Highlights
-
•
Miscanthus sinensis, an invasive plant species as potential source of commercial dyes.
-
•
Microwave extraction can yield dye extracts from M. sinensis.
-
•
Optimum condition is at 15 s −150 mL solvent - 540 W microwave power setting.
-
•
Extraction using 1:1 ethanol-water mixture had highest yield and color-fastness.
-
•
Mordant application improves binding of flavonoid-based M. sinensis dyes on fabric.
Keywords: Color fastness, Extract, Flavonoid, Microwave-assisted extraction, Miscanthus sinensis, Mordant
Abstract
The study was designed to devise a high-yielding, microwave-assisted extraction of the colored material from the core tissue of runo (Miscanthus sinensis Andersson) stem. Soxhlet extraction of M. sinensis core tissue gave yields ranging from 1.04 % with dichloromethane to 11.91 % from 1:1 ethanol-water mixture. Folin-Ciocalteau tests showed that the extracts were primarily flavonoid compounds, accounting for 66.05 ppm of the total 1:1 ethanol-water extractable material. Initial application trials of the ethanol, ethyl acetate, and ethanol-water extracts followed by color fastness tests showed poor retention on both paper and cotton fabric, suggesting the need for a mordant. Subsequent trials with aluminum acetate as mordant showed greatly improved binding of the ethanol-water extracted dye onto the cotton fabric following wash, rubbing, and light fastness tests. A two-level, full factorial model extraction procedure to determine the effects of extraction time (15 s – 90 s), solvent volume (50 mL – 150 mL), and microwave power level (90 W – 540 W) was done for all solvents used. All three factors had a significant effect on the dye extraction yield, along with the interactions between duration-power level and volume-power level. The highest yield for microwave assisted extraction was at 15 s −150 mL – 540 W setting. Results suggest that microwave extraction can potentially produce dye extracts from M. sinensis core material with a faster throughput than simple soaking and Soxhlet extraction.
1. Introduction
The use of natural dyes to render color to fabrics, paper and to cosmetics preceded human use of synthetically made dyes, which are easy to standardize and more economical to use. However, the discovery that synthetic dyes were toxic, and that they cause allergic reactions or that some were carcinogenic [1] fueled the return to natural dyes or colorants. Natural dyes derived from animals and plants have a non-toxic, non-carcinogenic and biodegradable nature [2]. Moreover, the production of natural dyes in comparison with synthetic dyes, produces less pollutants that trigger wastewater problems [1,3]. Nowadays, there are many kinds of dyes that can be used differently depending on their production, making the extraction process and its characterization an important part of industrial procedures.
The genus Miscanthus from the Poaceae family has approximately 17 species that are classified as perennial, non-wood, rhizomatous, tall grasses existing between the tropical and subtropical regions of Asia. In the Philippines, Miscanthus sinensis Andersson, known locally as runo or tanlak, is found in high elevation areas with low temperature such as the highlands of Laguna, Cavite, and Nueva Vizcaya provinces. However, the plant is considered as invasive (https://www.cabi.org/isc/datasheet/34269). It grows with a woody stem covered with leaf sheaths and with a foam-like core material. Local people make house walls, curtains, and handicrafts from the stem, but high-value products have not yet been explored. The occurrence of red-colored foam-like interior in the stem of M. sinensis indicates the presence of chromophores which can be extracted as dye.
Microwave-assisted extraction (MAE) method is a relatively new extraction technique, which combines microwave technology with traditional solvent extraction [4]. Electromagnetic waves cause vibrations of polar molecules in the cells resulting in intermolecular and intramolecular friction from the collisions of charged ions, heating up substrates in the cellular membrane and increase pressure that ruptures the cell membrane [5]. The phytochemical constituents can then be extracted out of the plant cell because the heat and mass transfer gradients are in the same direction [4]. Thus, microwave extraction times are shorter, which can be done in less than 5 min instead of 4 h onwards [6].
The primary aim of this study is to devise a high-yielding, short-duration, microwave-assisted dye extraction (MAE) process for extracting dyes for use in fabric and paper. The specific objectives are to: a) determine which solvent among hexane, dichloromethane, ethyl acetate, ethanol, and 1:1 ethanol-water mixture will provide the highest extractive yield from M. sinensis; b) test its application and color-fastness as dye for cotton fabric and paper; and c) determine which parameter (power, duration, and solvent-to-material ratio) greatly affects the yield during microwave-assisted extraction (MAE). The findings will benefit the industry looking for renewable resources as a source of dyes. Runo can be transformed from an abundant invasive plant species to one that will provide greater gain as an industrially important raw material.
2. Material and methods
2.1. Chemicals and reagents
Dichloromethane, ethanol, hexane, ethyl acetate, anhydrous sodium acetate, copper (II) sulfate pentahydrate, aluminum potassium sulfate dodecahydrate, calcium carbonate, methanol, aluminum chloride, potassium acetate, Folin-Ciocalteau reagent, sodium carbonate, quercetin, and gallic acid were purchased from Sigma Chemical Co. (Philippines). All the chemicals and reagents were of analytical grade for this study.
2.2. Harvesting and preparation of M. sinensis for extraction
Runo stems were harvested from Barangay Cueva, a highland village in Sta. Maria, Laguna, Philippines without considering the age and cultivation practices of the plant. The plants were chosen randomly, and the leaves, flowers, and roots were cut off, and only the stems were used. The stems’ nodes were removed with a band saw. The internodes were then split in half, and the soft, reddish, foam-like interior core was scraped off carefully with a stainless-steel knife to avoid contaminating the sample with rust and other minerals.
The collected core samples were then air-dried. The moisture content (MC) of the samples was measured by weighing 1 g of the sample and oven-drying at 105°C (in triplicate) and calculating by gravimetric method.
2.3. Dye extraction
To test which solvent will give the highest yield and the strongest binding capacity with the paper and fabric, Soxhlet extraction was done initially. Approximately 1 g of the sample was wrapped in filter paper and placed into the Soxhlet apparatus, containing separately the different solvents as follows: hexane, dichloromethane, ethyl acetate, ethanol and 1:1 ethanol-water. Triplicate extraction with each solvent was done for 4 h after the first siphoning. The solvent was removed by rotary evaporation from the resulting solution containing the extract, and percent (%) yield was calculated based on the dry mass of the starting material. All extracts obtained with the different solvents were applied to both the fabric and paper. The best solvent system was chosen based on the most intense color change and colorfastness, and subsequently used in optimization of the microwave assisted extraction (MAE) method.
For the MAE, 1 g amount of the core material in a 250 mL beaker with the specified volume of 1:1 ethanol:water was placed inside a microwave oven. The different combinations of duration, sample-to-solvent ratio and microwave power setting were calculated using Design Expert 11. The resulting solutions were filtered, the solvent removed by evaporation in a rotary evaporator, and yield was determined based on the oven-dry mass of starting material.
Using a Hitachi UH5300 UV–vis Spectrophotometer, the wavelength of maximum absorbance of the extract for each solvent was measured at a concentration of 400 ppm.
2.4. Application of dye to paper and cotton fabric
The dye extract from the Soxhlet extraction was re-dissolved with the corresponding solvent at a 1:100 extract-to-solvent ratio. The resulting mixture was used as a dye bath for application to the fabric as follows: A 1.5″ × 1.5″ cotton fabric was first soaked in hot water to remove any impurities and to soften the fibers before application of mordants. The fabric used was cotton flannel since it is sturdier than cotton and can withstand higher temperature treatments than traditional cotton cloth. After soaking, the fabric is then dried before the actual dyeing. A mixture of Alum (KAl(SO4)2) and sodium acetate (CH3COONa) with a ratio of 1:1 was used as primary mordant. The mordant bath was based on a 20 % weight of fabric (WOF) ratio at a temperature of 70 °C for a 1-hr duration. After the primary mordant application, a calcium carbonate (CaCO3) bath was prepared with 2 g of CaCO3 in 200 mL distilled water at a temperature of 60 °C within 1 h.
Dye extracts were re-dissolved in their respective solvents at a 1:100 extract-to-solvent. Fabric samples were dyed by soaking in the extract solution for 100 min at 70 °C. Afterwards, alternating cold (20 °C) and hot wash (80 °C) procedure with distilled water was performed. This was done in 3 replicates. After drying, the dyed fabric was soaked in a 2% copper sulfate (Cu2SO4) (based on WOF) at a temperature of 70 °C for 1 h.
For application of the dye on paper, Whatman No.1 filter paper was used, which was cut to a 1.5″ × 1.5″ size. Pure methanol was first applied for 20 s. After air-drying, the filter paper was soaked in a dye bath with a 1:100 extract-to-solvent ratio for 30 min. at room temperature. The dyed paper was then washed with distilled water and air-dried for 15 h. This was done with 3 replicates.
2.5. Color fastness and color analysis
The color fastness test was performed to determine the effectiveness of the fabric and paper application of the dye using wash fastness, rubbing fastness, and light fastness tests. The relative color value before and after the fastness test were measured and compared using the James Heal gray scale [14] for both shade and staining, as described by Subtirica et al. [7].
The wash fastness test was done by stitching the dyed fabric between two cotton fabric. This was then washed in a 500 mL beaker with commercial-grade Wings powdered detergent (with the following components: sodium carbonate, non-ionic detergents, sodium silicate, soil suspending agents, enzyme, and fragrance), at a 5:500 soap-to-water ratio at 60 °C for 30 min on a hot plate stirrer. The rubbing fastness test was done with a 4.5″ × 1.5″ of dyed fabric sample, following ISO 105-X12 method [8] which went along one direction of warp//length and weft/ width of the fabric. The specimen was rubbed back and forth over a straight track for 10 times at 1 cycle/second. For light fastness, the dyed fabric and paper were exposed under UV light for 12 h.
2.6. Total phenolic and flavonoid content
The total phenolic content was determined by reacting the dye solution with Folin-Ciocalteau reagent and 75 g/L Na2CO3 solution. Absorption was detected using the UH5300 UV–vis Spectrophotometer at 760 nm after allowing the mixture to stand in the dark for 2 h. Gallic acid was used as a standard and total phenolics were expressed as gallic acid equivalent (GAE). For the total flavonoid content, this was determined by mixing aluminum chloride and the sample with methanol-potassium acetate solution. The absorbance was read at 430 nm after 10 min. Quercetin was used as a standard and total flavonoid content was expressed as quercetin equivalent (QE).
2.7. Statistical analyses
Parametric analysis was employed for three parameters (power setting, duration, and solvent volume) using Design Expert 11. The difference to detect (Delta-“Signal”) was set to 1 and the estimated standard deviation (Sigma-“Noise”) was set to 0.73 based on past studies [9]. ANOVA and Tukey’s HSD post-hoc test was also employed to test the significance of the yield results of each solvent used for extraction.
3. Results and discussion
3.1. Soxhlet extraction yield in different solvents
The yield of the extracted material from the Soxhlet extraction of the soft inner core material of M. sinensis stem in different solvents is shown in Table 1. Yield was highest for 1:1 ethanol-water mixture at 11.91 %, followed by hexane, ethanol, ethyl acetate, and dichloromethane gave the lowest yield at 1.04 %. The results indicate that most of the extractable materials are polar, as ethanol:water is the most polar among the solvents used. Results of the ANOVA and Tukey’s HSD post-hoc test revealed that the solvents gave statistically significant yield variations, except between ethyl acetate and dichloromethane. Reddish extracts were obtained using 1:1 ethanol-water mixture, ethyl acetate, and ethanol, while an oily mixture was obtained with hexane and dichloromethane. Interestingly, the 1:1 ethanol-water extract was also the darkest in color, followed by the ethyl acetate extract.
Table 1.
M. sinensis core material extract yield in different solvents (triplicate) by Soxhlet extraction.
| Solvent/Solvent system | Average Percent Yield of Extract (%) |
|---|---|
| 1:1 Ethanol-Water | 11.91a |
| Hexane | 5.49b |
| Ethanol | 3.22c |
| Ethyl Acetate | 1.16d |
| Dichloromethane | 1.04d |
Note: Means with the same letter are not significantly different from one another according to Tukey’s HSD post-hoc test.
3.2. Color analysis of dye extract
The individual extracts from different solvents were analyzed at a basis concentration of 400 ppm by determining the wavelengths of maximum absorbance under UV–vis (Table 2). The dye extract with ethanol transmits an orange to near-red color. On the other hand, ethyl acetate dye extract transmits a red color. The extract with the 1:1 ethanol-water mixture was expected to transmit a darker shade of red, however, it was transmitting green color based on the UV spectrogram.
Table 2.
Wavelength of maximum absorbance at 400 ppm of M. sinensis core material extractives in different solvents.
| Solvent | Wavelength (nm) |
|---|---|
| Ethanol | 472 |
| Hexane | 299 |
| Dichloromethane | 314 |
| 1:1 Ethanol-Water | 382 |
| Ethyl Acetate | 499 |
3.3. Color fastness
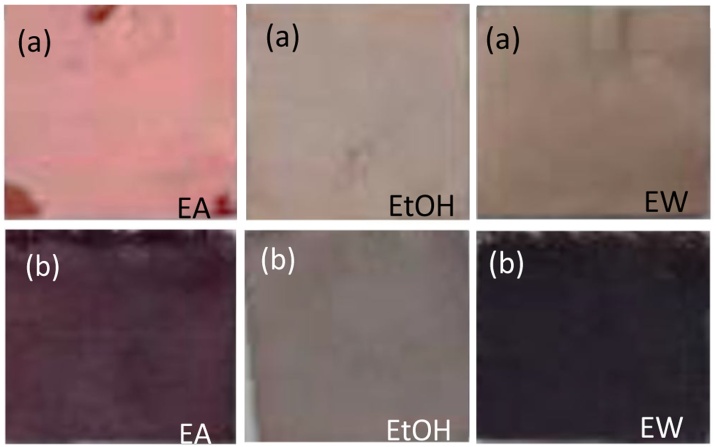
The extracts from M. sinensis core material with different solvents were applied as dye on paper and cotton fabric and the results are shown in Fig. 1. Preliminary trials showed poor color retention on the fabric by the pure extract, thus mordanting with aluminum acetate was done in subsequent dyeing treatments. This improved fastness, but also darkened the resulting color on the fabric.
Fig. 1.
Photographed images of (a) Whatman no. 1 filter paper and (b) aluminum acetate-mordanted cotton fabric treated with ethyl acetate (EA), ethanol (EtOH), and 1:1 ethanol-water (EW) extracts of Miscanthus sinensis.
Wash fastness is the resistance of a material to change its color when subjected to washing. Table 3 shows the results of the wash fastness test of the M. sinensis extracts on the cotton fabric. Fig. 2A shows the color changes after washing, which were ranked for shade as follows: 1:1 ethanol-water mixture > ethyl acetate > ethanol. The corresponding staining test (Fig. 2B) showed that the staining grades of ethanol and 1:1 ethanol-water mixture are similar with a value of 5, which means that no staining is evident in fabrics dyed with the said solvents. The ethyl acetate extract is the only one to exhibit staining in the wash fastness test.
Table 3.
Wash fastness on cotton fabric dyed with M. sinensis extract using different solvents.
| Solvent/Solvent System | Trial | Shade Gradea | Staining Gradeb |
|---|---|---|---|
| Ethyl acetate | 1 | 2−3 | 4 |
| 2 | 2−3 | 4 | |
| 3 | 2 | 4 | |
| 1:1 Ethanol: Water | 1 | 3 | 5 |
| 2 | 3 | 5 | |
| 3 | 3 | 5 | |
| Ethanol | 1 | 1−2 | 5 |
| 2 | 1−2 | 5 | |
| 3 | 1−2 | 5 |
Based on James Heal gray scale for shade (5-highest fastness; 1- lowest fastness).
Based on James Heal gray scale for staining (5-highest fastness; 1- lowest fastness).
Fig. 2.
A. Results of the wash fastness test of cotton fabric treated with ethyl acetate (EA), ethanol (EtOH), 1:1 ethanol-water (EW) extracts from Miscanthus sinensis. B. Wash fastness staining test on cotton fabric cloth treated with ethyl acetate (EA), ethanol (EtOH), and 1:1 ethanol-water (EW) Miscanthus sinensis extracts.
For the light fastness test, bleaching is caused by the impact of UV radiation on the color-rendering chemical structure of the molecules or chromophores. The light-induced changes in the chemical bonds of the pigment is called photodegradation [2]. Exposure to UV light is done in a dark environment to ensure that only the UV light affects the color of the samples. As seen in Fig. 3A, light fastness of the ethyl acetate and 1:1 ethanol:water extract on paper was high on UV light treatment, compared with the ethanol extract. On the other hand, cotton fabric light fastness is high for all extracts, in the following order: 1:1 ethanol-water mixture > ethyl acetate > ethanol. Table 4 shows the grades of the light fastness test for both paper and fabric.
Fig. 3.
A. Light fastness test of filter paper treated with ethyl acetate (EA), ethanol (EtOH), and 1:1 ethanol-water (EW) extracts of Miscanthus sinensis. B. Light fastness test of cotton fabric treated with ethyl acetate (EA), ethanol (EtOH), and 1:1 ethanol-water (EW) extracts of Miscanthus sinensis.
Table 4.
Light fastness grade of the shade of M. sinensis extracts from 3 different solvents in paper and cotton fabric.
| Solvent Trial | Paper | Fabric | |
|---|---|---|---|
| Ethyl Acetate | 1 | 3 | 3 |
| 2 | 4 | 3 | |
| 3 | 3 | 3 | |
| 1:1 Ethanol-Water | 1 | 3 | 3 |
| 2 | 4 | 3−4 | |
| 3 | 3 | 3−4 | |
| Ethanol | 1 | 2 | 3 |
| 2 | 2−3 | 2−3 | |
| 3 | 3 | 3 | |
*5-highest fastness grade; 1- lowest fastness grade.
The rubbing fastness test determines how well the dye will resist removal from the fabric by rubbing action. Table 5 shows the grade of the dry and wet rubbing fastness of cotton fabric treated with M. sinensis extracts from the 3 solvents. The wet rubbing fastness was the same for all solvents, which means that extracts obtained from the 3 solvents can resist staining from the rubbing action. On the other hand, for the dry rubbing fastness test, the resistance to rubbing action is greater for ethanol than for ethyl acetate and 1:1 ethanol-water mixture extracts. For the shade rubbing fastness grade, the order is 1:1 ethanol-water mixture > ethyl acetate > ethanol. The results of the dry rubbing fastness test can be seen in Figs. 4A-B.
Table 5.
Rubbing fastness grade for wet and dry staining and shade of cotton fabric treated with M. sinensis extracts with different solvents.
| Solvent | Wet (staining) | Dry (staining) | Shade | |
|---|---|---|---|---|
| Ethyl Acetate | Trial 1 | 2 | 2 | 4 |
| Trial 2 | 3 | 2−3 | 4 | |
| Trial 3 | 2−3 | 3 | 3−4 | |
| 1:1 Ethanol-Water | Trial 1 | 3 | 3 | 4−5 |
| Trial 2 | 2−3 | 2 | 4 | |
| Trial 3 | 2 | 3 | 4−5 | |
| Ethanol | Trial 1 | 2 | 3−4 | 3 |
| Trial 2 | 2−3 | 3 | 3 | |
| Trial 3 | 3−4 | 4 | 3 | |
*5-highest fastness grade; 1- lowest fastness grade.
Fig. 4.
A. Dry rubbing fastness test of cotton fabric treated with ethyl acetate (EA), ethanol (EtOH), 1:1 ethanol-water (EW) extracts from M. sinensis. B. Dry rubbing fastness stain test of cotton fabric treated with ethyl acetate (EA), ethanol (EtOH), 1:1 ethanol-water (EW) of M. sinensis.
Overall, the color fastness tests indicate that M. sinensis extracts obtained using 1:1 ethanol-water was the most stable dye in both fabric and paper. With this result and along with the yield, subsequent trials for microwave-assisted extraction was done with 1:1 ethanol-water and the determination of total phenolic and flavonoid content was performed for the M. sinensis extract with this solvent.
3.4. Microwave-assisted extraction (MAE)
Microwave-assisted extraction (MAE) uses microwave energy to produce the necessary heat for extraction with the added disintegration of cell walls of the plant material [9]. The presence of a solvent leaches out the necessary extract. In this study, the closed-vessel type of MAE was employed where the equipment essentially causes high temperature and pressure to build-up within the closed system, causing dehydration of the cell wall. This generates tremendous pressure within the cell wall causing it to swell and rupture. The phytochemical constituents will then be leached out of the plant cell. This can also be aided by solvents having higher heating efficiency [6].
The parameters that were investigated were duration in seconds (D), volume of solvent in mL (V), and power, watts (P). Design Expert 11 runs were simulated for a total of 24 runs in various combinations of the lowest and highest values of the parameters, and 4 runs for the center points of the factors. The highest values analyzed for the parameters were as follows: volume (150 mL), duration (90 s), and power (540 W). On the other hand, the corresponding lowest values were as follows: volume (50 mL), duration (15 s), and power (90 W). Two-level full factorial model was used for the parametric analysis. Average percent extract yields of the runs are shown in Table 6. The highest average extract yield was acquired using a 15-150-540 (DVP) run setting, resulting in 8.7255 % yield, while the lowest average extract yield was acquired with 15-50-90 (DVP) at 1.5762 %. Comparing the one-and-a-half minute-long MAE with Soxhlet extraction, the MAE gave a lower yield than Soxhlet extraction method, suggesting that short duration of extraction with the MAE cannot remove all possible extractable components from the source material. Soxhlet extraction is a standard procedure for extracting components from biological materials that is normally done for as long as 8 h [10] to remove most of the extractable materials present. Solvent:material ratios are high, ranging from 50 to 200 times the material to be extracted [11].
Table 6.
Average percent yield of experimental runs (triplicates) of microwave assisted extraction of M. sinensis with 1:1 ethanol:water.
| Duration (S) | Volume (mL) | Power (W) | Average Percent Yield (%) |
|---|---|---|---|
| 15 | 50 | 90 | 1.5762 |
| 90 | 50 | 90 | 6.1026 |
| 15 | 150 | 90 | 1.6482 |
| 90 | 150 | 90 | 5.7252 |
| 15 | 50 | 540 | 4.5328 |
| 90 | 50 | 540 | 3.9898 |
| 15 | 150 | 540 | 8.7255 |
| 90 | 150 | 540 | 8.5550 |
| 52.5 | 100 | 315 | 4.1855 |
The results imply that at an industrial scale, the main advantage of the MAE method is that it can extract the dye at shorter extraction times, and with less solvent volume. For the experiments done, MAE yielded 8.5 % extracts within 90 s (or 1 and ½ min), while 8 h of extraction was required to obtain the 11.9 % yield in Soxhlet extraction. This could mean significant cost reduction when MAE is employed in industry.
The analysis of variance (ANOVA) of the factorial model shows that the model is significant with an F-value of 51.76, which means that it can be used for further analysis of the interactions of the parameters within the boundaries used. ANOVA shows that all three individual parameters were significant model terms, but in terms of interaction between parameters, only volume-power and duration-power interactions were significant. On the other hand, the R2 of the model has a value of 0.9477 with a coefficient of variation of 13.23 %.
The ANOVA also shows there is a significant effect on the percent yield coming from the combined models of duration-power level and volume-power level.
Longer extraction time gives more time for the solvent to penetrate the material at a high-power level. However, higher duration also means higher exposure to heat which can degrade most of the dye extracts. This is shown in the decrease of values of percent yield with increasing power level while a continuous increase in values for increasing duration.
On the other hand, the more solvent at a higher power level, the greater cell swelling that creates higher pressure on the cell wall. This causes stretching and facilitates breakdown of cell walls. This releases the dye into the solvent. Moreover, the higher the power of the microwave used, then the temperature of the extraction process will approach the boiling point of the solvent more rapidly. Attaining the boiling point at a faster time results in faster solvent evaporation, which then reduces solvent volume that penetrates into the material
The 1:1ethanol: water extract using MAE shows a relatively lighter shade of red compared to the dye extracted using Soxhlet extraction method. By spectrophotometric analysis, on a basis of 400 ppm, the wavelength of maximum absorbance was at 297 nm which is outside the visible spectrum. This can be due to the burning of dye particles during the MAE method. The burnt particles from the extraction process could interfere with the spectrophotometric analysis giving a lower wavelength of maximum absorbance.
3.5. Total phenolic and flavonoid content
To determine the possible source of the dye’s color and provide a basis for its composition, two colorimetric methods were done: Folin-Ciocalteau (FCR) test [12] and another test using aluminum chloride (AlCl3) reagent [13]. The FCR method measures the total phenolic compound in the sample. On the other hand, AlCl3 method measures the total flavonoid content of the sample [13]. Some phenolic compounds possess color-producing compounds, and flavonoids are naturally occurring phenolic compounds. Plant dyes containing flavonoids (flavonoid-dyes) are used as mordant-dyes.
The absorbance curve obtained using FCR colorimetric assay indicates an R2 of 0.9233 with the linear regression equation, y = 0.023x-0.0535. The gallic acid equivalent (GAE), which pertains to the total phenolic compound found in the dye extract of 1:1 ethanol-water mixture has an average value of 113.61 ppm. Presence of phenolic compounds in the extract can mean the presence of flavonoids responsible for its color.
The absorbance curve obtained in the aluminum chloride colorimetric test indicates an R2 of 0.9895 for the plot of absorbance vs. concentration with the linear regression equation, y = 0.0079x-0.0314. The quercetin equivalent (QE), which indicates the total flavonoid content in the 1:1 ethanol-water extract, gave an average value of 66.05 ppm. From the total phenolic compound, more than half, i.e. approximately 58 % are flavonoid compounds, which account for the observed color of the dye.
4. Conclusion
Soxhlet extraction of Miscanthus sinensis core material yielded average values of 3.22 %, 5.49 %, 1.16 %, 1.04 % and 11.91 % for solvents ethanol, hexane, ethyl acetate, dichloromethane, and 1:1 ethanol-water mixture, respectively. From all the solvents, only ethyl acetate, ethanol and 1:1 ethanol-water mixture gave colored extractives. Spectrophotometric analysis of the dye-yielding solvents shows that the ethanol extract produces an orange to a near-red color, while ethyl acetate extract produces a red color, and 1:1 ethanol-water mixture extract produces a green color.
For the application to paper and fabric, the 1:1 ethanol-water extract produces the darkest color while the ethanol extract gives the lightest color. Wash fastness, light fastness, and rubbing fastness tests showed that 1:1 ethanol-water mixture extract has the highest color fastness among the three solvents used for dye extraction. Mordanting with aluminum acetate was needed because of the low ability of the extract to bind with the fabric. Characterization of the dye extract shows that total phenolic content amounts to 113.6087 ppm and total flavonoids content have a value of 66.0506 ppm which gives the dye its color.
Parametric analysis of MAE shows an R2 of 0.9477 for the model created using Design Expert 11. ANOVA shows that all three parameters (duration, volume, and power) are significant along with the interaction of duration with power, volume with power, and the interaction among the 3 factors. The study shows that a 15−250-540 (DVP) run setting produced the highest average extractive yield at 8.73 %. The potential advantage of microwave assisted extraction in industry was demonstrated because of the significant extraction time reduction, albeit the yield is slightly lower than that of Soxhlet extraction.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Ivan Anthony P. Pinzon: Writing - original draft, Writing - review & editing, Investigation, Data curation. Ramon A. Razal: Conceptualization, Methodology, Resources, Supervision, Writing - review & editing. Rosalie C. Mendoza: Visualization, Formal analysis, Writing - review & editing, Software. Rowena B. Carpio: Validation, Software, Formal analysis. Ramon Christian P. Eusebio: Formal analysis, Software, Validation.
References
- 1.Baliarsingh S., Panda A., Jena J., Das T., Das N. Exploring sustainable technique on natural dye extraction from native plants for textile: identification of colorants, colorimetric analysis of dyed yarns and their antimicrobial evaluation. J. Clean. Prod. 2012;37:257–264. doi: 10.1016/j.jclepro.2012.07.022. [DOI] [Google Scholar]
- 2.Siva R. Status of natural dyes and dye-yielding plants in India. Curr. Sci. 2007;92(7) [Google Scholar]
- 3.Ali S., Hussain T., Nawaz R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009;17:61–65. doi: 10.1016/j.jclepro.2008.03.002. [DOI] [Google Scholar]
- 4.Beoletto V., Oliva M., Marioli J., Carezzano M., Demo M. Vol. 1. 2016. Antimicrobial Natural Products Against Bacterial Biofilms; pp. 291–307. (Antibiotic Resistance Mechanisms and New Antimicrobial Approaches). Chapter 14. [DOI] [Google Scholar]
- 5.Nautiyal P., Subramanian K., Dastidar M. Reference Module in Earth Systems and Environmental Sciences. 2014. Recent advancements in the production of biodiesel from algae: a review. [DOI] [Google Scholar]
- 6.Chemat F., Cravotto G. Springer New York Heidelberg Dordecht; London: 2012. Microwave –assisted Extraction for Bioactive Compounds: Theory and Practice. [DOI] [Google Scholar]
- 7.Subtirica A., Taskoparan F., Ghituleasa C., Vamesu M. 2020. Color Stability of Naturally Dyed Denim Fabrics. Retrieved July 14, 2020 from http://textile.webhost.uoradea.ro/ [Google Scholar]
- 8.ISO . 2019. International Organization for Standardization 59.080.001 – Textiles in General. Retrieved November 16 2018 from https://www.iso.org/ics/59.080.01/x. [Google Scholar]
- 9.Sinha K., Das Saha P., Datta S. Extraction of natural dye from petals of Flame of forest (Butea monosperma) flower: process optimization using response surface methodology (RSM) Dye. Pigment. 2012;94:212–216. doi: 10.1016/j.dyepig.2012.01.008. [DOI] [Google Scholar]
- 10.De Boer J. Encyclopedia of Analytical Science. 2nd ed. 2005. Polychlorinated Biphenyls; pp. 214–225. [DOI] [Google Scholar]
- 11.Ridgway K., Smith R., Lalljie S. Vol. 3. 2012. Sample preparation for food contaminant analysis; pp. 819–833. (Comprehensive Sampling and Sample Preparation). [DOI] [Google Scholar]
- 12.Blainski A., Lopes G., De Mello J. Application and analysis of the folin ciocalteau method for the determination of the total phenolic content from Limonium Brasiliense L. Molecules. 2013;(18):6852–6865. doi: 10.3390/molecules18066852. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H., Song Y., Zhang X. Determination of total flavonoids in leek by AlCl3 colorimetric assay. Chem. Eng. Trans. 2017;59(2017):775–780. doi: 10.3303/CET1759130. [DOI] [Google Scholar]
- 14.TEXANLAB (n.d.). Color Fastness to Rubbing. Texanlab Laboratories Pvt. Ltd.. Colorfastness Testing Series. Retrieved September 4, 2018 from http://www.texanlab.com/documents/downloads/4.pdf.