Highlights
-
•
This case highlights two serious side effects of empagliflozin occurring concurrently. Those being diabetic ketoacidosis and Fournier’s gangrene.
-
•
Diabetic ketoacidosis is an important cause of perioperative high anion gap metabolic acidosis in the context of SGLT2 inhibitors and needs to be carefully managed.
-
•
SGLT2 inhibitors should be held up to 48 h prior to surgery to minimize the risk of diabetic ketoacidosis perioperatively.
Abbreviations: DKA, diabetic ketoacidosis; HAGA, high anion gap metabolic acidosis; SGLT2, sodium glucose cotransporter-2; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus
Keywords: Sodium-glucose cotransporter-2 (SGLT2) inhibitor, Euglycemic diabetic ketoacidosis, Type 2 diabetes mellitus, Fournier’s gangrene, Case report
Abstract
Introduction
Sodium glucose cotransporter-2 inhibitors (SGLT2) are an increasingly administered class of medication used to lower blood glucose levels in patients with type 2 diabetes mellitus. Diabetic ketoacidosis (DKA) and Fournier’s gangrene are rare, but potentially catastrophic side effects of SGLT2 inhibitors. This manuscript reports a case of both DKA and Fournier’s gangrene in the context of SGLT2 inhibitor use.
Presentation of case
A 51-year-old morbidly obese man with hypertension and poorly controlled Type 2 Diabetes Mellitus presented to the emergency department with a clinical presentation consistent with Fournier’s gangrene. He was promptly taken to the operating room by the urology team where he had extensive debridement of the perineum and abdomen. Intra-operatively he was found to have DKA, which was managed appropriately. The acidosis and Fournier’s gangrene were deemed a likely side effect of SGLT2 inhibitor use. After a thirty-day hospital admission, the patient was discharged to a rehabilitation facility where he is progressing well. His SGLT2 inhibitor was discontinued upon admission to hospital.
Discussion
Perioperative providers should have a high index of suspicion for diabetic ketoacidosis (DKA) and Fournier’s gangrene in patients prescribed SGLT2 inhibitors. Prompt treatment of DKA through correction of underlying triggers, aggressive fluid resuscitation, insulin to close the anion gap, and appropriate potassium repletion is vital to optimize patient outcomes.
Conclusion
The use of SGLT2 inhibitors among surgical populations is increasing. This case highlights the importance of being aware of the mechanism and side effects of SGLT2 inhibitors, and the management of DKA.
1. Introduction
Diabetic ketoacidosis (DKA) is an acute complication of diabetes mellitus characterized by hyperglycemia and ketoacidosis that may be encountered in the perioperative setting. If not recognized and treated early, DKA can lead to life-threatening complications such as metabolic acidosis, electrolyte abnormalities, and cerebral edema [1]. While DKA is commonly seen in patients with type 1 diabetes mellitus (T1DM), it is rare in patients with type 2 diabetes mellitus (T2DM). Sodium glucose cotransporter-2 (SGLT2) inhibitors, including empagliflozin, canagliflozin, and dapagliflozin, are an increasingly administered class of medications for the treatment of T2DM [2]. These medications have gained popularity for their positive metabolic, renal, and cardiovascular effects in patients with T2DM and have proven mortality benefit [3]. Euglycemic DKA is a well-described side effect associated with SGLT2 inhibitors that presents as DKA with normal or near-normal blood glucose levels [4]. Given the increased prescription of SGLT2 inhibitors, perioperative providers must be familiar with the diagnosis and management of euglycemic DKA.
Patients on SGLT2 inhibitors are also at risk for Fournier’s gangrene, a life-threatening and rapidly progressive type of necrotizing soft tissue infection affecting the external genitalia or perineum [5]. Patients presenting with Fournier’s gangrene are often taken emergently to the operating room for surgical debridement. These patients may be hemodynamically unstable, often in septic shock. Because hyperglycemia interferes with immune responses, diabetic patients, including those on SGLT2 inhibitors, are at an elevated risk for developing DKA. Failure to recognize DKA in this setting places the patient at risk for further clinical deterioration.
In this case, we describe a patient with poorly controlled T2DM treated with empagliflozin who presented with Fournier’s gangrene and DKA. This case report is in line with the SCARE 2018 criteria [6].
2. Presentation of case
A 51-year-old morbidly obese man with hypertension and poorly controlled T2DM (HbA1c of 9.0) presented to the emergency department with an eight-day history of malaise and perianal pain. His home medications included empagliflozin 25 mg daily, metformin 1000 mg twice daily, lisinopril 10 mg daily, atorvastatin 20 mg daily and aspirin 81 mg daily. These were last taken the day prior to his presentation. All of his home mediations were taken orally, with no home insulin requirements. There was no relevant family, genetic or psychosocial history. He denied any alcohol or smoking history.
On initially presentation he was tachycardic with a heart rate of 120 beats/min, but other vital signs were within normal limits. His initial serum laboratory values were notable for an elevated white blood cell count (20,000/μL), low bicarbonate level (6 mmol/L), elevated creatinine (1.64 mg/dL), elevated glucose (315 mg/dL), and normal lactate. Of note, the patient’s HbA1c of 9.0 corresponded to an average serum glucose level of 212 mg/dL. The sepsis protocol was initiated, and he was treated promptly with fluid resuscitation and administration of broad-spectrum antimicrobials. When a computed tomography scan demonstrated inflammatory changes and subcutaneous gas in the perineum, he was taken emergently to the operating room for surgical debridement by the urology team. Shortly after the induction of general anesthesia with etomidate 10 mg and rocuronium 100 mg, the patient became hemodynamically unstable with escalating vasopressor requirements. An arterial blood gas revealed a profound high anion gap metabolic acidosis (HAGA) (pH 7.12, anion gap 31), which was initially presumed to result from a lactic acidosis in the setting of an aggressive necrotizing soft tissue infection. However, the patient’s lactate, renal function and osmolality were within normal limits. Therefore, other etiologies of HAGA were explored. The managing team noted that the patient was being treated with empagliflozin, which can cause DKA. A urine analysis was then obtained, which was positive for ketones. Given the patient’s normal urine output and serum potassium of 3.5 mmol/L, the patient was promptly treated with an insulin infusion, potassium replacement, and aggressive fluid resuscitation with crystalloid solution containing dextrose. The patient’s anion gap quickly closed, his serum potassium remained within normal limits, and his glucose levels fell. As his HAGA resolved, the patient’s vasopressor requirements decreased markedly for the remainder of the procedure.
He was taken to the ICU post-operatively where his vasopressor requirement rapidly resolved. He returned to the operating room on five more occasions for further debridement of the perineum and abdomen. After a thirty-day hospital admission, including a ten-day ICU admission, the patient was discharged to a rehabilitation facility where he is progressing well. He will follow up with his family doctor for ongoing management of his T2DM. His SGLT2 inhibitor was discontinued upon admission to hospital.
3. Discussion
We describe a case of an acute presentation of Fournier’s gangrene and DKA in a patient on SGLT2 inhibitors. The Food and Drug Administration has issued warnings regarding an increased risk of both Fournier’s gangrene and DKA with the use of SGLT2 inhibitors prescribed for T2DM [7]. Risk factors for Fourniers gangrene include diabetes mellitus, hypertension, obesity and immunosuppression [8]. Risk factors for the development of SGLT2 inhibitor-associated DKA include reduced carbohydrate intake, volume depletion, surgical stress, and infection [9].
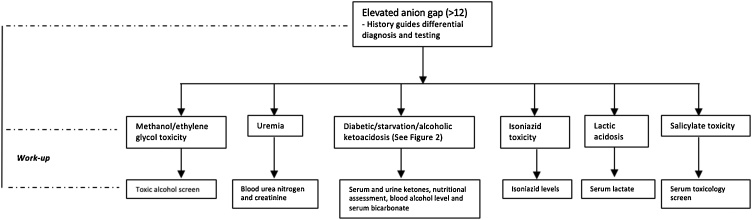
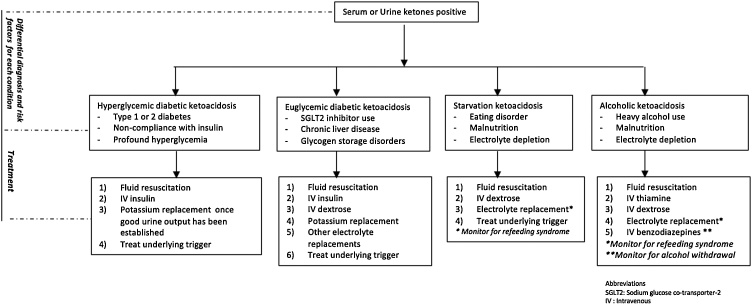
SGLT2 inhibitors maintain euglycemia by inhibiting glucose reabsorption at the proximal renal tubules and reduce the stimulation for pancreatic beta cell production of insulin [9]. In the setting of acute illness or infection, cortisol promotes further insulin resistance, and pancreatic alpha cells are stimulated to release glucagon. This initiates lipolysis and ketogenesis from fatty acids, as well as gluconeogenesis and glycogenolysis by the liver, ultimately increasing the risk for ketoacidosis. Our patient had an active infection and volume depletion secondary to decreased oral intake, placing him at risk for SGLT2 inhibitor associated DKA. Although this diagnosis was likely, there is no definitive diagnostic test for SGLT2 inhibitor associated DKA. As a result, the diagnosis should be suspected in diabetic patients on SGLT2 inhibitors who present with a HAGA and elevated plasma or urine ketones, with or without marked hyperglycemia. Common signs and symptoms of DKA include nausea, vomiting, tachycardia, polyuria, and altered mental status. However, in the perioperative setting, the diagnosis may be challenging with patients having a number of confounding factors. Prior to making a diagnosis of SGLT2 inhibitor associated DKA, other etiologies of ketoacidosis and HAGA must be excluded (Fig. 1). Common etiologies for HAGA encountered in surgical patients include lactic acidosis (secondary to sepsis), uremia and starvation ketosis. With all ketosis not being DKA, other etiologies of ketosis should be ruled out (Fig. 2).
Fig. 1.
Approach to diagnosis and management of elevated anion gap metabolic acidosis.
Fig. 2.
Approach to the management of ketoacidosis.
Identifying DKA in this case was challenging for a number of reasons. First, the patient presented with a rapidly progressing necrotizing soft tissue infection, and therefore, his HAGA may have been a result of lactic acidosis or uremia. Second, DKA is rare in patients with a diagnosis of T2DM or without a prior insulin requirement. His HbA1c of 9.0 corresponded to an average serum glucose level of 212 mg/dL. Although his diabetes was not well controlled, we assumed that he likely had reasonable insulin sensitivity prior to his acute illness. Finally, because of his SGLT2 inhibitor use, the patient’s blood glucose levels were less elevated than those typically associated with DKA. Fortunately, the perioperative team identified empagliflozin as a medication that can precipitate euglycemic DKA, with DKA treatment initiated immediately. Had DKA not been readily identified, the patient’s HAGA might have worsened despite supportive measures, potentially leading to irreversible hemodynamic collapse and severe multiorgan failure. Instead, the patient’s clinical condition improved rapidly with the administration of insulin, potassium, and dextrose infusions.
SGLT2 inhibitor-associated DKA should be managed similarly to classic DKA by the identification and correction of underlying triggers, aggressive fluid resuscitation, insulin to close the anion gap, and appropriate potassium repletion [10]. In euglycemic DKA, it is particularly important that blood glucose levels be monitored closely, and that dextrose be administered in conjunction with insulin to prevent hypoglycemia. Clinicians must monitor electrolytes closely when managing DKA, as in spite of elevated or normal potassium levels, there is typically a total body potassium deficit. This deficit results from a shift of potassium from intracellular to extracellular, resulting in loss of potassium via urine output. The initiation of insulin can lead to significant hypokalemia through intracellular shift of potassium, exacerbating the total body loss of potassium.
In patients presenting for elective surgery, recommendations offered by the American College of Endocrinology are to hold SGLT2 inhibitors for at least 24 h prior to surgery based on their elimination half-lives of approximately 13 h [11]. However, some expert groups recommend holding SGLT2 inhibitors up to 48 h prior to surgery [12]. For outpatient procedures, SGLT2 inhibitors can often be safely restarted 24 to 72 hours post-operatively once normal oral intake resumes [11]. Following these guidelines will help minimize the risk of SGLT2 inhibitor associated DKA.
4. Conclusion
The use of SGLT2 inhibitors among surgical populations is increasing, and despite cardiovascular benefits, there are serious side effects including DKA and Fournier’s gangrene. This case highlights these side effects occurring concurrently, in addition to the management of DKA intra-operatively. Given the potential catastrophic consequences of DKA, it is crucial that perioperative physicians maintain appropriate clinical suspicion for DKA in patients on SGLT2 inhibitors.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
No sources of funding to be declared.
Ethical approval
No ethical approval was required for this case report.
Consent
Patient provided written consent for this case report. All identifying details have been omitted.
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Name: Patrick Lindsay, MD.
Contribution: This author helped care for the patient and write the manuscript.
Name: Lauren Gibson, MD.
Contribution: This author helped write the manuscript.
Name: Edward A Bittner, MD, Ph.D.
Contribution: This author helped write the manuscript.
Name: Sheri Berg, MD.
Contribution: This author helped write the manuscript.
Name: Marvin G. Chang, MD, Ph.D.
Contribution: This author helped write the manuscript.
Registration of research studies
N/A.
Guarantor
Patrick Lindsay: Massachusetts General Hospital, Fruit Street, Boston, Massachusetts, USA,
Email: Patrick.lindsay@mail.utoronto.ca.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Wachtel T.J., Tetu-Mouradjian L.M., Goldman D.L., Ellis S.E., O’Sullivan P.S. Hyperosmolarity and acidosis in diabetes mellitus: a three-year experience in Rhode Island. J. Gen. Intern. Med. 1991;6(6):495–502. doi: 10.1007/BF02598216. [DOI] [PubMed] [Google Scholar]
- 2.Burke K.R., Schumacher C.A., Harpe S.E. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187–194. doi: 10.1002/phar.1881. [DOI] [PubMed] [Google Scholar]
- 3.Minze M.G., Will K.J., Terrell B.T., Black R.L., Irons B.K. Benefits of SGLT2 inhibitors beyond glycemic control - a focus on metabolic, cardiovascular and renal outcomes. Curr. Diabetes Rev. 2018;14(6):509–517. doi: 10.2174/1573399813666170816142351. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg R.M., Berard L.D., Cheng A.Y.Y., Gilbert J.D., Verma S., Woo V.C. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin. Ther. 2016;38(12) doi: 10.1016/j.clinthera.2016.11.002. 2654-64 e1. [DOI] [PubMed] [Google Scholar]
- 5.Bersoff-Matcha S.J., Chamberlain C., Cao C., Kortepeter C., Chong W.H. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann. Intern. Med. 2019;170(11):764–769. doi: 10.7326/M19-0085. [DOI] [PubMed] [Google Scholar]
- 6.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A.J., Orgill D.P. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 7.FDA . 2015. FDA Revises Labels of SGLT2 Inhibitors for Diabetes to Include Warnings About Too Much Acid in the Blood and Serious Urinary Tract Infections. [cited 2015 December 4]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about. [Google Scholar]
- 8.Voelzke B.B., Hagedorn J.C. Presentation and diagnosis of Fournier gangrene. Urology. 2018;114:8–13. doi: 10.1016/j.urology.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Meyer E.J., Gabb G., Jesudason D. SGLT2 inhibitor-associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care. 2018;41(4):e47–e49. doi: 10.2337/dc17-1721. [DOI] [PubMed] [Google Scholar]
- 10.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handelsman Y., Henry R.R., Bloomgarden Z.T., Dagogo-Jack S., DeFronzo R.A., Einhorn D. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of Sglt-2 inhibitors and diabetic ketoacidosis. Endocr. Pract. 2016;22(6):753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 12.Bardia A., Wai M., Fontes M.L. Sodium-glucose cotransporter-2 inhibitors: an overview and perioperative implications. Curr. Opin. Anaesthesiol. 2019;32(1):80–85. doi: 10.1097/ACO.0000000000000674. [DOI] [PubMed] [Google Scholar]