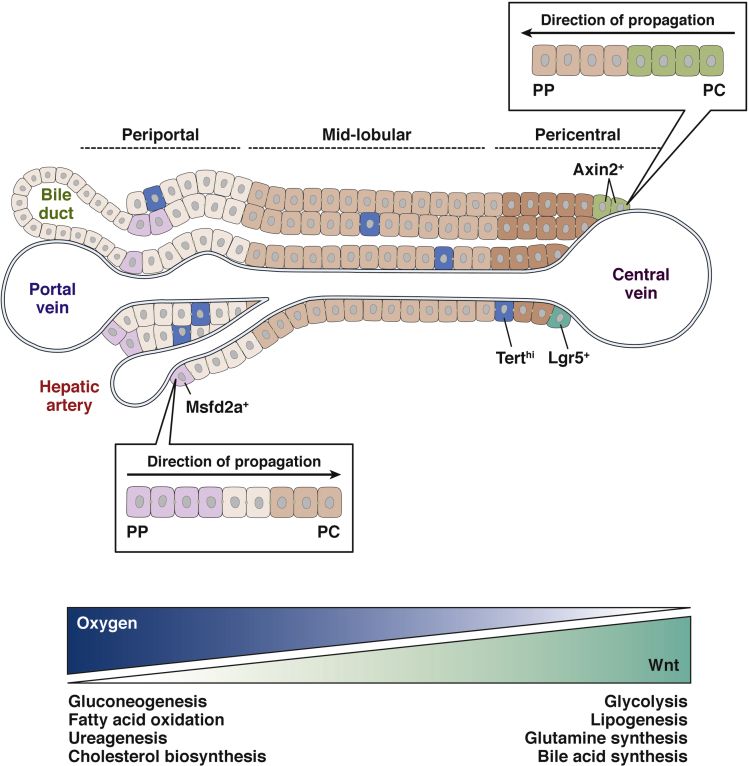
As one of the largest organs in the human body, the adult liver comprises approximately 300 billion cells.10 More than 70% of cells within the liver are epithelial cells known as hepatocytes, which are heavy weightlifters of the liver’s complex metabolic functions. Hepatocytes are organized in reiterated functional units shaped as hexagons, which are known as lobules. Each lobule comprises hepatocytes arranged in linear cords; at one pole is the central vein, and at the opposite is the so-called portal triad (bile ducts, hepatic artery, and portal vein) at the periphery. Hepatocytes located at different positions along the lobule were named on the basis of their proximity to these various blood vessels.2 At the periphery of the lobule are periportal hepatocytes, which receive blood carrying nutrients and oxygen from the hepatic artery and portal vein (Figure 1). Encircling the central vein are radial layers of concentric hepatocytes known as pericentral or perivenous hepatocytes.4 Hepatocytes in the mid-section of the liver lobule (between the central and portal veins) are referred to as mid-lobular hepatocytes.
Figure 1.
Sources of liver regeneration during homeostasis. Hepatocytes in the liver are spatially organized into periportal (PP), mid-lobular (ML), and pericentral (PC) “zones” depending on their proximity to the portal or central vein. PP and PC hepatocytes execute distinct metabolic functions in the liver. An oxygen gradient is established as blood flows from the portal vein to the central vein. Wnt signals are concentrated in the PC region. During homeostasis, Axin2+ (green) and Lgr5+ (teal) cells found in the pericentral region, can regenerate the liver. They propagate from the PC to the PP region. Terthi hepatocytes (blue) are distributed throughout the liver lobule and can clonally expand to regenerate the liver. Msfd2a+ hepatocytes (pink) are found at the PP region.
Hepatocytes across the liver lobule appear morphologically similar to one another, which belies the extent of their heterogeneity. There is a heterogenous continuum of different hepatocyte subtypes. Depending on their proximity to central veins or portal veins, hepatocytes perform distinct metabolic roles. This phenomenon is called zonation,4,7 which explains how the liver paradoxically manages opposing metabolic functions (eg, fatty acid oxidation vs lipogenesis, as well as glycolysis vs gluconeogenesis). These disparate metabolic labors are divided among subpopulations of hepatocytes. Pericentral hepatocytes mainly perform glutamine synthesis, glucose breakdown (glycolysis), lipogenesis, bile acid synthesis, and xenobiotic metabolism. By contrast, periportal hepatocytes are more active in other functions including urea, glucose, and glycogen synthesis, cholesterol biosynthesis, and fatty acid oxidation.4
Besides diversity in function, individual hepatocytes also differ in their number of chromosomes (ploidy). A significant fraction of the hepatocyte population is polyploid (with 1 or 2 nuclei, each containing 2, 4, 8, or even more sets of chromosomes). In the adult rodent liver, more than 75% of hepatocytes are polyploid with at least 4n DNA content.11, 12, 13 In the adult human liver, approximately 30%–50% of hepatocytes are polyploid.13,14 The abundant polyploidy evidenced among hepatocytes is quite unlike what is found in some other diploid cell types, and the specific physiological importance of hepatocyte polyploidy (if any) remains enigmatic. It has been suggested that polyploidization increases metabolic capacity, channeling resources via gene duplication to increase protein production and metabolism.15,16 Polyploidization has also been proposed to defend hepatocytes against injury or tumorigenesis, which this review will discuss later.
New technologies such as single molecule fluorescence in situ hybridization17 and single-cell RNA sequencing18,19 have captured differences in ploidy and gene expression among hepatocytes at unprecedented resolution.20, 21, 22, 23 For example, single molecule fluorescence in situ hybridization revealed that multinucleated polyploid hepatocytes can be found in all zones of the liver lobule.23 Hepatocytes are currently classified into 9 layers of cells spanning from the central vein to portal vein, with each layer defined by a unique gene expression signature.20 Pericentral hepatocytes express Wnt target gene Axin2 and Glutamine Synthetase, mid-lobular hepatocytes express peak levels of Hamp and Hamp2, and periportal hepatocytes preferentially express Ass1 and Asl1.20,21 Consistent with RNA profiling, protein expression analyses using mass spectrometry confirmed zonated expression of proteins across these liver lobule layers.24 This intricate spatial pattern implies a great degree of spatial organization and biological control.
The remarkable spatially-layered organization of hepatocytes in the liver lobule and their unique gene expression signatures begets a question: how is liver zonation established and regulated during liver development and homeostasis? Interestingly in mouse livers, zonation is first detected after birth as Glutamine Synthetase expression becomes restricted to pericentral hepatocytes25,26 (Figure 1).
In adults, the zonal pattern of hepatocyte functions is likely maintained by gradients of signaling proteins, nutrients, and oxygen established across the liver lobule as blood flows from the portal to central zones.9,27 Single-cell RNA sequencing showed that expression of genes associated with the hypoxia and Wnt signaling pathways is concentrated in the pericentral zone, whereas the expression of genes associated with the Ras pathway is enriched in the periportal zone20 (Figure 1). Notably, Ras is an essential downstream component of a number of signaling pathways including hepatocyte growth factor (HGF)/mesenchymal-epithelial transition (MET),28 fibroblast growth factor (FGF),29 and insulin signaling.30 Besides Ras signaling, other signaling pathways including the Hedgehog signaling pathway have been increasingly implicated in regulating metabolic zonation of the liver, and thus warrant further investigation.7,31
Wnt signals are among the most well-studied factors that zonate the adult liver; they are low at the periportal region and higher in the pericentral region.32, 33, 34 Wnt signaling ligands (eg, Wnt2 and Wnt9b) and Wnt co-agonist R-spondin3 are produced by endothelial cells of the central vein.35,36 These ligands are short-range37 and signal to the adjacent pericentral hepatocytes, which express the Wnt receptors Fzd7 and Fzd8 and Wnt co-agonist receptors leucine rich repeat containing G protein-coupled receptor 4 (Lgr4), Lgr5, and Lgr6.24,32 Consequently, this short-range Wnt signaling activates expression of Wnt target gene Axin2 in pericentral hepatocytes.35 Wnt is crucial to instill pericentral hepatocyte identity, as exemplified by the perturbed zonation and loss of pericentral marker Glutamine Synthetase observed when Wnt signaling is suppressed through deletion of its transcriptional effector beta-catenin.26,34
Although not often viewed as a classic morphogen, oxygen itself also shows graded levels across the liver lobule, being higher periportally and lower pericentrally; hypoxia pathway signaling thus shows an inverse gradient of activity.8,9,38 In line with this, hypoxia-inducible factors (HIFs), key transcription factors regulating molecular responses to oxygen, are highly enriched at the pericentral region.38 Deletion of HIF1a in hepatocytes led to impaired glucose metabolism during liver regeneration39 and high-fat induced obesity or diabetes.40 Inactivation of HIF2a suppressed liver steatosis in mice, whereas constitutive expression of HIF2a led to lipid accumulation in hepatocytes.41 However, zonation markers were not examined in these studies. Future studies may interrogate the effects of deleting HIFs on liver zonation by examining whether pericentral or periportal hepatocyte markers are still expressed in their respective zones.
Altogether, although liver zonation has now been described at high resolution, the upstream drivers of zonation await further definition. It is likely that a combination of signaling and metabolic pathways are operative, but how these pathways intersect to implement the finely graded spatial zonation observed in the adult liver is less well-understood. A common feature in developmental biology is that countervailing gradients of opposing signaling molecules can “sharpen” gene expression boundaries to achieve greater spatial precision.42 How this may similarly apply to the zonated adult liver remains underexplored. Candidate molecules beyond Wnt that similarly preserve liver zonation may emanate from the non-hepatocyte lineages within the liver that surround hepatocytes. Comprehensive profiling of mouse and human hepatocytes and other non-hepatocytes (eg, endothelial cells, immune cells, and stellate cells in the liver) at the single cell level could infer candidate zonation signals showing graded expression across the liver lobule.36 Then spatial visualization and targeted genetic studies could be used to unravel mechanisms that orchestrate liver zonation.
Controversies Surrounding Hepatic Stem Cells and Their Roles in Regeneration
In addition to performing an extraordinary range of functions to sustain the body, the liver also possesses an immense and remarkable regenerative capacity. On surgical resection, the liver can grow quickly to restore its original mass.43,44 Interestingly, the mode of regeneration differs on the basis of the degree of injury. When ∼30% of the liver is resected, hepatocytes within the liver undergo hypertrophy (or cellular enlargement). If ∼70% of the liver is resected, hepatocytes rapidly divide to compensate for the decline in liver function.45 How regenerating hepatocytes decide to undergo hypertrophy vs proliferation in response to different extents of liver injury is fascinating and not well-understood.
Various other sources of cells including Foxl1+,46 MIC1-1C3+,47 or Ck19+48 cells have also been proposed to yield hepatocytes during injury. Despite some evidence demonstrating that Ck19+ bile duct cells can transdifferentiate into hepatocytes under circumstances of severe injury,48 others proposed that liver regeneration is predominantly enacted by hepatocytes rather than non-hepatocytes.49,50 Ck19+ bile duct cells can proliferate during sustained diet-induced injury46,49 or when genetic ablation of β1-integrin was combined with artificially enforced cell-cycle arrest of hepatocytes to prevent hepatocyte-driven regeneration.48 Taken together, bile duct cells are thought to transdifferentiate into hepatocytes when native hepatocytes cannot enter cell cycle. On the other hand, others showed that mainly hepatocytes proliferate substantially to yield new hepatocytes during regeneration,51 as exemplified by lineage tracing of albumin-expressing hepatocytes.50 To address this dichotomy, a direct side-by-side comparison of the regenerative ability of bile duct cells and hepatocytes in the same mouse and under the same injury conditions would be worthwhile. This could be achieved using orthogonal genetic lineage tracing strategies52 to separately label bile duct cells and hepatocytes and to simultaneously track their respective homeostatic and regenerative behaviors in the same mouse. In sum, hepatocytes can self-replicate and give rise to additional hepatocytes.53 However, are hepatocytes equal in generating new hepatocytes? Is a select minority responsible? Is there a distinct group of hepatic “stem cells”?
Hepatic Stem Cells and Homeostasis
Currently, controversy surrounds whether there are specialized hepatic stem cells that can self-renew and generate the full zonated repertoire of mature hepatocytes in the liver lobule. Another outstanding question is whether a particular local microenvironment or a specialized niche can confer “stem cell” activity to a certain group of hepatocytes in one part of the liver lobule but not in other regions.
In recent years, it has been proposed that a special subset of hepatocytes localized in the pericentral zone seems more apt at proliferating and yielding new hepatocytes.35 Lineage tracing of hepatocytes expressing Wnt target gene Axin2 showed these hepatocytes can yield up to 30% of total hepatocytes within the liver lobule after 1 year. These pericentral hepatocytes are diploid, long-lived, and responsive to Wnt signals produced by pericentral endothelial cells.35 This is conceptually appealing because diploid hepatocytes should expectedly harbor more extensive proliferative capacity than most polyploid hepatocytes.
Although Wnt-responsive Axin2+ pericentral hepatocytes can clonally renew during homeostasis and persist for a long time, the extent of their proliferative capacity is variable across different studies. A subsequent study found Axin2+ hepatocytes contribute sparsely to liver turnover, mostly remaining in the pericentral area without yielding periportal hepatocytes.54 Similarly, lineage tracing of other Wnt-responsive hepatocytes (that express R-spondin receptors Lgr4 or Lgr5) for 18 months during homeostasis found that single hepatocytes yielded clones of few cells (eg, 1–5 cells), indicating a low degree of cell division during homeostasis.32,55 These divergent observations may be due to use of different lineage tracing strategies. In the first study, CreERT2 recombinase was knocked into the Axin2 locus, thus inactivating one allele of Axin2.35 However, Axin2 is a negative regulator of Wnt signaling, and therefore Axin2CreER/+ mice might have enhanced Wnt signaling.54 This perhaps explains the substantial growth of pericentral hepatocytes, because Wnt is a mitogen in the liver35 and elsewhere.37 In subsequent lineage tracing studies,32,55 Axin2-CreERT2 and Lgr5-CreERT2 recombinase cassettes were introduced as transgenes and were randomly integrated into the genome. A limitation of this approach is the risk of disrupting endogenous mouse genes, which could influence liver regeneration. Together such caveats of these lineage tracing approaches may obfuscate the extent of regenerative capacity harbored by Wnt-responsive hepatocytes. Ideally, future lineage-tracing studies would perform targeted knock-ins of recombinase proteins into endogenous genes without disrupting their coding sequence, for instance, by using 2A or internal ribosome entry site linkers56,57; this type of approach is increasingly adopted as the method of choice in the stem cell field.58
Viral-mediated labeling of single hepatocytes found no evidence for “zonal dominance”; rather, hepatocytes strewn across the liver lobule appeared competent to proliferate and expand.51 One potential caveat is that viral-mediated labeling of hepatocytes could alter the hepatocytes themselves, but this study detected no changes in cell proliferation after viral infection.51 Likewise, lineage tracing of a subset of hepatocytes expressing high levels of telomerase reverse transcriptase (Terthigh), scattered across the liver lobule, showed these cells can grow clonally to repopulate the liver for about 1 year.59 However, it was not mentioned whether the antibiotic selection cassette was removed in the generation of this TertCreER allele,59 which could alter expression of the recombinase. Nevertheless, these studies pose an alternative view: that liver homeostasis could be maintained by multiple populations of renewing hepatocytes without any specific zonal dominance.
Because multiple populations of hepatocytes in their respective zones can maintain and/or regenerate the liver during homeostasis (Figure 1), this still raises the question whether hepatocytes are equally proliferative or whether some are more proliferative than others during homeostasis. Randomly distributed Terthigh hepatocytes proliferated faster than Tertlow hepatocytes.59 Similarly, Axin2+ hepatocytes are more proliferative than Axin2– hepatocytes around the pericentral vein area.35,60 With multiple proliferative populations of hepatocytes, one population could conceivably persist longer than others. This has not yet been addressed by direct comparisons of Terthigh vs Axin2+ hepatocytes. However, periportal hepatocytes that express major facilitator superfamily domain containing 2A (Mfsd2a) eventually wane and reduce in numbers during homeostasis.60 By contrast, pericentral hepatocytes are long-lived; Lgr5+ hepatocytes maintain their own lineage during homeostasis and persist long-term.55
To date, how hepatocytes compare in maintaining the healthy, uninjured liver is an open question. Development of multiple genetic tools allowing long-term, simultaneous tracing and visualization of the different zones of the lobule, without interfering with hepatocyte proliferation and signaling pathways, will help elucidate this question and overcome inherent limitations associated with past approaches.
Versatile Responses to Injury and Interconversion of Hepatocytes
During homeostasis, the liver consistently maintains its mass with a slower turnover rate relative to other proliferative organs such as the intestines.61 Even then, the liver is vulnerable and exposed to excess fats, alcohol, viruses, toxins, and harmful metabolites; it has built-in failsafe mechanisms. During injury, hepatocytes can undergo massive compensatory proliferation and express genes normally detected in other zones of hepatocytes (Figure 2).
Figure 2.
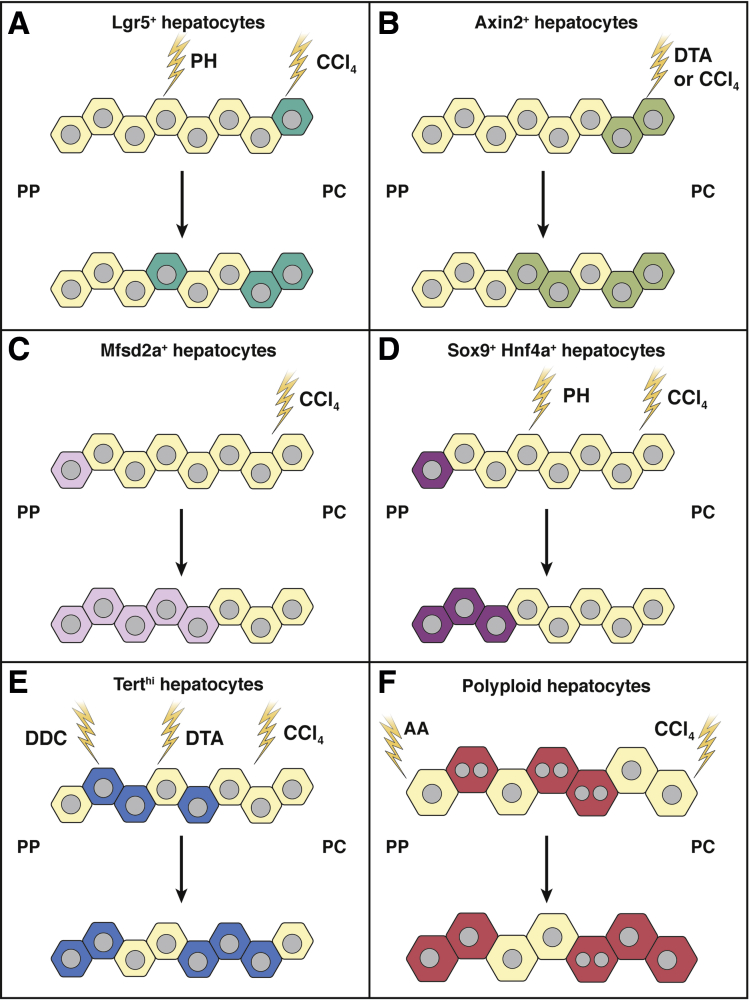
Liver regeneration responses by different hepatocytes during injury. (A) During partial hepatectomy (PH) injury and injury to pericentral (PC) hepatocytes, Lgr5– hepatocytes in other liver zones convert to Lgr5+ (green) to promote regeneration and repair hepatocytes. (B) Axin2– hepatocytes showed a similar pattern of regeneration, converting to Axin2+ (light green) hepatocytes after Cre-mediated expression of diphtheria toxin A (DTA). (C) Msfd2a+ (pink) hepatocytes repopulate the liver by proliferating from the periportal (PP) to the PC region on carbon tetrachloride (CCL4)-induced injury to PC hepatocytes. (D) Sox9+ Hnf4a+ (purple) hepatocytes contribute to regeneration in response to PH and CCL4-induced injuries. (E) 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), CCL4-induced injuries, and Cre-mediated expression of DTA promote proliferation of Terthi hepatocytes (blue) to regenerate PC hepatocytes. (F) Polyploid cells (red, 2 grey nuclei) contribute to liver recovery in response to arachidonic acid-induced (AA) PP injury and (CCL4)-induced PC injury. During liver regeneration, polyploid cells can reduce their ploidy status (red, 1 grey nucleus).
Wnt signaling is essential for hepatocyte regeneration after liver injury.62 Although Wnt signaling is typically concentrated in pericentral hepatocytes, on injury, mid-lobular hepatocytes that were originally Wnt-inactive can become Wnt-active.62 For example, Axin2-negative hepatocytes within other zones (eg, the mid-lobular zone) express Axin2 after diphtheria toxin A-mediated ablation of Axin2+ hepatocytes54 (Figure 2B). A similar phenomenon was observed on pericentral injuries, Lgr5-negative hepatocytes express Lgr555 (Figure 2A). How do these Wnt target genes become activated in hepatocytes that are farther away from the Wnt ligand-expressing central veins35 is unclear. One hypothesis is that Wnt-expressing immune cells, such as macrophages, infiltrate the injured liver and activate Wnt signaling more broadly across the lobule.63 Indeed, macrophage-specific deletion of the Wnt secretion factor Wntless in mice lowered hepatocyte proliferation 40 hours after partial hepatectomy, suggesting an early role of macrophage-derived Wnt signals in driving liver regeneration.34 Activation of Wnt signaling in hepatocytes during injury may act as a prerequisite for cells to respond to liver injury. This is supported by how liver-specific deletion of Lgr532 or ablation of Axin2-expressing hepatocytes54 significantly impaired regeneration. Taken together, the extent and spatial reach of Wnt signaling are altered after liver injury, and this enables liver regeneration.
Several lines of evidence indicate that damage to one zone of hepatocytes triggers a compensatory proliferative response in noninjured hepatocytes at other zones. When pericentral hepatocytes are damaged by CCl4 treatment, periportal hepatocytes (expressing Mfsd2a) grow and expand within the liver60 (Figure 2C). Lineage tracing of periportal hepatocytes (expressing both Sox9 and hepatocyte nuclear factor 4 alpha [Hnf4a]) also showed they replenish liver mass during chronic injuries to pericentral hepatocytes64,65 and injuries induced by partial hepatectomy60 (Figure 2D). Conversely, damage of periportal hepatocytes stimulates compensatory proliferation of pericentral and mid-lobular hepatocytes.51,59
From a pragmatic standpoint, having multiple zones of hepatocytes that participate in liver regeneration and generate new hepatocytes51 is advantageous. When injury is inflicted to hepatocytes in a given zone, it would be beneficial that other zones of hepatocytes can respond to the damage and execute regeneration. By contrast, having regenerative capacity concentrated only in one zone would likely render the liver prone to failure if that zone became damaged. Broadly distributed across the liver lobule, Terthi hepatocytes respond to periportal and/or pericentral-specific liver injuries,59 expanding and crossing zonal boundaries. On CCl4-induced pericentral injury or 3,5-diethoxycarbonyl-1,4-dihydrocollidine–induced injury, Terthi hepatocytes can expand and repopulate across the liver lobule. Genetically ablating Terthi hepatocytes with diphtheria toxin A dramatically reduced the extent of regeneration by the injured liver (Figure 2E).
Second, different parts of the liver lobule are susceptible to different drug-induced toxicities.66 Acetaminophen and alcohol inflict damages to predominantly pericentral hepatocytes as they are metabolized by P450 cytochrome enzyme Cyp2e1.67 Conversely, allyl alcohols and viral hepatitis damage periportal hepatocytes and cause inflammation of mostly the periportal tracts.66,68,69 The susceptibility of different parts of the liver lobule to different toxins reiterates the importance of a flexible system whereby multiple hepatocyte compartments can proliferate and compensate for injuries occurring in other regions.
Taken together, the wide ability of hepatocytes to proliferate and replenish other hepatocytes suggests complex regulatory and compensatory mechanisms at the lobule level. This also implies a tight coordination to reestablish metabolic zonation and size of the liver on injury. Notably, the proliferative hepatocytes discussed above appear “functional” (ie, they express genes related to liver physiology and metabolism). This contrasts with other tissues such as the blood and intestines, wherein proliferative stem cells and progenitors are “undifferentiated” and apparently lack clear physiological functions.70, 71, 72 On the other hand, it is also possible that hepatocytes “dedifferentiate” as they proliferate during injury, before repairing the liver.
Ploidy Status, Proliferative Zones, and Tumorigenesis
Adding to the complex discussion on the identity of hepatic stem cells is the question whether proliferating hepatocytes have a preferred ploidy status. This is relevant; after all, hepatocyte polyploidy has long been associated with terminal differentiation and senescence in pioneering studies.14 This section of the review will discuss (1) the intriguing link between hepatocyte ploidy status and proliferative capacity during liver regeneration and (2) the paradoxical relationship between polyploidization and uncontrolled proliferation/tumorigenesis.
In light of the substantial degree of polyploidy in the adult liver, can polyploid hepatocytes nonetheless divide or undergo reductive mitosis to reduce their ploidy status on demand? Contrary to the notion that polyploid hepatocytes are senescent and incapable of proliferation, a recent study genetically traced polyploid hepatocytes in vivo using Rosa26-Confetti multicolor reporter mice and showed that multicolored polyploid hepatocytes can replicate extensively during homeostasis and regeneration.73 Polyploid hepatocytes can engraft injured mouse livers and reduce their ploidy status to become diploid hepatocytes.73 Then diploid hepatocytes were serially transplanted into new host mouse livers and were found to reconstitute polyploid hepatocytes, significantly regenerating the recipient livers.73 However, it was unclear whether polyploid hepatocytes undergo ploidy reduction before proliferating and then reacquire polyploidy after liver repair was accomplished73 (Figure 2F). If polyploid hepatocytes are important to liver recovery, then one would expect mice with reduced hepatic polyploidy to be less capable of liver repair. Indeed, deletion of polyploidy regulators such as E2F transcription factors74 in mice reduced hepatic polyploidy, and the mice became more susceptible to morbidities associated with liver injuries.75
Nevertheless, polyploid hepatocytes were observed to have lower growth advantage than diploid hepatocytes.76 Mouse livers lacking E2f7 and E2f8 have more diploid hepatocytes, which proliferate faster than controls because of rapid transits through the cell cycle.76 Interestingly, in a recent lineage tracing study whereby hepatocytes were randomly labeled, diploid hepatocytes proliferated more efficiently than polyploid hepatocytes in injured liver; most of the large clones were diploid after injury unlike in the normal liver.51
The enhanced proliferation of diploid hepatocytes may contribute to age-dependent liver regeneration; neonatal livers (where hepatocytes are mostly diploid) can regenerate from injury more efficiently than adult livers. For example, neonatal livers can regrow locally at the injured lobe, without compensatory growth from adjacent lobes and without fibrosis. Similarly, neonatal hepatocytes engraft to a larger extent than adult hepatocytes on transplantation into Gunn rats.77 However, this period of expanded regeneration potential of hepatocytes is transient and fades with age.78 Unlike neonatal livers, adult livers recover from partial hepatectomy by compensatory hypertrophy and cell division across the remaining lobes,45 with a permanent change in liver architecture and development of fibrotic scars.78 Together, this suggests that intrinsic properties of neonatal hepatocytes (eg, their diploidy) influence their abilities to engraft and regenerate host livers. However, the full set of mechanisms underlying the advanced regenerative prowess of the neonatal liver remains to be detailed.
Diploid hepatocytes proliferate quickly and also appear more prone to tumorigenesis than polyploid hepatocytes.76 E2f7/8-/- mice that have predominantly diploid hepatocytes are vulnerable to spontaneous tumorigenesis. Similarly, when these mice were challenged with tumor-promoting stimuli, their livers developed numerous tumors.76 This suggests that those tumors were driven, at least in part, by diploid hepatocytes capable of rapid proliferation.76 Consistently, diploid Lgr5+ pericentral mouse hepatocytes are also susceptible to neoplastic transformation triggered by activation of the Erbb pathway and thus give rise to diethylnitrosamine-induced hepatocellular carcinoma.55 Therefore, diploidy may be both a boon and a bane, conferring enhanced hepatocyte proliferation in both health and disease.
Conversely, polyploidy plays a tumor suppressive role in the liver. Ploidy reduction by premature weaning or transient knockdown of E2f8 increased incidences of tumor formation.79 Furthermore, tumorigenesis is suppressed in mice with higher number of polyploid hepatocytes. In contrast, mice with mostly diploid hepatocytes developed tumors earlier in mutagen- and high fat-induced models.79 One explanation is that polyploid hepatocytes are more recalcitrant to tumorigenesis because they possess multiple copies of tumor suppressor genes, thus buffering them from spontaneous genetic loss of tumor suppressors.
Paradoxically, although the polyploid state confers protection against tumor formation, it has also been thought to accelerate progression of tumorigenesis.15,80, 81, 82 Genome doubling is among the most common events in cancer.80 In fact, polyploidy has been associated with aggressive, difficult-to-treat solid tumors, and highly polyploid hepatocellular carcinomas (HCCs) have poorer prognosis than HCCs with a lower degree of polyploidy.14 Thus, polyploidy in hepatocytes may be a double-edged sword, with divergent roles in tumorigenesis depending on the cellular context.
A fascinating question is what regulates the polyploidization of hepatocytes? Although embryonic hepatocytes are diploid, polyploid hepatocytes emerge in postnatal rodent livers during the suckling-to-weaning transition. This suggests a link between the onset of dietary intake and profound changes of hormonal (ie, insulin) production and liver polyploidization.83,84 Indeed, insulin/phosphoinositide-3-kinase (PI3K)/protein kinase B (Akt) signaling has been found to be critical for the genesis of binucleated hepatocytes.85,86 Destruction of insulin-producing rat pancreatic beta cells by streptozotocin before weaning reduced circulating levels of insulin and reduced the number of polyploid hepatocytes.86 Conversely, injecting insulin increased numbers of binucleated hepatocytes. Insulin signaling acts via PI3K/Akt, which also leads to incomplete cytokinesis.86 Besides being a downstream target of insulin signaling, Akt is also a downstream target of Yes-associated protein (Yap) signaling. Yap activation induces Akt phosphorylation, which cooperates with p300 to retain S-phase kinase associated protein 2 (Skp2) in the cytoplasm. Then Skp2 causes cyclin-dependent p27 to hyperaccumulate, leading to mitotic arrest and hence polyploidy.87 Whether additional signaling pathways regulate the ploidy status of hepatocytes is not well-explored.
Together, this suggests that signaling pathways and ploidy play important roles regulating the proliferation of hepatocytes during liver damage through robust checks and balances. However, how proliferative signals and ploidy interact and are dynamically modulated to regulate liver regeneration and prevent uncontrolled tumor growths remains to be addressed. Importantly, deciphering the connections among ploidy status, tumor formation, and signaling control will help shed new light on mechanisms underpinning liver tumorigenesis. Ultimately, understanding this could better inform targeted therapies that inhibit tumor growth and cancer progression.
Propagation of Primary Hepatocytes in Vitro: Common Signaling Themes
Because hepatocytes in various zones of the liver lobule proliferate in response to physical resection and chemical insults, primary hepatocytes should theoretically proliferate ex vivo within the right environment. The ability to culture primary hepatocytes in large numbers would overcome the shortage of hepatocytes for various practical applications including cellular transplantation therapies, drug toxicology testing, and disease modeling.88,89 However, historically it has been difficult to culture hepatocytes for extended amounts of time, while preserving their functionality.89,90
Propagation of Hepatocytes Using Signals Promoting Regeneration
Because hepatocytes operate in a complex signaling environment in vivo and divide infrequently during homeostasis, one can imagine the difficulty in preserving these states in vitro. For a long time, methods to culture primary hepatocytes have not been able to expand these cells in large numbers.91, 92, 93 Beyond just hepatocytes, most adult cells appear to have a finite proliferative capacity and eventually senesce, unable to re-enter cell cycle.94,95
Another major hurdle in primary hepatocyte expansion in vitro is that once hepatocytes are removed from the host, their signaling environment becomes altered, they begin to lose important hepatic metabolic gene expression and rapidly "dedifferentiate".90,91 Here the term dedifferentiation is used loosely to refer to the functional regression of hepatocytes, although it is unclear whether this same phenomenon occurs in vivo or whether it is an in vitro artifact. The loss of hepatic functionality also correlates with an increase in proliferation of the "dedifferentiated" hepatocytes. Some approaches to propagate primary hepatocytes reduced liver marker ALBUMIN96 and increased immature marker alpha-fetoprotein (AFP) or even biliary markers SRY-box transcription factor (SOX9) or cytokeratin 19 (CK19) (Table 1).
Table 1.
Protocols to Expand Primary Human and Rodent Hepatocytes in Vitro
| Article | Hepatocyte source | Types of cell culture | Signals used to expand hepatocytes | Expansion period | Gene expression of expanded hepatocytes | In vivo experiment |
|---|---|---|---|---|---|---|
| Hu et al, 2018 | Human and mouse | 3D organoid cell culture | HGF, EGF, WNT agonist (R-spondin 1, CHIR99021), TGFβ inhibitor (A83-01), ROCK inhibitor (Y27632), fibroblast growth factor 7 (FGF7), FGF10, TGFα | Mouse: 2–3 months Human: 2.5–11 months |
Mouse: Afp, Alb, Hnf4a, Cyp1a2, Cyp3a11 Human: ALB, HNF4A, CYP2E1, AFP |
Hepatocytes were expanded as single cells and transplanted into immunodeficient FRG mice via splenic injection. Engrafted hepatocytes expressed ALBUMIN, MRP2, CYP2E1, and secreted human serum albumin |
| Fu et al, 2018 | Human | 2D cell culture | HGF, EGF, WNT agonist (CHIR99021), TGFβ inhibitor (A83-01), ROCK inhibitor (Y27632), lysophosphatidic acid, sphingosine-1-phosphate | 10 days | CK19 (cytokeratin 19), CK7 (cytokeratin 7), HNF4A, SOX9, HNF1A, CK18 (cytokeratin 18), EpCAM, CD24 | Hepatocytes were transplanted via intrasplenic injection into Fah−/−Rag2−/− mice; 7.2%–16.1% engraftment was observed; the cells expressed ALBUMIN and were able to secrete ALBUMIN and alpha-1-antitrypsin |
| Garnier et al, 2018 | Human | 3D organoid cell culture | HGF, EGF, Wnt3a, TGFβ inhibitor (A83-01), forskolin, R-spondin 1, FGF10, Noggin, ROCK inhibitor (Y27632) | 2 months | CK19, EpCAM, SOX9, Ki67 | N/A |
| Katsuda et al, 2019 | Human | 2D cell culture | Wnt agonist (CHIR99021), TGFβ inhibitor (A83-01), ROCK inhibitor (Y27632) | 14–18 days | CK19, EPCAM, CD44, CD133, CD24, integrin subunit alpha 6 (ITGA6), SOX9, Prospero Homeobox (PROX1), AXIN2 | Hepatocytes were transplanted via intrasplenic injection into chronic injured livers of cDNA-μPA/SCID mouse. 10–11 weeks after transplantation, 17%–39% of transplanted cells engrafted the liver, expressed CYP2C, and secreted ALBUMIN |
| Katsuda et al, 2017 | Rat and mouse | 2D cell culture | Wnt agonist (CHIR99021), TGFβ inhibitor (A83-01), ROCK inhibitor (Y27632) | 15 days | Ck19, Forkhead Box M1(Foxm1), proliferating cell nuclear antigen (PCNA), Afp, Sox9, Epcam, Cd90, Forkhead Box J1 (Foxj1), Hnf1b, Hnf6, Cd44, Itga6 | Hepatocytes were transplanted via intrasplenic injection into cDNA-μPA/SCID mouse model and observed that 75%–90% of the liver comprised of transplanted rat cells. Engrafted cells secreted Albumin and expressed hepatocyte markers (Cyp1a2, Cyp3a1, Cyp7a1, Mrp2, Hnf4a) |
| Zhang et al, 2018 | Human | 2D cell culture | HGF, EGF, Wnt3a, FGF10, TGFβ inhibitor (A83-01), ROCK inhibitor (Y27632) | 1 month | CK19, CK7, SOX9, CD133, CYP1A2, ALB, AAT | Hepatocytes were transplanted via intrasplenic injection into Fah−/−Rag2−/−IL2rg-/- mouse model. The engrafted cells increased survival after 4 months of transplantation, reduced serum levels of aspartate transaminase, alanine transaminase, and total bilirubin, and increased serum levels of ALBUMIN. Engrafted cells repopulated approximately 64% of the mouse liver and expressed ALBUMIN and HNF4A 4 months after transplant |
| Xiang et al, 2019 | Human | 2D cell culture | Forskolin, TGF inhibitor (SB431542), Wnt inhibitor (IWP2), Notch inhibitor (DAPT), bone morphogenetic protein inhibitor (LDN193189) | 2 months | ALB, AAT, carbamoyl-phosphate synthase 1(CPS1), CYP3A4, CYP1A2, CYP2C9 CYP2D6, CYP2B6 | N/A |
| Peng et al, 2018 | Mouse | 3D cell culture | HGF, EGF, Wnt agonist (CHIR99021), TNFα | 6 months | Hnf4a, Yap, NF-kB, Ki67, Alb, transthyretin (Ttr), tyrosine aminotransferase (Tat), Fah | Hepatocytes were transplanted via intrasplenic injection into Fah−/−Rag2−/−IL2rg-/- mouse model. 80% repopulation was observed 103 days after transplantation, and the engrafted cells expressed FAH, pericentral genes (Glt1, Gs, Cyp2e1), and Cps1 |
| Huch et al, 2013 | Mouse | 3D cell culture | HGF, EGF, R-spondin 1, Wnt3a, FGF10 | 8 months | Ck19, Lgr5, Sox9 | Hepatocytes were expanded as 3D aggregates and were transplanted via intrasplenic injection into Fah−/−Rag2−/−IL2rg-/- mice. Transplanted cells expressed Fah and Hnf4a and improved mice survival |
| Huch et al, 2015 | Human | 3D cell culture | HGF, EGF, Wnt conditioned medium, R-spondin 1, TGFβ inhibitor (A83-01), FGF10, forskolin, bone morphogenetic protein inhibitor (Noggin), ROCK inhibitor (Y27632) | 6 months | CK7, LGR5, SOX9, HNF4a, CK19, AXIN2 | Hepatocytes were transplanted via intrasplenic injection into CCl4-induced liver injury mouse model. Engrafted cells expressed ALBUMIN; secreted ALBUMIN and alpha-1-antitrypsin |
ROCK, rho associated kinase; TGFβ, transforming growth factor beta.
It is now possible to expand primary human hepatocytes in vitro to some extent by activating hepatocyte proliferation through the addition of exogenous signaling cues known to regulate liver regeneration in vivo. These signaling cues include HGF, epidermal growth factor (EGF), tumor necrosis factor (TNF), Wnt, and Yap signaling.97,98 Inactivation of these pathways in vivo impairs liver regeneration,32,98, 99, 100, 101, 102 reiterating their functional importance. In these recent studies, activators of HGF, EGF, and Wnt signaling, and inhibitors of transforming growth factor β (TGFβ) and rho-associated kinase (ROCK) signaling, are among the factors used to expand primary human hepatocytes in 2-dimensional (2D) or 3-dimensional (3D) cultures (Table 1).103, 104, 105, 106, 107, 108
For example, Wnt signals promote long-term culture of primary hepatocytes in most studies, echoing their function to sustain Wnt-responsive hepatocytes in vivo.35,54,96,104, 105, 106, 107,109 In vitro, media containing WNT3A resulted in hepatocytes expanding 10,000-fold without genetic manipulation,96 similar to how Wnt signaling sustains hepatocytes in vivo. However, Wnt alone appears insufficient to expand primary hepatocytes.96,104,105
Another important signal is TNFα, an injury-induced inflammatory cytokine that has been shown to promote expansion of mouse hepatocytes in 3D cultures.107 In vivo, TNFα is responsible for the regeneration of the adult liver after injury and also the liver’s developmental growth.110 The exact function of TNFα remains unclear, although it is reported to promote DNA replication in hepatocytes through interactions with nuclear factor kappa B signaling.111 By adding TNFα as well as other factors to primary mouse hepatocyte cultures, 3D aggregates of mouse adult hepatocytes could be cultured for more than 6 months, while maintaining expression of certain important hepatic genes.107 Remarkably, these TNFα-expanded hepatocytes could also robustly engraft the injured mouse liver, attesting to their value.107 However, whether TNFα or other signals will prove equally effective in maintaining human hepatocytes remains an outstanding question.
In addition to the above signals, oxygen levels were modulated to mimic hypoxia and promote proliferation of hepatocytes.96 However, normoxia appears sufficient to expand hepatocytes in various other studies (Table 1). Nevertheless, hypoxia-regulated genes are enriched in the pericentral hepatocytes than in periportal hepatocytes.20 The mechanisms by which hypoxia is beneficial to proliferation are unclear.
Although the aforementioned signaling cues enhance the proliferation of hepatocytes in vitro, they also lead to loss in hepatocyte functionality and mature gene expression. However, such altered states appear reversible; after expanding primary hepatocytes in vitro, they can be “redifferentiated” with differentiation-inducing factors including dexamethasone and oncostatin M or transplanted into mouse liver, where they adopted higher expression of hepatocyte genes and showed enhanced liver functions96,105, 106, 107,109,112 (Table 1). For example, "redifferentiation" of expanded hepatocytes increased ALBUMIN and HNF4A105,113 expression and enhanced p450 cytochrome activities.96 This suggests that these hepatocytes are poised to differentiate in response to differentiation cues. However, it remains unclear whether the "redifferentiated" hepatocytes also recapitulate the zonated properties of hepatocytes in vivo; for example, how similar are these hepatocytes to the pericentral, mid-lobular, or periportal hepatocytes in the liver? A future experiment will be to determine how "dedifferentiation" in culture affects global hepatocyte gene expression and whether "redifferentiation" reinstates the original expression profile.
Preventing Hepatocyte Dedifferentiation and Hippo/Yap Signaling
Altogether, it appears that upon in vitro culture, proliferating hepatocytes express lower levels of metabolic genes, potentially temporarily losing their key metabolic functions. For example, mouse hepatocytes regenerating injured livers have increased chromatin accessibility in loci of genes driving proliferation and reduced accessibility to regulatory regions of genes controlling metabolism.114
Hepatocytes down-regulate functional genes (eg, ALBUMIN) and express immature markers (eg, alpha-fetoprotein) during in vitro propagation and become "dedifferentiated" as they transit to a more immature, proliferative state. Interestingly, hepatocyte "dedifferentiation" is associated with activated YAP signaling; proliferating hepatocytes have a higher expression of YAP target genes.90,96 Knocking out Yap in these hepatocytes prevented the loss of ALBUMIN expression in culture.90 As a parallel approach, small molecules inhibiting actomyosin contraction, actin polymerization, and Yap signaling also helped preserve the expression of hepatocyte genes.90 Together, this demonstrates the importance of Yap signaling in hepatocyte expansion.
In vivo, the role of Yap signaling in promoting proliferation of hepatocytes is also evident. Hippo/Yap signaling controls organ growth, and its dysregulation leads to uncontrolled proliferation and hepatocellular carcinogenesis in the liver.115, 116, 117, 118 Liver-specific overexpression of Yap led to hepatomegaly (with 4-fold increase in liver size within weeks),116 and persistent expression of Yap results in tumorigenesis.117,119 Acute postnatal loss of upstream regulators of Yap, including Mst1/2120 in mice and Ww45 in Drosophila,121 led to increased YAP levels, resulting in hepatomegaly and eventually liver cancer. Conversely, knocking out Yap target NUAK2 in a Doxycycline-inducible YAP mouse model reduced tumor growth.98,122
In vitro, Yap signaling and Yap nuclear localization are affected by cell density, cell shape, extracellular matrix stiffness, and actomyosin cytoskeleton.123, 124, 125, 126, 127 Indeed, hepatocytes often "dedifferentiate" as they become more spread out in the cell culture dish (as visualized by F-actin expression).90 When spreading of primary hepatocytes was limited by growing them on micropatterned devices, these constrained hepatocytes did not "dedifferentiate"; they have cytoplasmic Yap localization and retained expression of ALBUMIN.90 This novel notion linking cell spreading to cell fate is important and can have implications in cultures of other epithelial cell types beyond hepatocytes.
In sum, the ability to culture primary hepatocytes for long periods is an important step, but a number of questions remain to be addressed. One question is whether all expanded hepatocytes are conducive to "redifferentiation", or if there are any cells refractory to "redifferentiation" after in vitro expansion. Even though these hepatocytes may be "redifferentiated", another important question is whether prolonged expansion of hepatocytes in vitro using HGF, EGF, Wnt, and other signals will convert some of them into tumorigenic cells. After all, overactivation of a number of these pathways in vivo can result in uncontrolled proliferation and tumorigenesis.115,128, 129, 130 To address these questions, one can perform time course experiments to track molecular changes in hepatocytes during expansion at the single cell level and over a prolonged period of time.
Generation of Human Pluripotent Stem Cell–Derived Hepatocyte-like Cells: Progress and Possibilities
Although primary human hepatocytes are appealing sources of liver cells, these cells must be obtained from human liver tissues, which are often scarce. In addition, digestion of human liver tissue to isolate cells can be technically challenging. A parallel approach to generate hepatocytes in vitro is to differentiate human pluripotent stem cells (PSCs) into hepatocyte-like cells (HepLCs) through exposure to growth factors and small molecules.131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143 One major advantage of using human PSCs as a starting source of hepatocytes is that they are highly abundant144,145 and can be propagated to generate (theoretically) limitless numbers of hepatocytes. A key goal in this field is to generate highly pure and fully functional hepatocytes in vitro that are counterparts to hepatocytes in vivo.
Converting human PSCs into hepatocytes requires a series of intermediate steps that mimic embryonic development and liver organogenesis. In vivo, pluripotent epiblast cells sequentially turn into intermediate cell types (primitive streak, definitive endoderm, posterior foregut, and liver progenitors), before differentiating into hepatocytes. In some in vitro differentiation studies, human PSCs were exposed to different doses and combinations of signals to drive the formation of desired cell types at each lineage decision point. Importantly, generation of highly pure HepLCs involves suppressing the specification of unwanted lineages (ie, non-liver cell-types) at each step of the differentiation process.132,146 One challenge is that signaling cues operate with rapid temporal dynamics to guide the formation of specific cell types.132 Thus, the time of signal activation is important because prolonged addition of certain signaling factors over an unwarranted period of time may lead to formation of undesired lineages.
Remarkably, human PSC-derived HepLCs appear functional to some extent; they have been shown to be metabolically active in vitro, and they are capable of secreting ALBUMIN, metabolize lipids and glycogen, and have high levels of cytochrome P450 enzyme activities.132,134,140, 141, 142,147 These HepLCs are also able to engraft into mouse models of acute and chronic liver injury and retain some of their metabolic functionalities in vivo.132,148,149
However, akin to in vitro-expanded primary hepatocytes, human PSC-derived HepLCs are typically less functional and express lower xenobiotic metabolic genes than freshly isolated primary human hepatocytes. Some studies have shown that PSC-derived HepLCs exhibit fetal-like phenotypes, representing an immature cell state by comparison to fully-mature adult hepatocytes.135 The overall transcriptome profiles of human PSC-derived HepLCs are also distinct from that of primary human hepatocytes.141,150 More specifically, expression patterns of cytochrome P450 enzymes in human PSC-derived HepLCs appear more similar to fetal hepatocytes than adult ones.135,140 It remains challenging to generate hepatocytes that are functionally comparable to hepatocytes found in the adult liver. Hence, understanding how hepatocytes mature in vivo will be necessary to provide insights on how to achieve better in vitro generation of functionally mature hepatocytes.
Even though human PSC-derived HepLCs are less functional than hepatocytes in vivo, transplanted hepatocytes can engraft and repopulate the mouse liver over time in chronic mouse models of liver injury.132,148 In one study, the level of engraftment of human PSC-derived HepLCs in mouse models of liver injury was 10.2%.148 In addition, transplantation of human PSC-derived HepLCs into the liver of injured mice can improve the overall survival of these mice.131,132 These engrafted HepLCs expressed ALBUMIN and could secrete human ALBUMIN protein into the mouse bloodstream.132,151 Despite a lower degree of functionality, the less “mature” HepLCs demonstrate a certain capacity to proliferate in vivo under injury conditions. It remains to be determined whether human PSC-derived HepLCs after transplantation can adopt different hepatocyte subtype identities and become spatially zonated in vivo to perform a spectrum of functions, analogous to how authentic hepatocytes presumably regenerate the injured liver in vivo.
Another important consideration is what specific genetic or chemical mouse models of liver injury the HepLCs are transplanted into, as the type and extent of injury presumably alters the engraftment of transplanted HepLCs.131,132,148,151 For example, mice lacking a key tyrosine metabolic enzyme (fumarylacetoacetate hydrolase or Fah) can develop chronic liver failure, thereby providing a selective growth advantage for transplanted, wild-type (FAH)+ HepLCs.152 In particular, injuring the mouse liver provides "space" for transplanted cells to engraft, grow, and potentially restore liver functionality. However, a rigorous comparison of human PSC-derived HepLCs in both acute and chronic models of liver injury awaits.
It remains a major challenge to generate human PSC-derived HepLCs that are functionally on par with primary hepatocytes, and many outstanding questions remain.1 What are the proportions of pericentral-like or periportal-like hepatocyte subtypes within the pool of differentiated human PSC-derived HepLCs? Are they responsive to Wnt signaling activation in vitro, analogous to pericentral hepatocytes in vivo? Are these hepatocytes diploid or polyploid? Although it has been suggested in a study that pericentral HepLCs can be generated from human PSCs,135 further follow-up functional validations may shed light on how comparable are the hepatocytes generated in vitro to hepatocytes found in the liver. In sum, human PSC-derived HepLCs can also serve as valuable models to investigate molecular mechanisms regulating metabolic functions and proliferation.
Conclusion
Liver disease is one of the leading causes of mortality in the world, accounting for 2 million deaths per year.153 Even though the liver has an immense ability to regenerate, when the damage is too severe, liver failure occurs.154 One solution is liver transplantation, but the shortage of human liver donors makes this option unavailable to many people.105 Therefore, there is immense interest in understanding how the liver recuperates from toxic insults or physical injuries, which holds promise for developing new strategies to either protect the liver from injury or to enhance its regeneration.
The question of which population of cells within the liver have a superior ability to proliferate and regenerate the entire liver remains debatable. Some believe that liver stem cells reside mostly in one particular zone (pericentral or periportal), whereas others believe that liver stem cells are distributed randomly throughout the liver lobule. In addition, some groups have found that liver regeneration is based on innate characteristics of hepatocytes, such as their ploidy or telomerase activity.
By understanding the mechanisms regulating the liver's response to insults and metabolic zonation in vivo, one can harness and apply this knowledge to generate hepatocytes in vitro of therapeutic interest for drug testing or liver transplantation. Specifically, generating large numbers of hepatocytes from human PSCs or primary liver tissues is of growing interest because these hepatocytes hold promise for cell replacement therapy (for patients on the wait list for liver transplantation), drug testing, and modeling of liver diseases. Altogether, there is hope in the field of liver regeneration that the ability to generate abundant and pure hepatocytes in vitro can provide an alternative cell source for liver transplantation.
Acknowledgments
The authors thank Kyle M. Loh for editing the manuscript and for discussions.
CRediT Authorship Contributions
Nicole Min Qian Pek (Visualization: Lead; Writing – review & editing: Equal)
Kevin J. Liu (Visualization: Supporting; Writing – review & editing: Equal)
Massimo Nichane, PhD (Writing – review & editing: Equal)
Lay Teng Ang, PhD (Conceptualization: Lead; Funding acquisition: Lead; Writing – original draft: Lead; Writing – review & editing: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding L.T.A. is supported by the Maternal & Child Health Research Institute, California Institute for Regenerative Medicine (DISC2-10679 and DISC2-11105), and the Stanford-UC Berkeley Siebel Stem Cell Institute.
References
- 1.Chen C., Soto-Gutierrez A., Baptista P.M., Spee B. Reviews in basic and clinical gastroenterology and hepatology. Gastroenterology. 2018;154:1258–1272. doi: 10.1053/j.gastro.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Si-Tayeb K., Lemaigre F.P., Duncan S.A. Organogenesis and development of the liver. Developmental Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Guzmán M., Castro J. Zonation of fatty acid metabolism in rat liver. Biochem J. 1989;264:107–113. doi: 10.1042/bj2640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jungermann K. Zonation of metabolism and gene expression in liver. Histochem Cell Biol. 1995;103:81–91. doi: 10.1007/BF01454004. [DOI] [PubMed] [Google Scholar]
- 5.Jungermann K., Kietzmann T. Zonation of parenchymal and nonparenchymal metabolism in liver. Ann Rev Nutr. 1996;16:179–203. doi: 10.1146/annurev.nu.16.070196.001143. [DOI] [PubMed] [Google Scholar]
- 6.Oinonen T., Lindros K.O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem J. 1998;329(Pt 1):17–35. doi: 10.1042/bj3290017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- 8.Kietzmann T. Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biology. 2017;11:622–630. doi: 10.1016/j.redox.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kietzmann T. Liver zonation in health and disease: hypoxia and hypoxia-inducible transcription factors as concert masters. International Journal of Molecular Sciences. 2019;20:2347. doi: 10.3390/ijms20092347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianconi E., Piovesan A., Facchin F., Beraudi A., Casadei R., Frabetti F., Vitale L., Pelleri M.C., Tassani S., Piva F., Perez-Amodio S., Strippoli P., Canaider S. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463–471. doi: 10.3109/03014460.2013.807878. [DOI] [PubMed] [Google Scholar]
- 11.Duncan A.W., Taylor M.H., Hickey R.D., Newell A.E.H., Lenzi M.L., Olson S.B., Finegold M.J., Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreutz C., MacNelly S., Follo M., Wäldin A., Binninger-Lacour P., Timmer J., Bartolomé-Rodríguez M.M. Hepatocyte ploidy is a diversity factor for liver homeostasis. Frontiers in Physiology. 2017;8:70. doi: 10.3389/fphys.2017.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knouse K.A., Wu J., Whittaker C.A., Amon A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci U S A. 2014;111:13409–13414. doi: 10.1073/pnas.1415287111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donne R., Saroul-Ainama M., Cordier C., Celton-Morizur S., Desdouets C. Polyploidy in liver development, homeostasis and disease. Nat Rev Gastroenterol Hepatol. 2020;17:391–405. doi: 10.1038/s41575-020-0284-x. [DOI] [PubMed] [Google Scholar]
- 15.Davoli T., de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annu Rev Cell Dev Biol. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- 16.Lee H.O., Davidson J.M., Duronio R.J. Endoreplication: polyploidy with purpose. Genes Dev. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Femino A.M., Fay F.S., Fogarty K., Singer R.H. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 18.Tang F., Barbacioru C., Wang Y., Nordman E., Lee C., Xu N., Wang X., Bodeau J., Tuch B.B., Siddiqui A., Lao K., Surani M.A. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 19.Kolodziejczyk A.A., Kim J.K., Svensson V., Marioni J.C., Teichmann S.A. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58:610–620. doi: 10.1016/j.molcel.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Halpern K.B., Shenhav R., Matcovitch-Natan O., Tóth B., Lemze D., Golan M., Massasa E.E., Baydatch S., Landen S., Moor A.E., Brandis A., Giladi A., Stokar-Avihail A., David E., Amit I., Itzkovitz S. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352–356. doi: 10.1038/nature21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizarani N., Saviano A., Sagar, Mailly L., Durand S., Herman J.S., Pessaux P., Baumert T.F., Grun D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572:1–6. doi: 10.1038/s41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacParland S.A., Liu J.C., Ma X.-Z., Innes B.T., Bartczak A.M., Gage B.K., Manuel J., Khuu N., Echeverri J., Linares I., Gupta R., Cheng M.L., Liu L.Y., Camat D., Chung S.W., Seliga R.K., Shao Z., Lee E., Ogawa S., Ogawa M., Wilson M.D., Fish J.E., Selzner M., Ghanekar A., Grant D., Greig P., Sapisochin G., Selzner N., Winegarden N., Adeyi O., Keller G., Bader G.D., McGilvray I.D. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nature Communications. 2018;9 doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanami S., Ben-Moshe S., Elkayam A., Mayo A., Halpern K.B., Itzkovitz S. Dynamic zonation of liver polyploidy. Cell Tissue Res. 2016 doi: 10.1007/s00441-016-2427-5. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Moshe S., Shapira Y., Moor A.E., Manco R., Veg T., Halpern K.B., Itzkovitz S. Spatial sorting enables comprehensive characterization of liver zonation. Nature Metabolism. 2019 doi: 10.1038/s42255-019-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiojiri N., Wada J.I., Tanaka T., Noguchi M. Heterogeneous hepatocellular expression of glutamine synthetase in developing mouse liver and in testicular transplants of fetal liver. Lab Invest. 1995;72:740–747. [PubMed] [Google Scholar]
- 26.Burke Z.D., Reed K.R., Yeh S.-W., Meniel V., Sansom O.J., Clarke A.R., Tosh D. Spatiotemporal regulation of liver development by the Wnt/β-catenin pathway. Scientific Reports. 2018 doi: 10.1038/s41598-018-20888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jungermann K., Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255–260. doi: 10.1002/hep.510310201. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Xia M., Jin K., Wang S., Wei H., Fan C., Wu Y., Li X., Li X., Li G., Zeng Z., Xiong W. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ornitz D.M., Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdisciplinary Reviews: Developmental Biology. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddle K. Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances. Frontiers in Endocrinology. 2012;3:34. doi: 10.3389/fendo.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matz-Soja M., Aleithe S., Marbach E., ttger J.B., Arnold K., Schmidt-Heck W., Kratzsch J.r., Gebhardt R. Hepatic Hedgehog signaling contributes to the regulation of IGF1 and IGFBP1 serum levels. Cell Commun Signal. 2014;12:11. doi: 10.1186/1478-811X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planas-Paz L., Orsini V., Boulter L., Calabrese D., Pikiolek M., Nigsch F., Xie Y., Roma G., Donovan A., Marti P., Beckmann N., Dill M.T., Carbone W., Bergling S., Isken M. Mueller, Kinzel B., Yang Y., Mao X., Nicholson T.B., Zamponi R., Capodieci P., Valdez R., Rivera D., Loew A., Ukomadu C., Terracciano L.M., Bouwmeester T., Cong F., Heim M.H., Forbes S.J., Ruffner H., Tchorz J.S. The RSPO–LGR4/5–ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–479. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 33.Benhamouche S., Decaens T., Godard C., Chambrey R., Rickman D.S., Moinard C., Vasseur-Cognet M., Kuo C.J., Kahn A., Perret C., Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Developmental Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Yang J., Mowry L.E., Nejak-Bowen K.N., Okabe H., Diegel C.R., Lang R.A., Williams B.O., Monga S.P. Beta-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halpern K.B., Shenhav R., Massalha H., Tóth B., Egozi A., Massasa E.E., Medgalia C., David E., Giladi A., Moor A.E., Porat Z., Amit I., Itzkovitz S. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nature Biotechnology. 2018;36:962–970. doi: 10.1038/nbt.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clevers H., Loh K.M., Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 38.Kietzmann T., Cornesse Y., Brechtel K., Modaressi S., Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem J. 2001;354:531–537. doi: 10.1042/0264-6021:3540531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tajima T., Goda N., Fujiki N., Hishiki T., Nishiyama Y., Senoo-Matsuda N., Shimazu M., Soga T., Yoshimura Y., Johnson R.S., Suematsu M. HIF-1alpha is necessary to support gluconeogenesis during liver regeneration. Biochem Biophys Res Commun. 2009;387:789–794. doi: 10.1016/j.bbrc.2009.07.115. [DOI] [PubMed] [Google Scholar]
- 40.Ochiai D., Goda N., Hishiki T., Kanai M., Senoo-Matsuda N., Soga T., Johnson R.S., Yoshimura Y., Suematsu M. Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem Biophys Res Commun. 2011;415:445–449. doi: 10.1016/j.bbrc.2011.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rankin E.B., Rha J., Selak M.A., Unger T.L., Keith B., Liu Q., Haase V.H. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zagorski M., Tabata Y., Brandenberg N., Lutolf M.P., Tkačik G., Bollenbach T., Briscoe J., Kicheva A. Decoding of position in the developing neural tube from antiparallel morphogen gradients. Science. 2017;356:1379–1383. doi: 10.1126/science.aam5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagasue N., Yukaya H., Ogawa Y., Kohno H., Nakamura T. Human liver regeneration after major hepatic resection: a study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30–39. doi: 10.1097/00000658-198707000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pack G.T., Islami A.H., Hubbard J.C., Brasfield R.D. Regeneration of human liver after major hepatectomy. Surgery. 1962;52:617–623. [PubMed] [Google Scholar]
- 45.Miyaoka Y., Ebato K., Kato H., Arakawa S., Shimizu S., Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;CB 22:1166–1175. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Shin S., Upadhyay N., Greenbaum L.E., Kaestner K.H. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology. 2015;148:192–202.e193. doi: 10.1053/j.gastro.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarlow B.D., Pelz C., Naugler W.E., Wakefield L., Wilson E.M., Finegold M.J., Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raven A., Lu W.-Y., Man T.Y., Ferreira-Gonzalez S., O’Duibhir E., Dwyer B.J., Thomson J.P., Meehan R.R., Bogorad R., Koteliansky V., Kotelevtsev Y., ffrench-Constant C., Boulter L., Forbes S.J. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547:350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanger K., Knigin D., Zong Y., Maggs L., Gu G., Akiyama H., Pikarsky E., Stanger B.Z. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15:340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Huang X., He L., Pu W., Li Y., Liu Q., Li Y., Zhang L., Yu W., Zhao H., Zhou Y., Zhou B. Genetic tracing of hepatocytes in liver homeostasis, injury, and regeneration. J Biol Chem. 2017;292:8594–8604. doi: 10.1074/jbc.M117.782029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen F., Jimenez R.J., Sharma K., Luu H.Y., Hsu B.Y., Ravindranathan A., Stohr B.A., Willenbring H. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell. 2020;26:27–33.e24. doi: 10.1016/j.stem.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu K., Jin H., Zhou B. Genetic lineage tracing with multiple DNA recombinases: a user’s guide for conducting more precise cell fate mapping studies. J Biol Chem. 2020;295:6413–6424. doi: 10.1074/jbc.REV120.011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanger B.Z. Cellular homeostasis and repair in the mammalian liver. Annu Rev Physiol. 2015;77:179–200. doi: 10.1146/annurev-physiol-021113-170255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun T., Pikiolek M., Orsini V., Bergling S., Holwerda S., Morelli L., Hoppe P.S., Planas-Paz L., Yang Y., Ruffner H., Bouwmeester T., Lohmann F., Terracciano L.M., Roma G., Cong F., Tchorz J.S. Axin2+ pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell. 2020;26:97–107.e106. doi: 10.1016/j.stem.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Ang C.H., Hsu S.H., Guo F., Tan C.T., Yu V.C., Visvader J.E., Chow P.K.H., Fu N.Y. Lgr5 +pericentral hepatocytes are self-maintained in normal liver regeneration and susceptible to hepatocarcinogenesis. Proc Natl Acad Sci U S A. 2019;116:19530–19540. doi: 10.1073/pnas.1908099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.H., Lee S.-R., Li L.-H., Park H.-J., Park J.-H., Lee K.Y., Kim M.-K., Shin B.A., Choi S.-Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan H.Y., Sivakamasundari, Xing X., Kraus P., Yap S.P., Ng P., Lim S.L., Lufkin T. Comparison of IRES and F2A-based locus-specific multicistronic expression in stable mouse lines. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kretzschmar K., Watt F.M. Lineage tracing. Cell. 2012;148:33–45. doi: 10.1016/j.cell.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Lin S., Nascimento E.M., Gajera C.R., Chen L., Neuhöfer P., Garbuzov A., Wang S., Artandi S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/s41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pu W., Zhang H., Huang X., Tian X., He L., Wang Y., Zhang L., Liu Q., Li Y., Li Y., Zhao H., Liu K., Lu J., Zhou Y., Huang P., Nie Y., Yan Y., Hui L., Lui K.O., Zhou B. Mfsd2a+ hepatocytes repopulate the liver during injury and regeneration. Nature Communications. 2016;7:13369. doi: 10.1038/ncomms13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iismaa S.E., Kaidonis X., Nicks A.M., Bogush N., Kikuchi K., Naqvi N., Harvey R.P., Husain A., Graham R.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regenerative Medicine. 2018;3:6. doi: 10.1038/s41536-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao L., Jin Y., Donahue K., Tsui M., Fish M., Logan C.Y., Wang B., Nusse R. Tissue repair in the mouse liver following acute carbon tetrachloride depends on injury-induced Wnt/β-catenin signaling. Hepatology. 2019;15:340. doi: 10.1002/hep.30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell J.O., Monga S.P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annual Review of Pathology: Mechanisms of Disease. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M.A., Ye L., Kopp J.L., Sander M., Carter H., Deisseroth K., Verma I., Karin M. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han X., Wang Y., Pu W., Huang X., Qiu L., Li Y., Yu W., Zhao H., Liu X., He L., Zhang L., Ji Y., Lu J., Lui K.O., Zhou B. Lineage tracing reveals the bipotency of SOX9(+) hepatocytes during liver regeneration. STEMCR. 2019;12:624–638. doi: 10.1016/j.stemcr.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindros K.O. Zonation of cytochrome P450 expression, drug metabolism and toxicity in liver. Gen Pharmacol. 1997;28:191–196. doi: 10.1016/s0306-3623(96)00183-8. [DOI] [PubMed] [Google Scholar]
- 67.Lee S.S., Buters J.T., Pineau T., Fernandez-Salguero P., Gonzalez F.J. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 68.Goodman Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Dhingra S. Liver pathology of hepatitis C, beyond grading and staging of the disease. World J Gastroenterol. 2016;22:1357. doi: 10.3748/wjg.v22.i4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logan C.Y., Desai T.J. Keeping it together: pulmonary alveoli are maintained by a hierarchy of cellular programs. Bioessays. 2015;37:1028–1037. doi: 10.1002/bies.201500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weissman I.L., Shizuru J.A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chacón-Martínez C.A., Koester J., Wickström S.A. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 2018;145:dev165399. doi: 10.1242/dev.165399. [DOI] [PubMed] [Google Scholar]
- 73.Matsumoto T., Wakefield L., Tarlow B.D., Grompe M. In vivo lineage tracing of polyploid hepatocytes reveals extensive proliferation during liver regeneration. Cell Stem Cell. 2020;26:34–47.e33. doi: 10.1016/j.stem.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandit S.K., Westendorp B., Nantasanti S., van Liere E., Tooten P.C.J., Cornelissen P.W.A., Toussaint M.J.M., Lamers W.H., de Bruin A. E2F8 is essential for polyploidization in mammalian cells. Nat Cell Biol. 2012;14:1181–1191. doi: 10.1038/ncb2585. [DOI] [PubMed] [Google Scholar]
- 75.Wilkinson P.D., Alencastro F., Delgado E.R., Leek M.P., Weirich M.P., Otero P.A., Roy N., Brown W.K., Oertel M., Duncan A.W. Polyploid hepatocytes facilitate adaptation and regeneration to chronic liver injury. AJPA. 2019;189:1241–1255. doi: 10.1016/j.ajpath.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilkinson P.D., Delgado E.R., Alencastro F., Leek M.P., Roy N., Weirich M.P., Stahl E.C., Otero P.A., Chen M.I., Brown W.K., Duncan A.W. The polyploid state restricts hepatocyte proliferation and liver regeneration in mice. Hepatology. 2019;69:1242–1258. doi: 10.1002/hep.30286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolosa L., López S., Pareja E., Donato M.T., Myara A., Nguyen T.H., Castell J.V., Gómez-Lechón M.J. Human neonatal hepatocyte transplantation induces long-term rescue of unconjugated hyperbilirubinemia in the Gunn rat. Liver Transplant. 2015;21:801–811. doi: 10.1002/lt.24121. [DOI] [PubMed] [Google Scholar]
- 78.Tsai J.M., Koh P.W., Stefanska A., Xing L., Walmsley G.G., Poux N., Weissman I.L., Rinkevich Y. Localized hepatic lobular regeneration by central-vein–associated lineage-restricted progenitors. Proc Natl Acad Sci U S A. 2017;114:3654–3659. doi: 10.1073/pnas.1621361114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang S., Zhou K., Luo X., Li L., Tu H.-C., Sehgal A., Nguyen L.H., Zhang Y., Gopal P., Tarlow B.D., Siegwart D.J., Zhu H. The polyploid state plays a tumor-suppressive role in the liver. Dev Cell. 2018;44:447–459.e5. doi: 10.1016/j.devcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bielski C.M., Zehir A., Penson A.V., Donoghue M.T.A., Chatila W., Armenia J., Chang M.T., Schram A.M., Jonsson P., Bandlamudi C., Razavi P., Iyer G., Robson M.E., Stadler Z.K., Schultz N., Baselga J., Solit D.B., Hyman D.M., Berger M.F., Taylor B.S. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet. 2018;50:1189–1195. doi: 10.1038/s41588-018-0165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coward J., Harding A. Size does matter: why polyploid tumor cells are critical drug targets in the war on cancer. Frontiers in Oncology. 2014;4:123. doi: 10.3389/fonc.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dewhurst S.M., McGranahan N., Burrell R.A., Rowan A.J., Gronroos E., Endesfelder D., Joshi T., Mouradov D., Gibbs P., Ward R.L., Hawkins N.J., Szallasi Z., Sieber O.M., Swanton C. Tolerance of whole-genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discovery. 2014;4:175–185. doi: 10.1158/2159-8290.CD-13-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang S., Lin Y.-H., Tarlow B., Zhu H. The origins and functions of hepatic polyploidy. Cell Cycle. 2019;18:1302–1315. doi: 10.1080/15384101.2019.1618123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Margall-Ducos G., Celton-Morizur S., Couton D., Bregerie O., Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci. 2005;120:3633–3639. doi: 10.1242/jcs.016907. [DOI] [PubMed] [Google Scholar]
- 85.Celton-Morizur S., Merlen G., Couton D., Desdouets C. Polyploidy and liver proliferation: central role of insulin signaling. Cell Cycle. 2014;9:460–466. doi: 10.4161/cc.9.3.10542. [DOI] [PubMed] [Google Scholar]
- 86.Celton-Morizur S., Merlen G., Couton D., Margall-Ducos G., Desdouets C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. J Clin Invest. 2009;119:1880–1887. doi: 10.1172/JCI38677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang S., Chen Q., Liu Q., Li Y., Sun X., Hong L., Ji S., Liu C., Geng J., Zhang W., Lu Z., Yin Z.-Y., Zeng Y., Lin K.-H., Wu Q., Li Q., Nakayama K., Nakayama K.I., Deng X., Johnson R.L., Zhu L., Gao D., Chen L., Zhou D. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell. 2017;31:669–684.e667. doi: 10.1016/j.ccell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strom S.C., Fisher R.A., Thompson M.T., Sanyal A.J., Cole P.E., Ham J.M., Posner M.P. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 89.Bhatia S.N., Underhill G.H., Zaret K.S., Fox I.J. Cell and tissue engineering for liver disease. Science Translational Medicine. 2014;6 doi: 10.1126/scitranslmed.3005975. 245sr242-245sr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun P., Zhang G., Su X., Jin C., Yu B., Yu X., Lv Z., Ma H., Zhang M., Wei W., Li W. Maintenance of primary hepatocyte functions in vitro by inhibiting mechanical tension-induced YAP activation. Cell Reports. 2019;29:3212–3222.e3214. doi: 10.1016/j.celrep.2019.10.128. [DOI] [PubMed] [Google Scholar]
- 91.Cascio S.M. Novel strategies for immortalization of human hepatocytes. Artif Organs. 2001;25:529–538. doi: 10.1046/j.1525-1594.2001.025007529.x. [DOI] [PubMed] [Google Scholar]
- 92.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 93.Ramboer E., De Craene B., De Kock J., Vanhaecke T., Berx G., Rogiers V., Vinken M. Strategies for immortalization of primary hepatocytes. J Hepatol. 2014;61:925–943. doi: 10.1016/j.jhep.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 95.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 96.Zhang K., Zhang L., Liu W., Ma X., Cen J., Sun Z., Wang C., Feng S., Zhang Z., Yue L., Sun L., Zhu Z., Chen X., Feng A., Wu J., Jiang Z., Li P., Cheng X., Gao D., Peng L., Hui L. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018;23:806–819.e804. doi: 10.1016/j.stem.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 97.Böhm F., Köhler U.A., Speicher T., Werner S. Regulation of liver regeneration by growth factors and cytokines. EMBO Molecular Medicine. 2010;2:294–305. doi: 10.1002/emmm.201000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pepe-Mooney B.J., Dill M.T., Alemany A., Ordovas-Montanes J., Matsushita Y., Rao A., Sen A., Miyazaki M., Anakk S., Dawson P.A., Ono N., Shalek A.K., Van Oudenaarden A., Camargo F.D. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Stem Cell. 2019;25:23–38.e28. doi: 10.1016/j.stem.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borowiak M., Garratt A.N., Wüstefeld T., Strehle M., Trautwein C., Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci U S A. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Natarajan A., Wagner B., Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu L., Finegold M.J., Johnson R.L. Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Nature. 2018;50:e423–e428. doi: 10.1038/emm.2017.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamada Y., Webber E.M., Kirillova I., Peschon J.J., Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X., Xiang C., Du Y., Meng G., Soon Yi L., Sun S., Song N., Xiao Y., Wang J., Yi Z., Liu Y., Xie B., Wu M., Shu J., Sun D., Jia J., Liang Z., Sun D., Huang Y., Shi Y., Xu J., Lu F., Li C., Xiang K., Yuan Z., Lu S., Deng H. Long-term functional maintenance of primary human hepatocytes in vitro. Science. 2019;364:399–402. doi: 10.1126/science.aau7307. [DOI] [PubMed] [Google Scholar]
- 104.Hu H., Gehart H., Artegiani B., LÖpez-Iglesias C., Dekkers F., Basak O., van Es J., de Sousa Lopes S.M.C., Begthel H., Korving J., van den Born M., Zou C., Quirk C., Chiriboga L., Rice C.M., Ma S., Rios A., Peters P.J., de Jong Y.P., Clevers H. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175:1591–1606.e19. doi: 10.1016/j.cell.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 105.Garnier D., Li R., Delbos F., Fourrier A., Collet C., Guguen-Guillouzo C., Chesne C., Nguyen T.H. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-26584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu G.-B., Huang W.-J., Zeng M., Zhou X., Wu H.-P., Liu C.-C., Wu H., Weng J., Zhang H.-D., Cai Y.-C., Ashton C., Ding M., Tang D., Zhang B.-H., Gao Y., Yu W.-F., Zhai B., He Z.-Y., Wang H.-Y., Yan H.-X. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2018;29 doi: 10.1038/s41422-018-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng W.C., Logan C.Y., Fish M., Anbarchian T., Aguisanda F., Álvarez-Varela A., Wu P., Jin Y., Zhu J., Li B., Grompe M., Wang B., Nusse R. Inflammatory cytokine TNFalpha promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018;175:1607–1619.e1615. doi: 10.1016/j.cell.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M.A., Ellis E., van Wenum M., Fuchs S.A., de Ligt J., van de Wetering M., Sasaki N., Boers S.J., Kemperman H., de Jonge J., Ijzermans J.N.M., Nieuwenhuis E.E.S., Hoekstra R., Strom S., Vries R.R.G., van der Laan L.J.W., Cuppen E., Clevers H. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S.W., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., Haft A., Vries R.G., Grompe M., Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamada Y., Kirillova I., Peschon J.J., Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wullaert A., van Loo G., Heyninck K., Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-κB: effects on liver homeostasis and beyond. Endocr Rev. 2007:28365–28386. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- 112.Katsuda T., Kawamata M., Hagiwara K., Takahashi R.-u., Yamamoto Y., Camargo F.D., Ochiya T. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 2017;20:41–55. doi: 10.1016/j.stem.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 113.Katsuda T., Matsuzaki J., Yamaguchi T., Yamada Y., Prieto-Vila M., Hosaka K., Takeuchi A., Saito Y., Ochiya T. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. eLife. 2019;8 doi: 10.7554/eLife.47313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang A.W., Wang Y.J., Zahm A.M., Morgan A.R., Wangensteen K.J., Kaestner K.H. The dynamic chromatin architecture of the regenerating liver. Cell Mol Gastroenterol Hepatol. 2020;9:121–143. doi: 10.1016/j.jcmgh.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yimlamai D., Christodoulou C., Galli G.G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B.Z., Camargo F.D. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Camargo F.D., Gokhale S., Johnnidis J.B., Fu D., Bell G.W., Jaenisch R., Brummelkamp T.R. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 117.Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S.A., Gayyed M.F., Anders R.A., Maitra A., Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu L., Li Y., Kim S.M., Bossuyt W., Liu P., Qiu Q., Wang Y., Halder G., Finegold M.J., Lee J.S., Johnson R.L. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Nat Acad Sci U S A. 2020;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel S.H., Camargo F.D., Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152:533–545. doi: 10.1053/j.gastro.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou D., Conrad C., Xia F., Park J.-S., Payer B., Yin Y., Lauwers G.Y., Thasler W., Lee J.T., Avruch J., Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee K.-P., Lee J.-H., Kim T.-S., Kim T.-H., Park H.-D., Byun J.-S., Kim M.-C., Jeong W.-I., Calvisi D.F., Kim J.-M., Lim D.-S. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Nat Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan W.-C., Pepe-Mooney B., Galli G.G., Dill M.T., Huang H.-T., Hao M., Wang Y., Liang H., Calogero R.A., Camargo F.D. NUAK2 is a critical YAP target in liver cancer. Nature Communications. 2018 doi: 10.1038/s41467-018-07394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 124.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 125.Wada K.I., Itoga K., Okano T., Yonemura S., Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 126.Sansores-Garcia L., Bossuyt W., Wada K.-I., Yonemura S., Tao C., Sasaki H., Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fernandez B.G., Gaspar P., Bras-Pereira C., Jezowska B., Rebelo S.R., Janody F. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 128.Wang R., Ferrell L.D., Faouzi S., Maher J.J., Bishop J.M. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borlak J., Meier T., Halter R., Spanel R., Spanel-Borowski K. Epidermal growth factor-induced hepatocellular carcinoma: gene expression profiles in precursor lesions, early stage and solitary tumours. Oncogene. 2005;24:1809–1819. doi: 10.1038/sj.onc.1208196. [DOI] [PubMed] [Google Scholar]
- 130.Colnot S., Decaens T., Niwa-Kawakita M., Godard C., Hamard G., Kahn A., Giovannini M., Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.-R., Ueno Y., Zheng Y.-W., Koike N., Aoyama S., Adachi Y., Taniguchi H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 132.Ang L.T., Tan A.K.Y., Autio M.I., Goh S.H., Choo S.H., Lee K.L., Tan J., Pan B., Lee J.J.H., Lum J.J., Lim C.Y.Y., Yeo I.K.X., Wong C.J.Y., Liu M., Oh J.L.L., Chia C.P.L., Loh C.H., Chen A., Chen Q., Weissman I.L., Loh K.M., Lim B. A roadmap for human liver differentiation from pluripotent stem cells. Cell Reports. 2018;22:2190–2205. doi: 10.1016/j.celrep.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Agarwal S., Holton K.L., Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- 134.Avior Y., Levy G., Zimerman M., Kitsberg D., Schwartz R., Sadeh R., Moussaieff A., Cohen M., Itskovitz-Eldor J., Nahmias Y. Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology. 2015;62:265–278. doi: 10.1002/hep.27803. [DOI] [PubMed] [Google Scholar]
- 135.Baxter M., Withey S., Harrison S., Segeritz C.-P., Zhang F., Atkinson-Dell R., Rowe C., Gerrard D.T., Sison-Young R., Jenkins R., Henry J., Berry A.A., Mohamet L., Best M., Fenwick S.W., Malik H., Kitteringham N.R., Goldring C.E., Hanley K.P., Vallier L., Hanley N.A. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Basma H., Gutiérrez A.S., Yannam G.R., Liu L., Ito R., Yamamoto T., Ellis E., Carson S.D., Sato S., Chen Y., Muirhead D., Álvarez N.N., Wong R.J., Chowdhury J.R., Platt J.L., Mercer D.F., Miller J.D., Strom S.C., Kobayashi N., Fox I.J. Differentiation and transplantation of human embryonic stem cell–derived hepatocytes. Gastroenterology. 2009;136:990–999.e994. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cai J., Zhao Y., Liu Y., Ye F., Song Z., Qin H., Meng S., Chen Y., Zhou R., Song X., Guo Y., Ding M., Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- 138.Carpentier A., Nimgaonkar I., Chu V., Xia Y., Hu Z., Liang T.J. Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res. 2016;16:640–650. doi: 10.1016/j.scr.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Song Z., Cai J., Liu Y., Zhao D., Yong J., Duo S., Song X., Guo Y., Zhao Y., Qin H., Yin X., Wu C., Che J., Lu S., Ding M., Deng H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 140.Kondo Y., Iwao T., Nakamura K., Sasaki T., Takahashi S., Kamada N., Matsubara T., Gonzalez F.J., Akutsu H., Miyagawa Y., Okita H., Kiyokawa N., Toyoda M., Umezawa A., Nagata K., Matsunaga T., Ohmori S. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metabolism and Pharmacokinetics. 2014;29:237–243. doi: 10.2133/dmpk.dmpk-13-rg-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kvist A.J., Kanebratt K.P., Walentinsson A., Palmgren H., O’Hara M., Björkbom A., Andersson L.C., Ahlqvist M., Andersson T.B. Critical differences in drug metabolic properties of human hepatic cellular models, including primary human hepatocytes, stem cell derived hepatocytes, and hepatoma cell lines. Biochem Pharmacol. 2018;155:124–140. doi: 10.1016/j.bcp.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 142.Du C., Feng Y., Qiu D., Xu Y., Pang M., Cai N., Xiang A.P., Zhang Q. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem Cell Res Ther. 2019;9 doi: 10.1186/s13287-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thi V.L.D., Wu X., Belote R.L., Andreo U., Takacs C.N., Fernandez J.P., Vale-Silva L.A., Prallet S., Decker C.C., Fu R.M., Qu B., Uryu K., Molina H., Saeed M., Steinmann E., Urban S., Singaraja R.R., Schneider W.M., Simon S.M., Rice C.M. Stem cell-derived polarized hepatocytes. Nature Communications. 2020 doi: 10.1038/s41467-020-15337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 145.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 146.Loh K.M., ANG L.T., Zhang J., Kumar V., Ang J., Auyeong J.Q., Lee K.L., Choo S.H., Lim C.Y.Y., Nichane M., Tan J., Noghabi M.S., Azzola L., Ng E.S., Durruthy-Durruthy J., Sebastiano V., Poellinger L., Elefanty A.G., Stanley E.G., Chen Q., Prabhakar S., Weissman I.L., Lim B. Efficient endoderm induction from human pluripotent stem cells by logically directing signals controlling lineage bifurcations. Cell Stem Cell. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kiamehr M., Alexanova A., Viiri L.E., Heiskanen L., Vihervaara T., Kauhanen D., Ekroos K., Laaksonen R., Käkelä R., Aalto-Setälä K. hiPSC-derived hepatocytes closely mimic the lipid profile of primary hepatocytes: a future personalised cell model for studying the lipid metabolism of the liver. J Cell Physiol. 2018;234:3744–3761. doi: 10.1002/jcp.27131. [DOI] [PubMed] [Google Scholar]
- 148.Tolosa L., Caron J., Hannoun Z., Antoni M., López S., Burks D., Castell J.V., Weber A., Gómez-Lechón M.J., Dubart-Kupperschmitt A. Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Research & Therapy. 2015;6:246. doi: 10.1186/s13287-015-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carpentier A., Tesfaye A., Chu V., Nimgaonkar I., Zhang F., Lee S.B., Thorgeirsson S.S., Feinstone S.M., Liang T.J. Engrafted human stem cell–derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953–4964. doi: 10.1172/JCI75456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao X., Liu Y. A transcriptomic study suggesting human iPSC-derived hepatocytes potentially offer a better in vitro model of hepatotoxicity than most hepatoma cell lines. Cell Biol Toxicol. 2017;33:407–421. doi: 10.1007/s10565-017-9383-z. [DOI] [PubMed] [Google Scholar]
- 151.Nagamoto Y., Takayama K., Ohashi K., Okamoto R., Sakurai F., Tachibana M., Kawabata K., Mizuguchi H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068–1075. doi: 10.1016/j.jhep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 152.Azuma H., Paulk N., Ranade A., Dorrell C., Al-Dhalimy M., Ellis E., Strom S., Kay M.A., Finegold M., Grompe M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nature Biotechnology. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Asrani S.K., Larson J.J., Yawn B., Therneau T.M., Kim W.R. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145:375–382.e372. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tan A.K.Y., Loh K.M., Ang L.T. Evaluating the regenerative potential and functionality of human liver cells in mice. Differentiation. 2017;98:25–34. doi: 10.1016/j.diff.2017.09.003. [DOI] [PubMed] [Google Scholar]